CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae
2022-10-25FabianovoraAnnecileMeunierAuroreVernetMuriellePortefaixJolleMilazzoHenriAdreitDidierTharreauOctvioFrancoAngelaMehta
Fabiano T. P. K. Távora, Anne Cécile Meunier, Aurore Vernet, Murielle Portefaix, Joëlle Milazzo, Henri Adreit, Didier Tharreau, Octávio L. Franco, Angela Mehta
Research Paper
CRISPR/Cas9-Targeted Knockout of Rice Susceptibility GenesandReveals Alternative Sources of Resistance to
Fabiano T. P. K. Távora1, 2, Anne Cécile Meunier3, 4, Aurore Vernet3, 4, Murielle Portefaix3, 4, Joëlle Milazzo5, 6, Henri Adreit5, 6, Didier Tharreau5, 6, Octávio L. Franco7, 8, Angela Mehta2
(lant Health Institute of Montpellier, CIRAD, Montpellier 34398, France; University of Montpellier, National Institute of Agronomic Research, Research Institute for Development, Montpellier SupAgro, Montpellier 34398, France)
Rice genesand, encoding a chaperone protein and an APETELA2/ ethylene-responsive factor, respectively, are strongly induced in a compatible interaction with blast fungus, and also have function in plant susceptibility validated through gene silencing. Here, we reported the CRISPR/Cas9 knockout ofandgenes resulting in considerable improvement of blast resistance. A total of 15(62.5%) and 17(70.8%) T0transformed lines were identified from 24 regenerated plants for each target and used in downstream experiments. Phenotyping of homozygous T1mutant lines revealed not only a significant decrease in the number of blast lesions but also a reduction in the percentage of diseased leaf area, compared with the infected control plants. Our results supported CRISPR/Cas9-mediated target mutation in rice susceptibility genes as a potential and alternative breeding strategy for building resistance to blast disease.
gene editing; plant-pathogen interaction;; plant immunity; blast resistance;-gene; rice
Rice (L.), the staple food for more than half of humankind, is a crucial crop for food security, feeding more people than any other cereal crop (Fukagawa and Ziska, 2019). However, rice plants have to deal with(synonym), a hemibiotrophic fungus responsible for rice blast, one of the most ubiquitous and destructive diseases affecting rice production globally (Jain et al, 2017). The cultivation of rice resistant varieties, harboring single or a couple of major resistance () genes, is the most used and environment-friendly approach to cope withinfection (Ahn and Seshu, 1991). Nonetheless, along with being a labor- intensive technique, conventional breeding aiming atgene-mediated resistance is race-specific and partially efficient. Moreover, resistance is often broken down within a few years after its commercial use (Bonman et al, 1992).
Alternatively to the resistance governed bygenes, the genetic manipulation of host susceptibility () genes represents a powerful source towards a more durable rice-blast resistance (Zaidi et al, 2018). Although the plant and pathogen arms race has forced pathogens to continuously evolve new strategies to evade or suppress plant immunity, most pathogens require host cooperation for the establishment of a compatible interaction, and typically exploit hosts’genes to facilitate their nutrition and proliferation (Win et al, 2012). Hence, all plant genes that somehow facilitate infection and/or support compatibility can be considered as angene (van Schie and Takken, 2014).
Further investigations of different pathosystems assisted by omics (e.g., proteomics and transcriptomics)together with gene silencing technologies (e.g., antisense oligonucleotide, host-induced gene silence and RNAi) have expanded our understanding of the molecular basis of pathogenicity, revealing crucial players (potential candidate-genes) engaged with the infection process, and notably contributed to the ever-expanding host-gene repertoire. More recently, CRISPR/Cas genome editing technology has offered new frontiers to overcome plant-pathogen compatibility by targeting-genes in a very precise manner(Jinek et al, 2012), enabling the development of transgene-free disease- resistant varieties, with several such cultivars already commercialized worldwide (Parisi et al, 2016).
In a previous shotgun proteomics study (Távora et al,2021), we identified() with a remarkably increased expression in a susceptible interaction at 12 h post-infection (hpi) with. Aiming to reinforce the set of candidate target genes, as well as to broaden the frame of prospection, a second potential candidate was picked from a transcriptomics study performed by Bevitori et al (2020).() is the most notable differentially expressed gene, identified at 24 hpi within the same susceptible interaction. Further, we successfully characterized their function in rice susceptibility through an antisense gene silencing assay, where treated plants show a notable decrease in foliar blast disease symptoms compared with control plants (Távora et al, 2021).
Here, the CRISPR/Cas9-target knockout (KO) ofandgenes in the modelrice variety Nipponbare has reported. Homozygous mutant lines of T1progeny carrying edited forms of each targeted gene displayed enhanced resistance to blast disease. Therefore, although the molecular mechanism of rice susceptibility tois far from being fully captured, the precise and rational manipulation of host susceptibility genes can contribute to the development of effective disease management strategies, making it an interesting alternative and/or complementary approach togenes in breeding programs.
RESULTS
ex-vivo assessment of sgRNA gene-editing activity
To evaluate the efficacy of our CRISPR vectors in generating double-stranded break at target sites, rice protoplasts were independently transformed with both constructs (and) (Fig. 1-A and -B), and the results showed that our expressing vectors exhibited suited gene-editing activity on the target sites of rice protoplast DNA (Fig. 1-C), hence, supporting their use for creating rice mutant plants.
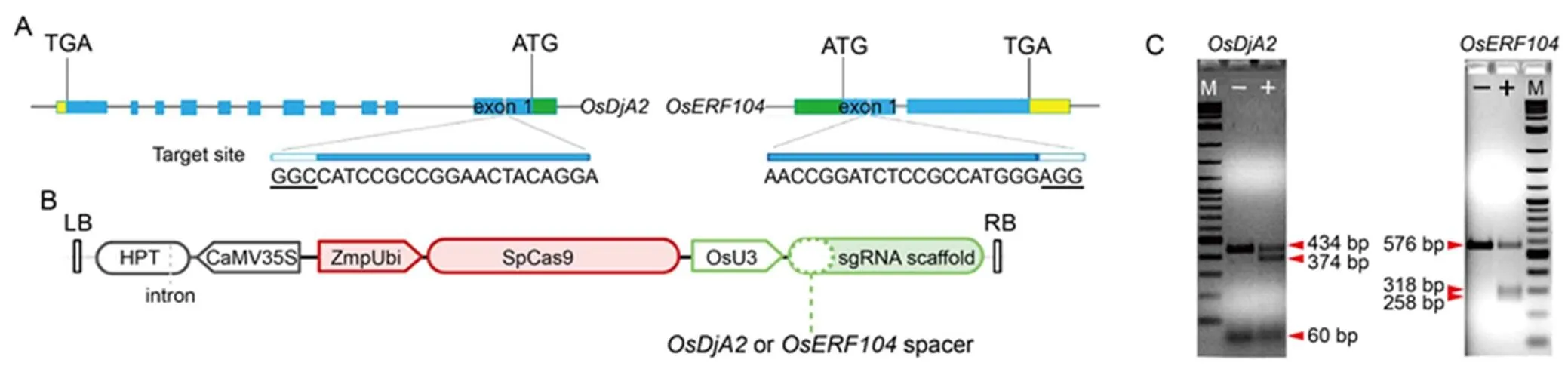
Fig. 1. CRISPR/Cas9 design and T7EI assay for sgRNA gene-editing activity.
A, Schematic map of gRNA target sites on genomic regions ofand. Exons are indicated as blue boxes, interspaced by introns shown as lines. Promoter and transcription termination sites are represented by green and yellow boxes, respectively. Protospacer adjacent motif is underlined and represented as white boxes. ATG and TGA represent start codon and stop codon, respectively.
B, Simplified schematic representation of CRISPR/Cas9 T-DNA structure. LB and RB, T-DNA left and right borders, respectively; HPT, Hygromycin resistance gene; CaMV35S,35S promoter; ZmpUbi, Maizepromoter; SpCas9,Cas9 gene; OsU3,PolII U3 promoter sequence.
C, Assessment of gRNA cleavage activity of rice protoplast genomic DNA via T7EI assay. ‘–’ means non-cleaved PCR product derived from wild type protoplast transformed with a control plasmid; ‘+’ means cleaved PCR product derived from protoplasts transformed with CRISPR/Cas9 final vector. M, Marker.
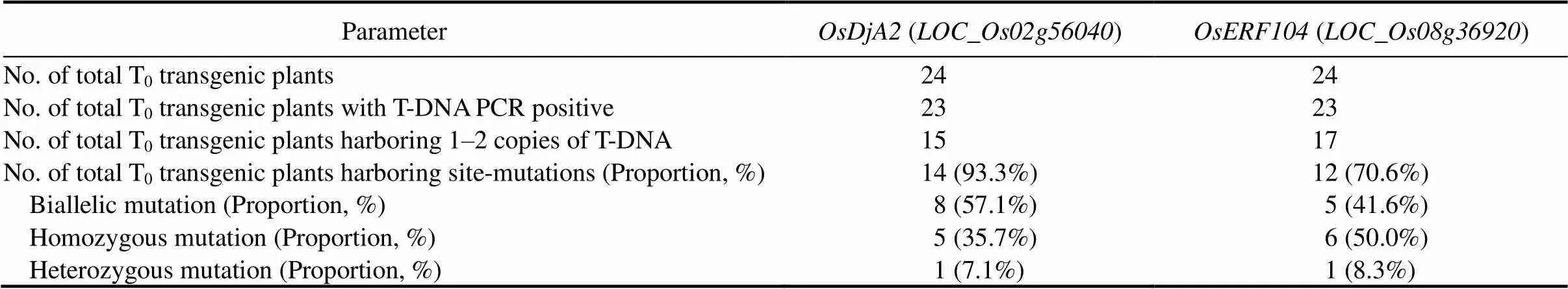
Table 1. Efficiency of CRISPR/Cas9-mediated genome editing of target genes and ratios of mutant genotypes in T0 plants.
Generation of OsDjA2 and OsERF104 rice mutant plants by CRISPR/Cas9 mutagenesis
We obtained 24 primary transformant (T0) plants for each targeted gene. A total of 23 (95.83%) T0recovered plants of bothandwere T-DNA PCR positive. The screening for T-DNA copy number integrated into their genomes by qPCR revealed 15 (62.5%)and 17 (70.8%)T0plants containing one or two transgene copies (Table S1), of which only the single copy lines were selected for further analysis. In those plants, CRISPR/ Cas9-target mutagenesis was remarkably efficient: 93.3% and 70.6% of(14) and(12) primary transformants, respectively, exhibited insertion/ deletion (InDel) mutations in the sgRNA target regions upon Sanger sequencing (Table 1). Regarding the nature of CRISPR/Cas9-induced mutations, further examination of sequence chromatograms revealed that amongtargeted alleles, there were 8 (57.1%) harboring biallelic mutations, 5 (35.7%) homozygous, and 1 (7.1%) heterozygous. Likewise, amongmutant lines, there were 5 (41.6%) harboring biallelic mutations, 6 (50.0%) homozygous, and 1 (8.3%) heterozygous (Table 1).
Assessment of InDel impacts on both open reading fragments (ORFs) and targeted gene products
outcomes of ExPasy Translate tool revealed that allandhomozygous T0mutant lines exhibited a premature stop-codon on their ORFs (Fig. S1). The InDel mutation inT0homozygous mutant lines (i.e.: -1[G]bp), despite generating great predicted amino acid deletion (proportion up to 77%), has conserved 90 amino acids of the native protein, comprising a great portion of the N-terminal conserved domain (known as ‘J’ domain), and the nascent part of the glycine-rich region (‘G’ domain) (Fig. S2-A). On the other hand, the frameshift mutations observed onT0homozygous mutant lines (i.e.: +1[A]bp and +1[T]bp) resulted in an extensive deletion of 185 amino acids (84% of the total protein content), vanishing with the whole transcriptional factor AP2/ERF domain, laying from 75 to 132 amino acid (Fig. S2-B). For this reason, we performed a complementary analysis to check the InDel impacts on the targeted-gene cognate residual OsDjA2 proteinat the biological activity level. According to PROVEAN (protein variation effect analyzer) scores, generated based on query sequences of the twohomozygous T0mutant lines, the large majority of observed amino acid deletions/ substitutions were predicted as being deleterious (Fig. S3), which means that although OsDjA2remained a truncated-protein, CRISPR/Cas9 mutagenesis probably led to the full knockout of the targeted-genes through loss-of-function (null) mutations.
Recovery of T1 progeny homozygous mutant lines
A total of five independent T0mutant lines,L1 (_20.1), L2 (_24.1), L3 (_1.1), L4 (_5.1) and L5 (_6.1) harboring homozygous and predicted loss-of-function mutations, were selected and self-pollinated(Fig. 2-A). T1progeny plants (= 6 of each mutant line as #1, #2, #3, #4, #5 and #6) were firstly screened for the presence of T-DNA (Fig. 2-B). All T1mutant lines were homozygous for the same mutations observed in bothandT0lines (Table S2). It is noteworthy that no unintended mutation was identified in the potential predicted off-target loci of our homozygous T1mutant lines (Table S3). Therefore, we were able to recover a sufficient number of suitable homozygous mutant rice plants to subject to the blast resistance assay.
Improved resistance to blast disease in CRISPR/Cas9-edited rice mutants
Wild type Nipponbare and T1homozygous mutant plants of each target gene, with no detectable vegetative development defects under normal growth conditions, were tested for blast disease resistance(Fig. S4). All plants at the fourth-leaf stage were inoculated with the fungal pathogencompatible isolate GY0011. At 7 d post-inoculation (dpi), the number of blast lesions and the percentage of the diseased area on the fourth leaf of each mutant line were notably decreased in comparison with the control plants (Fig. 3-A). All tested replicates per mutant line of both targetgenes were used for blast symptoms’ quantification (Fig. S5). The quantification of both disease severity parameters was further evaluated using a post-hoc Student’s-test, which pointed to a statistical significance (< 0.05) for the number of blast lesions on the leaves of_24.1 and_5.1 mutant lines (Fig. 3-B), and likewise for the percentage of the foliar lesioned area on_24.1,_1.1 and_6.1 mutant lines (Fig. 3-C), in comparison with the control plants. Although some events, from the same mutant line (_20.1 and_24.1;_1.1 and_5.1), harboring the same type of frameshift InDel mutations (-1[G]bp and +1[A]bp, respectively), showed a subtle deviation in disease severity phenotypes, such differences were not statistically significant (< 0.05) by ANOVA test.
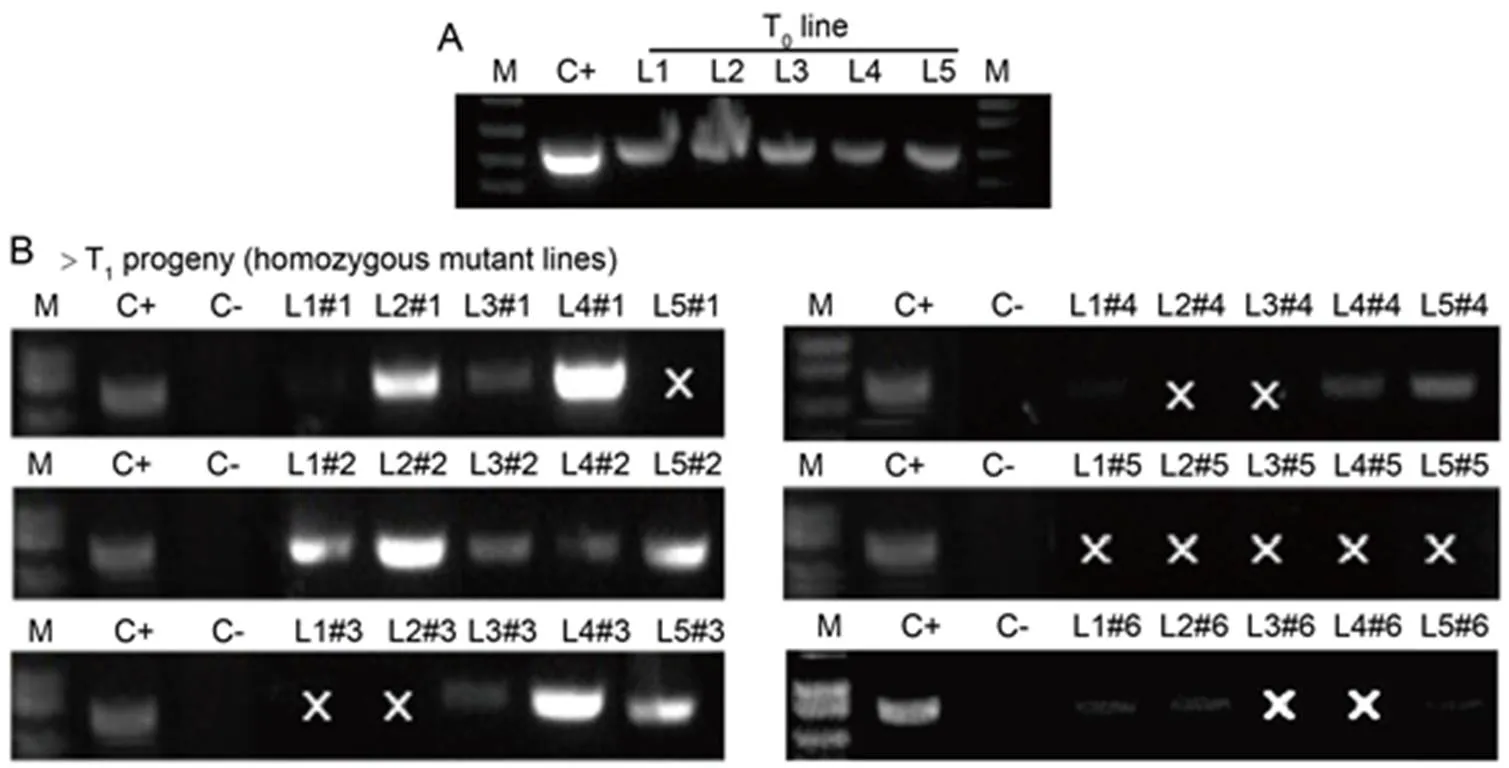
Fig. 2. PCR-based screening for presence of T-DNA in rice mutant plants.
A, T0homozygous primary transformants L1 (_20.1), L2 (_24.1), L3 (_1.1), L4 (_5.1) and L5 (_6.1).
B, T1progeny plants (= 6 of each independent mutant line as #1, #2, #3, #4, #5 and #6), using specific Cas9 primer pair.
M, DNA molecular ladder; C+, CRISPR plasmid; C-, Genomic DNA of wild type Nipponbare; ‘×’ indicates PCR negative for T-DNA.
DISCUSSION
Targeting S-genes as an alternative approach to R-gene building plant resistance
The plant pathogenposes a major threat to rice productivity worldwide. The fitness of susceptible rice cultivars is seriously impaired under disease pressure, leading to yield reduction or complete crop losses (Jain et al, 2017). To mitigate these negative impacts in agriculture, the usage of-gene-containing cultivars has been for a long time the most effective measure for rice crop protection against blast disease (Li et al, 2019). Nevertheless, dominant resistance governed by singlegenes entangles several limitations (Stam and McDonald, 2018). In this way, targeting host-gene alleles re-flourished along with the recent advancements in new breeding techniques, as an effective strategy to build a more durable and broad-spectrum disease resistance. Indeed, several case studies have been reported in this direction by mutagenesis of-genes (Streubel et al, 2013; Hong et al, 2019; Oliva et al, 2019). For instance, a well-known and long-stablished host-genes,, encodes a membrane-anchored protein that acts by supporting the establishment of fungus haustoria penetration structure facilitating the invasion of plant epidermal cells (Büschges et al, 1997).mutants represent the potential robustness of-gene strategy, of which a recessive mutation was shown to confer powdery mildew (PM) resistance in barley seven decades ago and it continues to be employed and still confers durable resistance to all PM races in the field (Kusch and Panstruga, 2017).
From a total of 24 primary transformant recovered plants for each target gene, we achieved 15 and 17 one or two transgene copies T-DNAandevents, respectively, of which 14/15 and 12/17 exhibited InDel mutations at their respective sgRNA target-sites, implying a prominent efficiency of CRISPR/Cas9-target mutagenesis. We obtained 5and 6homozygous T0mutant lines, harboring desirable frameshift InDel mutations (e.g., -1[G]bp, +1[A]bp, and +1[T]bp), which were self-pollinated and generated Cas9-positive plants as well as transgene-free T1progeny. The presence of the same InDel mutations at the target sites of T0-derived plants and also the expected Mendelian segregation ratios of the transgene in the single copy mutant lines ultimately suggested stability of the inherited zygosity on the following generation. Therefore, we were able to recover a sufficient number ofandhomozygous T1mutant lines showing on-target frameshift mutations on both alleles, the most suitable mutant rice plants for the phenotyping.
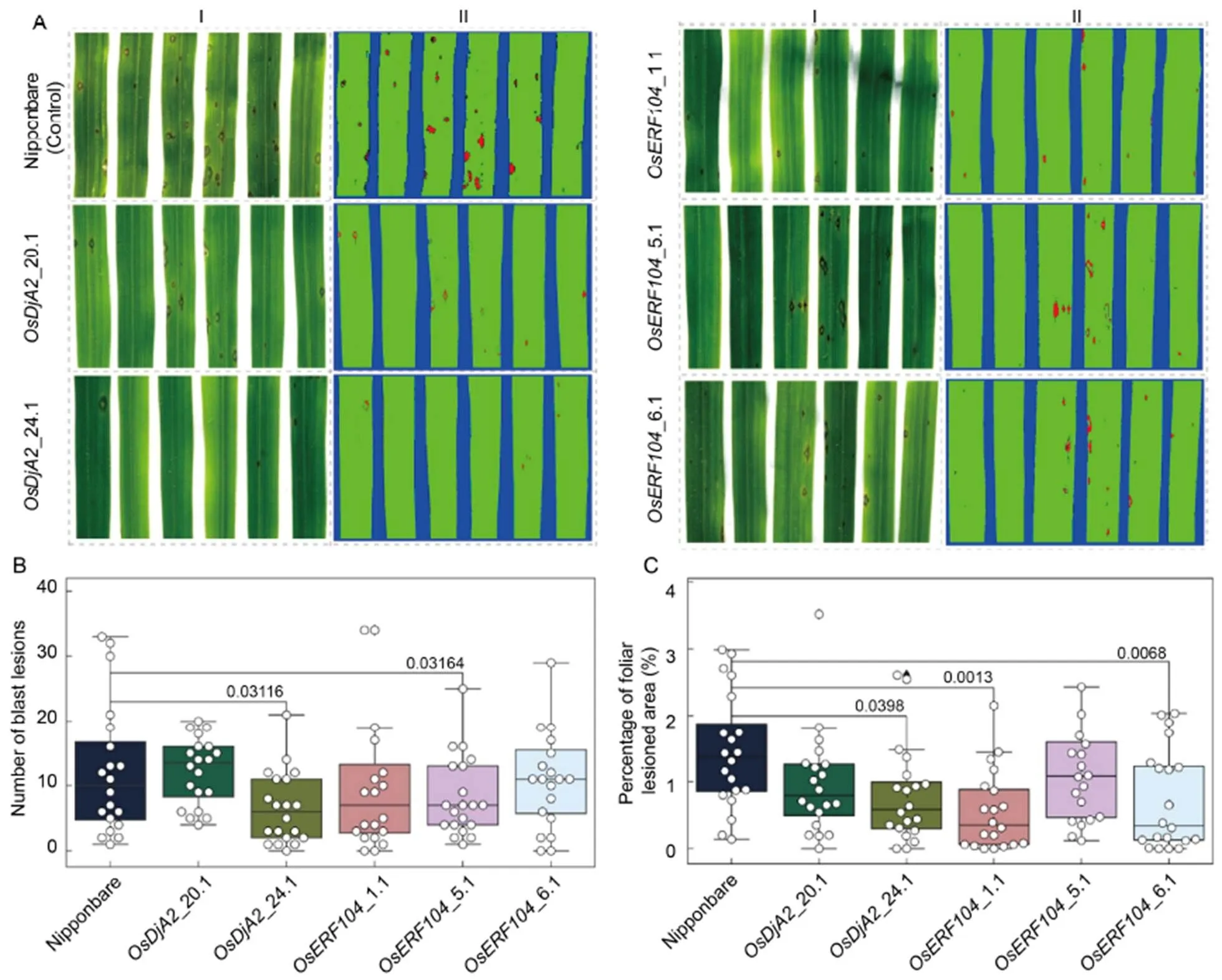
Fig. 3. Identification of blast resistance in CRISPR/Cas9-edited rice mutant plants.
A, Phenotypes upon blast infection of wild type Nipponbare, and T0homozygous mutant plants (_20.1 and_24.1;_1.1,_5.1 and_6.1) of each target gene. The fourth leaves of each line were detached at 7 d post-infection, scanned and analyzed for the number of blast lesions (A-I) and the percentage of the lesioned foliar area (A-II) using the software Quant®.
B and C, Boxplot merged with swarmplot data representation for the number of blast lesions (B) and the percentage of foliar lesioned area (C), respectively, observed on each of the 20 leaves. The numbers above the boxplot indicate statistical significance (< 0.05, two-sample-test).
For, 1/2 of the mutant lines showed significantly (< 0.05) improved disease-resistance [in terms of both analyzed parameters for disease symptoms (i.e. number of blast lesions and percentage of foliar lesioned area)]. For, 1/3 of the mutant lines displayed a significant improvement in disease resistance in terms of the number of blast lesions, and 2/3 of mutants showed a significant decrease in the percentage of foliar lesioned area. Ultimately, inoculated mutant plants (= 20 independent replicates per event) for both targeted-gene displayed a general trend of blast resistance in comparison with the wild type plants. Regarding the analyzed disease parameters in terms ofpattern of infection, the observed results indicate an acquired blast resistance (partially, at least) probably due to an impairment of both fungus penetration and growth phases (inferred from the number of blast lesions and the percentage of foliar lesioned area parameters, respectively). Furthermore, especially in terms of the percentage of foliar lesioned area, the reduction of blast disease symptoms was significantly more pronounced (< 0.05) on the-KO plants, suggesting a more critical role of this-gene in host susceptibility, and probably reflecting the ubiquity of AP2/ERF transcription factor in plant stress responses and its broader engagement with rice-triggered susceptibility. Lastly, our results indicate that bothandrice-genes seem to negatively regulate rice resistance to.
Molecular chaperones as key players in rice-blast susceptibility
Exposed to an ever-changing environment, deluged by biotic and abiotic stressors, plants must be able to maintain cellular proteostasis for its proper growth, development, and survival (Park and Seo, 2015). This requires a fine-tune orchestration of a squad of molecular chaperones. Originally referred to as ‘heat shock proteins’ (Hsps) (Boston et al, 1996), these Hsps are indeed implicated in a myriad of functions in diverse plant species, playing also an essential and regulatory role in plant innate immune response. Hsp70s and their obligate co-chaperones, known as J-domain proteins (JDPs), are arguably the most ubiquitous components of the cellular chaperone network (Verma et al, 2019). In addition, JDPs represent the largest family of Hsp70 co-chaperones and are decisive for functionally specifying and directing Hsp70 functions. Rice genome counts for 115 J-protein family genes, randomly distributed on all twelve chromosomes, and classified into three classes (corresponding to types A, B and C) according to both domain organization and conserved signature sequences (Sarkar et al, 2013). Type A J-proteins, such as our-gene target, are characterized by a 70 amino acid long J-domain, which is mostly present near the N-terminus, followed by a stretch of glycine/phenylalanine (G/F)-rich region, four repeats of a cysteine-rich CxxCxGxG-type zinc-finger motif, and a C-terminal domain involved in dimerization and substrate binding. In addition, the presence of a tripeptide motif His-Pro-Asp (HPD) is a highly conserved feature of the J-domain, argued to be essential for the stimulation of the ATPase activity of Hsp70s (Kampinga et al, 2019). Interestingly, ourprediction results regarding the impacts of InDel-induced frameshift mutations on protein domains oftargeted gene showed that the remained protein residues lost their conserved HPD motif, as well as a great portion of the J-domain (Fig. S2-C). The roles of HSP40/DnaJ proteins have been well studied in plant growth, development, and abiotic stress tolerance in plants. Regarding its function during biotic stress factors, we have pieces of evidence that in viral pathogenesis, for example, the silencing of diverse J-domain-containing protein can lead to resistance or susceptible outcomes (Ko et al, 2019; Luo et al, 2019). However, there are still large gaps in the understanding of how these DnaJ proteins negatively modulate plant immune response mechanisms during pathogen infection, in terms of PAMP sensing, signal transduction, and transcriptional activation/ repression of stress-related genes, to trigger disease susceptibility, especially in crop plants.
Modulation of AP2/ERF plant-specific TF and the triggering of plant-immunity suppression
Another great player in the tangled modulation of plant immunity is plant hormones. Upon pathogen attack, ethylene phytohormone production typically raises and its complex signaling network can contribute positively or negatively to resistance depending on the enemy’s lifestyle and tactics of infection (Wen, 2015). Phytohormone responses are often regulated by a large number of transcription factors (TF), with APETALA2/ethylene responsive factor (AP2/ERF) family being the most conservatively widespread in the plant kingdom (Feng et al, 2020). According to Rashid (2012), there are 170 AP2/ERF plant-specific TF family genes in the rice (L. spp.) genome and they are divided into a total of 11 groups, including the three most studied groups AP2, ERF, and DREB. The members of AP2/ERF gene family participate in different pathways in response to hormones and biotic/abiotic stresses, such as salicylic/jasmonic acid, abscisic acid, drought, salinity, cold, disease, and flooding stress (Phukan et al, 2017). Our CRISPR-edited rice geneis classified into the phylogenetic group IIIc of the rice ERF family (Nakano et al, 2006; Rashid et al, 2012), which is composed of 16 genes. The majority of its members have been found to integrate metabolic, hormonal and environmental signals in the biotic stress responses.encodes a plant-specific TF, containing only one APETALA2 (AP2) domain (of about 60 amino acids) that plays decisive regulatory functions in controlling the transcription of downstream target genes by directly binding with-acting regulatory elements (called a GCC-box containing the core 5′-GCCGCC-3′ sequence) in their promoters. Interestingly, thecomputational prediction of the induced-mutation impacts ongene product showed that the KO vanished with the whole transcriptional factor AP2/ERF domain (Fig. S2-A), argued to be vital for protein function in biotic stress regulatory networks (Abiri et al, 2017). It is important to emphasize that the present targeted genewas selected as a potential-gene candidate from previous transcriptomics results,where it showed to be the most differentially-expressedgene at 4 hpi in the susceptible interaction. In addition, it exhibited a notable differential increase at 12 hpi, and scored the highest fold-change 24 hpi in the susceptible interaction, compared with control plants (Bevitori et al, 2020). Althoughalso showeda differential increase in the resistant interaction, it is well known that pathogen-responsive genes are commonly expressed in compatible and incompatible interactions and are related to common defense pathways triggered by the pathogen (Ribot et al, 2008). The ERF genes are ubiquitous transcriptional factors, well-known for their plasticity and association with complex signaling networks, and roughly classified as activators or as repressors depending on whether they activate or suppress transcription of specific target genes (Srivastava and Kumar, 2018).
Taken together, the appropriate manipulation of Type A J-domain and AP2/ERF TFs, suggested to be intricate with negative regulation of plant immune responses, has the potential to improve rice disease resistance. Our results revealed that CRISPR/Cas9- targeted KO ofandpointed to an enhanced resistance to, and also corroborated the findings of our previous work that suggested the ability of the blast fungus to modulate the expression of a subset of rice-genes, key players in the negative regulation of basal and innate plant-immune responses, favoring infection and host colonization.Lastly, the results of this study not only provide potential and alternative targets for fighting rice-blast disease but also strengthens CRISPR/Cas9-mediated knockout of rice susceptibility genes as a useful strategy for improving blast resistance.
METHODS
Rice materials and growth conditions
Rice cultivar Nipponbare (L. spp.) plants were grown in a greenhouse facility at Cirad, France, under the following conditions: temperature of 28 ºC during the day and 24 ºC at night with 60% humidity. The natural light was complemented by artificial sodium light [700 μmol/(m2∙s)]. For blast inoculation, rice seeds from wild type Nipponbareand T0progeny homozygous mutant lines were sown in rows (20 seeds per row) in 60 cm × 30 cm × 5 cm plastic seedling-nursing trays and maintained in greenhouse optimal conditions at the Joint Research Unit, Genetic Improvement and Adaptation of Plants, French Agricultural Research Centre for International Development, France.
Design of CRISPR/Cas9 sgRNAs and construction of T-DNA vectors
Gene-specific spacers (20 nt sgRNA templates) for each targetand(Fig. 1-A) were designed using CRISPR-assisted website (http://crispor.tefor.net/) (Concordet and Haeussler, 2018). We then inserted the sgRNAs into an entry vector derived from Miao et al (2013) by minor modifications/improvements. Briefly, single-stranded gRNAs (20 nt oligos) were synthesized as spacer-containing primers (Table S4) and cloned intoI-digested pENTRY vectors. Subsequently, the resulting sgRNAs were cloned into the T-DNA region of a destination binary vector (Fig. 1-B). The final CRISPR constructs for each target gene (and) were confirmed by Sanger sequencing using specific primers (Table S4). Potential off-target mutations in CRISPR/Cas9-induced mutant plants were predicted by the CRISPOR tool (http://crispor.tefor.net/). For each of our target genes, we designed specific primers (Table S4) to amplify a genomic region (about 600 bp) flanking one top-ranking off-target site showing a higher likelihood to cause unintended mutations, and the resulting PCR products were analyzed by sequencing.
Rice protoplast for ex-vivo editing assay
Rice protoplast isolation and transformation were performed as described by Bes et al (2021). Briefly, Nipponbare seeds were sterilized in a 70% ethanol, 2.5% hypochlorite solution for 15 min under agitation, then washed five times in distilled water, and sown on 0.5× Murashige and Skoog solid medium (4.5 g/L phytagel) in rectangular (40 cm × 30 cm) Petri dishes. The seedlings were grown in the dark for 7 to 11 d at 26 ºC in a growth chamber. Further protoplast isolation, purification, and transformation steps are detailed in File S1.
Rice stable transformation
Rice transformations were carried out as described by Hiei et al (1994), with modifications. Briefly,strain EHA105 was transformed, independently, with one of our previously described binary vectors (and)by electroporation and then used for coculture with embryo-derived secondary calli tissue induced from mature seeds of wild type Nipponbare. We transformed a total of 30 calli per construction. Hygromycin-containing medium was used to select hygromycin-resistant calli that were then transferred onto regeneration medium for the regeneration of potentially transformed (edited) plants. After rooting and acclimationperiods (approximately 3 months) into glass tubes, rice seedlings were transferred to soil in greenhouse optimal conditions.
Molecular characterization of CRISPR mutant events
We generated 24 hygromycin-resistant calli-derived regenerated plants for each CRISPR construction (and). Firstly, the genomic DNA of all primary transformants (T0) was extracted by MATAB (Mixed Alkyl Trimethyl Ammonium Bromide) method and the presence of Cas9 in primary transformants and its segregation to the progeny was ascertained using the primers listed in Table S4. Transfer DNA (T-DNA) copy number was estimated by a DNA-based quantitative PCR (qPCR) optimized method (Yang et al, 2005) usingII-specific primers. The reaction and real-time fluorescence readings were carried out using a Light Cycler 480™ (Roche®, Shanghai, China). The copy number of the transgene was estimated after normalizing the amount of DNA using the reference gene and the DNA from a T0plant containing only one copy (verified by Southern blot) of thegene as a comparison. The single-copy T-DNA sample served as a reference (for which it was assigned the value 1) and the transgene number of copies was estimated in relation to this reference value. T0plants harboring only one T-DNA copy were subjected to PCR using on-target specific primer pairs (Table S4) to amplify DNA fragments across both gene-target sites and amplicons subjected directly to Sanger sequencing. The generated chromatograms were explored and deconvoluted using CRISP-ID web-based tool (http://crispid. gbiomed.kuleuven.be/) (Dehairs et al, 2016) and CRISPR- mediated InDels on alleles of each mutant event were decoded. Lastly, we employed the ExPasy Translate tool (https://web. expasy.org/translate/) (Gasteiger et al, 2003) to provide a computational prediction of the impacts of CRISPR/Cas9- induced InDels on both ORFs of targeted-genes, and PROVEAN (Choi et al, 2012) algorithm, developed by Institute Craig Venter (http://provean.jcvi.org/index.php), to assess the variation effects caused by altered amino acid composition/ chain structure on the biological function of its cognate- expressed proteins.
Pathogenicity assay
To evaluate the CRISPR-target KO mediated resistance to, the inoculation of rice blast funguswas performed as described by Sallaud et al (2003). Briefly,isolate GY0011, virulent (compatible) to Nipponbare, was cultured on oatmeal medium (20 g of oatmeal, 15 g of agar, 10 g of sucrose, and 1 L of distilled water) for 7 d in a dark incubator at 25 ºC. Conidia were harvested by flooding the plate with distilled water and softly scraping the medium surface. The concentration of conidial suspension inoculum was adjusted to 5 × 104conidia/mL. Rice seeds of the control line and three independent homozygous T1mutant lines of each target gene were sown in trays of 20 cm × 12 cm × 5 cm filled with compost. Except for one independent homozygous mutant line (from thetarget gene) that did not germinate, all plants at the fourth-leaf stage were inoculated withby spraying with 20 mL conidial suspension per tray. The inoculated rice plants were stored for one night in a controlled dark chamber at 25 ºC with 95% relative humidity and then transferred back to the greenhouse. Disease severity was evaluated considering both blast lesion number per leaf and the percentage of lesioned foliar area, observed on the fourth leaves of 20 plants (i.e., independent biological replicates) of each line at 7 dpi using the software QUANT®, according to do Vale et al (2003). Statistical analysis was performed using one-way ANOVA, followed by a post hoc two-sample-test for average comparison between mutants and control line.
ACKNOWLEDGEMENTS
This study was financially supported by Brazilian Agricultural Research Corporation (Embrapa)-Coordination for the Improvement of Higher Education Personnel, National Council for Scientific and Technological Development, Federal District Research Support Foundation, and Foundation for Scientific and Technological Development of Mato Grosso do Sul State.We thank the Joint Research Unit, Genetic Improvement and Adaptation of Plants, French Agricultural Research Centre for International Development, and Dr. Léo Herbert from Genetics and Variety innovation team for sharing his great expertise on CRISPR experiments involving rice protoplasts.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1.outcomes of ExPasy Translate tool.
Fig. S2. InDel-induced frameshift mutations on protein domains ofandT0homozygous mutant lines.
Fig. S3. PROVEAN scores for query sequences ofandhomozygous T0mutant linesgenerated based on CRISPR-mediated InDel mutations.
Fig. S4. Overview pictures ofandmutant lines growing in standard greenhouse conditions.
Fig. S5. Raw images capture by scanning of all 20 foliar replicates of wild type and all T1homozygous mutant rice lines of both targeted genes tested for blast resistance.
Table S1. CRISPR/Cas9-induced InDel mutations at bothandsgRNA cleavage sites on primary T0transformants.
Table S2. Segregation of CRISPR/Cas9-induced InDel mutations in sgRNA target regions ofandT1progeny that were submitted to phenotyping.
Table S3. Analysis of off-target sites of homozygous T1mutant lines.
Table S4. Primers used in this study.
File S1. Protocol ofrice protoplast forediting assay.
Abiri R, Shaharuddin N A, Maziah M, Yusof Z N B, Atabaki N, Sahebi M, Valdiani A, Kalhori N, Azizi P, Hanafi M M. 2017. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions., 134: 33–44.
Ahn S W, Seshu D V. 1991. Blast reaction of durably resistance rice cultivar in multiplication trials., 81(10): 1150.
Bes M, Herbert L, Mounier T, Meunier A C, Durandet F, Guiderdoni E, Périn C. 2021. Efficient genome editing in rice protoplasts using CRISPR/CAS9 construct., 2238: 173–191.
Bevitori R, Sircar S, de Mello R N, Togawa R C, Côrtes M V C B, Oliveira T S, Grossi-de-Sá M F, Parekh N. 2020. Identification of co-expression gene networks controlling rice blast disease during an incompatible reaction., 19(3): gmr18579.
Bonman J M, Khush G S, Nelson R J. 1992. Breeding rice for resistance to pests., 30: 507–528.
Boston R S, Viitanen P V, Vierling E. 1996. Molecular chaperones and protein folding in plants., 32(1/2): 191–222.
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Töpsch S, Vos P, Salamini F, Schulze-Lefert P. 1997. The barleygene: A novel control element of plant pathogen resistance., 88(5): 695–705.
Choi Y, Sims G E, Murphy S, Miller J R, Chan A P. 2012. Predicting the functional effect of amino acid substitutions and indels., 7(10): e46688.
Concordet J P, Haeussler M. 2018. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens., 46(W1): W242–W245.
Dehairs J, Talebi A, Cherifi Y, Swinnen J V. 2016. CRISP-ID: Decoding CRISPR mediated indels by Sanger sequencing., 6: 28973.
do Vale F X R, Fernandes Filho E I, Liberato J R. 2003. A Software for Plant Disease Severity Assessment. Christchurch, New Zealand: 8th International Congress of Plant Pathology: 105.
Feng K, Hou X L, Xing G M, Liu J X, Duan A Q, Xu Z S, Li M Y, Zhuang J, Xiong A S. 2020. Advances in AP2/ERF super-family transcription factors in plant., 40(6): 750–776.
Fukagawa N K, Ziska L H. 2019. Rice: Importance for global nutrition., 65: S2–S3.
Gasteiger E, Gattiker A, Hooland C, Ivanyi I, Appel R D, Bairoch A. 2003. ExPASy: The proteomics server for in-death protein knowledge and analysis., 31(13): 3784–3788.
Hiei Y, Ohta S, Komari T, Kumashiro T. 1994. Efficient transformationof rice (L.) mediated byand sequence analysis of the boundaries of the T-DNA., 6(2): 271–282.
Hong Y B, Liu Q N, Cao Y R, Zhang Y, Chen D B, Lou X Y, Cheng S H, Cao L Y. 2019. Thenegatively regulatesanddisease resistance via SA and JA signaling pathway in rice., 10: 752.
Jain P, Singh P K, Kapoor R, Khanna A, Solanke A U, Krishnan S G, Singh A K, Sharma V, Sharma T R. 2017. Understanding host-pathogen interactions with expression profiling of NILs carrying rice-blast resistancegene., 8: 93.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna J A, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity., 337: 816–821.
Kampinga H H, Andreasson C, Barducci A, Cheetham M E, Cyr D, Emanuelsson C, Genevaux P, Gestwicki J E, Goloubinoff P, Huerta-Cepas J, Kirstein J, Liberek K, Mayer M P, Nagata K, Nillegoda N B, Pulido P, Ramos C, de Los Rios P, Rospert S, Rosenzweig R, Sahi C, Taipale M, Tomiczek B, Ushioda R, Young J C, Zimmermann R, Zylicz A, Zylicz M, Craig E A, Marszalek J. 2019. Function, evolution, and structure of J-domain proteins., 24(1): 7–15.
Ko S H, Huang L M, Tarn W Y. 2019. The host heat shock protein MRJ/DNAJB6 modulates virus infection., 10: 2885.
Kusch S, Panstruga R. 2017.-based resistance: An apparently universal “weapon” to defeat powdery mildew disease., 30(3): 179–189.
Li W T, Chern M, Yin J J, Wang J, Chen X W. 2019. Recent advances in broad-spectrum resistance to the rice blast disease., 50: 114–120.
Luo Y, Fang B H, Wang W P, Yang Y, Rao L Q, Zhang C. 2019. Genome-wide analysis of the rice J-protein family: Identification, genomic organization, and expression profiles under multiple stresses., 9(10): 358.
Miao J, Guo D S, Zhang J Z, Huang Q P, Qin G J, Zhang X, Wan J M, Gu H Y, Qu L J. 2013. Targeted mutagenesis in rice using CRISPR-Cas system., 23(10): 1233–1236.
Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice., 140(2): 411–432.
Oliva R, Ji C H, Atienza-Grande G, Huguet-Tapia J C, Perez-Quintero A, Li T, Eom J S, Li C H, Nguyen H, Liu B, Auguy F, Sciallano C, Luu V T, Dossa G S, Cunnac S, Schmidt S M, Slamet-Loedin I H, Vera Cruz C, Szurek B, Frommer W B, White F F, Yang B. 2019. Broad-spectrum resistance to bacterial blight in rice using genome editing., 37(11): 1344–1350.
Parisi C, Tillie P, Rodríguez-Cerezo E. 2016. The global pipeline of GM crops out to 2020., 34(1): 31–36.
Park C J, Seo Y S. 2015. Heat shock proteins: A review of the molecular chaperones for plant immunity., 31(4): 323–333.
Phukan U J, Jeena G S, Tripathi V, Shukla R K. 2017. Regulation of Apetala2/ethylene response factors in plants., 8: 150.
Rashid M, He G Y, Yang G X, Hussain J, Yan X. 2012. AP2/ERF transcription factor in rice: Genome-wide canvas and syntenic relationships between monocots and eudicots., 8: 321–355.
Ribot C, Hirsch J, Balzergue S, Tharreau D, Nottéghem J L, Lebrun M H, Morel J B. 2008. Susceptibility of rice to the blast fungus,., 165(1): 114–124.
Sallaud C, Lorieux M, Roumen E, Tharreau D, Berruyer R, Svestasrani P, Garsmeur O, Ghesquiere A, Notteghem J L. 2003. Identification of five new blast resistance genes in the highly blast-resistant rice variety IR64 using a QTL mapping strategy., 106(5): 794–803.
Sarkar N K, Thapar U, Kundnani P, Panwar P, Grover A. 2013. Functional relevance of J-protein family of rice ()., 18(3): 321–331.
Srivastava R, Kumar R. 2018. The expanding roles of APETALA2/Ethylene Responsive Factors and their potential applications in crop improvement., 18(4): 240–254.
Stam R, McDonald B A. 2018. When resistance gene pyramids are not durable: The role of pathogen diversity., 19(3): 521–524.
Streubel J, Pesce C, Hutin M, Koebnik R, Boch J, Szurek B. 2013. Five phylogenetically close ricegenes confer TAL effector-mediated susceptibility topv.., 200(3): 808–819.
Távora F T P K, Bevitori R, Mello R N, Cintra M M D F, Oliveira-Neto O B, Fontes W, Castro M S, Sousa M V, Franco O L, Mehta A. 2021. Shotgun proteomics coupled to transient- inducible gene silencing reveal rice susceptibility genes as new sources for blast disease resistance., 241: 104223.
van Schie C C N, Takken F L W. 2014. Susceptibility genes 101: How to be a good host., 52: 551–581.
Verma A K, Tamadaddi C, Tak Y, Lal S S, Cole S J, Hines J K, Sahi C. 2019. The expanding world of plant J-domain proteins., 38: 382–400.
Win J, Chaparro-Garcia A, Belhaj K, Saunders D G O, Yoshida K, Dong S, Schornack S, Zipfel C, Robatzek S, Hogenhout S A, Kamoun S. 2012. Effector biology of plant-associated organisms: Concepts and perspectives., 77: 235–247.
Wen C K. 2015. Ethylene in Plants. Berlin: Springer: 286.
Yang L T, Ding J Y, Zhang C M, Jia J W, Weng H B, Liu W X, Zhang D B. 2005. Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR., 23(10/11): 759–763.
Zaidi S S E A, Mukhtar M S, Mansoor S. 2018. Genome editing: Targeting susceptibility genes for plant disease resistance., 36(9): 898–906.
7 January 2022;
24 April 2022
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.04.001
Angela Mehta (angela.mehta@embrapa.br)
(Managing Editor: Wang Caihong)
杂志排行
Rice Science的其它文章
- Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels
- QTL Mapping for Plant Height Using Introgression Lines Derived from Zhonghui 8015 and Wild Rice (Oryza rufipogon)
- Genetic Dissection of Quantitative Trait Loci for Panicle Traits and Heat Tolerance by High-Density Bin Map in Rice
- Improvement of Rice Production under Drought Conditions in West Africa:Application of QTLs in Breeding for Drought Resistance
- Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding
- Identification of Potential Zinc Deficiency Responsive Genes and Regulatory Pathways in Rice by Weighted Gene Co-expression Network Analysis
