A review of effective strides in amelioration of the biocompatibility of PEO coatings on Mg alloys
2022-10-24ArshFtthlhosseiniRziehChhrmhliKzemBeiMeismNouriMohsenKeshvrzMosKseem
Arsh Ftth-lhosseini ,Rzieh Chhrmhli ,Kzem Bei ,Meism Nouri ,Mohsen K.Keshvrz ,Mos Kseem
a Department of Materials Engineering,Bu-Ali Sina University,Hamedan 65178-38695,Iran
b Department of Mechanical and Mechatronics Engineering,University of Waterloo,Waterloo,ON N2L 3G1,Canada
c Department of Nanotechnology and Advanced Materials Engineering,Sejong University,Seoul 05006,Republic of Korea
Abstract Recently,developing bioactive and biocompatible materials based on Mg and Mg-alloys for implant applications has drawn attention among researchers owing to their suitable body degradability.Implementing Mg and its alloys reduces the risk of long-term incompatibility with tissues because of their close mechanical properties and no need for re-operation to remove the implant.Nevertheless,the degradation rate of the implant needs to be controlled because production of hydrogen gas and accumulation of its bubbles increases local pH around the implants.To confine the integrity of implants and the body,the corrosion concern in the body fluid requires to be addressed.Surface modification as one of the effective strategies can improve corrosion resistance.Besides,it creates a suitable surface for bone grafting and cell growth.The development of proper surface-coated implants needs appropriate techniques and approaches.Plasma electrolytic oxidation(PEO) coating can provide long-term protection by providing a ceramic layer and improving the implant’s biocompatibility.Herein,a general review of in-vivo and in-vitro evaluation of PEO coatings on Mg and Mg-alloys has been carried out.Recent advances in surface modification on Mg and Mg-alloys have been discussed,however,the need for reliable laboratory models to predict in-vivo degradation is still valid.
Keywords: Mg alloys;Biodegradation;Surface modification; In-vivo; In-vitro;PEO coatings.
1.Introduction
Orthopedic biomaterials must have many capabilities to perform well in-service conditions and play a significant role in recovering the patients’ health [1,2].For instance,they must exhibit great bioactivity within implementation and degradation procedures to avoid problems including allergy and inflammation in neighboring tissues.Considering the application and the surrounding tissues,the biodegradable implants are required to be corroded uniformly during the healing and growth of the tissues [3–7].The latest investigations on bioabsorbable metals have proved that Mg alloys are good options in order to develop the latest generation of biodegradable implants due to their distinctive benefits [8–15].
Rapid corrosion in Mg alloys is a major hurdle to their vast usage.It is well known that Mg corrosion resistance in physiological media increases through an electrochemical reaction with water in order to produce a passivating Mg hydroxide(Mg(OH)2) film [16–18].Based on the Pourbaix diagram,Mg hydroxide film is stable only inside alkaline solutions (pH values above 10.3),and acidic or neutral media declines the stability of this layer [19–21].Thus,the corrosion kinetic is accelerated by decreasing in the pH of the environment and Mg degradation occurs at a much faster rate[22–28].On other hand,the evolution of hydrogen may result in the formation of gas packets exacerbating the necrosis and inflammation in the living tissues that delay the healing procedure at the implantation sites.Declining corrosion rate of Mg is possible by the addition of alloying elements [29–34].Tailoring the surface properties is another successful strategy to improve the corrosion behavior of Mg [7,35–40].
The design and selection of a coating material used in biomaterials applications depends on the in-service conditions and poorly selected coatings can result in severe clinical issues [41–43].To be used in biodegradable applications,a coating material requires to have different criteria.First,the coating material is supposed to be biocompatible chemically,physically,and mechanically with the surrounding environment.Also,the coating is required to function properly without generating any unpleasant symptoms in the patient.Moreover,it should have a useful tissue or cellular response in addition to cell proliferation[44–47].One technique to tailor the biodegradability and bioactivity of biometals is to treat their surface by plasma electrolyte oxidation (PEO) process that helps to insert various implants safely in the body [48–53].Through the PEO procedure,the bioactivity of the implant could be boosted by the embedment of bioactive components into the passive film formed on the outer surface and controlling the degradation rate via modifying the surface characteristics such as porosity and roughness [54–59].PEO coatings,including silicate-and phosphate-based coatings,are biocompatible and facilitate cell adhesion of Mg alloy [60,61].Mg alloy covered with silicate-based PEO coating advanced cell differentiation and proliferation of osteoblasts and so showed proper biocompatibility [62].
Wu et al.[51] indicated that the PEO-coated Mg is better for cell proliferation.Results of micro-computed tomography(micro CT) showed that many new bones were produced and primarily bridged a 15 mm gap at 2 months.Histological findings showed that the newly produced bone consisted of large maturation within 12 weeks.Pan et al.[63] produced calcium phosphate (Ca-P) on ZK60 applying PEO.They utilized a solution that comprised disodium hydrogen sulfate and calcium stat monohydrate.Rising concentration of electrolyte resulted in an increase in elements absorption in the electrolyte.In the same investigation,an immersion measurement was done in a simulated body fluid (SBF) to evaluate the biodegradability and biocompatibility of the coatings.After immersion in the SBF electrolyte for 1 month,Ca-P coating efficiently declined the rate of degradation.And after 1 month the surface was covered with a film of hydroxyapatite (HAp).Yang et al.[64] studied the biocompatibility properties of the coatings produced at distinct HAp concentrations within an SBF.They realized when the HAp concentration augmented,on the surface of the coating the capability of producing biological apatite augmented and the porosities were totally sealed that improved the corrosion behavior.
The characterization of PEO coatings inin-vivomedium is still limited.There are few general types of biological techniques utilized in finding and development of new products:techniques and holistic bioassays that assess biological mechanisms.For each,there are some instances ofin-vitroandin-vivotesting [65].Fig.1 shows the comparison betweeninvitroandin-vivotechniques [9,66,67].The following section provides a comprehensive overview of the latest investigations onin-vivoandin-vitroperformance of PEO-coated Mg alloys.
2.Mg and its alloy as a biodegradable implant material
Biodegradable metals are a popular category of implants to achieve complement tissue regeneration.Mg,Fe,and Zn are three of the most well-studied biodegradable metals and are highly utilized for orthopedic and cardiovascular uses[68–73].These metals appear to have excellentin-vivobiocompatibility,the mechanical strength required to sustain bone undergoing regeneration,and a controlled degradation profile.The biodegradable implant can remain in-service in the human’s body and after that can be gradually absorbed,dissolved,or excreted.Basically,in the case of biodegradable implants,the occurrence of corrosion is pleasant.Moreover,biodegradable implants are a proper choice for fixing the problems related to metallic implants in the human body [74–77].Due to their high biocompatibility,Mg alloys were known as implant materials for trauma and orthopedic surgery in the 1930s [78–81].Recently,Mg has successfully been chosen for human trials (Fig.2) [82].As shown in Fig.2,Mg has been utilized for human trials since 1900.It is astonishing to note that in the fifty-year period between 1948 and 2010,there had not been clinical trials of Mg for orthopedic uses [5,83].The utilization of Mg-based implants in cardiovascular,general,and musculoskeletal surgery has been studied for more than a century [3].Some examples of the utilizations are screws or intramedullary fixator in orthopedic applications,plates,stents for revascularization,suture staples,scaffolds for tissue engineering,and wires [69,84–86].The metallic mesh implant for the generation of the jaw bone is a new application in dental uses that has been aimed at.Fig.3 shows some instances of Mg implants [87].
2.1.The benefits of Mg as a biodegradable implant material
Mg is the most used biodegradable metallic material because of its low elastic modulus (45 GPa) and density (1.74 g/cm3) that are close to the modulus and density of the human bone (elastic modulus: 2–20 GPa;density 1.8 g/cm3)[69,88–92].Table 1 [69] compares the mechanical properties of Mg with other metals used as implants.Another signifciant benefit of Mg is its high biocompatibility property [93–97].Mg has a great potential in biomedicine and it is one of the best materials for biodegradable implant usages [78,98].Mg ion is the fourth major metallic ion participating in the formation and remodeling of bone and therefore,Mg is one of the most significant elements in the health of bone [99].Castellani et al.[100] reported that the measured biodegradable Mg implant is better than the titanium control considering the strength of the bone-implant interface and osseointegration.Biodegradable Mg alloy stimulates the formation of bone and has a great interfacial strength which both are two critical needs for bone implant usages.

Fig.1.Comparison of in-vitro and in-vivo approaches.

Fig.2.Case studies on Mg-based orthopedic implant materials in humans [82].(With permission from Ref.[82];License Number: 5331170759612,Jun 17,2022).

Table 1 Mechanical properties of bone tissues and materials for orthopedic implants [69].(With permission from Ref.[69];License Number: 5331190716293,Jun 17,2022).
Chaya et al.[101] investigated the treatment effect of Mg screws and plates used to fix rabbit ulna fractures.The formation of new bone near the degrading Mg implants was observed.Fracture treatment was seen within 2 months and remodeled or matured after 4 months.Bending measurements divulged there was no distinction in flexural load between the intact ulna and healed ulna offering that Mg implants provided enough stability for fracture healing via its degraded ions.Mg has great biocompatibility comparing with other metallic implants [102,103].Mg composites are valuable materials among other implant materials such as Co–Cr,stainless steel,and titanium alloys which are shown in Table 2 [104,105].

Table 2 Implant metals applications,advantages,and disadvantages [104,105].
2.2.Drawbacks of utilizing Mg as a biodegradable implant material
Despite its many benefits,the application of Mg as a biodegradable material in implant is limited due to several important restrictions [106–108].Generally,Mg is corroded quickly in body fluid that results in the formation of a large quantity of hydrogen gas and a significant increase in the local alkalinity of the body fluid.The mechanism of corrosion in Mg in an aqueous medium can be described as the following reactions [109–111]:

The Mg2+ions freed into the body fluid take part in different physiological reactions and are stored into adjacent bone tissues [112],whereas the additional content of Mg2+will be excreted through feces or urine [98,113].
The accumulation of tin gas pockets of hydrogen near the implant makes a delay in curing of the wound and result in tissue necrosis [70,98,106,114,115].In extreme circumstances,such as the situations of very large hydrogen bubbles,there is an augmented risk of blood stream blockage that can even make the patient to die.The local alkalization is able to unbalance the pH-based physiological reaction around the Mg implant and may even cause alkaline poisoning in situations the localin-vivopH content surpasses 7.8[109–111,116].Quick corrosion,production of a large amount of hydrogen gas,accumulation of the hydrogen bubbles close to the implant,and the elevated pH value of the body fluid are the major restrictions in utilizing Mg in implants.
Controlling the corrosion rate of Mg in body fluid is an important step in expanding the application of biodegradable Mg-based implants [117].By using Mg as a biodegradable implanting material,it is supposed to meet the following conditions:
·Maintain its integrity and mechanical requirements until the damaged organ is healed.
·Low rate of corrosion in the body during the early steps of the implantation and be a controllable and steady rate of corrosion.
·Corrosion products left from the corrosion reaction must not go above the threshold of the body absorption.
3.Adopted strategies for the development of Mg-based implants
Declining the corrosion rate of Mg is the most effective since a slow rate results in a decline in the quantity of hydrogen alkalization and evolution that let the body to be prepared to absorb or digest the corrosion products.There are two main choices for dealing with this: (i) alloying,and (ii) coating or surface treatment [42,118–121].

Fig.3.(a) Several implants made from Mg alloys for fixation of bone,(b)bioabsorbable orthoperiodic implants,(c) microsurgical clips for laryngeal use,(d) wound-closing device,and (e) MGANEZIX dental screw [87].(With permission from Ref.[87];License Number: 5331170936892,Jun 17,2022).
3.1.Using alloying elements to control the corrosion rate of Mg
Alloying Mg can considerably decline the rate of corrosion in body fluid.Elements such as Al,Zn,Mn,Li,Ca,Zr,and rare earth (RE) are currently utilized to make Mg alloys and each has distinct effects on the corrosion behavior of Mg alloys [78,122–125].The mechanical behavior of Mg alloys is also affected by these elements.The influences of alloying elements on the cathodic and anodic kinetics of Mg are schematically depicted in Fig.4.The role of the elements alloyed with Mg in the polarization performance of Mg alloys is described by their distance related to relative movement [126].Therefore,the type and amount of alloying elements in the composition of Mg-based alloys must be carefully opted in order to maintain their biocompatibility and corrosion resistance.Following,a general explanation of some popular alloying elements and their influence on Mg alloys are presented [8,122]:
·Al: Metals alloyed with Al possess a good mixture of diecastability and mechanical properties.Al amends hardness and strength [99,124].

Fig.4.Schematic illustration of the electrochemical factors due to chemical composition [126].(With permission from Ref.[126];License Number:5331171057302,Jun 17,2022).
·Zn: Similar to Al,Zn is able to improve mechanical properties in Mg alloys for instance,strengthening via solidsolution hardening.Mg is commonly alloyed with Zn that raises its yield strength [127].
·Ca: Studies showed that adding Ca improves mechanical properties,works as a grain refinement agent,and can improve rollability of Mg sheets [128].
·Mn: It is a very popular alloying element and is utilized to neutralize the impact of Fe and to modify the morphology.Modifciations in the morphology amend elongation,tensile strength,and ductility.Adding Mn can also raise creep resistance and high-temperature strength [129].
·Li: Adding Li declines strength but raises ductility.Mg-Li Alloys are also susceptible to age hardening [130].
·RE metals: These elements have a powerful grain-refining impact on Mg-based alloys.Adding the REs raises diecastability and strength [131,132].
Fig.5 demonstrates a summary of effects of various alloying elements on properties of Mg alloys [20].

Fig.5.Summary of Mg alloys development [20].
Some commercial Mg alloy systems were chosen as biodegradable Mg alloys early on due to their combination of good mechanical qualities and corrosion resistance.The WE (Mg-RE-Zr),AZ (Mg-Al-Zn),and ZK (Mg-Zn-Zr) series alloys are commercial Mg alloys utilized in biological research.In recent years,AZ series alloys,particularly AZ91(Mg-9Al-1Zn) and AZ31 (Mg-3Al-1Zn) alloys,have been intensively investigated bothin-vitroandin-vivo[133,134].It has been reported that when AZ91 and AZ31 alloys degrade in physiological conditions,they release hydrogen,resulting in a considerable increase in both pH and Mg ion concentration.The AZ31 alloy degrades more slowly than the AZ91 alloy in Hank’s solution,but there is no substantial differencein-vivo[109,135].Short-termin-vivoinvestigations of the AZ31 and AZ91 alloys indicated that a biocompatible Ca phosphate protective film layer covers their surfaces and promotes the creation of new bone mass surrounding the implants[136].WE series alloys are resistant to biocorrosion because they form a rare-earth (RE) oxide layer in aqueous environments.Witte et al.[136] investigated thein-vivodegradation of four different Mg alloys and found that WE43 is biocompatible.However,after the administration of RE components such as Ce,Y,and Pr,serious hepatotoxicity has occurred[137].Because of the good biocompatibility of the component elements,ZK series alloys,particularly ZK60 (Mg-6Zn-0.5Zr) and ZK40 (Mg-4Zn-0.5Zr),have recently piqued the interest of researchers [138,139].Mg-Zn-Zr alloys are more appealing in terms of element biocompatibility and biosafety than Mg-Al-Zn and Mg-RE-Zr alloys,and they are candidate biodegradable metals for use in bone healing devices [140].However,the exceptionally high degradation rates of Mg-Zn-Zr alloys are concerning and limit their further growth.In addition to the commercial Mg alloy systems listed above,novel Mg alloys for orthopedic applications have been created,including Mg-Ca,Mg-Zn,Mg-Sr,and Mg-RE alloy systems.
3.2.Available coatings to control the corrosion rate of Mg
Coatings are helpful in decreasing the primary localized corrosion of Mg and its alloys.Normally,the thin coatings applied on Mg-based implants are able to protect the substrate from severe corrosion,particularly in the initial steps after the implantation.Furthermore,as a temporal surface,the appropriate produced coating can slightly vanishin-vivoand will not make deleterious affections on the neighboring tissues [141–143].Generally,the fabrication methods of the coatings can fall into several groups as illustrated in Table 3.From Table 3,it can be observed that the fabrication methods contain physical vapor deposition (PVD) [144,145],thermal spray [146,147],chemical vapor deposition (CVD) [148],sol-gel[149,150],hydrothermal[151,152],electrophoretic deposition (EPD) [153],PEO [154–158].Each coating method has its benefits and restrictions.Choosing a specific approach mainly depends on several parameters including characteristics of the substrate and aimed properties of the coating.For biomedical applications,improving biocompatibility or osseointegration in orthopedic applications,antibiotic ability,bioactivity,or local drug delivery ability besides are also important [69,159,160].Furthermore,the coatings must support biodegradation at an acceptable rate.Other review papers have addressed the various surface treatment techniques and benefits for biomedical utilization of Mg alloys [6,161,162].

Table 3 Common techniques used to develop metal oxide coatings.
Among coating techniques to boost corrosion resistance of Mg alloys,PEO which is also well known as the microarc oxidation (MAO) process,is a popular method due to its environmental friendliness and high efficiency [163].The principal benefit of the PEO method is that the formation ofthe oxide layer occurs in a short time within a single stage.Within the PEO procedure,the discharge characteristics including chemical,electrochemical,plasma-chemical,and also thermal reactions happen at discharge sites,specify the thermal and chemical status of the oxide surface that play a significant role in the structural composition,morphology,and phase production of the developed oxide film [164–166].This method presents significant benefits in comparison to conventional surface treatment techniques owing to its high production output,uniform thickness of the layer,and great adhesion to the substrate in addition to the strong mechanical properties [167,168].Particularly,this technology has been widely utilized in the field of anti-corrosion coating of metallic substrates owing to thein-situproduction of the compact protective ceramic film [169,170].This makes PEO technology an interesting choice for producing bio-ceramic coatings [171].
4.Surface modification of Mg alloys by PEO process
PEO is one of the most typical methods utilized to passivate surfaces of Mg.PEO is a conversion coating procedure that is used for metallic materials which tend to have passivity in proper aqueous solutions [172–174].The important parameters in determining the properties of PEO coating are shown in Fig.6.These include electrolyte composition[175–177],electrical parameters [178–181],type and content of additives [23,182–184],the alloy nature,and electrolyte temperature [185].

Fig.6.The important parameters in the PEO coating process.
4.1.Formation mechanism of PEO coatings
The mechanism of forming plasma electrolyte oxide coatings is complex since it contains plasma chemical,electrochemical and thermal chemical [186–189].The formation of oxide coating,dissolution of pre-formed layer,dielectric breakdown,and gas evolution are the competing and dominant chemical processes.The supremacy of each of these processes is determined by the composition of the studied Mg alloy,the type of the solutions,the concentration of different constituents in the solution,and the applied current density[190].
The PEO setup includes a potential source,an electrolytic bath,a counter electrode (cathode),and a working electrode(anode).Fig.7 shows the schematic of the PEO setup.The applied potential should be more than the dielectric breakdown voltage of the oxide film that usually grows between 300 and 500 V.In this process,plasma is formed on the surface of the substrate and produces an oxide film.Melting,melt flow,and solidification of the oxide film are part of this process.The use of high currents leads to the production of little pores,normally of few microns,on the surface [191,192].Metal dissolution and barrier film growth occur at the start of the PEO process on the substrate[193–195].By increasing the time,in the first step,the voltage increases.Noticeable sparks on the metal surface cannot be observed in this step and a clear,thin,and passive film is formed.Furthermore,oxygen formed by water oxidation is continuously adsorbed on the metal surface along with hydroxyl anions.The surface of the anode is surrounded by a thin dielectric layer.The strong electric field shows the driving force needed for moving anions and cations that is present in the solution across the barrier layer.This step is indeed showing a general anodizing procedure[196].Tiny sparks might be seen all over the metal surface in the second stage.A steady sparking state is produced in the third step and a reasonably constant value is achieved by cell potential.White sparks are replaced by orange ones that can be observed on specific sites.These sparks move slowly across the surface of the metal.The density and size of the sparks decline slightly (Fig.7).
The different possible reactions that may happen during the PEO of Mg/Mg alloys are as below [197,198]:


In addition to these general reactions,the particular anions in the solution may react with MgO at high temperatures of spark discharge to produce Mg compounds.At such high temperatures,some compounds may undergo phase transformation and once they cool down,changing of the initial phase can be possible.The spark discharge phenomena characteristics specify the resultant coating quality according to the formation mechanism of PEO coatings [199–202].Researchers have impressively utilized spectroscopic investigations to deeply understand the properties of spark discharge.

Fig.7.Illustration of formation mechanism in PEO process.
For PEO treatment of Mg alloys,alkaline electrolytes are usually utilized[175,182,203,204].When the breakdown voltage is attained,micro-regional instability produces a high number of dispersed discharge channels.Because of the high temperature (20000 °C) at or near the center of discharge and the high pressure (100 MPa) in less than 1 μs,the generated electron collapse effect causes the coating materials to flow into the discharge channels quickly [205–207].In the PEO treatment of Mg alloys,common electrolytes include silicates,aluminates,and phosphates.Anionic components such asenter the channels via electrophoresis when subjected to a strong electric field force.At the same time,passages allow alloying components of the substrate to melt or diffuse into the channels due to the effects of high pressure and high temperature [208–210].The oxide products solidify as the proximity electrolyte rapidly cools,increasing the coating thickness in the local area around the discharge channels.When the discharge channels cool,the reaction products deposit on the channel walls,causing the discharge channels to close.The created gases are forced to escape from the discharge channels,preserving the remaining blind holes with ’volcano’ shapes.As the oxidation process continues,the aforesaid process is repeated in the comparatively weak portions of the entire coating surface,encouraging overall uniform coating thickness.Many research have demonstrated the effects of various anions in solutions on coating characteristics.These electrolytes may cause significant changes in coating characteristics.It was demonstrated that anions play a direct role in the coating development process.Aside from MgO,which is present in all types of coatings,specific phases such as Mg3(PO4)2,MgSiO4,and MgAl2O4are produced with P-,Si-,and Al-containing electrolytes [211].
4.2.Microstructure of PEO coating on Mg alloys
Microstructural properties of PEO coatings depend on different stages of the process.The coating thickness can be in the range of 5–200 μm.All PEO coatings have a really thin barrier film that ranges from some nanometers up to 2 μm [213,214].By increasing the procedure time,the ceramic oxide film on top of the barrier film expands and thickens.A few pores are created in the coating as the ceramic film develops through the electrical discharge procedure.The electrical discharge procedure’s intensity and duration affect the porosity content in the PEO coating.Pores having diameters ranging from 0.5 μm to 50 μm are seen in various kinds of PEO coatings on Mg alloys.SEM micrographs of the top surface of PEO coatings indicate that the structures have a mixture of uniform and fine pores and uneven and big pores on the surface.The obtained characteristics mainly depend on the electrolyte and conditions of the procedure [215–218].
4.3.Coating requirements for biomedical applications
The selection and design of the coating materials used in biomedical applications are vitally dependent on predestined use.Biomaterials could typically demonstrate varying degrees of compatibility in the medium of the human body.There are various properties required for a coating material to be used as biodegradable implants.First,the material for the coating must be biocompatible [6,219–221].The coating must do the required functions with eliciting no unpleasant effects in the patient.It should have useful cellular or tissue response in addition to cell proliferation.Coatings are faced human fluids and tissues;so,it is important to be aware of the possible interactions between the material and the host medium.The coating’s main mission is to delay the degradation of the Mg-based implant materials.Therefore,it is important that the coating itself must be non-degradable within the body fluid [7,97,222,223].The coating is supposed to possess a predictable rate of degradation to prepare progressive loading of bone and to prohibit stress shielding for helping better cure of bone.The coating must be dense and uniform.Micro-pores that form because of micro-discharge during the coating process are a major issue of PEO coatings.The pores make the bare substrate to face the fluid of the body by causing the fluid to penetrate via the pores.As a result,the performance of the coating is declined.Thus,controlling the porosity of the coating is important.Attempts have been made to reduce the porosity of PEO coatings [224,225],particularly for engineering usages.Changing the procedure parameters has been helped declining the PEO coatings porosity,however,it is fascinating to notice that biomaterials having porous structures are deliberately produced for scaffolding.The porous structures are important in production process of bone.The pores let the graft grow into new blood capillaries that expand inside the curing process.Higher porosity and pore size have been shown to contribute in a greater growth of bone.The pH of the body fluid is normally between 7.4 and 7.6.The body fluid pH around the implant must not change considerably for two purposes: (i) alkalinity or acidity would influence the live cells in that area (i.e.,Biocompatibility may be influenced by an unbalanced pH),and (ii) the substrate Mg material dissolution is affected by the pH of the ambiance [74].Corrosion of Mg accelerates in a low pH environment and it passivates as the ambiance is alkaline.
5.In-vitro
In-vitrotests are performed outside the living body system by keeping environmental conditions such as pH and temperature at certain conditions.In-vitrotechniques are mainly inexpensive and quick to implement.In these tests,the effects of the environment and production methods on the material and how to change them are investigated.The principal component of the human body is water,so the solubility of biomaterials in buffer solution with physiological conditions at 37 °C and a pH of 7.4 is studied and characterized.Typically,thein-vitrotest minimizes the use of animals and allows assessing the response and reaction of substances to cells.
The biological characteristics of coatings are significant factors in their biomedical uses.Biocompatibility is the first needed characteristic of any implantable biomaterial.The success of an implant in the body depends on its biocompatibility,design,and properties of the material used.Biocompatibility,according to Williams in 1992,is defined as the ability of a material to be put next to a host in specific applications.Generally,the biocompatibility of a material is defined by the surrounding tissue and ultimately the whole human body[226–230].In the following,the biocompatibility properties of PEO coatings on Mg alloys are studied.
5.1.Biomaterial destruction
Biomaterial stability in a biological environment is one of the most important biocompatibility factors.Indeed,the first step in choosing the right material in a biomaterial application is its resistance against degradation.Sometimes,biomaterial degradation causes the release of degradation products and may have a negative effect on the surrounding tissues,locally or supra-locally,but in some cases the release of degradation products is useful and desirable.Corrosion is the electrochemical behavior of biomaterials at the moment they are put in the electrolyte that surrounds them in the body and corrosion results in their destruction through the production of different hydroxy,oxides,and other compounds [231].
The polarization method is utilized to assess metal corrosion potential in a different period.This test is carried out by using electrochemical measurements at 37 °C in SBF electrolyte (pH=7.4) to imitate the human blood plasma.Tafel extrapolation and linear fits to the anodic and cathodic parts of the polarization plots can be used to measure corrosion current density (icorr).Electrochemical impedance spectroscopy(EIS) is a great tool utilized to assess biosensory and conductivity in addition to corrosion resistance of various metals.Table 4 provides a summary of the obtained results from the corrosion tests [51,180,232–247].

Table 4 Corrosion properties of produced coatings for biologic uses.
Li et al.[248] proposed a mechanism for PEO coating degradation in SBF.The PEO coating generated on the Mg alloy was generally made up of two layers: a porous outer layer and a dense inner layer.There are multiple deep and large-sized pores in the porous outer layer.The inner layer,on the other hand,was denser and had smaller pores.After immersion in SBF,the PEO-coated samples corroded as follows:
Anodic reaction:

Cathodic reactions:
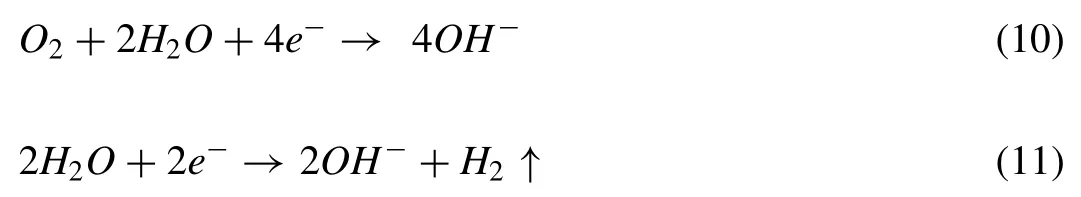
As a result,the following reaction occurs:

Based on the aforesaid reactions,the Mg was corroded and transferred into Mg(OH)2film during the initial stage of immersion time,with SBF rapidly entering into the porous outer layer.The corrosion production of Mg(OH)2increased as the immersion period rose,and certain pores were filled by these precipitates,reducing the porosity of the covering.As a result,SBF would take a long time to pass through the outer surface layer and into the interior layer.The results showed that PEO coating had a protective function in the SBF corrosion process.The Mg(OH)2film produced in SBF,on the other hand,was porous due to SBF diffusion into the PEO coating.As the immersion period was increased,Cl-from corrosive media adsorbed on the sample surface and dissolved the Mg(OH)2coating as the following reaction occurred:
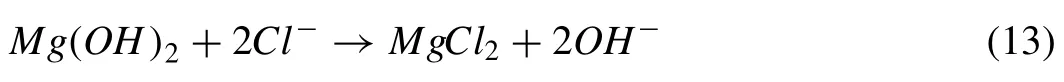
As a result,Cl-could hasten PEO coating corrosion by producing MgCl2,as well as impair PEO coating corrosion resistance.The pH of SBF increased early on due to the degradation of the PEO-coated Mg alloy,which was attributed tothe precipitation of Mg(OH)2during this course.Meanwhile,Cl-ions might dissolve the Mg(OH)2deposit,acting as a barrier to prevent Mg alloy degradation.More Ca-P minerals collected on the surface after prolonged immersion in SBF,forming a thin layer.The layer became substantially thicker,forming a barrier that prevents corrosive SBF from accessing the inner layer of PEO coating.When PEO-coated Mg alloys are immersed in SBF,the degradation rate is reduced greatly due to the barrier effect of both the PEO coating and the precipitation layer.
Bordbar-Khiabani et al.[239] showed that the coatings developed from the solutions with 0,1,2,3 g/l ZnO nanoparticles (Z0,Z1,Z2,Z3) indicated higher corrosion resistance in comparison to the unmodified coatings after immersing for a long time in SBF.The corrosion behavior of PEO-coated specimens tested by polarization and EIS methods is shown in Fig.8(a and b).The results showed that the coatings with different concentrations of nanoparticles had better corrosion resistance in comparison to the substrates which shows that PEO coatings can provide better protection for biological applications.Furthermore,the best corrosion resistance performance of the coatings was obtained by 3 g/l of nanoparticles since the obtained dense coating was free of microstructural defects and provided excellent protection compared to other specimens.Fig.8(c–g) shows pictures of the coated specimens after 2 weeks of immersion in SBF.On the surface of the uncoated AZ91 alloy,significant localized attack damage,perforation,peeling,and deterioration of the PEO coating were observed after immersion (Fig.8(c)).All the PEO-coated specimens were more resistant to corrosion than the uncoated specimen.The dissolution of the PEO coating at the edges shows the progression of corrosion (Fig.8(d)).Specimens Z1 and Z2 indicated less localized attack damage(Fig.8(e and f)) but some corrosion products (white zones)and perforation were present on the surface of specimen Z1.In the case of specimen Z3,there is no significant corrosion product on the surface but discoloration of the surface after 2 weeks of immersion is observed,emphasizing that the longterm corrosion resistance of the PEO coating was considerably enhanced by the addition of 4.5 g/l ZnO nanoparticles into phosphate-based solution.
Zhang et al.[249] carried out a PEO procedure on AZ31 at 250,300,and 350 V in a phosphate-based solution to boost corrosion resistance.The influence of voltage on the corrosion performance of layers was studied by EIS and polarization in an SBF after 1 week of immersion.Pan et al.[250] produced a ceramic coating on ZK60 alloy using the PEO procedure under constant voltage at various concentrations of P and Ca.The biodegradability and biocompatibility of the coatings were measured by immersion in an SBF.Micrographs indicated that pores with various sizes were spread throughout the surface.In addition,the thickness of the layers is augmented by the rising ratio of calcium-to-phosphorous.Also,XRD tests approved formation of bioactive phases such as Ca-P and HAp on the coatings that were immersed in the SBF.This is because PEO coatings on Mg are extremely bioactive.The degradation rate of the coating declines by rising the ratio of calcium to phosphorous.
Pan et al.[251] applied Ca-P layers on Mg-Zn-Zr alloy using the PEO method in solutions with calcium stat monohydrate and different phosphates such as sodium phosphate,hydrogen disodium dodecahydrate phosphate,and sodium hexametaphosphate.It was concluded that ions of phosphate and calcium were successfully added to the coatings.The Ca-P layer efficiently boosted corrosion resistance.The coating generated in the solution with sodium hexametaphosphate was more adhesive,thicker,and more biocompatible.In addition,it had a smaller rate of degradability and a greater capability of generating HAp.

Fig.8.(a) EIS and (b) polarization plots of AZ91 and the specimens treated in electrolytes containing different concentrations of ZnO NPs,Optical images of the (c) uncoated AZ91,(d) Z0,(e) Z1,(f) Z2 and (g) Z3 coatings after 2 weeks immersion in SBF [239].(With permission from Ref.[239];License Number: 5331171277404,Jun 17,2022).
The effect of time on thein-vitrobioactivity of PEO layers added to AZ31 Mg alloy inside an SBF electrolyte was investigated by Gu et al.[60].The procedure was carried out in 1,3,5,and 8 min.The highest impedance and least corrosion current density were observed for the formed layer at 5 min.Hap formed on surface of the layer after immersion in the SBF electrolyte.Indeed,HAp formation is attributed to the significant role of SBF in simulating the bioactivity of PEO coatings in the human body.Because of the high bioactivity and biocompatibility of HAp,it is known as a great material for medical implants.
Ghasali at el.[252] studied the effect of two types of porous Mg-metal matrix nanocomposites separately reinforced using Al2O3(specimen A) whiskers and Si3N4(specimen S) particles onin-vitrobioactivity.In order to test the rate of degradation and the prepared composites bioactivity,composites-A and S were immersed in SBF electrolyte for 2 weeks.Fig.9(a) illustrates the weight loss of the composite specimens and as depicted weight loss rises by extending time.After 2 weeks (Fig.9(b)),specimen S was degraded more than the other specimen.Composite-A showed the lower corrosion and the moderately corroded zone was observed on the surface.It is thought that micro-galvanic corrosion happened between electrochemically noble intermetallics and Mg matrix that increased the degradation of composite-S.The low porosity of composite-A prohibited the diffusion of aggressive electrolyte inside composites so the integrity of the composite-A was maintained.Fig.9(c)indicates the change in the pH value of the SBF electrolyte after 2 weeks of soaking the composites.It can be observed that all samples resulted in the alkalization of the SBF electrolyte due to the degradation of corrosion products in the Mg matrix.The pH of the SBF electrolyte with the composite-S augmented from 7.4 to 9.6 after the first day of immersion and then augmented gradually with immersion time and was stable after 4 days of immersion,likely owing to the coverage of the corrosion products including Mg(OH)2and Ca-P compounds as studied by Witte et al.[253].The evolution of hydrogen from the composites during 2 weeks is indicated in Fig.9(d).Up to 24 h,the specimens presented no evolution of hydrogen.Hydrogen was released from composite-S much more than from composite-A which showed a lower rate of corrosion.
5.2.Bioactivity
The basic need for a biomedical implant is to be bioactive.Bioactivity is the property of a material that,once put in the body,reacts with hard bone tissue and in some cases even the soft tissue around it [254].When a bioactive material is implanted in a living bone,a bond is formed at the tissue-implant interface.The formed bond prevents motion between the two materials.Natural materials are created by themselves.Calcium phosphates have the highest bioactivity with the highest structural and chemical composition similarity to the mineral part of bone [255–258].The family of calcium phosphates cannot be used for applications that need load-bearing due to their poor mechanical properties but can be used as coating materials on metals [259–261].If the metals are coated with calcium phosphates,the bone tissue itself binds to the implant.These coatings can stabilize the implant in the bone and can cause a chemical bond with the bone,thus preventing it from loosening.In addition,these coatings reduce the release of metal ions from the implants into the body environment and protect the metal surface from the invasion of the environment [262].

Fig.9.(a) Specimens weight loss being immersed within SBF,(b) specimens camera picture after immersion for 14 days within SBF,(c) changes of pH value in SBF solutions having specimens,and (d) evolution of hydrogen from specimens [252].(With permission from Ref.[252];License Number: 5331171417448,Jun 17,2022).
HAp can develop calcification and resorption of bone because of its similarity to the natural bone apatite.Tricalcium phosphate (TCP,Ca3(PO4)2) also has substantial bioactivity,which is due to the fact that in a biological environment,TCP can convert to HAp [263,264].In addition to its bioactivity,HAp also has high stability in human body fluid so it can protect implants by banning the corrosive solution to reach the substrate.It was previously mentioned that the existence of phosphorous calcium in PEO coatings on Mg is the principal factor in creating biodegradable coatings [265–268].For adequate bonding of the metal implants with bone,the development of a film formed from bone-like apatite on the implant surfaces is of particular importance [235,269].The SBF can be used to study the formation of the apatite film on the surfaces in the implantation medium.With the consumption of calcium and phosphate ions present in the SBF,apatite buds begin to grow spontaneously on the surface of biomaterials in the organism.In order to form an apathetic film,it is recommended to use body simulation solutions (Table 5) [119].

Table 5 Ionic composition of different media used for Mg biodegradation testing compared to body plasma and whole blood.Units are in mmol/L,unless otherwise indicated [119].(With permission from Ref.[119];License Number: 5331190820971,Jun 17,2022).
SBF is a solution that has an ionic concentration similar to blood plasma and is prepared under temperature and physiological pH conditions of the body.This solution was first used by Kokubo et al.[270] to study changes in the surfaces of bioactive glass-ceramic.Testing in SBF is one of the common tests for study of bioactivity of biomaterials and owing to its simplicity,it has a wide range of uses and provides helpful information.The test time is declined by changing the concentration of ions in the electrolyte [271].By immersing the specimens in the SBF,P atoms help increasing the ionic activity between the surface and the surrounding liquid and form PO43-ions.Ca2+ions are positively charged in the solution,then as the concentration increases,Ca2+ions accumulate on the coating surface and the PEO surface becomes positively charged.PO43-ions migrate to the positively charged surface and then,Ca2+and PO43-ions react with each other.After the evolution of secondary apatite over the surface,larger numbers of Ca2+and PO43-ions are dispersed in SBF.Then,apatite structures grow spontaneouslyas more ions enter and cover the whole surface.A schematic depiction of apatite formation on PEO coatings is given in Fig.10 [233,272].

Fig.10.Schematic diagram of the apatites formation on CaP-PEO coating surface in SBF [272].(With permission from Ref.[272];License Number:5331180026675,Jun 17,2022).
Pan et al.[63] produced Ca-P on ZK60 Mg alloy via PEO.They utilized a solution containing disodium hydrogen sulfate and calcium stat monohydrate.Analysis of XRD divulged production ofβ-TCP in the coating.It was also proved thatβ-TCP is a very biocompatible phase that is dissolved in the human body [273].Raising the concentration of the solution resulted in an increase in elements absorption inside the electrolyte.In a similar study,an immersion measurement was done in an SBF to evaluate the biodegradability and biocompatibility of the coatings.After 1 month of immersion in SBF electrolyte,Ca-P coating efficiently declined the rate of degradation.The coating of Ca-P was covered by a film of HAp after 1 month.
Seyfoori et al.[233] applied nanocomposite and PEO coatings on AZ31 alloy via an electrolyte containing HAp nanoparticles to improve corrosion and biomedical behavior of Mg.Higher corrosion resistant nanocomposite coatings were achieved owing to blocking of the corrosive environment penetration into the coating by nanoparticles.Moreover,on the surface nanocomposite coating,more apatite was produced than the pure PEO coating.The ability of apatite production for both coatings after 72 h of immersion in SBF electrolyte is indicated in Fig.11.It can be seen that more apatite was produced over the nanocomposite specimen.This is mostly owing to the bioactive nature of the apatite in the structure of the oxide film and the high surface roughness of the nanocomposite coating [274].
Tang and Wang [275] generated CaTiO3-containing PEO layers on AZ31 Mg.The corrosion resistance of the layers in the SBF electrolyte was analyzed using an electrochemical technique.Apatite formation degree and corrosion resistance were boosted using the PEO films deposited in the existence of CaTiO3.The surfaces of the coated and the uncoated specimens after 1 week of immersion in SBF electrolyte are indicated in Fig.12.It might be concluded that PEO augmented the degree of apatite formation on AZ31 Mg alloy.A list of performed studies on the bioactivity of PEO coatings is given in Table 6 [232–234,237,244,276–280].

Table 6 Studying the bioactive behavior of coatings and their microstructures.

Fig.11.Apatite forming ability of (a) pure PEO,and (b) nanocomposite layers after immersion in SBF for 3 days [233].(With permission from Ref.[233];License Number: 5331180230709,Jun 17,2022).
5.3.Antibacterial
The bacterial infection associated with implants is a growing concern that must not be ignored [281].This type of infection has caused suffering to the patients and has made big economic damages to medical resources.Bacteria can adhere strongly to the surface of biomaterial because biofilm which is a broad extracellular matrix.Biofilm help the bacteria to avoid the attack of the immune system and antibiotics.This makes treatment of the infection more difficult and eventually results in the failure of the implantation [282].

Fig.12.Morphologies of the surface (a) bare Mg,and (b) PEO coating after immersion SBF for 1 week [275].(With permission from Ref.[275];License Number: 5331180899495,Jun 17,2022).

Fig.13.Schematic representation of the growth from the beginning bacterial adhesion to the grown bacterial layer [283].(With permission from Ref.[283];License Number: 5331181070250,Jun 17,2022).
The infections associated with implant typically contain five steps from primary bacterial attachment to formation of mature biofilms (Fig.13) [283].In the first step,via electrostatic and hydrodynamic interactions,planktonic bacteria easily tolerate reversible adhesion activity on material’s surface and the force of the adhesion quickly rises.In the second step,the adherent bacteria communicate with each another via signal molecules and secrete sticky protein parts which produce a permanent adhesion causing the adhesion to become permanent in a few hours.Sessile bacteria colonize and self-secrete matrices to encapsulate bacterial communities in the third stage,leading to the primary production of three-dimensional architecture.In stage four,bacterial colonies continue to selfsecrete matrices of extracellular polymeric matters and evolve into mature biofilms which is a more complex organism and is primarily composed of polysaccharides,proteins,bacteria,and extracellular DNA.Biofilms make cavities in the final stage that cause channels to form and expand,liberating planktonic bacteria and prompting a new cycle [282,284–286].

Fig.14.Factors influencing bacterial adhesion and bio-layer growth.
The composition of biofilm varies from site to site.The formation of a bacterial biofilm is a complicated procedure that begins with bacterial adhesion.Material properties,bacterial properties,and environmental conditions are the three main factors that control bacterial adhesion (Fig.14).These factors give an idea of how to control biofilm formation better.Thus,implant materials having strong antibacterial behavior are immediately required.Fortunately,research has discovered that Mg has good antibacterial properties that are largely because of the high alkalinity formed by its degradation [287–289].
According to Eq.1 with the corrosion progression,Mg hydroxide (Mg(OH)2) precipitates on the surface of the Mg alloy [290,291].As we know,the Mg hydroxide layer cannot protect Mg alloys against corrosion in physiological environments.Chloride ions can be adsorbed on the surface of Mg and convert Mg hydroxide to soluble MgCl2and by this pH rises [78].Mg corrosion raises alkalinity,resulting in a pH of 9-10 [292,293].Most bacteria are able to live only in a suitable range of pH (6-8) [294].Thus,high alkalinity due to Mg degradation may explain its antibacterial properties.
Staphylococcus aureus (S.aureus) proliferation epidermidis and Pseudomonas aeruginosa (P.aeruginosa) were repressed in the extracts of Mg corrosion [295].By increasing the pH of the corrosion supernatants,the antibacterial abilities were improved,whereas by neutralizing the supernatants the antibacterial affections were totally lost.Thus,the antibacterial influence raised by the high alkalinity of Mg degradation products will decrease by implantation into the body.So,it is important to take steps to improve thein-vivoantibacterial characteristics of Mg alloys.However,when considering the antibacterial role of Mg alloys,the high rate ofin-vivocorrosion cannot be overlooked.To boost the corrosion performance of Mg,surface coating and alloying have been applied [296,297].The declined corrosion rate of Mg makes a low alkaline pH that will lead to a decline in the antibacterial characteristics [298].

Fig.15.Simple illustration of physicochemical policies and their influence on bacterial adhesion.
Doping antibacterial metallic elements into coatings or alloying with these elements to improve the antibacterial behavior of Mg and its alloys could be a potential technique for developing more sophisticated implants.Fattah-Alhosseini et al.[299]investigated the antibacterial properties of PEO coatings on Mg alloys.PEO coatings made from antibacterial agentdoped solutions are thought to be a good choice for improving the antibacterial protection of Mg-based biomaterials.During the culture phase,antibacterial compounds such as Ag+and Cu2+ions released from PEO coatings have an inhibitory impact against bacteria [300,301].Nanoscale additions with a wide surface area,strong reactivity,and easy penetrability into cell membranes can improve the antibacterial efficacy of PEO coatings by eliminating the biofilm.
Generally,change in the bacterial adhesion is related to the physicochemical properties (including surface composition,free surface energy,topography,and surface roughness)illustrated in Fig.15.However,it is not always applicable in all cases.It varies depending on the environmental conditions,properties of the material,and the type of adhesive bacteria.Changes in these physicochemical behaviors prevent bacterial adhesion more but do not kill the bacteria.These behaviors will be changed with any level modification.So,they always have a common effect in controlling the formation of biofilm on the surface of the material.

Fig.16.Photographs of the culture plates after re-cultivation: (a) E.coli plates after PEO cultivation on surface,(b)E.coli plates after silver-deposited PEO treatment cultivation,(c) S.aureus plates after PEO cultivation on surface,and(d)S.aureus plates after silver-deposited PEO treatment cultivation,(e) Active colony ratios of S.aureus and E.coli on PEO and silver-deposited PEO treatment after re-cultivation [280].(With permission from Ref.[280];License Number: 5331181322610,Jun 17,2022).
Silver nanoparticles are the most famous inorganic antimicrobial particles.Corrosion-resistant and antibacterial Mg implants can be made possible by doping Ag elements in coatings [302].Aktug et al.[280] evaluated the antibacterial behavior of doped silver in PEO coatings applied on Mg alloys.Fig.16 (a–e) shows the antibacterial reaction of PEO coating and silver-doped PEO coating to gram-positive and gram-negative bacteria.The antibacterial ability was clearly raised in the presence of silver particles where the ratios of active colony ofS.aureusandE.coliwere declined by 74.6%and 87.3%,respectively.The decrease in the formation of bacterial colony could be clarified by the inhibitor role of silver on the bacteria.The interaction of silver with bacterial cells could inactivate cellular proteins and could prevent DNA replication.Cell proliferation and division were considerably declined or even stopped,as a result of declining formation of the colony[303].These effects fully led to major antibacterial characteristics of Ag-deposited PEO surfaces.The research indicated stronger antibacterial activity in opposition to the gram-negativeE.colithan the gram-positiveS.aureus.The observation may be associated with the thick-structured peptidoglycan film of gram-positive bacteria inhibiting the motion of silver ions by the wall of the bacterial cell,and declining the inhibitory influence of silver [304].Similar to silver,copper is also desirable due to its low cytotoxicity and antibacterial activity.Copper can inactivate the central catabolic and biosynthetic pathways called catalytic clusters of dehydratases that provide copper with strong antibacterial properties [305].
Chen et al.[306] studied the effect of copper oxide nanoparticles on the antibacterial behavior of PEO coatings applied on Mg alloy for 6,12,and 24 h.They calculated the antibacterial rate (R) that was very strong when antibacterial activity was R≥99%.The antibacterial ability was good when R≥90 [307].The results of this study indicated that the uncoated alloy has suitable antibacterial properties because of the high pH level occurred by its rapid degradation in the solution.The antibacterial properties of the PEO-coated alloy were negligible at first 6 h.Then,the antibacterial ability increased as the simultaneous implantation time increased due to the rise in pH level.The Cu+PEO coated sample presented the highest antibacterial properties with the least amount of corrosion.For this sample,the antibacterial content was around 93 and 98% after 6 and 12 h,respectively.The antibacterial activity of the Cu+PEO coating was satisfactory.The antibacterial rate in the three different types of samples was more than 99% after 24 h of implantation.
Zinc oxide (ZnO) nanoparticles have proven antibacterial properties against Enterotoxigenic Escherichia coli (ETEC)bacteria.ZnO prevents ETEC adhesion and their internalization into enterocytes [308].Also,zinc oxide treatment has an impact on the produced biofilm.Adding zinc oxides led to a biofilm structure that is disintegrated and intractable.This biofilm effect was not only seen in its growth but also in a declined cell surface hydrophobicity easing the ZnO nanoparticles diffusion via this biofilm.
Daroonparvar et al.[309] studied the antibacterial behavior of coatings by adding ZrO2nanoparticles.The principal reason for good antibacterial performance of PEO/nano-ZrO2coated sample was attributed to the presence of ZrO2nanoparticles that reduced the growth ofE.colias a result ofE.colimembranes.The escalated permeability of the membrane led to the deposition of the nanoparticles in the cytoplasmic areas of the cells and bacterial membrane [310].Table 7 shows a summary of the published articles in this field[243,280,306,309,311–314].

Table 7 A summary of performed research on the antibacterial properties of coatings.

Table 8 Types of cells and results of cell culture on the coatings.
5.4.Cell culture
The most significant step in a cell interaction with a biological material is cell adhesion because it has a large impact on other cellular functions.Cells cannot attach and spread well on the surface of uncoated specimens.Higher cell transplantation and proliferation in PEO coatings compared with uncoated specimens is due to less degradation of uncoated specimens that provide proper places for bonding,proliferation,and growth of cell,while producing lower amounts of corrosion products may cause cellular toxicity [315].
In the Mg alloys substrate,the cell attachment is prevented by a smooth substrate surface and so the cell proliferation is declined (Fig.17(a)) [316,317].In biodegradable Mg implants,the untreated bare substrates showed several roundshaped cells on their surfaces.These were influenced by manyparameters that mostly indicate corrosion with mixed hydrogen gas and compel toxicity to neighboring tissues.On the other hand,PEO coating had a high roughness that provided a more suitable situation for attachment and proliferation of cell (Fig.17(b)).According to the morphology of cell,on the smooth surface of Mg alloy the cell is in a close contact with the base alloy via a lot of filopodia,and is dispersed randomly in different directions (Fig.17(c)).The hypothesis by Anselme can clarify this as the cell tended to find a good state in order to adjust the external and internal forces [318,319].In the case of PEO coating (Fig.17(d)),the rough surface of the coating made the cell to have a round shape [320].

Fig.17.Schematic representation of the morphology and cell proliferation of (a and c) AZ31 substrate,(b and d) PEO coating [317].

Fig.18.Response of MG63 cells to (a) bare AZ31 alloy and PEO coated sample after 1 and 3 days of incubation;of the surface micrographs of (b-1) AZ31 sample and (b-2) PEO coated sample after 1 day of incubation;Live/Dead of MG63 cells after 3 day of incubation: (c-1) bare AZ31 sample and (c-2) PEO coated sample [317].
Zhang et al.[317] developed PEO coatings in silicate solution containing potassium fluoride and sodium hydroxide and then studied the cellular behavior of the coatings compared with Mg substrate.Fig.18(a) shows the counting the cells at two various periods,24 and 72 h.The researchers saw for the uncoated sample the number of cells decreased after 72 h due to the rapid degradation of the Mg alloy and the increase in pH that prevented cell proliferation [321].The results indicated that PEO coatings had good bioactivity and show good cell surviving ability because PEO coatings were mainly composed of MgO [320].
Fig.18(b) shows the morphology of MG63 cells.The cells were spherical on the coated surface and firmly attached to the surface of the sample,while on the Mg substrate,the cells formed a thin flat layer.Also,the authors performed live/dead painting of cells after 72 h of implantation in order to test the proliferation of MG63 cells on the specimens (Fig.18(c)).
Table 8 presents the executed research in this field and the obtained results[51,241,245,246,277,279,322–324].In another study,Tian et al.[246] concluded that oxide films were formed on AZ31 by the PEO process in a solution of silicate via the addition of potassium fluoride (0.05,0.1,and 0.2 M).The fluoride-embedded coating is provided on the AZ31 via the PEO procedure.The coating was mostly consisted of MgF2and MgO.The fluoride embedment in the coating improved itsin-vitrocorrosion resistance.As a small amount of fluoride was embedded into the PEO coating,the number of living cells was augmented and that of the dead cells was declined.When the fluoride was too much,it was a drawback to the living cells.The observation could be described by the similar purpose specified above.The cells flourish better on a surface with better corrosion behavior.Corrosion behavior it could enhance by a little amount of fluoride but became weaker with too much fluoride.
Zhang et al.[245] produced calcium coatings on Mg alloys.In their study,cell adhesion and differentiation were evaluated over a period of 21 days.They observed that the uncoated sample indicated just a few numbers of cells on its surface after 7 days.The number of cells increased by rising time and HAp was formed after 14 days.A thin film of HAp was formed on the PEO coating samples after 7 days and the results showed that the sample cells increased significantly over time and resulted in complete cell coating after 21 days.The researchers concluded that the formed HAp was observed just locally.Moreover,the coating had cell proliferation effect and the cells soon started to differentiate into a spherical morphology and continued to increase in 21 days.Wu et al.[325] showed that the osteoblast cells well adhered to the surface of bio-ceramics and spread on the surface,by the formation of an apatite-shape layer and calcium ions from calcium phosphate.

Fig.19.DAPI staining of MG63 osteoblasts cells on (a) bare Mg alloy,(b) TM coated sample,(c) ZH coated sample,and (d) Cell viability of MG63 osteoblast cells after incubation on bare Mg alloy and coated Mg alloy samples for 48,96 and 168 h [243].(With permission from Ref.[243];License Number: 5331190071086,Jun 17,2022).
Bakhsheshi-Rad et al.[243] studied the biocompatibility behavior of the coatings created by the addition of titanium oxide (TM) nanoparticles and doping zinc ions (ZH) onto Mg substrates.DAPI staining of cell nuclei on the untreated and treated Mg alloy specimens in Fig.19(a–c) showed a remarkable change from the untreated samples to the treated on as fewer cells were adhered to the untreated Mg whereas a large number of cells were identified on the surface of the treated sample.This shows that the MG63 osteoblast cells had an excellent dependence to the TM and ZH-coated samples.Fig.19(d) indicates the viability of cells cultured on the uncoated and coated Mg alloy specimens for 2,4,and 7 days.Specifically,the MG63 cells in the ZH coating extracts showed the highest biocompatibility.The uncoated specimen cytotoxicity was owing to the high rate of degradation that resulted in the production of gas pockets near the implant and high cell osmolality.This was indicated as a remarkable discrepancy between the neighboring medium of the cells and the cells that resulted in death of the cells [326].Fig.19(d)indicates that the TM coating had higher viability of cell than the uncoated sample due to the slower rate of degradation and pH value that was more desirable for bonding and growth of cell [62].Introducing Zn into the coatings improved the viability of cell as the discharge of Zn ions can keep the development of osteoblast-like cells.This investigation showed that the ZH-coated Mg alloy not only owned a smaller rate of degradation but also showed more cell viability and dependence on the cells.
Zomorodian et al.[327] depicted that the viability of cell was considerably influenced by the transition happening on the surface of Mg alloy owing to the quick corrosion procedure.They concluded that corrosion process of the Mg alloy led to the production of Mg(OH)2and rising the pH that made the cell adhesion to be harder.According to the investigations,it can be concluded that PEO coatings successfully cause cell proliferation and growth in comparison to the substrate.Indeed,adhesion and cell growth are improved by controlling the parameters in the coating process.
6.In-vivo
In-vivotests play a vital role in the development and implementation of implants to be used in the human body after the success ofin-vitrotests.The purpose of these tests is to place the biomaterial next to a living physiological system and examine its biocompatibility with the tissue in an animal body to determine if it is not harmful to the body.Animals have many differences in anatomy,physiology,and biochemistry.Thus,the animal model should be opted which is most similar to the behavior of anatomy,physiology,and biochemistry of the human body.In-vitrotests are categorized into two types of non-functional and functional.The implant that is suspended in the tissue is evaluated in non-functional tests and the implant that reacts with the surrounding tissue and has biochemical behavior is studied by a functional test.In tests of biomaterial implantation and substitution in the body,the interactions of the surrounding tissue with biological coincidences are carried out by histological tests (using a microscope).
In-vivocorrosion assessment of the PEO coatings formed in base solutions with those additives coupled with certain current regimes shows that the corrosion rate of Mg in an SBF could be declined considerably in comparison to that of the bare substrate with an appropriate current regime and composition of electrolyte [61,328,329].Nevertheless,these PEO coatings can only provide a temporary protection against corrosion attack and after penetration of the solution into the defects of the coatings,the rate of corrosion will be considerably accelerated [330].

Fig.20.Micro CT images of implanted ZX50 pins after various implantation times,with and without PEO coatings [331].(With permission from Ref.[331];License Number: 5331190180237,Jun 17,2022).
Fig.20 [331] demonstrates thein-vivodegradation procedure of ZX50 implant pins with time;in the first month,the pins with the PEO coating performed much better (larger volume left).Then,the rate of degradation for the PEO-coated alloy augmented and the specimen entirely disappeared within 3 months.Thein-vivocharacterization of HAp-coated Mg-Zn-Ca alloy performed by Wang et al.[332] revealed accelerated bone production and declined rate of degradation.P and Ca are the principal elemental constituents of TCP and HAp.So,P and Ca incorporation into the PEO coatings is a prerequisite to the production of TCP or Hap that could boost the bioactivity of the PEO coatings applied on Mg alloys.

Fig.21.SEM photos (BSE mode) of (a,b) Cpol-AZ31,(c–e) OCP-AZ31,and (f–h) HAp-AZ31 immersed or mounted in the mouse body.(c,f) as-prepared sample,(c,d,g) immersed in the environment and (b,e,h) mounted in the mouse body [333].(With permission from Ref.[333];License Number:5331190280387,Jun 17,2022).

Fig.22.(a) Postoperative x-ray study of rabbits bone after 2,4,8,and 12 weeks;(b) micro CT images of rabbits in various classes with residual implants after 4,8,and 12 weeks;(c) residual implants of different groups after 4,8 and 12 weeks;(d) surface morphologies of the taken out implants after 2,4,8 and 12 weeks [51].(With permission from Ref.[51];License Number: 5331190420917,Jun 17,2022).
Hiromoto et al.[333] produced octacalcium phosphate(OCP) and HAp coatings on Mg alloy.AZ31 (Cpol-AZ31),HAp-coated AZ31 (HAp-AZ31),and OCP-coated AZ31(OCP-AZ31)were immersed in an environment for 13 months or mounted in transgenic mouse for 4 months to testin-situinflammation behavior and longtime corrosion.Fig.21 illustrates SEM backscattered mode images of the surface for OCP-AZ31,Cpol-AZ31,and HAp-AZ31samples immersed inside the environment or mounted in the mouse body.The thickness of the OCP plate-like crystals augmented (Fig.21(c and d)) and the precipitates were accumulated next to the HAp rod-like crystals (Fig.21(f and g)).In the body of the mouse,the OCP plate-like crystals were shortened and diluted(Fig.21(c and e)).HAp rod-like crystals did not alter significantly as part of the rod-like crystals vanished in very small zones (Fig.21(h)).It was believed that the rod-like crystals in the small zones were dissolved since high contents of Ca and P were identified in these zones.Cpol-AZ31 sample was covered by the products of corrosion in both the mouse body and the medium (Fig.21(a and b)).
Wu et al.[51] studied thein-vivotreatment of the coatings with thickness of 10 μm and 20 μm and uncoated Mg.Fig.22(a) illustrates the X-ray analysis of the rabbits 2 and 4 weeks and 2 and 3 months after the surgery.It is clear based on the figure,bone callus was not present in the no implanted control categories within the 3 months.Nevertheless,the other three implanted categories show slight bone defect curing.Two weeks after surgery,there was no bone callus among three implanted categories,and scaffolds of Mg were seen.One month after surgery,a small bone callus was produced on the bare Mg as there was no bone callus within the PEO10 and PEO20 categories.The Mg scaffold experienced high corrosion degradation and some corrosion pitting was present on the bare Mg in comparison to the low corrosion scaffold in PEO10 and PEO20 samples.Fig.22(b)illustrates the micro CT photos of the rabbits in various categories of the implants after 4,8,and 12 weeks.A large amount of bone callus was produced at 8 weeks after the surgery on all of these three implanted categories.The newly produced bone callus was available at both sides of the damaged bone and environed the implants.Fig.22(c) illustrates the remnant implants volume for each group after 4,8,and 12 weeks.The scaffold of bare Mg was totally dissolved after 8 weeks of surgery.Fig.22(d) shows the surface morphologies of the removed implants for the samples after 2,4,8,and 12 weeks,respectively.The bare Mg implant suffered from severein-vivocorrosion in 14 days with many globular corrosion products of Mg(OH)2formed over the surface.In 1 month,the products were peeled off from the surface and corrosion developed toward the interior side.After 2 months,the bare Mg implant was degraded.In 2 and 4 weeks,only micro-cracks were present on the PEO10 and PEO20 implants surface.Severe corrosion reactions happened after 2 months and large areas of the coating were peeled off from the surface on both implants of PEO10 and PEO20 due to the generated corrosion products.After 3 months,more corrosion products,mainly Mg(OH)2,formed and covered the remained substrate.

Fig.23.Optical photos for AZ31 and PEO-AZ31 after 0,4,8,and 12 weeks of in-vitro and in-vivo study [238].(With permission from Ref.[238];License Number: 5331190522168,Jun 17,2022).
Jang et al.[238]studied the biocompatibility and biodegradation behaviors by immersion in Hanks’ solution and also implantation of the samples in mice body.Fig.23 exhibits optical images afterin-vitroandin-vivomeasurements of PEOAZ31 and AZ31 in Hanks’ solution and in mice body for 12 weeks.The distinctions betweenin-vitroandin-vivotests were that more severe localized attack happened inin-vitroin comparison toin-vivoand steady corrosion progressed into the alloy duringin-vivotest rather than ofin-vitro.In the case of immersion in Hanks’ solution,in first 7 days of immersion AZ31 was coated by a brown layer and then white corrosion product appeared on the surface.The PEO coating started to get degraded near the edge of the specimen after 4 weeks and continuous corrosion process was clear after 8 weeks.A localized attack happened near the wire connecting point and the edge when the test was performed.Consideringin-vivoexposure,both PEO-AZ31 and AZ31surfaces were coated by a brown layer.On the other hand,a localized attack that happened in immersion test was not discovered even at the wire connecting point in PEO.The main corrosion type of AZ31 and PEO-AZ31 immersed in Hanks’ solution seemed to be localized and uniform corrosion while the type of corrosion afterin-vivotest appeared to be uniform corrosion.
The mechanism of local corrosion is due to the formation of a uniform layer of Mg(OH)2that converts to MgCl2in the presence of chlorine in the solution by chemical reaction.Nevertheless,it still has not been clearly understood why the corrosion rate and also localized corrosion are retarded underin-vivomedium compared toin-vitromedia.It has been suggested that this observation is due to the prevention of corrosion by adsorption of cells and proteins on the surface of Mg alloys as well as the production of insoluble corrosion products.
7.Conclusions
The present work is a comprehensive review about thein-vivoandin-vitrobehavior of the coatings produced by PEO on Mg substrates and their alloys.In-vitrotests are used to sieve the biomaterials used as implants or other parts of medical equipment.Once the toxicity of the material has been assessed,characterization and application tests can be performed on the samples.In general,in-vitrotest methods are highly sensitive to toxicity and interact well with animal methods.The main drawback of these tests is that only the toxicity of the material is checked and the subsequent destructive effects of the metabolic products are not assessed.But the fact is that the soluble chemicals enter the tissue dermis and have an active metabolism and biological effects.Another drawback of these tests is the lack of local rotation of the cell that causes it not to move less than 100 micrometers from the surface and these tests have been designed to examine the initial and sometimes acute behaviors of materials as well as reducing the use of various animals.
PEO coatings possess great potential for surface modification for Mg-based biodegradable materials.The composition and properties of the coatings can be widely adjusted in order to control degradation and achieve biocompatibility.The coatings show that Mg-based implants have good biocompatibility,biodegradability,and corrosion retardation and can therefore be used to modify the surface.
The primary aim of using PEO coating is to delay the deterioration of Mg-based implants.Therefore,it is very important to have a lower degradation rate for the coating material than for the substrate material.It is equally critical that there is no decomposition of the coating material inside body fluids.The coating must have predictable damage to provide gradual loading of the bone and prevent pressure protection to help better cure of bone.The coating must be uniform and dense for optimum protection of the base material against corrosion.Micropores that are created by micro-discharge within the coating can be a problem arising from PEO coatings.
Declaration of Competing Interest
The authors declare that they have no known competingfinancial interests or personal relationships that could haveappeared to influence the work reported in this paper.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Novel Mg-Bi-Mn wrought alloys: The effects of extrusion temperature and Mn addition on their microstructures and mechanical properties
- Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform
- Passivation of corrosion product layer on AM50 Mg by corrosion inhibitor
- A crystal plasticity based approach to establish role of grain size and crystallographic texture in the Tension–Compression yield asymmetry andstrain hardening behavior of a Magnesium–Silver–Rare Earth alloy
- Developing polydopamine modified molybdenum disulfide/epoxy resin powder coatings with enhanced anticorrosion performance and wear resistance on magnesium lithium alloys
- Novel extended C-m models of flow stress for accurate mechanical and metallurgical calculations and comparison with traditional flow models
