Developing polydopamine modified molybdenum disulfide/epoxy resin powder coatings with enhanced anticorrosion performance and wear resistance on magnesium lithium alloys
2022-10-24ShioChnChangqingYinYiWangShuangYiXiangGaoXujuanZhangQiyuLiaoYuxinZhangXiaZhaoJinsongRaoBaorongHoua
Shio Chn ,Changqing Yin ,Yi Wang ,Shuang Yi ,Xiang Gao ,Xujuan Zhang ,Qiyu Liao,Yuxin Zhang,,g,Xia Zhao,Jinsong Rao,*,Baorong Houa,,**
aSchool of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,China
b College of Material Science and Engineering,Chongqing University,Chongqing 400044,China
c Pilot National Laboratory for Marine Science and Technology,Open Studio for Marine Corrosion and Protection,Qingdao 266237,China
d Weifang East Steel Pipe Co.LTD.,Weifang 261055,China
e College of Mechanical and Vehicle Engineering,Chongqing University,Chongqing 400044,China
fChina Academy of Space Technology (Xi’an),Xi’an 710100,China
g State Key Laboratory of Mechanical Transmissions,Chongqing University,Chongqing 400044,China
Abstract Epoxy resin powder coating has been successfully applied on the corrosion protection of magnesium lithium alloys.However,poor wear resistance and microcracks formed during the solidification have limited it extensive application.There are limited approaches to exploit such anti-corrosion and mechanical properties of magnesium lithium alloys.Herein,the epoxy resin powder coating with polydopamine modified molybdenum disulfide (MoS2@PDA-EP powder coating with 0,0.1,0.2,0.5,1.0 wt.% loading) was well prepared by melt extrusion to investigate its anticorrosion performance and wear resistance.The results revealed that the addition of MoS2@PDA enhanced the adhesion strength between coatings and alloys,wear resistance and corrosion protection of the powder coatings.Among them,the optimum was obtained by 0.2 wt.% MoS2@PDA-EP powder coating which could be attributed to well dispersion and efficient adhesion with coating matrix.To conclude,MoS2@PDA-EP powder coating is meaningfully beneficial for the anticorrosive and wear performance improvement of magnesium lithium alloys.
Keywords: Magnesium lithium alloys;Epoxy resin powder coating;Molybdenum disulfide;Polydopamine;Anticorrosion performance;Wear resistance.
1.Introduction
Magnesium lithium alloys show significant application prospects in the field of automotive,telecommunications and aerospace industries as ultra-lightweight alloys,attributed to the high specific tenacity,outstanding malleability,impact resistance,excellent damping coefficient and energetic particles intrusion resistivity of magnesium lithium alloys in comparison with other structural metallic materials [1–5].Magnesium lithium alloys enhance the advantages of low density,improve the characteristics of poor ductility,but amplify the disadvantages that magnesium alloys possess inferior property of corrosion protection and weak wear resistance owing to high chemical activity and low hardness of lithium [6–8].In addition,magnesium lithium alloys present high sensitivity to the corrosive circumstances such as hyperthermia and humid atmosphere.Moreover,the corrosion damage of magnesium lithium alloys was awfully severe and the decomposition of Li phases and Mg phases continuously proceeds due to the loose formed powdery substance on the interface which is tough to prevent the constant corrosive process [9–11].Besides,poor wear resistance also limits the applications of magnesium lithium alloys under some special conditions owing to the reduction of strength through wear failure[12].Therefore,it is essential to enhance the anticorrosion performance and wear resistance to extend the applications and exert the advantages of magnesium lithium alloys.
Organic/inorganic hybrid coatings play a significant role in the domain of metallic materials protection due to their excellent mechanical properties,corrosion resistance and chemical stability [13–16].The organism reduces the brittleness of the inorganic component,which improves the durability of the coating,meanwhile,the inorganic component can enhance the strength and hardness of the organism,which renders the coating possesses better wear resistance [17].Among the organic coatings,epoxy resin powder coatings are developed owing to the merits of stability in corrosive media,good adhesion strength on metallic substrates,thermal properties and environmental friendly requirement [18–20].Besides,to enhance the anticorrosion property and improve the abrasion resistance of powder coatings,the addition of inorganic nanomaterials which possess proper morphology and structure,excellent thermal and chemical stability and efficient adhesion with epoxy resin powder is beneficial.MoS2has a unique lamellar construction similar to graphene,in which the internal atoms are bonded with covalent bond and the mezzanines are stacked vertically and loosely which are integrated together by weak van der Waals forces [21–23].This special chemical structure endows MoS2unique morphology and properties.Covalent bond of MoS2guarantees the excellent thermal and chemical stability and MoS2enhances the mechanic properties of powder coatings as reinforcing additive [24,25].Two-dimensional planar layered morphology of MoS2amplifies the barrier effectiveness of the powder coating,as well as offsets the microcracks in the powder coating [26-–28].Consequently,MoS2added in epoxy resin powder coating decreases the penetration possibility of corrosive media through the coating and enhances the anticorrosion performance of the powder coating.Moreover,the MoS2interlamellar weak van der Waals forces cause facile slide of MoS2layers which generates splendid lubricity of MoS2[29,30].Therefore,the additive of MoS2increases the wear resistance of the powder coating and completes the reduction of friction failure.Whereas,diverse problems about the application of MoS2in the epoxy resin powder coating still exist.MoS2possesses awful adhesion with epoxy resin powder coatings.Moreover,the agglomeration phenomenon of MoS2nanosheets is susceptible in the coating owing to the interface effect of MoS2[25,31,32].This phenomenon significantly influences of the dispersion of MoS2in the coating and impairs the realization of function of MoS2.To solve the issues of poor adhesion and dispersion [30,31],as a result,the modification of MoS2is crucial before the application in the powder coating.
Herein,to enhance the adhesion with epoxy resin powder coatings and prevent the agglomeration phenomenon of MoS2nanosheets,the modification of MoS2is accomplished by PDA.In addition,the MoS2@PDA is mixed with epoxy powder by melt extrusion which further improves the dispersion that is a dilemma of the electrostatic spraying.The aim of this work is to enhance the anticorrosion property and wear resistance of magnesium lithium alloy through homogeneous dispersion of MoS2@PDA added in the epoxy resin powder coating and probe the regularity of the amount of additive on the capability of the powder coating.And according the results of electrochemical measurement,the anticorrosion of magnesium lithium alloys is improved by several orders of magnitude.
2.Experimental
2.1.Materials
MoS2powder and dopamine hydrochloride employed in this research were procured from Shanghai Aladdin Biochemical Technology Co.,Ltd.Hydroxymethyl aminomethane(TRIS) was purchased from Beijing Solarbio Science and Technology Co.,Ltd.Sodium chloride (NaCl),hydrochloric acid (HCl) and ethanol absolute were acquired from Sinopharm Chemical Reagent Co.,Ltd.Benzoin was gained from Ningbo South Sea Chemical Co.,Ltd.Epoxy resin(Amanda 118 BHT),curing agent (Amanda 969 T02),2-Methylimidazole,leveling agent(PV88),mica powder,wollastonite,bariumsulfate,fumed silica and titanium dioxide(R960) were purchased from Daqing Qing Lu Long Green Technology Co.,Ltd.All commercial chemical agents were analytically pure and utilized without any further refinement in this study.Magnesium lithium alloy (LZ91) substrates (Li:8.5–9.5%;Zn: 0.5–1.5%;Mg: balance) were used in the form of panels with dimensions of 50 × 50 × 2 mm.
2.2.Coating preparation procedure
2.2.1.Preparation of MoS2@PDA composites
Firstly,TRIS (6.057 g) was dissolved in 750 ml of deionized water.The pH value of the solution was adjusted to 8.5 with HCl (1 M) and the solution was stirred for 20 min.Then,MoS2powder (5 g) and ethanol absolute (250 ml) were dispersed into the mixed solution to form homogeneous mixture through continuous ultrasonic stirring (60 min).Next,dopamine hydrochloride (2 g) was added in the mixture and the above mixed solution was magnetic stirred for 12 h at 20 °C.Finally,the blended solution was centrifuged and the precipitates were rinsed three times with deionized water and ethanol.The desiccation of sediments was conducted in vacuum oven at 80 °C for 12 h to acquire MoS2@PDA composites.
2.2.2.Preparation of MoS2@PDA-EP powder coating
The epoxy resin powder,curing agent and other additives were added and mixed according to the formulation ofTable 1.The extrusion of the mixture was completed in a double screw extruder with a thread diameter of 20 mm and a speed screw of 40 rpm.This facility has two heating sections.The temperature of the feeding area is 95 °C,and the extruding section is 110°C.The aim of the feeding area is to obtain molten state epoxy and the higher temperature of extruding section makes the mixture extruding smoother and dispersing better.The machined slices are triturated in a bench-level knife grinder (OMC) and screened (120 mesh),with a mean granularity of 70 μm.It was essential that the magnesium lithium alloy substrates were pre-treated before coating.The planes were polished with sandpaper(#400,#800 and#1200),washed with acetone and ethanol and treated with silanization which could significantly increase the adhesion between the metal matrix and the epoxy composite through the generation of Si-O-Me (-Si(OR)3+3H2O=Si(OH)3+3ROH and SiOH+MeOH=SiOMe+H2O),respectively.The powder were applied on alloy panels by using electrostatic spray gun (Gema,Swiss) with an output voltage of 60 kV.Subsequently,the planes were put into an air circulation oven (200 °C,15 min) for curing.The thickness of ultimate magnesium lithium alloy planes was 150 ± 20 μm.

Table 1 Different formulations of epoxy powder nanocomposite coatings.
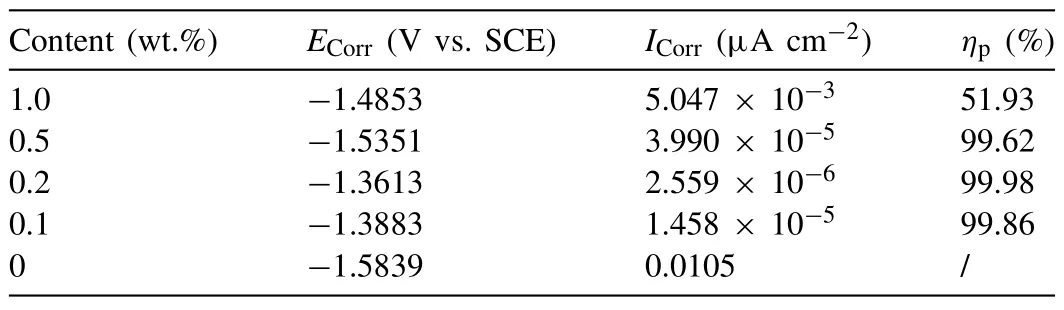
Table 2 Corrosive data calculated from the polarization curves in Fig.10.
2.3.Materials characterization
The lattice structure of MoS2and MoS2@PDA were characterized by X-ray diffraction (XRD,Max-3C,Rigaku D,Japan) with Cu-Kαradiation at scanning rate of 5°min-1in a range of 10–90° In addition,the organic functional groups of MoS2@PDA,epoxy resin powder coating and MoS2@PDAEP powder coating were detected by Fourier transform infrared spectra (FTIR,Frontier,PerkinElmer,USA).And the surface element and valence states were estimated by X-ray photoelectron spectroscopy (XPS,ESCALAB 250XI,Thermo Scientific Co.) using Al Kαradiation.The morphology observation of the coatings was accomplished by scanning electron microscopy (SEM;ULTRA 55,Zeiss,German).The structural and morphology of MoS2@PDA were investigated by transmission electron microscopy (TEM,Zeiss Model Libra 200).
2.4.Friction performance test
The friction performance of coating on magnesium lithium alloy was tested by UMT-5 tribotester (Bruker,USA) with a rotation mode of ball-on-disk.The loading rotation velocity was ascended from 5 to 900 rpm with an applied load of 3 N,correspondingly,the sliding veloity grew from 0.002 to 0.377 m/s.During each test,the coefficient of friction (COF)was measured.To guarantee the veracity,each test was carried out at room temperature with a relative moisture of 20% and each test was repeated three times.
2.5.Adhesive strength test
The adhesive strength between the coating and the magnesium lithium alloy matrix was measured by the ISO 4624–2002 standard using a full automated draw machine PosiTestAT-A manufactured by Delelsko,USA.To ensure the accuracy of the results,each group was tested more than six times and the mean value was reported as the adhesion consequence.
2.6.Electrochemical measurements
All electrochemical measurements were conducted in 3.5 wt.% NaCl aqueous solution with a PARSTAT 4000A electrochemical workstation at ambient temperature.A typical three-electrode cell was used in these measurements with a 1 cm2exposed region of the coating sample employed as the working electrode.Meanwhile,the saturated calomel electrode (SCE) and a platinum plate were performed as the reference electrode and the counter electrode,respectively.To ensure the accuracy,all samples were immersed till the open circuit potential was stable prior to the measurements of the electrochemical impedance spectroscopy (EIS) and the polarization curve.
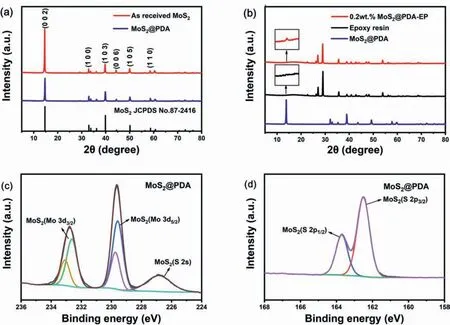
Fig.1.XRD patterns of (a) as received MoS2,MoS2@PDA and (b) epoxy resin,MoS2@PDA and MoS2@PDA-EP powder coating and high resolution XPS spectra of MoS2@PDA: (c) Mo 3d,(d) S 2p.
3.Results and discussion
3.1.Microstructure and morphology of MoS2@PDA and coatings
For the sake of investigating the influence of modification on the growth of MoS2crystals and judge if the MoS2@PDA was added into the epoxy resin,the crystalline structure was probed by XRD.In addition,to research the valence of MoS2@PDA,the XPS spectra were performed.Fig.1 displays the XRD patterns recorded for MoS2,epoxy resin and MoS2@PDA,and MoS2@PDA-EP powder coating and high resolution XPS spectra of MoS2@PDA.
According to Fig.1(a),diffraction peaks in both MoS2and MoS2@PDA could be found at 14.4,32.9,39.5,44.5,50.1and 58.0°,which reveal the crystal orientation of (002),(100),(103),(006),(105)and(110)from the typical 2H phase structures of MoS2[33].There is no change in the diffraction peak before and after modification.It indicates that the modification had not changed the crystal structure of MoS2.However,for the intensity of the peak,all peak strength of MoS2@PDA is lower than that of MoS2,which may be due to the effect of the coverage by PDA.As shown in Fig.1(b),owing to the little addition of MoS2@PDA,there is no obvious distinction between the XRD patterns of epoxy resin and MoS2@PDA-EP powder coating,but a small prominence at 14.4°,which represents the (002) lattice plane of MoS2and verified the existence of MoS2@PDA in epoxy resin powder.
According to Fig.1(c) and (d),Mo 3d and S 2p spectra acquired from horizontally aligned MoS2modified by PDA sample are displayed.The binding energy (BE) values of 229.6 eV (Mo 3d5/2) and 162.5 eV (S 2p3/2) are observed for the dramatically intense Mo 3d and S 2p doublets.The weaker doublets at higher BE in the Mo 3d spectrum at 229.75 eV(Mo 3d5/2) evidence that intermediate oxidation states of nonstoichiometric MoxS2may also be present [34,35].In addition,the peak of N 1s is detected in the XPS spectra of PDA@MoS2displayed in Fig.S1,and the intensity of Mo 3d and S 2p peak weakens that implies PDA coated successfully on the appearance of MoS2[36].
FTIR spectroscopy of MoS2,MoS2@PDA,epoxy resin and MoS2@PDA-EP powder coating and the Raman spectra of the prepared MoS2and MoS2@PDA (Fig.2),are utilized to further prove the modification of MoS2by PDA and the existence of MoS2@PDA in the composite coatings.According to the FTIR spectra in Fig.2(a),the weak absorption peaks at 593 and 1120 cm-1could be discovered in both FTIR spectroscopy of MoS2and MoS2@PDA,which are attributed to Mo-S stretching vibrations and S=O asymmetric stretching vibration [37].Meanwhile,the characteristic peaks at 1260 and 1490 cm-1of the FTIR spectra of MoS2@PDA manifest the appearance of the C–C vibrational bond of the benzene ring and the C–O bond of phenol,which demonstrates the successful modification on MoS2by PDA.Fig.2(b) exhibits primary absorption peaks of epoxy resin,which represent O–H,C–H,benzene skeleton vibration,C–O and epoxy group.Whereas,the amplification of the absorption peaks of O–H,benzene skeleton vibration and C–O,which is due to the existence of PDA,certifies the existence of MoS2@PDA in the composite coatings.

Fig.2.FTIR spectroscopy of (a) as received MoS2,MoS2@PDA and (b) epoxy resin,MoS2@PDA-EP powder coating and Raman spectra of MoS2,MoS2@PDA: (c) inorganic section and (d) organic section.
According to Fig.2(c),the two intense characteristic peaks at 385 and 409 cm-1of inorganic section reveal a Raman pattern deriving from phonons with E21 g symmetry and a Raman mode from phonons with A1 g symmetry,respectively [38–40].However,in comparison with the organic section (Fig.2(d)) between MoS2and MoS2@PDA,it can be found that two broad characteristic peaks emerge at 1353 and 1583 cm-1,attributed to the aromaticν(C–N) stretching vibration blended with indole ring stretching [41] and the aromaticν(C–C)accompanied with pyrrole ring stretching vibration [42] which are from PDA.In conclusion,the consistence signifies the success that PDA was efficiently synthesized and evenly modified on MoS2.
The microstructure of the prepared materials was characterized by TEM.Fig.3 displays the morphologies of MoS2and MoS2@PDA.As shown in Fig.3(a),it can be found that MoS2was presented in two-dimensional flakes.And PDA modified MoS2reserves its original morphology as shown in Fig.3(b).However,in comparison with the images,it is found that the thin edge of MoS2@PDA is smoother than the thin edge of MoS2and has a film-like structure.And that is because the nanosheets edge has clear cover layers(the section between yellow lines shown in Fig.3(b-2)) after modified by PDA.Meanwhile,MoS2@PDA retains the original crystal structure by measuring the D-spacing which revealed the crystal orientation of(002),(100)from the matrix of MoS2and MoS2@PDA.And the result is compatible with that of XRD patterns.Moreover,the TEM images could evaluate if the modified nanosheets have good dispersion quality in the organic solvent,since the prepared nanosheets were dispersed in ethanol for TEM.As shown in Fig.S2,although,the two dimensional MoS2nanosheets are prone to conglomerate owing to the surface effect,the MoS2@PDA is dispersed better in the organic solvent.This behavior could be attributed to the presence of PDA modified on MoS2nanosheets which enhance their dispersion in organic media.
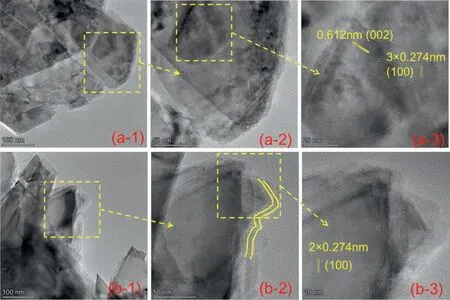
Fig.3.Different magnification TEM images of (a) MoS2 and (b) MoS2@PDA.
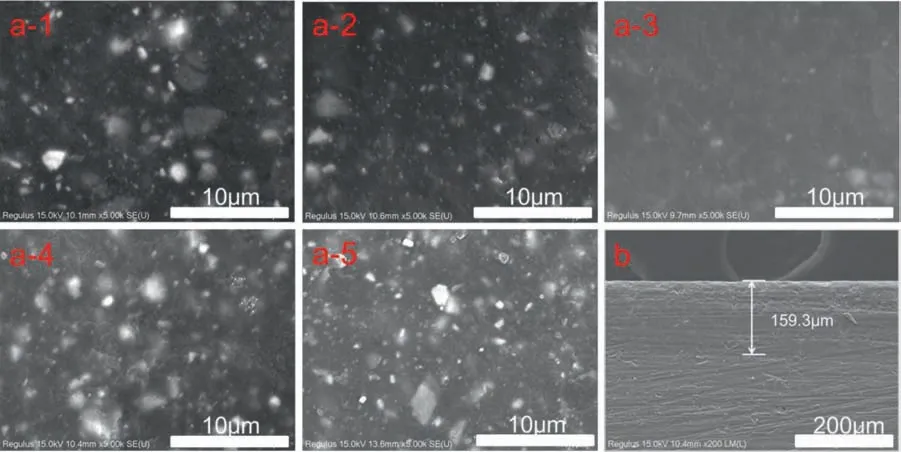
Fig.4.(a) The SEM of different coating surface sections: (1) 0 wt.%,(2) 0.1 wt.%,(3) 0.2 wt.%,(4) 0.5 wt.%,(5) 1.0 wt.% and (b) the SEM of cross section of 0.2 wt.% composite MoS2@ PDA -EP powder coating.
To research the impact of different content of applied MoS2@PDA on the surface morphology of powder coating,different coating surfaces were characterized by SEM.Fig.4 shows SEM images of the surface pure EP coating and different composite PDA@MoS2-EP powder coatings and the cross section of the powder coating on the alloy matrix.There are microcracks with different sized on the surface of epoxy powder coating (Fig.4(a-1)).And as the gradually increase of MoS2@PDA,as shown in Fig.4(a-2) and (a-3),the microcracks on the surface of powder coating decrease significantly,indicating that the nanocomposite has strong cohesion with epoxy resin matrix.Whereas,with the constantly increasing of MoS2@PDA,as shown in Fig.4(a-4) and (a-5),the surfaces of powder coatings become rougher and rougher which would immensely influence the wear resistance of the coating.The phenomenon could be attributed to the agglomeration of the other fillers,which is because that the MoS2@PDA becomes the center of the aggregate due to its high interface energy.What needs illustration is that microcracks regarded as defects in the coatings are reduced.Hence,the efficiency of barrier prevention and anticorrosion property of coating amplifies by means of reducing the possibility of the permeation of the corrosion media.Therefore,high interfacial interactions between the nanofillers and the polymer matrix with proper amount addition in the coating are critical factors for improving the anticorrosion performance of polymer coatings [43].In principle,the 0.2 wt.% composite MoS2@ PDA -EP powder coating displays the best filling effect that there was a few cracks and the surface is smoothest in these powder coatings.The interface between the 0.2 wt.% composite MoS2@ PDA-EP powder coating and the surface of the magnesium lithium alloy matrix is further characterized by SEM (Fig.4(b)).The result of that the coating thickness is consistent with the measured thickness is verified by the SEM of the cross-sectional morphology.In addition,the image reveals well combination between the powder coating and alloy substrate owing to the pre-treatment of silanization on the appearance of magnesium lithium alloys.
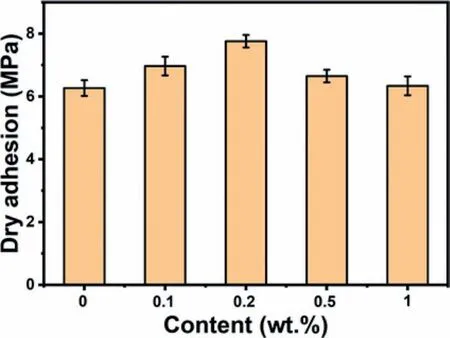
Fig.5.Adhesion strength of powder coating with different content of MoS2@PDA.
3.2.Adhesion of different powder coatings
The interface adhesion between coating and magnesium lithium alloy matrix is a crucial parameter for the property of corrosion protection and mechanism performance.Strong adhesion amplifies the effect of barrier resistance of the coating.Hence,the property of corrosion resistance could be improved.Moreover,it could also avoid the peeling of coating because of long-term flushing by some intruders,which enhances the mechanism property therefore.And the pull-off adhesion test is executed to evaluate the bonding strength between the prepared coatings and alloy substrates.Fig.5 exhibits the adhesion strength of powder coating with different content of MoS2@PDA.And the real morphologies of coating peeling from the alloy matrix are displayed in Fig.S3.It could be found that with the content of MoS2@PDA increasing,the adhesion strength is gradually enhanced.And the highest adhesion strength of 7.76 ± 0.2 MPa is obtained at the addition of 0.2 wt.% MoS2@PDA.Comparing with the adhesion strength (6.27 ± 0.25 MPa) of the neat epoxy powder coating,it could be predicted that the protective performance of 0.2 wt.% MoS2@PDA-EP powder coating would be significantly improved.However,as higher amounts of MoS2@PDA added,the adhesion strength decreases,and the bonding strength of 1.0 wt.% MoS2@PDA-EP powder coating is 6.34 ± 0.3 MPa,which may be attributed to the poor dispersion of MoS2@PDA in the polymer coating.Nevertheless,the application of the MoS2@PDA is certified that the adhesion strength between coating and alloy matrix could be enhanced.
3.3.Wear resistance of different powder coatings
The characteristic of low hardness of magnesium lithium alloy determines its weak resistance to wear and impact,and it is easy to cause scratch and wear failure of components under heavy load or impact load [44].Hence,this defect immensely limits the application of magnesium lithium alloy in some special work environment.Fig.6 displays the coefficient of friction (COF) of different content MoS2@PDA-EP,which could be utilized to assess the abrasion performance of the powder coating.As the Fig.6 shown,the COF decreases obviously with the application of MoS2@PDA in the powder coating.And the least COF of 0.15–0.2 is obtained by 0.2 wt.% MoS2@PDA-EP.This is because MoS2with hexagonal crystal system has a laminated structure,hence,this structure slides easily that generates the COF reduction of powder coating.Besides,the ion bond between the atom of Mo and S endows MoS2@PDA-EP higher strength.However,the continuous addition of MoS2@PDA increases the COF of powder coating.Even,the COF of 1.0 wt.% MoS2@PDA-EP is higher than that of neat powder coating.With the amount of MoS2@PDA adding,the aggregation effect is more remarkable,which causes the aggregation of MoS2@PDA and other fillers on the surface of powder coating.This consequence is compatible with the coating images of SEM (Fig.4).
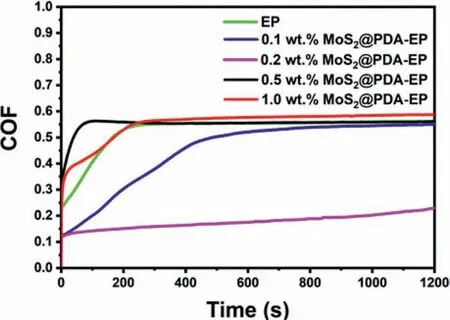
Fig.6.The COF of powder coatings with different content of MoS2@PDA.
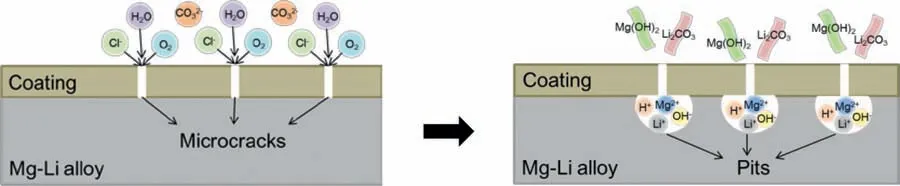
Fig.7.The corrosion behavior of magnesium lithium alloy with powder coating.
3.4.Anticorrosion properties of different powder coatings
3.4.1.Anticorrosion mechanism of MoS2@PDA-EP powder coating
The corrosive manifestation of magnesium lithium alloys in NaCl aqueous solution presents the morphology of corrosive pitting and filiform corrosion [45].As shown in Fig.7,the chloride ion would penetrate through microcracks in the powder coating which is owing to the small ion radius and arrive at the surface of alloy substrate.Subsequently,chloride ions initially adsorb on the alloy substrate.Then,the metal ions would be extruded from the matrix,and form a soluble salt combined with chloride ions,resulting in the generation of small pits at exposed substrate.
The front reactions of corrosion on the substrate Eqs.(1)-(8) are as follows:
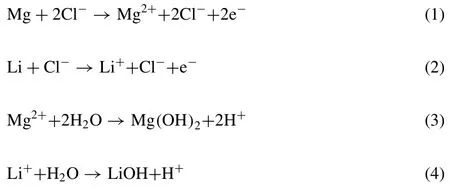
The tail end reactions of corrosion on the substrate are as follows:
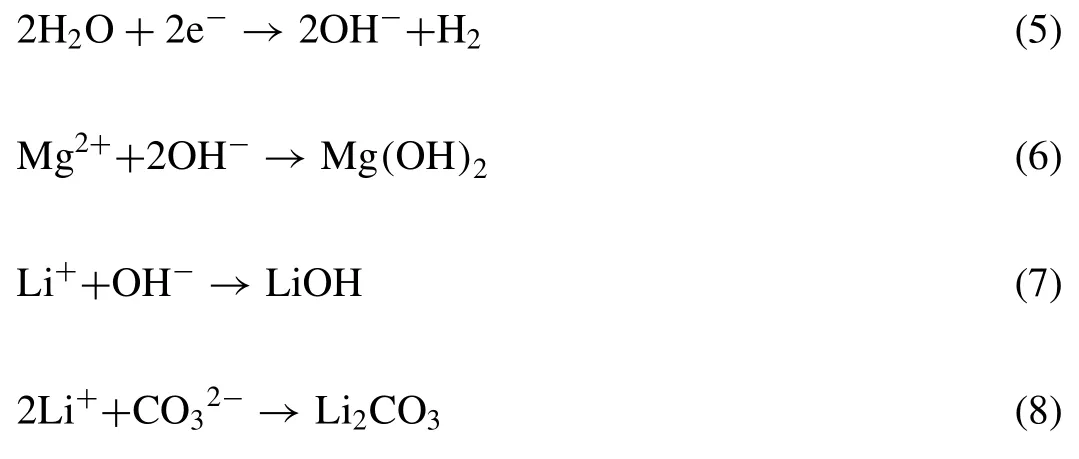
Fig.8.The schematic diagram of anticorrosion principle of MoS2@PDA in epoxy resin powder coating.
The generation of corrosion products significantly impairs the bonding strength between the powder coating and alloy matrix.The anticorrosion properties of coating dramatically decrease and even vanish due to the peeling of coating from the alloy substrate.There are some efficient methods reported for anticorrosion,such as fabricating a superhydrophobic surface (a stearic acid/CeO2bilayer coating) [16] and the self-healing anti-corrosion coatings (clinochrysotile-like magnesium silicate nanotubes loaded with corrosion inhibitor 2-mercaptabenzimidazole) [46].
The anticorrosion principle of MoS2@PDA is filling the microcracks in the powder coating and blocking the intrusion of corrosive media through the special two-dimensional layered structure of MoS2as displayed in Fig.8.However,the aggregation effect of MoS2inevitably occurs since the special structure and the bond between MoS2and epoxy resin is weak.Therefore,the modification of PDA is essential to enhance the binding force between MoS2and epoxy resin.Meanwhile,it transforms the edge morphology of MoS2and reduces the occurrence of the aggregation.
3.4.2.EIS of different powder coatings
One of the most effective and destruction-free measurement to evaluate the corrosion protection of the coating is EIS measurement [47].The corrosion protection properties of different content MoS2@PDA-EP powder coatings are estimated through the EIS after disparate immersion time in 3.5% NaCl solution and the consequences are displayed in Fig.9.The anticorrosion performance of the coating is positively associated with the radius of the semicircle in the Nyquist plots[48].In general,the anticorrosion property is more excellent with the larger radius of the semicircle.
Fig.9(a) shows the Nyquist plots of different coatings after 1d immersion.In the primal corrosive stage,the coatings maintain the integrity and corrosive media rarely intrude through the coating and arrive at the surface of the alloy matrix.Hence,it could be found that there is no obvious distinction between the impedance data of different powder coatings except for 0.2 wt.% MoS2@PDA-EP powder coating.The 0.2 wt.% MoS2@PDA-EP powder coating presents the best anticorrosion performance initially.
As immersion time increased,significant difference has emerged between the radius of the semicircle in the Nyquist plots which represents the performance of anticorrosion.Fig.9(b) displays the Nyquist plots of different coatings after 4d immersion.Compared with the plots of 1d immersion,the radiuses in the Nyquist plots of different coatings after 4d immersion increased with distinct levels except the neat coating.And this is because that the MoS2@PDA expanded and filled the microcracks while the solution penetrated the coatings.According to the graph,the value of|Z|0.01Hzpresents increase with varying degrees.It could be found that the anticorrosion performance improved with the addition of MoS2@PDA,and the best performance is obtained at the 0.2 wt.% MoS2@PDA-EP powder coating again.However,the corrosion resistance of powder coating recedes with the constant addition of MoS2@PDA which may be owing to the agglomeration of MoS2@PDA.
While immersion time reached at 7d,the consequences are shown in Fig.9(c).Gradual decline of the value of|Z|0.01Hzof all coatings indicates that the corrosion stage enters the medium-term phase.However,for the neat coating,the impedance spectrum manifests the basic failure of the barrier.And the results of other coatings reveal the similar trend of previous measurements.
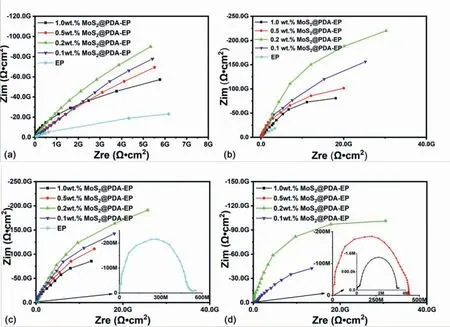
Fig.9.Impedance spectra of the different coatings prepared in 3.5 wt.% NaCl solution at different immersion time: (a) 1d,(b) 4d,(c) 7d,(d) 14d.
With the immersion time increasing,the consequences of EIS measurement at 14d are displayed in the Fig.9(d).After the constant intrusion of the solution,plentiful chloride ion reached at the surface of the alloy and caused the appearance of corrosive pitting and the peeling of the coatings.The diagram indicates that the 1 and 0.5 wt.% MoS2@PDAEP powder coatings failed and incapacitated the protection in succession.However,the other powder coatings still remain the barrier effect.In particular,the 0.2 wt.% MoS2@PDA-EP powder coating reveals excellent anticorrosion performance yet.
3.4.3.Polarization curves of different powder coatings
Polarization curves could evaluate the corrosive rate of different coatings more apparently.And the consequences are displayed in the Fig.10.By the method of extrapolation,corrosion current density could be quantitatively researched according to the polarization curves [49].The relevant corrosive data obtained from Fig.10 are listed in Table 2.ECorr,ICorrandηpin Table 2 represent the corrosive potential,corrosive current density and corrosion inhibition efficiency.Andηpis calculated by the Eq.(9) [50]:

TheI0CorrandICorrin the Eq.(9) represent the corrosive current density of neat coatings and other coatings,respectively.According to the data in the Table 2,the application of MoS2@PDA could efficiently amplify the barrier effect of the powder coating.Meanwhile,the polarization curves reveals similar trend on the addition of nanocomposite compared with the EIS.Among them,the 0.2 wt.% MoS2@PDA-EP powder coating obtain the maximum self-corrosive potential and minimum self-corrosive current density,which indicates that it possesses the best anticorrosion performance.Andηpin Table 2 demonstrate the same conclusion this which is compatible with the verdict summarized from the EIS.
To comprehensively understand functionality of the asprepared organic/inorganic powder coating in this study,a brief comparison of coatings (including prepared methods,main materials,electrochemical impedance andIcorr) on magnesium and lithium alloys corrosion protection previously reported in literatures was summarized in Table 3.From Table 3,it is easy to find out that the anticorrosion performance of the materials used here demonstrates satisfactory superiority in comparison with most of previously-reported coatings.
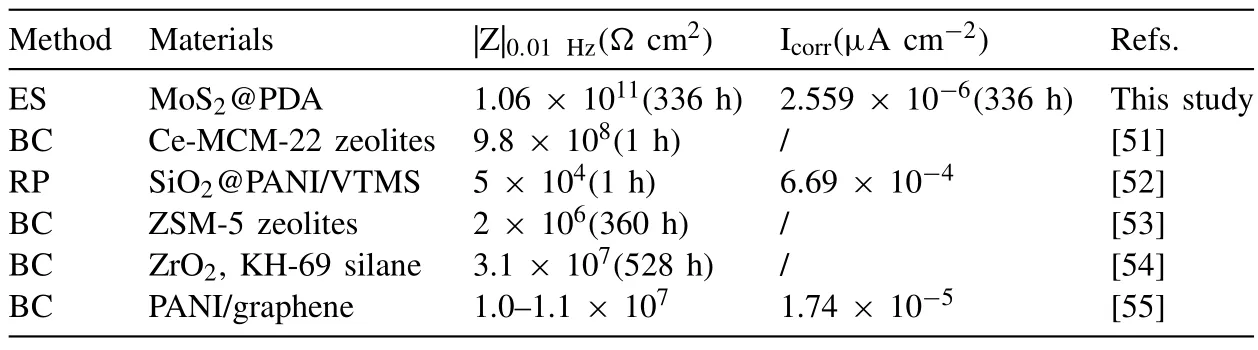
Table 3 Preparation method and performance comparison of coatings on magnesium lithium alloys corrosion protection.
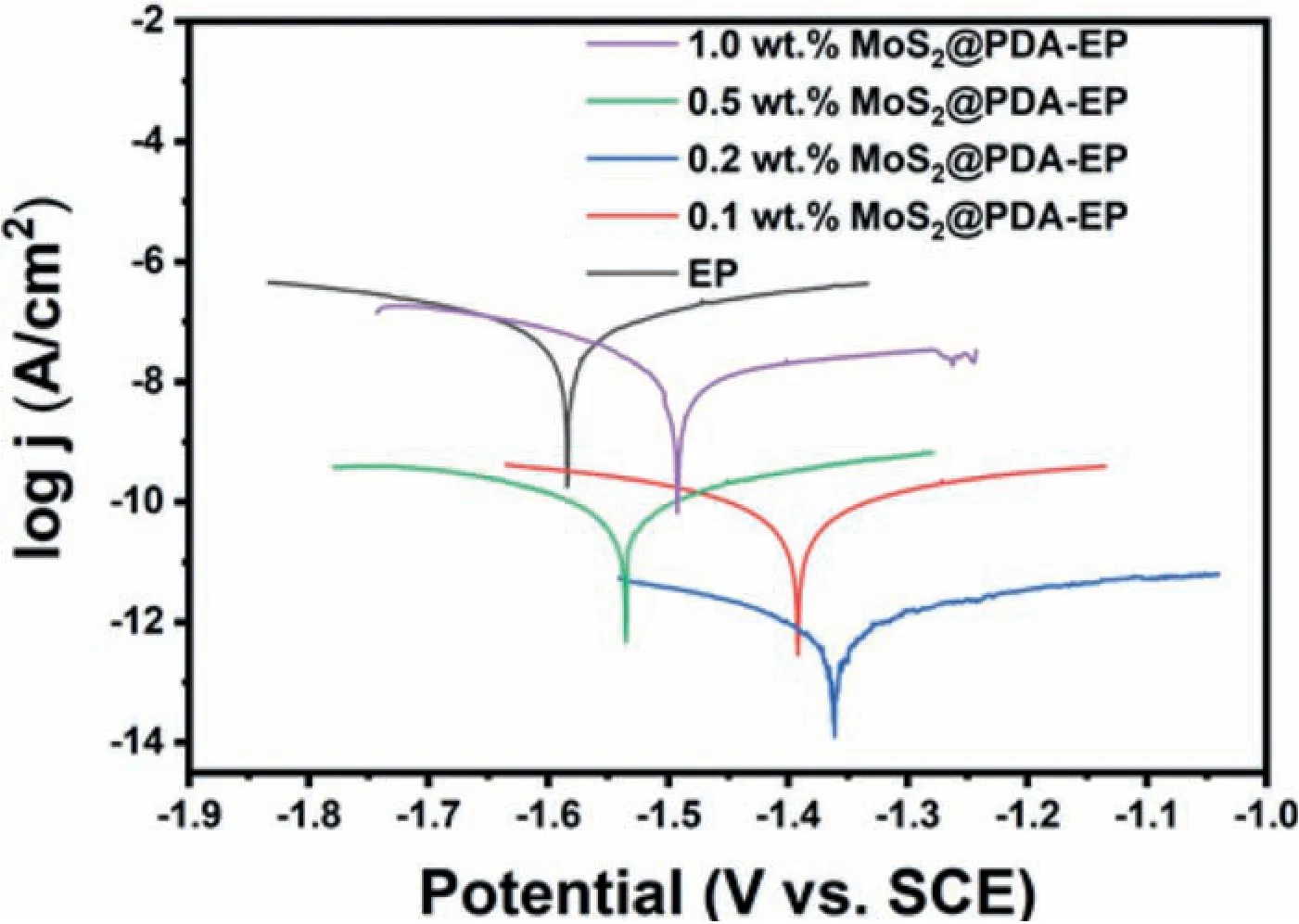
Fig.10.Polarization curves of different powder coatings after 14d immersion.
4.Conclusion
In this work,MoS2nanosheets modified with PDA were introduced in the epoxy powder coatings for the improvement of anticorrosion performance on the magnesium lithium alloys.And the barrier effect of different amount of nanofillers in the coatings has been researched.The modification of PDA alleviates the agglomeration of MoS2nanosheets,accomplishes well dispersion of MoS2nanosheets in the coatings and enhances the adhesion with the epoxy matrix.Consequently,it efficiently improves the anticorrosion performance of epoxy powder coating which could be concluded from the result the measurement of EIS and polarization curves.Meanwhile,contributed to the lamellar structure of MoS2,the property of wear resistance of powder coatings has been substantial improved.By comparing different amount of MoS2nanosheets,it can draw a conclusion that the 0.2 wt.% MoS2@PDA-EP powder coating possesses the optimal performance based on adhesion test,wear resistance test and electrochemical measurements.In conclusion,0.2 wt.%MoS2@PDA-EP powder coating could be applied on the magnesium lithium alloys for enhanced anticorrosion performance and wear resistance.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China(Grant No.U1806225),the National Natural Science Foundation of China (Grant No.51908092),the Joint Funds of the National Natural Science Foundation of China-Guangdong(Grant No.U1801254).The authors also thank the Electron Microscopy Center of Chongqing University for materials characterizations.
Supplementary material
Supplementary material associated with this article can be found,in the online version,at 10.1016/j.jma.2021.08.007.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Novel Mg-Bi-Mn wrought alloys: The effects of extrusion temperature and Mn addition on their microstructures and mechanical properties
- Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform
- Passivation of corrosion product layer on AM50 Mg by corrosion inhibitor
- A crystal plasticity based approach to establish role of grain size and crystallographic texture in the Tension–Compression yield asymmetry andstrain hardening behavior of a Magnesium–Silver–Rare Earth alloy
- Novel extended C-m models of flow stress for accurate mechanical and metallurgical calculations and comparison with traditional flow models
- Unexpected high-temperature brittleness of a Mg-Gd-Y-Ag alloy
