Passivation of corrosion product layer on AM50 Mg by corrosion inhibitor
2022-10-24YnLiXiopengLuDiMeiToZhngFuhuiWng
Yn Li ,Xiopeng Lu,* ,Di Mei ,To Zhng ,Fuhui Wng
a Shenyang National Laboratory for Materials Science,Northeastern University,3-11 Wenhua Road,Shenyang 110819,China
b School of Materials Science and Engineering and Henan Key Laboratory of Advanced Magnesium Alloy,Zhengzhou University,100 Kexue Road,Zhengzhou 450001,China
Abstract The influence of sodium dodecyl sulfate (SDS) on morphology and chemical composition of corrosion product layer formed on α-Mg matrix and cathodic Al-Mn intermetallic was systematically investigated by using FIB and TEM analysis for the first time to disclose the underlying inhibition mechanism.A porous corrosion bi-layer composed of crystalline MgO and Mg(OH)2 was observed on both of α-Mg and Al-Mn intermetallic.It was found that a passive inner layer was deposited on α-Mg after immersion in SDS-containing NaCl solution,which can be ascribed to steady-state growth of magnesium and aluminum oxide under the protection of hydrophobic group of SDS.The inhibition mechanism of the inhibitor was mainly associated with formation of dense oxide layer on α-Mg matrix and preferential adsorption of SDS on the corrosion layer deposited on Al-Mn intermetallic.
Keywords: Magnesium;TEM;FIB;Inhibition mechanism.
1.Introduction
Magnesium (Mg) and its alloys are promising structural materials in aerospace and automotive applications due to high strength-to-weight ratio and excellent damping behavior,which are restricted due to relatively poor corrosion resistance[1–6].Alloying is a practical approach for enhancement of the corrosion and mechanical properties of Mg alloys.The addition of Al is conducive to improvement of yield strength and corrosion performance by solid solution strengthening and formation of Al-containing secondary phases[7–10].The continuous distributed net-likeβ-phase(Mg17Al12) in Mg-Al alloys provide a barrier effect against corrosion propagation.However,the Mg17Al12secondary phases are also cathodic sites for initiating the pitting corrosion because of high volta potential difference (VPD)betweenα-Mg and secondary phase [11].Alloying with Mn enhances the corrosion resistance of Mg by capturing iron impurity and facilitates their formability during extrusion[12–14].In addition,Mn-containing precipitates exhibit apparent effect on grain size by suppressing the growth of crystallized grains [15,16].However,the co-alloying of Al and Mn leads to formation of Al-Mn intermetallics,such as Al8Mn5,Al6Mn and Al10Mn3[17,18],which show acceleration effect on Mg corrosion since the VPD between Al-Mn intermetallic and Mg matrix (800 mV) is much higher than that between Mg17Al12andα-Mg (400 mV) [19].Williams et al.[20] reported that noble Al-Mn intermetallic was the source of cathodic activation of the corroded surface.
The utilization of corrosion inhibitors has been regarded as an effective strategy to provide protection for Mg and its alloys [21–27].Hu et al.[25] found newly formed corrosion products with paeonol retarded dissolution of Mg.8-hydroxyquinoline (8-HQ),phosphatase,fluoridates and sodium dodecylbenzenesulfonate (SDBS) were found to suppress corrosion of ZK30 Mg alloy by forming Mg(8-HQ)2,Mg3(PO4)2·22H2O,MgF2and Mg-SDBS protective precipitates on Mg surface [28].Although formation of new precipitations improves the corrosion resistance,they cannot completely prevent penetration of aggressive Cl-ions due to existence of cracks and pores in corrosion layer.The synergy between phosphate with sodium dodecylbenzene sulfonate(SDBS)and sodium alginate(SA)modulated Mg corrosion by formation of a more compact and homogeneous layer[29,30].Surfactants are also considered to be effective inhibitors by forming a physical adsorption barrier that reduces corrosion.Dinodi and Shetty[31]showed alkyl carboxylates can be used as efficient corrosion inhibitors for ZK41 Mg alloy.The inhibition effect was ascribed to the compact corrosion layer by adsorption of alkyl carboxylates on Mg surface.Daloz et al.[32] also investigated sodium carboxylates as inhibitor and proposed that the decreased corrosion rate was a result of formation of Mg(CH3[CH2]nCOO)2.In recent years,it was found that the corrosion performance of Mg substrate can be enhanced by blocking the cathodic phases.Lamaka et al.[21,33,34] revealed that iron complex agents were capable of improving corrosion resistance of pure Mg by chelating iron impurities (the main cathodic phase in pure Mg).Yang et al.[35] also reported that 3-methylsalicylate effectively inhibit electrochemical activity of iron-rich particles in Mg.It is wellknown that the cathodic effect of the Al-Mn intermetallic in Mg-Al-Mn alloys is pronounced.Early studies have revealed that sodium dodecyl sulfate (SDS) can inhibit corrosion of AZ91 Mg alloy in NaCl solution [22,36].Nevertheless,the effect of SDS on the microstructure and composition of Mg surface,specifically corrosion products formed on top of anodic and cathodic phases,during corrosion test is not systematically elaborated,which is of great importance to reveal inhibition mechanism of the inhibitor.
In the present work,AM50 alloy was selected as Mg substrate to study the inhibition effect of SDS on the anodic dissolution and cathodic reaction kinetics to understand the underlying corrosion inhibition mechanism.FIB and TEM were performed to investigate the influence of corrosion inhibitor on the microstructure,morphology and chemical composition of the corrosion product layer formed onα-Mg matrix and cathodic Al-Mn intermetallic after immersion in NaCl solution.
2.Experimental
2.1.Material and electrolyte
Commercial AM50 Mg alloy was ground up to 2000 grit sandpaper,ultrasonically washed with alcohol and dried in cold air.The corrosive media are 3.5 wt.% NaCl solutions with and without addition of 0.05 mol/L SDS (Macklin Biochemical Co.,Ltd,China).
2.2.Corrosion measurements
Samples in size of 30 × 30 mm2were used for hydrogen evolution test for 48 h,which could reflect the general corrosion rate of Mg in aqueous solution [37–39].The inhibition efficiency (η) of SDS was calculated using Eq.(1).

in whichVHEandare volume of evolved hydrogen during immersion in blank NaCl solution and inhibitor containing solution,respectively.
Electrochemical corrosion measurements were performed using a Princeton V3F potentiostat (Ametek,USA).The exposed region of specimens for electrochemical corrosion test was 10×10 mm2.Mg samples were working electrode,saturated calomel electrode (SCE) was used as the reference electrode and the platinum plate was the counter electrode.After immersion 20 min for stabilization,potentiodynamic polarization was performed from open circuit potential (OCP) to the anodic and cathodic side at a rate of 0.333 mV/s.Electrochemical impedance spectroscopy (EIS) measurements were tested from 105to 10-2Hz with 10 mV sinusoidal perturbation.The obtained EIS data were analyzed by using Zsimp-Win software.
2.3.Microstructure and composition of the corroded specimens
The macroscopic and microscopic morphology of Mg alloy after 48 h immersion in different electrolytes were investigated by optical microscopy and scanning electron microscope (JEOL,Japan),respectively.In order to observe the cross-sectional morphology,the corroded samples were encapsulated in epoxy,ground using emery papers up to 2000 grit,polished and rinsed by ethanol.The surface roughness of the samples after removal of corrosion product was measured by means of laser confocal microscope (Olympus,FV1200,Japan).Elemental distribution on the metal surface was measured using electron probe microanalysis (EPMA,JEOL,Japan).The composition of the corrosion product on sample surface was analyzed by using X-ray photoelectron spectroscopy (XPS).The sample was cleaned by Ar+ion sputtering for 60 s with an ion energy of 3 keV to remove the contaminated layer on the surface.XPS measurements were performed with Al Kαanode on an ESCAALAB 250(hot VG) spectrometer.High resolution spectra of C 1s,Mg 1s,Al 2p,S 2p and Mn 2p were recorded and XPSPEAK software (version 4.1) was used for peak fitting.The binding energy scale was corrected with the C 1s peak at 284.6 eV as the reference.The deconvolution of high-resolution XPS peaks was carried out by mixed Gaussian-Lorentzian fit after the background was subtracted using the standard Shirley method [40–42].

Fig.1.Surface and cross section morphology of Mg alloy in (a,b,c and d) NaCl solution and (e,f,g and h) inhibitor containing NaCl solution after immersion for 48 h.(For interpretation of the references to color in this figure,the reader is referred to the web version of this article.)

Fig.2.Hydrogen evolution test of Mg alloy in various NaCl solutions (b) evolution of open circuit potential and (c) potentiodynamic polarization curves of the samples.
Samples were immersed 24 h in various NaCl solutions before performing focused ion beam (FIB,Crossbeam550,Germany) and transmission electron microscopy (TEM,JEM-ARM200F,Japan).The TEM lamellae were milled from the corroded specimen by using FIB with an acceleration voltage of 30 keV.A tungsten layer was deposited on the sample surface to prevent the influence of electron bombardment.The selected area electron diffraction (SAED) of specific regions was obtained.In order to prevent influence of ion beam,a tungsten protective layer was deposited on the sample surface.

Fig.3.EIS of the samples after immersion in (a) NaCl solution and (b) inhibitor containing NaCl solution for different times (1,24 and 48 h).Equivalent circuits used to fit EIS data (c) NaCl solution and (d) SDS containing NaCl solution.

Fig.4.SEM images and EDS analysis of the samples after immersion test for 48 h (a) NaCl solution and (b) SDS containing NaCl solution.
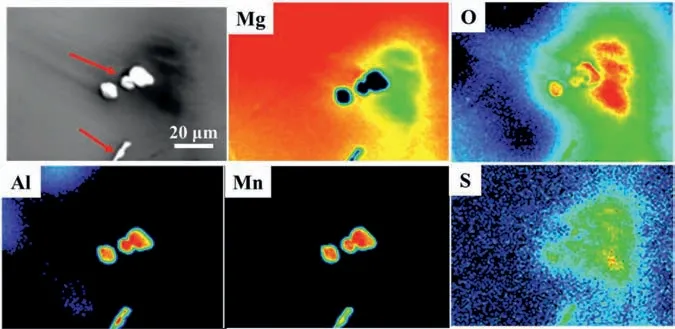
Fig.5.EPMA mapping on the surface of the corroded samples after immersion 48 h in inhibitor containing NaCl solution.
2.4.Quantum chemical calculation
The quantum mechanical calculation in this study was performed using the DMol3 module of Material Studio program (BIOVIA Company,San Diego,CA,USA),employing the B3LYP exchange-correlation functional.The properties of SDS molecule,such as the energy of HOMO (EHOMO) and LUMO (ELUMO),energy gapΔE (ΔE=ELUMO-EHOMO),and Fukui indices of the molecule,were calculated based on the optimized SDS molecule model.The convergence value for geometry optimization was 0.005 ˚A for displacement,2 × 10-5eV/atom for energy,0.004 eV/˚A for force.
3.Results
3.1.Microstructure and morphology of the corroded samples
Figure 1 displays the optical and micrographs of the samples after performing immersion test.The sample surface is heavily corroded and covered by corrosion products (Fig.1a and b).The cross-section micrograph of the corroded sample shows Mg suffers severe localized corrosion in NaCl solution (Fig.1c).Figure 1f and g show that the dense product layer is deposited on Mg surface after immersion into SDS containing solution.In addition,after 48 h immersion,the scratches generated during grinding process are still visible on the sample surface (Fig.1f),indicating that the corrosion layer is thin but protective after addition of corrosion inhibitors.The three-dimensional (3D) images and average roughness (Ra) are shown in Fig.1d and h.The roughness of the sample surface after removal of corrosion product was 22.48 ± 2.17 μm (NaCl solution) and 4.16 ± 0.66 μm (inhibitor containing solution),respectively.The general corrosion of Mg substrate can be reflected by the bumpy surface with blue valleys and red ridges (Fig.1d).The surface of sample was flat after immersion in inhibitor containing NaCl solution at the same magnification (Fig.1h).In general,the results indicate high inhibition efficiency of SDS on Mg corrosion,which are probably originated from the SDS adsorption layer on Mg surface.
3.2.Corrosion tests
Figure 2a shows the evolved volume of H2during 48 h immersion test.The samples immersed in blank solution show large volume of evolved hydrogen.The corrosion rate of AM50 Mg alloy has been greatly reduced in the presence of inhibitor.After 48 h test,the total amount of evolved hydrogen of Mg was 9.98 ± 0.82 and 0.21 ± 0.11 mL/cm2,respectively.According to Eq.(1),the inhibition efficiency of SDS is more than 97%.
The evolution of OCP of the samples during immersion in various electrolytes is depicted in Fig.2b.It is noteworthy that the open circuit potential of samples immersed in inhibitor containing NaCl solution is much lower than that in blank NaCl solution at the initial stage of corrosion test.However,OCP shifts towards noble potential and eventually reached -1.55 V (vs SCE) after 24 h The stable OCP indicates formation of a stable and protective corrosion product layer on the metal surface [43].The obvious difference is that OCP is constantly fluctuating in blank NaCl solution,suggesting high corrosion rate of the substrate.
Figure 2c demonstrates polarization curves of Mg samples after immersion in different NaCl solutions.Cathodic Tafel extrapolation was used to calculate corrosion current density.The corrosion current density of samples immersed in blank NaCl solution is 46.5 ± 5.3 μA/cm2,which is reduced to 17.6 ± 2.1 μA/cm2in presence of SDS.In addition,passivation region appears in the anodic branch because of formation of a protective corrosion product layer.With the addition of SDS,the cathodic branch is shifted to left direction,which implies the efficient inhibition effect of SDS on the cathodic reaction kinetics.The result suggests the inhibitor is effective to suppress both cathodic and anodic process of Mg corrosion.
The EIS spectra of the Mg samples during 48 h immersion in different corrosion solutions are presented in Fig.3.The capacitance loop gradually decreases and inductance loop is more obvious in blank NaCl solution (Fig.3a).Similarly,reduction of impedance modulus (|Z|) and phase angle is illustrated in Bode spectra.On the contrary,the Nyquist plots of the sample in inhibitor-containing solution comprise of two capacitive loops (Fig.3b).The continual increase of the impedance at low frequency indicates the inhibition effect of SDS on Mg corrosion[44].The widened phase angle together with the increased impedance suggest the enhanced corrosion performance of the corrosion layer.

Fig.6.XPS spectra of the samples after immersion 48 h in inhibitor containing NaCl solution (a) survey spectra,(b) C 1s,(c) Mg 1s,(d) Al 2p,(e) S 2p and (f) Mn 2p.
Equivalent circuits (Fig.3c and d) are used to fit the EIS data to explore the electrochemical corrosion reactions occurring at the metal-electrolyte interface.Rsis the solution resistance,Rcpis corrosion resistance and constant phase element (CPEcp) represents capacitance of the corrosion products.RctandCPEdlis the charge transfer resistance and double layer capacitance,respectively.RLandLare related to the inductive resistance and inductance of the samples in the later stage of corrosion test.The fitted data are showed in Table 1.In the initial stage,Rcpis 1287 ± 243Ωcm2in NaCl solution,while it decreases to 757.2 ± 122Ωcm2andRctis only 184.2 ± 32.1Ωcm2after immersion 48 h in the blank electrolyte.In contrast,RcpandRcthave been greatly increased (9440 ± 347 and 8525 ± 402Ωcm2) after immersion in SDS containing NaCl solution.

Table 1 Fitted results of the EIS data.
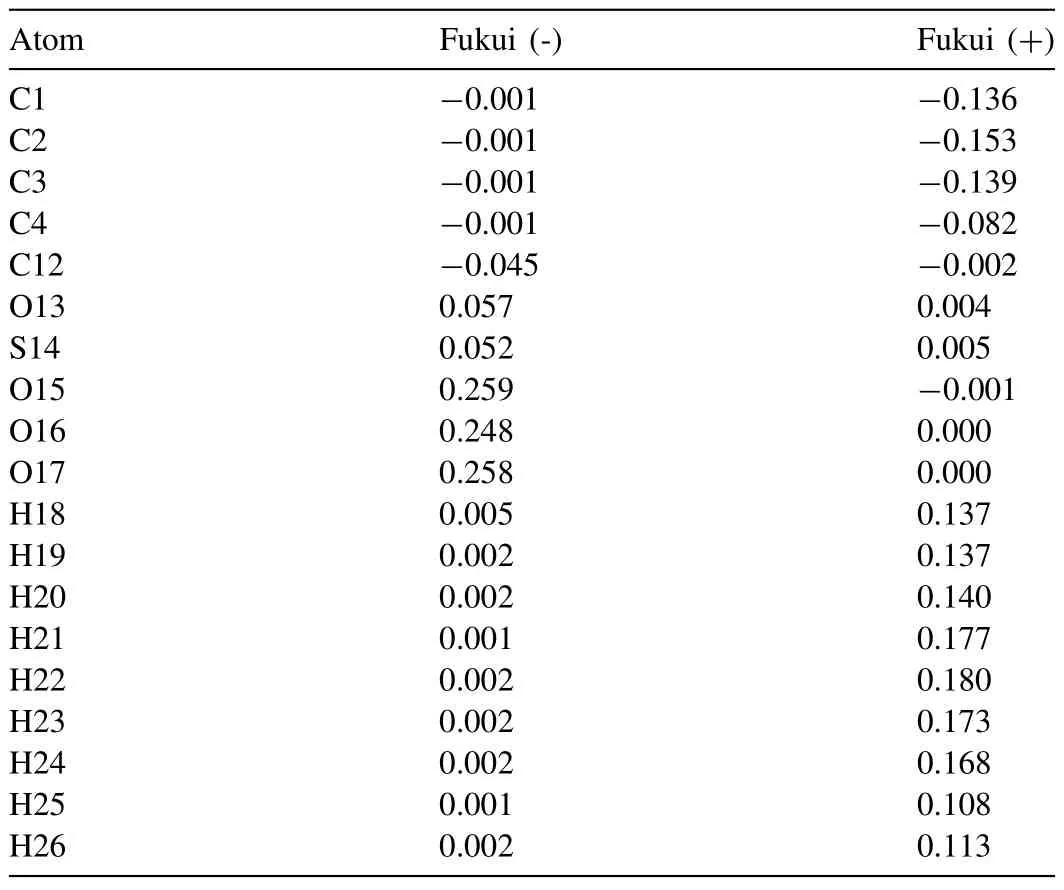
Table 2 Mulliken charges for SDS anion.
3.3.Characterization of the corrosion product layer
Figure 4 shows SEM images and elemental composition of the corrosion products formed on Al-Mn intermetallic after immersion in different NaCl solutions.The Al-Mn intermetallic behaves as cathode with respect to the matrix and thus induces micro-galvanic corrosion.Severe corrosion occurs around Al-Mn intermetallic and thick corrosion products are found on the sample surface.As the evidence of SDS,S is detected at the corrosion products formed on Al-Mn intermetallic after immersion in inhibitor containing solution.
EPMA was employed to analyze the distribution of inhibitor on the surface of corroded samples after immersion test (Fig.5).It can be seen that Al-Mn intermetallic is surrounded by large amount of corrosion products due to microgalvanic corrosion.The detection of S implies adsorption of corrosion inhibitors in the corrosion layer,which are only detected on top of Al-Mn intermetallic and the surroundingα-Mg.The entire oxidation area on the Mg surface seems to be much larger compared to the inhibitor containing region,indicating that the existence of SDS might inhibit the micro-galvanic corrosion of the substrate.
XPS spectra were carried out to study the composition of the corrosion products (Fig.6).In terms of C 1s,the peak is observed in 284.6 eV,which is related to -(CH2)n-[45].The Mg 1s spectrum is fitted with two components apart from Mg at 1302.7 eV for Mg(OH)2and 1304.1 eV for MgO[46,47].Figure 9e shows the S 2p spectra,indicating that SDS is present on the surface of corroded samples.In addition,Al2O3is detected in the spectra as part of the corrosion products.
Figure 7 displays overview of the cross section of the corrosion product formed on Mg sample after immersion in blank NaCl solution.The thickness of corrosion products on Al-Mn intermetallic is about 2 μm,which is much thicker than that on Mg matrix.Fig.7b demonstrates that the thickness of corrosion layer onα-Mg matrix is approximately 300 nm.The corrosion layer appears to be porous and nonuniform on bothα-Mg and Al-Mn intermetallic.To compare composition of corrosion products at different locations,SAED of corrosion products is performed and shown in Fig.8.The column on the left presents the location bright field corresponding to the SAED patterns.It is observable that the outer corrosion layer is mainly composed of Mg(OH)2,while the inner corrosion layer consists of Mg(OH)2and MgO onα-Mg matrix.As for corrosion products formed on Al-Mn intermetallic(Fig.8b),the corrosion bi-layer is much thicker compared to that formed onα-Mg and phase composition of the corrosion layer is a mixture of Mg(OH)2and MgO.

Fig.7.TEM images of the corrosion products of samples immersed in the blank NaCl solution.(a) TEM overview,(b) microstructure of the corrosion layer formed on α-Mg and(c)microstructure of the corrosion layer on Al-Mn intermetallic.
However,the microstructure and composition of the corrosion product layer on samples immersed in inhibitor containing solution show considerable difference.The corrosion product layer onα-Mg is composed of two sub-layers (a dense inner layer and a porous outer layer),while the corrosion products with single-layered structure forms on Al-Mn intermetallic.Fig.9b shows that the porous outer corrosion layer is irregularly shaped,while the inner corrosion layer is dense and defect-free.The SAED of the selected regions on the cross section of the corrosion layer is shown in Fig.10.Mg(OH)2is the primary corrosion product formed on the surface of Al-Mn intermetallic,while the corrosion product formed onα-Mg is mainly composed of amorphous phase (Fig.10a).
Figure 11 shows the HAADF-STEM images and EDS mappings of the corrosion product layer.For the specimens immersed in NaCl solution,Mg and O are the main elements in the corrosion layer (Fig.11a).The presence of inhibitor significantly influences the microstructure and composition of the corrosion product layer (Fig.11b and c).The inner layer is relatively compact on top ofα-Mg and is composed of O,Mg and Al.For the outer corrosion layer,S signal can be visible because of adsorption of corrosion inhibitor in the corrosion layer.As for the cathodic Al-Mn intermetallic(Fig.11c),the corrosion layer is porous and mainly composed of Mg,O,Al and S,which is similar to the elemental composition in the outer layer onα-Mg.Additionally,the signal of Mn distribution is relatively weak in the corrosion product layer.

Fig.8.High magnification and SAED ring of the corrosion products formed on samples immersed in 3.5 wt.% NaCl solution.(a) corrosion products formed on α-Mg and (b) corrosion products formed on Al-Mn intermetallic.

Fig.9.TEM images of corrosion products of samples immersed in SDS containing NaCl solution.(a) TEM overview,(b) microstructure of the corrosion layer on α-Mg and (c) microstructure of the corrosion products formed on Al-Mn intermetallic.
3.4.Quantum chemical calculation

Fig.10.High magnification and SAED ring of the corrosion products formed on samples immersed in SDS containing NaCl solution(a)corrosion products formed on α-Mg and (b) corrosion products formed on Al-Mn intermetallic.

Fig.11.STEM-HAADF micrograph and elemental distribution of the corrosion product layer.(a) corrosion products of samples immersed in NaCl solution,(b) corrosion products on α-Mg in SDS containing NaCl solution and (c) corrosion products on Al-Mn intermetallic in SDS containing NaCl solution.

Fig.12.(a) Optimized geometry structure of SDS anion,(b) HOMO and (c)LUMO.
Quantum chemical calculations were carried out to acquire more information between the reactive sites and corrosion inhibition efficiency.The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital(LUMO) take part in interactions between the corrosion inhibitor and Mg alloy based on the frontier molecular orbital theory [48–50].Figure 12 exhibits the optimized SDS anion structure,HOMO and LUMO images.It can be seen that the HOMO of SDS is mainly located on sulfate,while LUMO is detected in the tail of alkyl.This indicates that the alkyl chains play an important role in accepting electrons and the-SO4groups are able to donating electrons when SDS adsorbs on the corrosion product layer on Mg surface.Lower value ofΔE is prone to show higher inhibition efficiency [51].The Mulliken charges including Fukui(+)and Fukui(-)are calculated and shown in Table 2.The S and O atoms are locations for electrophilic attack,while H and C atoms are potential locations for nucleophilic attack.
4.Discussion
As mentioned above,the corrosion inhibition of SDS on AM50 Mg alloy is evidenced by electrochemical corrosion measurements and microscopic analysis of the corrosion product layer.The micro-galvanic corrosion between anodicα-Mg and cathodic Al-Mn intermetallic is greatly suppressed in the presence of corrosion inhibitor.The microstructure and composition of the corrosion layer are both influenced and modified by the corrosion inhibitor.On the one hand,the anion of SDS moves towards the metal surface and sulfate radical is preferentially adsorbed on the corrosion products.In the meanwhile,the adsorption of corrosion inhibitor is competitive with other anions from the electrolyte,e.g.,Cl-and OH-.One the other hand,the hydrophobic group of the surfactant is capable of suppressing adsorption of corrosive ions (Cl-).Therefore,the physical adsorption of corrosion inhibitors in the outer porous corrosion layer on bothα-Mg and Al-Mn intermetallic reduces anodic dissolution rate and cathodic reaction kinetics,leading to decreased deposition rate of Mg(OH)2and corrosion attack from the corrosive ions.It should be noted that the oxidation process of the Mg substrate still occurs due to existence of such passive and hydrophobic outer corrosion layer.Consequently,dissolution of substrate and deposition of Mg(OH)2on Mg surface have been inhibited,while formation of thick and protective inner oxide layer is facilitated.The active Mg surface is protected by a passive and compact corrosion product layer (mainly amorphous MgO and Al2O3) in the presence of SDS,resulting in the high inhibition efficiency.
5.Conclusion
1 A passive corrosion product layer is formed on surface of AM50 Mg alloy in sodium dodecyl sulfate containing NaCl solution.
2 The physical adsorption of corrosion inhibitors in the outer porous corrosion layer reduces the anodic dissolution rate and cathodic reaction kinetics.
3 The corrosion products of AM50 Mg alloy are composed of an outer porous Mg(OH)2layer and an inner MgO/Mg(OH)2corrosion layer when immersed in NaCl solution.Such porous layer is replaced by a passive and dense amorphous oxide layer,mainly MgO and Al2O3,when SDS is adsorbed onto outermost of the corrosion products.
Acknowledgments
The authors acknowledge Dr.Cong Han,School of Resources and Civil Engineering,Northeastern University,for the help and fruitful discussion on the quantum chemical calculations of inhibitor.The authors would like to acknowledge the financial support from National Natural Science Foundation of China (No.52071067) and the Fundamental Research Funds for the Central Universities (N2002009).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Novel Mg-Bi-Mn wrought alloys: The effects of extrusion temperature and Mn addition on their microstructures and mechanical properties
- Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform
- A crystal plasticity based approach to establish role of grain size and crystallographic texture in the Tension–Compression yield asymmetry andstrain hardening behavior of a Magnesium–Silver–Rare Earth alloy
- Developing polydopamine modified molybdenum disulfide/epoxy resin powder coatings with enhanced anticorrosion performance and wear resistance on magnesium lithium alloys
- Novel extended C-m models of flow stress for accurate mechanical and metallurgical calculations and comparison with traditional flow models
- Unexpected high-temperature brittleness of a Mg-Gd-Y-Ag alloy
