miR-3682-3p directly targets FOXO3 and stimulates tumor stemness in hepatocellular carcinoma via a positive feedback loop involving FOXO3/Pl3K/AKT/c-Myc
2022-08-01QianChenSiBoYangYeWeiZhangSiYuanHanLeiJiaBoLiYiZhangShiZuo
Qian Chen, Si-Bo Yang, Ye-Wei Zhang, Si-Yuan Han, Lei Jia, Bo Li, Yi Zhang, Shi Zuo
Qian Chen, Ye-Wei Zhang, Βo Li, Shi Zuo, Department of Hepatobiliary Surgery, Affiliated Hospital of Guizhou Medical University, Guiyang 550000, Guizhou Province, China
Si-Βo Yang, Department of Clinical Medicine, Guizhou Medical University, Guiyang 550000,Guizhou Province, China
Si-Yuan Han, Department of Infectious Diseases, SSL Central Hospital of Dongguan, Dongguan 523000, Guangdong Province, China
Lei Jia, Department of Organ Transplantation, Affiliated Hospital of Guizhou Medical University, Guiyang 550000, Guizhou Province, China
Yi Zhang, Department of Hepatobiliary Surgery, Guizhou Provincial People's Hospital, Guiyang 550000, Guizhou Province, China
Abstract BACKGROUND Cancer stem cells (CSCs) have been implicated in tumorigenesis and tumor recurrence and metastasis are key therapeutic targets in cancer treatment.MicroRNAs display therapeutic potential by controlling the properties of CSCs;however, whether an association exists between miR-3682-3p and CSCs is unknown.AIM To investigate the mechanism by which miR-3682-3p promotes stemness maintenance in hepatocellular carcinoma (HCC).METHODS MiR-3682-3p expression in HCC cell lines and 34 pairs of normal and HCC specimens was assayed by quantitative polymerase chain reaction. The functional role of miR-3682-3p was investigated in vitro and in vivo. Dual-luciferase reporter and chromatin immunoprecipitation assays were performed for target assessment, and western blotting was utilized to confirm miR-3682-3p/target relationships.RESULTS We found that miR-3682-3p plays a key role in HCC pathogenesis by promoting HCC cell stemness. The upregulation of miR-3682-3p enhanced CSC spheroid-forming ability, side population cell fractions, and the expression of CSC factors in HCC cells in vitro and the tumorigenicity of transplanted HCC cells in vivo. Furthermore, silencing miR-3682-3p prolonged the survival of HCC-bearing mice. Mechanistically, we found that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression through PI3K/AKT; c-Myc, in turn, activates miR-3682-3p, forming a positive feedback loop. Intriguingly, miR-3682-3p expression was induced by hepatitis B virus X protein (HBx) and was involved in HBx-induced tumor stemness-related pathogenesis.CONCLUSION Our findings reveal a novel mechanism by which miR-3682-3p promotes stemness in HCC stem cells. Silencing miR-3682-3p may represent a novel therapeutic strategy for HCC.
Key Words: miR-3682-3p; FOXO3; Cancer stem cells; Hepatocellular carcinoma
lNTRODUCTlON
Hepatocellular carcinoma (HCC) is a commonly diagnosed malignant tumor in China and is associated with very high morbidity and mortality[1,2]. Despite notable advances in multimodal treatment strategies over recent decades, such as radiotherapy, immunotherapy, and noninvasive surgical resection, the prognosis of patients with HCC, with a 5-year relative overall survival rate of approximately only 12%, remains unsatisfactory[3,4]. This highlights the urgent need to elucidate the mechanisms underlying HCC tumorigenicity and metastasis and identify reliable biomarkers to guide early HCC diagnosis and therapeutic intervention[5,6]. Increasing evidence has indicated that cancer stem cells (CSCs) underlie the insensitivity of HCC to radiotherapy and chemotherapy and play a key role in HCC metastasis and recurrence[7]. Like most stem cells, CSCs have the potential for self-renewal and the ability to differentiate into multiple cell types, resulting in tumor heterogeneity, and ultimately leading to tumor recurrence and metastasis[8]. Accordingly, targeting CSCs may represent a promising therapeutic target for cancer treatment[9,10].
The hepatitis B virus X protein (HBx) can reportedly disrupt several pathways and associated functions by regulating the expression and activity of numerous genes, epigenetics-related molecules[such as microRNAs (miRNAs)], and long non-coding RNAs (lncRNAs)[11]. MiRNAs are endogenous non-coding RNA molecules that can bind to the 3′-untranslated regions (3′-UTRs) of target mRNAs and regulate their expression at the post-transcriptional level[12]. More than 2500 miRNAs have been identified in humans to date, and many likely remain to be identified[13-15]. It is well documented that miRNAs play a significant role in regulating CSC function and cancer progression at multiple levels[16,17]. MiR-3682-3p exerts both oncogenic and tumor-suppressive functions, depending on the cancer type.For instance, high miR-3682-3p expression is correlated with esophageal cancer[18]. Exosomal miR-3682-3p targets angiopoietin 1 by altering Ras-MEK1/2-ERK signaling, suggesting that exosomalderived miR-3682-3p may act as a tumor suppressor in HCC[19]. However, a different study reported that miR-3682-3p induces the proliferation of HCC cells and inhibits their apoptosis through the FAS pathway, indicating that miR-3682-3p may instead exert oncogenic effects in HCC[20].
Given these conflicting reports on the role of miR-3682-3p in HCC, we performed early validation of miR-3682-3p expression using in situ hybridization, and found that the expression level of miR-3682-3p was significantly elevated in HCC tissue compared with that in controls, and was positively correlated with both malignancy and worse prognosis in HCC. Our data further indicated that high miR-3682-3p expression levels were positively correlated with hepatitis B virus (HBV) infection. However, whether miR-3682-3p also affects stemness in HCC remains unknown. In this study, we demonstrated that miR-3682-3p directly targets and inhibits the expression of FOXO3. The downregulation of FOXO3 Levels subsequently leads to the activation of a PI3K/AKT/β-catenin/c-Myc/miR-3682-3p positive feedback loop that promotes the stemness of HCC cells. In summary, our findings revealed a role for miR-3682-3p in promoting the stemness of HCC cells and suggested that this miRNA may represent a potential therapeutic target for the treatment of HCC.
MATERlALS AND METHODS
Clinical tissue specimens
A total of 34 patients with HCC who underwent surgical resection in the Department of Hepatobiliary Surgery of the Affiliated Hospital of Guizhou Medical University from June 2017 to June 2019 were selected for this study. All patients provided informed consent. The study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. HCC and paired paracancerous tissue specimens (at least 3 cm from the edge of the tumor) were collected after resection and were preserved in liquid nitrogen. HCC was pathologically confirmed in all cases. All the selected patients were undergoing first-time surgical resection of the primary lesion, and none had received radiotherapy, chemotherapy, or hormone therapy before surgery.
The HCC tissue microarray was purchased from the Shanghai Molecular Medicine Engineering Center (Shanghai, China) with approval from its Ethics Committee.
Cell culture
The cell lines used in this study (LO2, LM3, Huh7, Hep3B, HepG2, and MHCC97H) were provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone Laboratories, Inc., Logan, UT, United States)supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2.
RNA isolation and quantitative polymerase chain reaction
Total RNA was extracted from cultured HCC cells using Trizol reagent and reverse transcribed into cDNA, which served as a template for quantitative polymerase chain reaction (qPCR). Gene expression levels were detected separately using a standard SYBR Green RT-PCR kit (Thermo Scientific, Waltham,MA, United States) according to the manufacturer’s instructions. The sequences of the primers used are detailed in Supplementary Table 1. Relative gene expression levels were calculated using the 2-ΔΔCt method and are reported as fold changes.
Lentivirus production and infection
Purchased miR-3682-3p overexpressing lentivirus with green fluorescence constructed and synthesized by Ji kai (Shanghai, China) and the corresponding control lentivirus in vacuo. The virus dosage was calculated at a cell fusion rate of 60%. Lentivirus was transfected into HCC cells at an MOI of 80 using a transfection reagent. The medium was changed after 8 h of transfection. Green fluorescence could be detected after 72 h of transfection under a fluorescence microscope, at which point the cells were collected for PCR-based detection of the miR-3682-3p level to determine the transfection efficiency(Supplementary Table 2).
Sphere formation assay
A total of 5 × 103HCC cells were inoculated into six-well ultra-low adsorption plates (Corning, Painted Post, NY, United States) containing 2% B27 (BD PharMingen, Carlsbad, CA, United States), 20 ng/mL epidermal growth factor, 20 ng/mL fibroblast growth factor in DMEM/F12 serum-free medium(Invitrogen). The cells were cultured for 14 d and then imaged and counted under a light microscope.
CSC sorting
Cells in the logarithmic growth phase were digested into single-cell suspensions and washed twice with PBS. A total of 1 × 106cells/mL were resuspended in each of two tubes containing medium supplemented with 2% FBS. Verapamil (20 μL) was added to one tube (to inhibit dye efflux) and incubated for 30 min at 37 °C protected from light. Vybrant DyeCycle Violet stain (Sigma-Aldrich) at a final concentration of 5 µg/mL was then added to both tubes followed by incubation with shaking for 90 min at 37°C in the dark, and with mixing every 10 min. The samples were subsequently centrifuged at 1500 ×g,the supernatant was discarded, and the cells were resuspended in 1 mL of PBS. Finally, 5 μL of 7-ADD(BD PharMingen) was added to each tube followed by flow cytometric detection. Each set of experiments was repeated three times.
Dual-luciferase reporter assay
HCC cells were inoculated into 24-well plates, and the cells were fused to 70% with Lipofectamine 2000(Invitrogen, Guangzhou, China). Plasmids containing the wild-type or mutated (mut) FOXO3 3′-UTR were co-transfected with miR-3682-3p mimic/inhibitor and the cells were assayed for luciferase activity after 48 h. To investigate the effect of c-Myc on miR-3682-3p transcription, vectors containing mutated c-Myc binding sites were constructed and co-transfected with c-Myc expression plasmids into HCC cells,following which the cells were assayed for luciferase activity.
Western blot analysis
Total protein was extracted from each group of HCC cells. Protein concentrations were determined by BCA. Equal amounts of protein were separated by 10% SDS-PAGE, transferred to PVDF membranes(Millipore, Bedford, MA, United States), incubated overnight at 4 °C with primary antibodies, and then labeled with secondary antibodies at room temperature for 1 h. Bands were developed using luminolenhanced chemiluminescence (Thermo Scientific). The primary antibodies used for western blotting are described in Supplementary Table 3.
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed using a ChIP kit (Thermo Scientific)according to the manufacturer's instructions. Briefly, genomic DNA was extracted from HCC cells, the DNA was fragmented, and a c-Myc antibody was added to the reaction system for immunoprecipitation. IgG served as a negative control. The purified DNA was PCR-amplified and the products were separated on a 2% agarose gel. The primers used for PCR amplification are shown in Supplementary Table 1.
Co-immunoprecipitation
Total cellular protein was extracted using the Pierce Co-immunoprecipitation (Co-IP) Kit (Thermo Scientific). A total of 2 mg of protein was incubated with 5 μg of specific antibody or IgG (used as a negative control) overnight at 4 °C. After washing, the protein samples were subjected to western blotting.
Nude mouse tumor xenograft model
All animal experiments were performed according to the requirements of the Guizhou Medical University Animal Experiment Ethics Committee (2100555). Cyclophosphamide (200 μL) was injected daily for the first three days of the experiment to disrupt the immune system of BALB/c nude mice(SPF, Beijing, China). Then, the mice were divided into two groups of 20 mice each and administered HCC cells (1 × 106, 2 × 106, 4 × 106, and 8 × 106cells,n= 5 mice per concentration) overexpressing or not miR-3682-3p by subcutaneous injection. The mice were euthanized after 21 d and the tumors were excised. Equal amounts of HCC cells (8 × 106) were subcutaneously injected into four-week-old BALB/c nude mice (n= 10 per group) to establish a HCC xenograft model. After one week, the mice received either saline or 10 nmol of a miR-3682-3p antagomir (RiboBio, Guangzhou, China) intraperitoneally twice a week for three weeks. The Kaplan-Meier method was used to evaluate the survival of the mice.
Immunohistochemistry and evaluation of immunohistochemical staining
FOXO3, OCT4, and SOX2 protein expression levels were assessed in tissue microarrays (Proteintech,Wuhan, China) according to the manufacturer’s protocol as well as in animal tissues. The indirect streptavidin peroxidase method was used. Immunohistochemistry was scored by two registered pathologists from the Affiliated Hospital of Guizhou Medical University based on the intensity of coloration and area of the sections. The stained area was scored from 0 to 4 (0: < 5% staining; 1: 5%-25%staining; 2: 26%-50% staining; 3: 50%-75% staining; and 4: > 75% staining). The product of the two sets of scores was counted, and final staining scores of < 6 and ≥ 6 were considered to reflect low and high expression, respectively.
Statistical analysis
SPSS v.25.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Data were expressed as means ± standard deviation. Differences between two groups were compared usingt-tests. Patient clinicopathological parameters were analyzed using theχ2test, patient survival was analyzed using Kaplan-Meier survival curves, and comparisons of survival and recurrence rates between groups were performed using the log-rank test.Pvalues < 0.05 were considered significant. One-way Cox regression was used to analyze all clinicopathological indicators and factors withPvalues < 0.05 were used for multivariate regression analysis.
RESULTS
High expression of miR-3682-3p reflected poor prognosis of HCC patients
Data from The Cancer Genome Atlas (TCGA) were accessed to identify miRNAs related to the survival of HCC patients. We identified miR-3682-3p as being distinctly highly expressed in HCC specimens from TCGA datasets (P= 0.001) (Figure 1A). The results of our previous in situ hybridization analysis in primary HCC tissues from a cohort of 90 HCC patients had suggested that a significant correlation exists between miR-3682-3p expression and tumor size and stage, intrahepatic GGT levels, and HBV surface antigen status. Additionally, high miR-3682-3p expression levels were associated with a shorter survival time in HCC patients[21]. To verify the above results, we obtained HCC and adjacent paracancerous tissue from a different cohort of 34 patients. RT-qPCR analysis showed that miR-3682-3p expression was significantly higher in HCC tissue than in adjacent liver tissue (P= 0.001) (Figure 1B)and was also significantly higher in HCC cell lines than in an immortalized hepatocyte line (LO2).Intriguingly, the expression of miR-3682-3p was upregulated in HBV-positive HCC cells (Hep3B,MHCC-97H, and HCCLM3) compared with that in HBV-negative (Huh7 and HepG2) HCC cells(Figure 1C). Furthermore, survival analysis of TCGA dataset suggested that HCC patients with low miR-3682-3p expression survived longer than those with high miR-3682-3p expression (P< 0.001)(Figure 1D). In summary, our results suggested that miR-3682-3p expression is upregulated in HCC tissues and is positively correlated with poor prognosis in HCC patients.
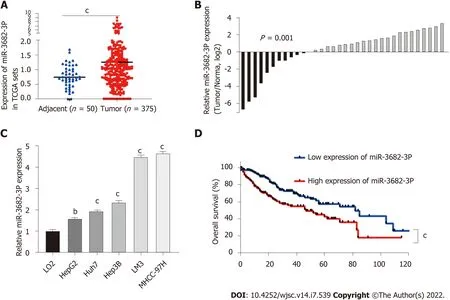
Figure 1 High expression of miR-3682-3p reflected poor prognosis of hepatocellular carcinoma. A: The expression levels of miR-3682-3p in normal and hepatocellular carcinoma (HCC) tissues in The Cancer Genome Atlas (TCGA) dataset; B: The expression of miR-3682-3p in 34 HCC and paired paracancerous tissue samples as determined by RT-qPCR; C: miR-3682-3p expression in HCC cells (HepG2, Huh7, Hep3B, LM3, and MHCC-97H) and normal liver epithelial (LO2) cells as determined by RT-qPCR; D: Analysis of Kaplan-Meier overall survival curves for HCC patients based on miR-3682-3p expression in TCGA dataset. bP < 0.01, cP < 0.001. Experiments were repeated three times.
miR-3682-3p promotes stem cell properties in HCC cells via the PI3K/AKT/c-Myc signaling pathway
To investigate the effect of miR-3682-3p on CSCs, miR-3682-3p mimics, miR-3682-3p inhibitors, or miR-3682-3p-expressing lentiviral vectors were transfected into HCC cells. We first examined the transfection efficiency of miR-3682-3p by RT-qPCR (Supplementary Figure 1A and B). Overexpression of miR-3682-3p increased the sphere-forming ability of HCC cells (Figure 2A and Supplementary Figure 1C) as well as the percentage of side population cells (Figure 2B and Supplementary Figure 1D). Immunofluorescence staining and RT-qPCR further confirmed that miR-3682-3p increased the stemness of HCC cells(Figure 2C-E). In contrast, miR-3682-3p downregulation in LM3 and MHCC-97H cells following treatment with the miR-3682-3p inhibitor elicited the opposite result (Supplementary Figure 1E-H).
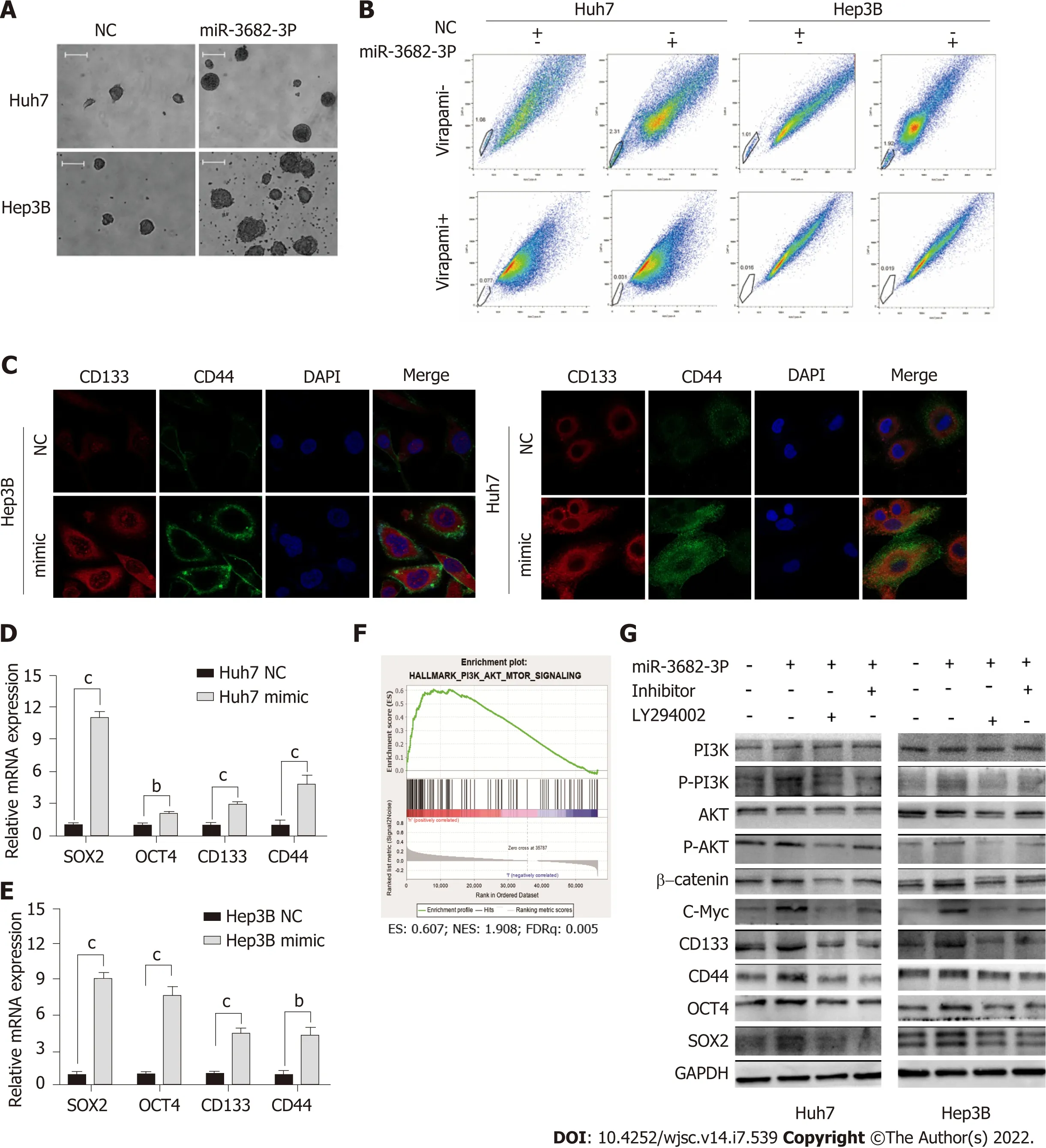
Figure 2 miR-3682-3p promotes the stem cell properties of hepatocellular carcinoma cells via the Pl3K/AKT/c-Myc signaling pathway. A:Representative images of formed hepatospheres (× 100 magnification); scale bar: 200 μm; B: Flow cytometric analysis of the proportion of side population cells; C:Immunofluorescence staining was performed to assess the expression levels of CD44 and CD133 in hepatocellular carcinoma cells (scale bar: 5 μm); D and E: RTqPCR analysis of SOX2, OCT4, CD133, and CD44 expression; F: Gene set enrichment analysis showing that miR-3682-3p regulates the PI3K/AKT signaling axis; G:Western blotting analysis of PI3K/AKT/c-Myc signaling- and stemness-related protein expression levels. bP < 0.01; cP < 0.001. Experiments were repeated three times. NC: Negative control.
To investigate whether miR-3682-3p modulated the signaling pathway involved in the promotion of tumor stemness, we performed gene set enrichment analysis (GSEA), and found that miR-3682-3p expression was closely associated with PI3K/AKT activation in HCC (Figure 2F). Western blot was further performed to confirm this possibility, with the results showing that miR-3682-3p overexpression led to the upregulation of the expression of PI3K/AKT pathway-related proteins, including c-Myc, as well as that of stemness-related molecules such as CD44, CD133, SOX2, and OCT4. The simultaneous application of a PI3K inhibitor (LY294002) and the miR-3682-3p inhibitor in HCC cells overexpressing miR-3682-3p reversed the above-mentioned changes in protein levels (Figure 2G). Together, these data demonstrated that miR-3682-3p promotes a CSC-like phenotype in HCC cells through the PI3K/AKT/c-Myc signaling axis, thereby further contributing to the progression of HCC.
MiR-3682-3p promotes the tumorigenicity of HCC cells in vivo
Because we found that MiR-3682-3p could promote a CSC-like phenotype in vitro, we next assessed whether miR-3682-3p exerted a similar effectin vivo. For this, different concentrations (1 × 106, 2 × 106, 4× 106, and 8 × 106) of HCC cells stably transfected with miR-3682-3p were subcutaneously injected into BALB/C nude mice. As shown in Figure 3A, the associated tumorigenicity rates were 20% (1/5), 60%(3/5), 100% (5/5), and 100% (5/5), respectively. For equal concentrations of control cells, the tumorigenicity rates were 0% (0/5), 20% (1/5), 60% (3/5), and 100% (5/5), respectively. Similarly, the tumor weight was greater in the miR-3682-3p overexpression group than in the control group (Figure 3B). RTqPCR results confirmed that the miR-3682-3p expression levels were higher in xenografts derived from cells stably overexpressing miR-3682-3p than in negative control cell-derived xenografts (Figure 3C).Additionally, Kaplan-Meier curve analysis indicated that miR-3682-3p antagomir administration prolonged the survival time of mice compared with that of control animals (Figure 3D). The expression of SOX2 and OCT4 in nude mouse xenograft tumors was then detected by immunohistochemistry, and the same results were obtained (Figure 3E). Overall, these results provided further evidence that miR-3682 -3p promotes the tumorigenicity of HCC cellsin vivo.
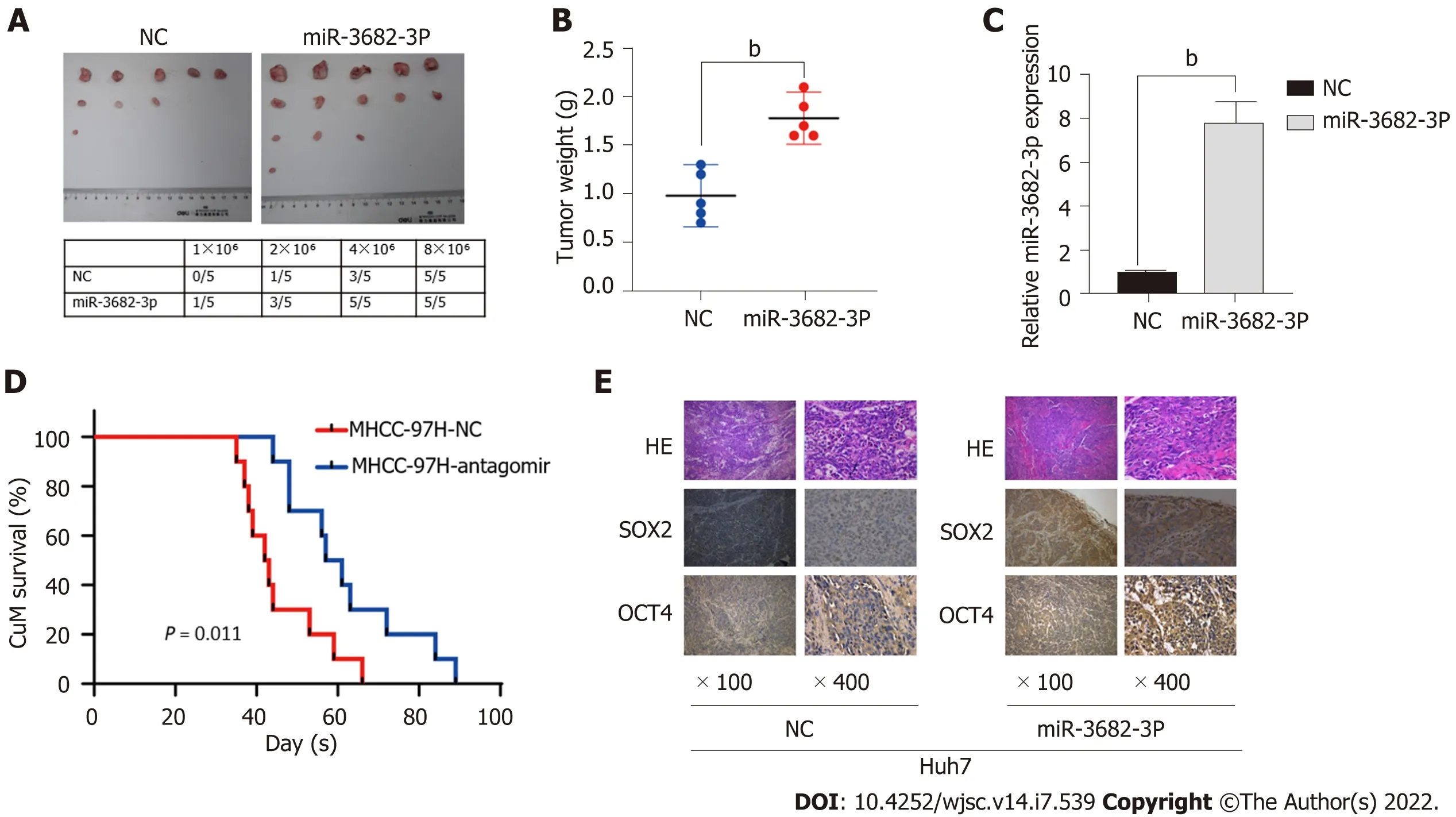
Figure 3 miR-3682-3p promotes the tumorigenicity of hepatocellular carcinoma cells in vivo. A: The tumorigenic rate of different concentrations of hepatocellular carcinoma cells (n = 5); B: Tumors in the miR-3682-3p overexpression group weighed significantly more than those of the control group (n = 5); C:Detection of miR-3682-3p expression in tumor tissues by RT-qPCR; D: Survival analysis revealed that miR-3682-3p antagomir prolongs the survival time of mice (n =10, log-rank test); E: Immunohistochemical staining for stem cell-associated markers (SOX2 and OCT4) in xenograft tumors (n = 5) (× 100 magnification scale bar:200 µm; × 400 magnification scale bar: 50 µm). bP < 0.01. NC: Negative control; HE: Hematoxylin and eosin.
FOXO3 is a direct target of miR-3682-3p
To understand how miR-3682-3p affects the stemness of HCC cells, miRNA walk 2.0 was used for the prediction of miR-382-3p targets, resulting in the identification of FOXO3 as a potential target gene of this miRNA (Figure 4A). MiR-3682-3p overexpression led to the suppression of FOXO3 protein levels,but did not affect FOXO3 transcript levels; meanwhile, the knockdown of miR-3682-3p elicited the opposite effect (Figure 4B-D). The interaction between miR-3682-3p and FOXO3 was further validated using a dual-luciferase reporter assay (Figure 4E). Additionally, immunohistochemical analysis of xenografts derived from miR-3682-3p-overexpressing HCC cells demonstrated that FOXO3 expression was downregulated in miR-3682-3p-overexpressing tumors (Figure 4F). To further demonstrate that FOXO3 mediates the oncogenic activity of miR-3682-3p, we evaluated the effect of FOXO3 on miR-3682-3p-modulated signals in HCC cells. We found that the overexpression of FOXO3 eliminated the promotive effects of miR-3682-3p on the expression of phosphorylated (p)-PI3K, p-AKT, β-catenin,SOX2, OCT4, CD133, CD44, and c-Myc (Figure 4G). Moreover, FOXO3 inhibited miR-3682-3p-induced stimulation of CSC-like phenotypes in HCC cells (Supplementary Figure 2A-D). Taken together, these results indicated that FOXO3 inhibits the miR-3682-3p-induced stemness of HCC cells.
It has been documented that FOXO3 interacts with β-catenin and plays a pivotal role in a variety of tumors[22,23]. Here, we identified a possible interaction between FOXO3 and β-catenin by CoIP(Figure 4H). Interestingly, we further found that FOXO3 co-localized with β-catenin in the cytoplasm(Figure 4I), while further immunofluorescence staining results showed that FOXO3 inhibited β-catenin nuclear translocation (Figure 4J).
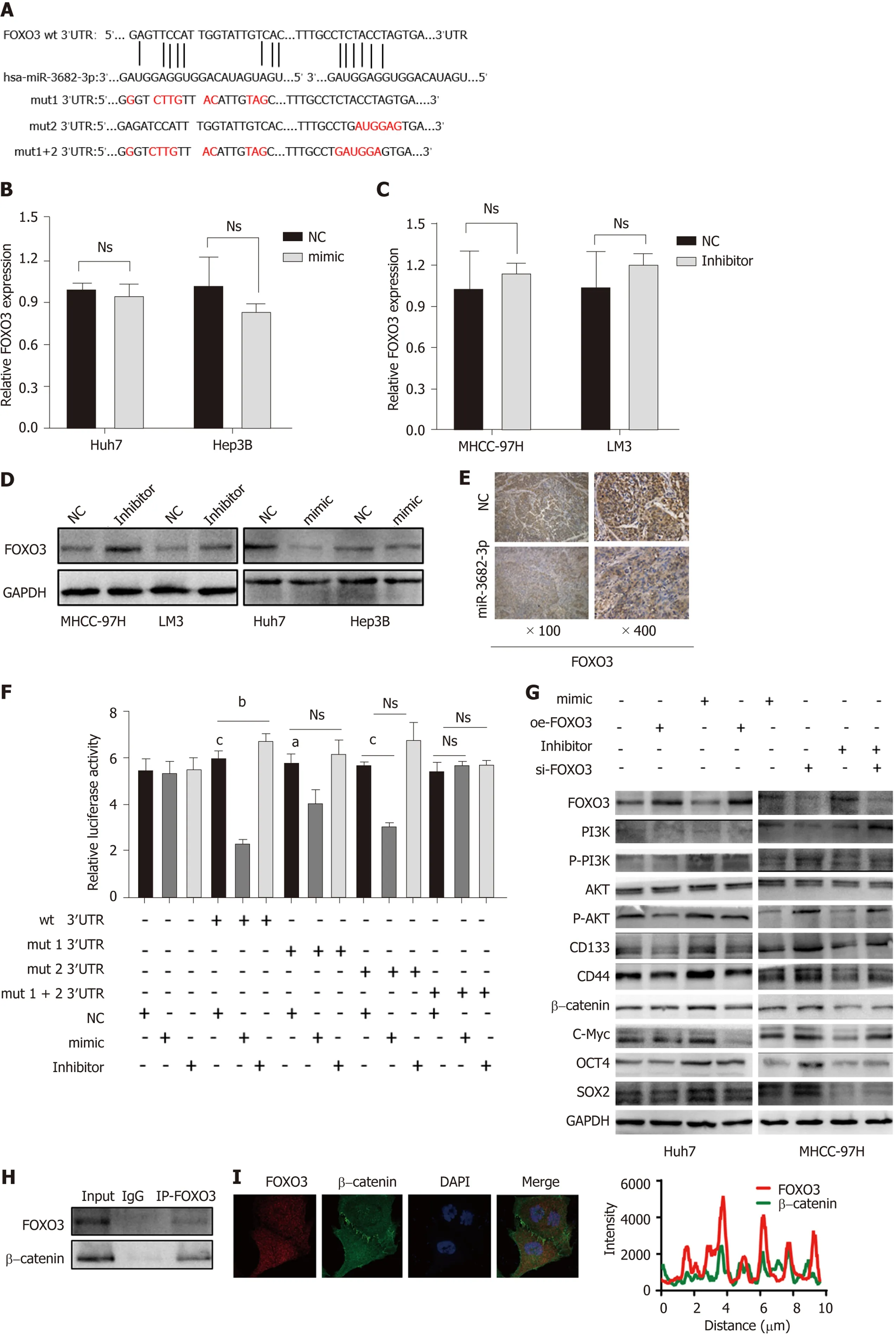
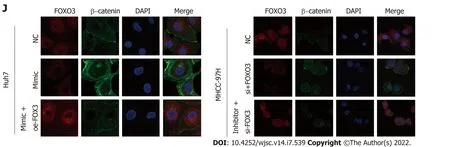
Figure 4 FOXO3 is a direct target of miR-3682-3p. A: Binding sites for miR-3682-3p in the 3′-UTR of FOXO3 were predicted using bioinformatics tools; B-D:The effect of miR-3682-3p on FOXO3 mRNA and protein levels as determined by RT-qPCR and western blot, respectively; E: Dual-luciferase reporter assay was used to detect the interaction between miR-3682-3p and the 3′-UTR of FOXO3; F: Immunohistochemical analysis of FOXO3 expression in xenograft tumors (n = 5) (×100 magnification scale bar: 200 µm, × 400 magnification scale bar: 50 µm); G: Western blot-based analysis of the effects of FOXO3 on stemness- and PI3K/AKT/c-Myc pathway-related proteins; H: Immunoprecipitation assays for the interaction between endogenous FOXO3 and β-catenin; I: Immunofluorescence staining-based assessment of FOXO3/β-catenin co-localization; J: Immunofluorescence staining of FOXO3 and β-catenin in hepatocellular carcinoma cells (scale bar: 5 μm). aP <0.05, cP < 0.001. Experiments were repeated three times. NC: Negative control; wt: Wild-type; mut: Mutant.
In brief, the above findings indicated that miR-3682-3p directly binds to the 3′-UTR of FOXO3,thereby activating the PI3K/AKT/β-catenin/c-MYC signaling axis and promoting the stemness of HCC cells.
c-Myc binds to the miR-3682-3p promoter region to promote its transcriptional activation
Transcription factors can promote the transcription of downstream miRNAs, which then bind to and regulate the expression of their target genes. To determine the mechanism underlying the upregulation of miR-3682-3p expression in HCC, we performed GSEA, and found that miR-3682-3p was positively correlated with c-Myc (Figure 5A). Transcription factor binding site prediction using the UCSC and JASPAR databases indicated that c-Myc can bind three regions in the miR-3682-3p promoter (Figure 5B).RT-qPCR analysis showed that upregulation of c-Myc increased the expression levels of miR-3682-3p,whereas c-Myc downregulation elicited the opposite result (Figure 5C and D). ChIP and luciferase reporter assays further confirmed that c-Myc can bind to the three sites on the miR-3682-3p promoter(Figure 5E-G). In summary, these data suggested that in HCC, c-Myc regulates miR-3682-3p transcription through binding to its promoter region.
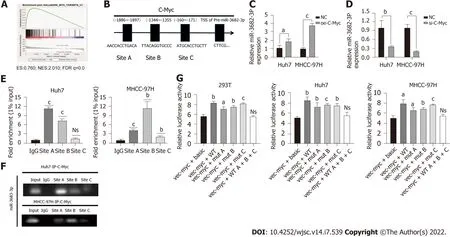
Figure 5 c-Myc binds to the miR-3682-3p promoter region and promotes its transcriptional activation. A: Gene set enrichment analysis revealed that miR-3682-3p is positively associated with c-Myc; B: Bioinformatic-based prediction of c-Myc binding sites in the miR-3682-3p promoter region; C and D: RTqPCR analysis of miR-3682-3p levels after c-Myc silencing and overexpression; E and F: Chromatin immunoprecipitation validated the binding of c-Myc to the miR-3682-3p promoter; G: Dual-luciferase reporter assay confirmed the binding of c-Myc to the miR-3682-3p promoter. aP < 0.05, bP < 0.01, cP < 0.001. Experiments were repeated three times. NC: Negative control.
HBx induces miR-3682-3p expression, thereby enhancing the cancer stem cell-like properties of HCC cells
HBx is an HBV-encoded protein that displays oncogenic properties and is known to play a critical role in the development of many tumors. We have previously shown that miR-3682-3p expression is positively correlated with HBV infection. Here, we sought to determine whether there is a correlation between HBx and the miR-3682-3p/FOXO3/PI3K/AKT/β-catenin/c-Myc feedback loop. Our results demonstrated that the overexpression or knockdown of the HBx-encoding gene respectively increased and decreased the expression level of miR-3682-3p (Figure 6A and B). We further showed that miR-3682-3p mediated the oncogenic effects resulting from the HBx-mediated induction of PI3K/AKT/c-Myc signaling (Figure 6C and D),i.e., HBx promoted stemness by upregulating miR-3682-3p expression.
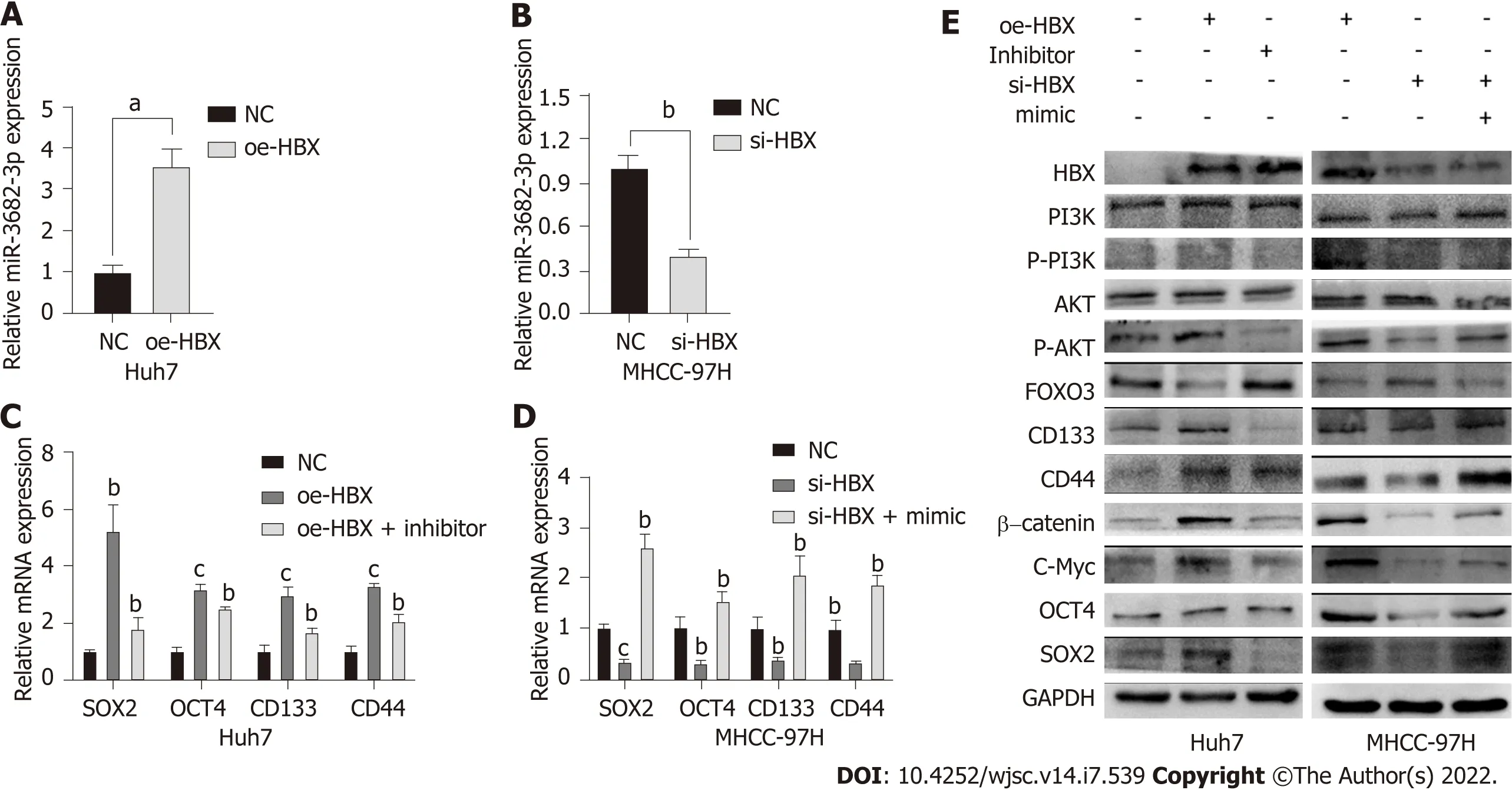
Figure 6 HΒx induces miR-3682-3p expression, thereby enhancing the cancer stem cell-like properties of hepatocellular carcinoma cells.A and B: The levels of miR-3682-3p after the overexpression and silencing of HBx were detected by RT-qPCR; C and D: RT-qPCR and western blot analysis of the effect of HBx on stemness markers. aP < 0.05, bP < 0.01, cP < 0.001. Experiments were repeated three times. NC: Negative control.
Pathoclinical features of FOXO3 expression
Finally, to obtain additional evidence to support our conclusions, tissue microarrays containing 90 HCC and paired adjacent non-tumor tissue samples were subjected to FOXO3 immunohistochemical analysis and scored for cell staining (Figure 7A and B). Survival analysis indicated that low FOXO3 expression correlated with reduced overall survival time in HCC patients (Figure 7C and D), while high FOXO3 expression elicited the opposite effect (Figure 7C and D). Subsequently, FOXO3 expression was evaluated in relation to the clinicopathological characteristics of HCC (Table 1). A one-step univariate Cox regression analysis indicated that AJCC staging (P= 0.022), relapse (P= 0.002), GGT (P= 0.035),Edmondson-Steiner grading (P< 0.001), and FOXO3 expression (P= 0.006). However, no differences were identified using multivariate Cox regression analysis (Table 2). Combined with our early fluorescence in situ hybridization analysis scores, our immunohistochemical analysis revealed that FOXO3 expression was negatively correlated with miR-3682-3p expression (Figure 7E).
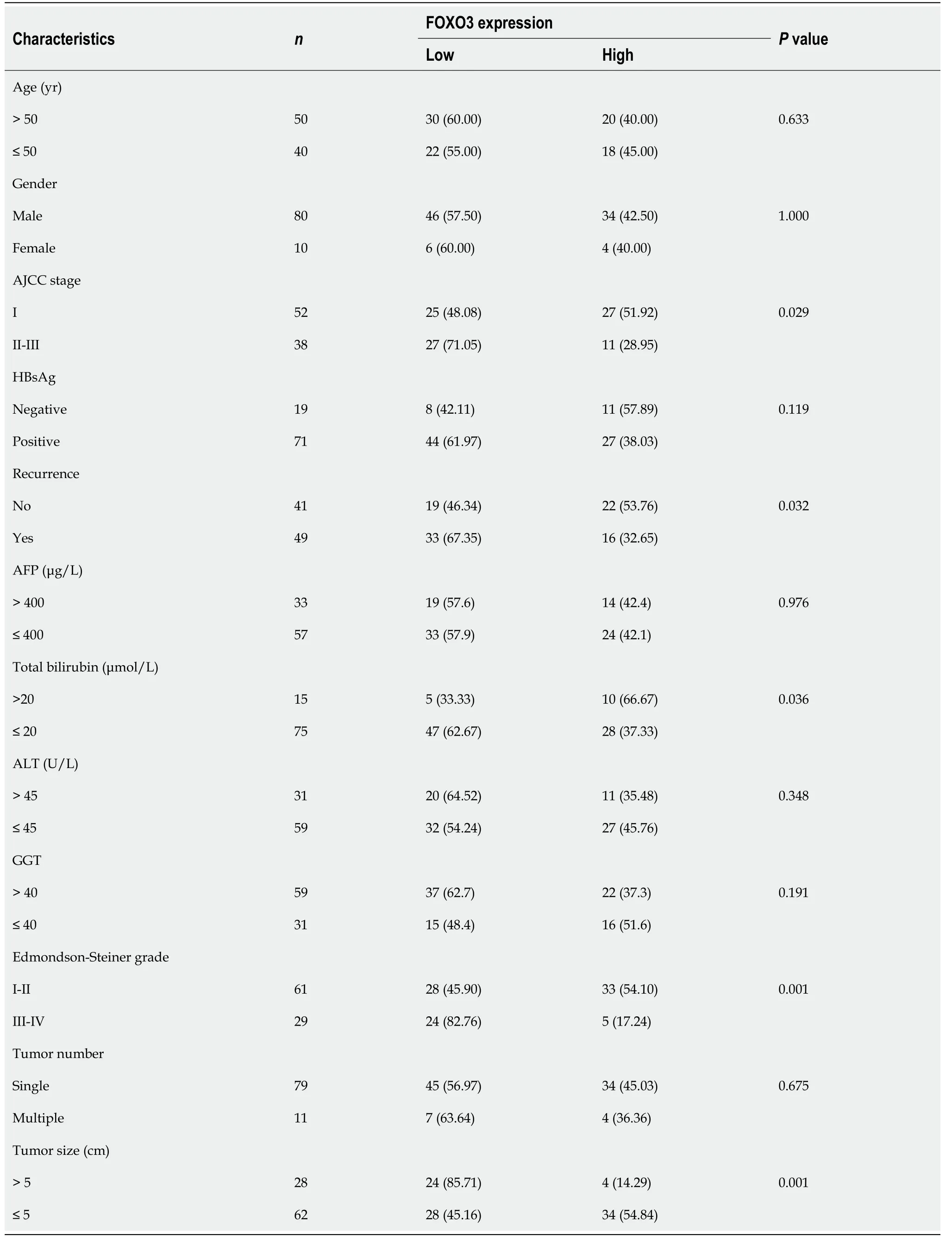
Table 1 Correlations between FOXO3 expression and the clinicopathological features of hepatocellular carcinoma patients, n (%)
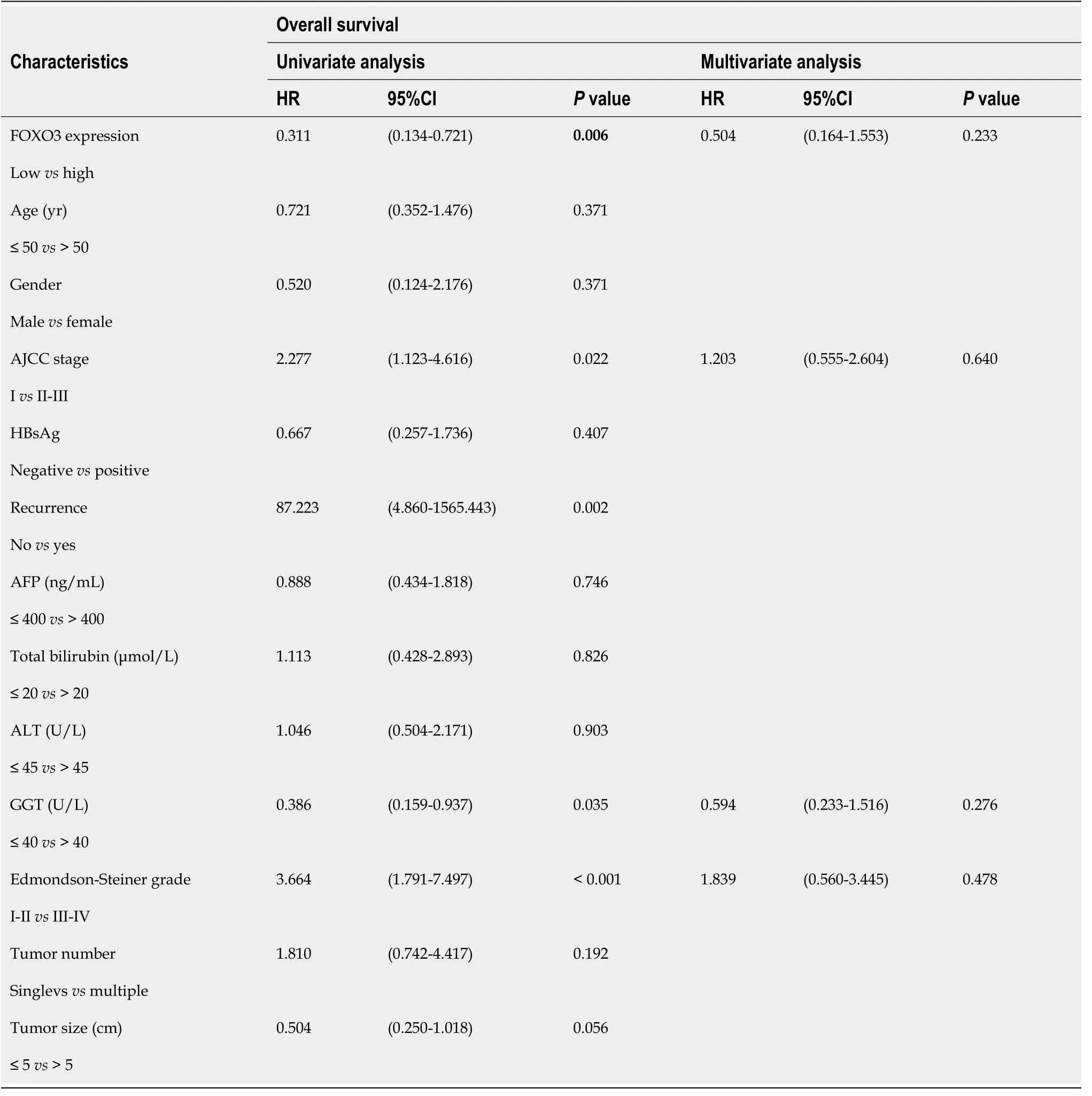
Table 2 Univariate and multivariate survival analysis of clinicpathological variables of hepatocellular carcinoma patients
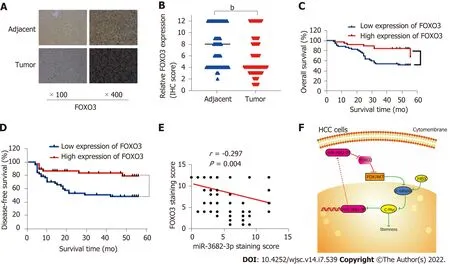
Figure 7 Pathoclinical features of FOXO3 expression. A: FOXO3 expression in hepatocellular carcinoma (HCC) tissue microarray (× 100 magnification scale bar: 200 µm; × 400 magnification scale bar: 50 µm); B: Immunohistochemical scoring of FOXO3 staining; C: Kaplan-Meier survival analysis showing the FOXO3 expression level-related overall survival in HCC; D: Kaplan-Meier survival analysis showing the FOXO3 expression level-related disease-free survival in HCC;E: Correlation between FOXO1 and miR-3682-3p expression (Spearman’s rank correlation test); F: Schematic representation of the HBx-induced miR-3682-3p/FOXO3/PI3K/AKT/c-Myc feedback loop that promotes HCC stemness. bP < 0.01.
DlSCUSSlON
HCC is one of the most commonly diagnosed cancers worldwide[24]. Despite improvements in the treatment of HCC, this disease continues to be associated with high morbidity and mortality[5,25]. CSCs play key roles in tumorigenesis and tumor metastasis, and targeting CSCs has the potential to treat a variety of cancers[26]. Multiple hepatic CSC biomarkers have already been identified. In this study, we have added a genetic signature of these liver CSCs and revealed the signaling pathways that regulate HCC[27].
Epigenetic modification is a key regulatory mechanism in tumor pathogenesis and involves the participationof numerous miRNAs[28]. Recent studies have shown that specific miRNAs exhibit good therapeutic potential associated with the control of cancer cell stemness[29]. In addition, strategies to upregulate or inhibit miRNA levels in tumors, such as the design of miRNA mimics or antagomiRs,have shown promising preliminary clinical results in tumor treatment, especially those targeting CSCs.Studies have revealed that miR-3682-3p plays a major role in a variety of cancers, including HCC. In addition, miR-3682-3p has been implicated as a key player in bladder cancer drug resistance by inhibiting its activation by BML1 and thereby affecting[30]. However, no study to date has investigatedstemness in HCC or whether miR-3682-3p has a role in this process. To address this, we investigated the role of miR-3682-3p in regulating the properties of HCC stem cells as well as the underlying mechanism.We found that the inhibition of miR-3682-3p suppressed the development of HCC, demonstrating that miR-3682-3p may serve as a prognostic marker for this cancer.
FOXO3 is a key member of the large FOX family of transcription factors[31]. In mammals, FOXO3 is known to have a wide range of biological functions[32]. Studies have confirmed that FOXO3 exerts an oncogenic role in a variety of cancers, including breast cancer[33], through the promotion of tumor cell proliferation, metastasis, stemness, and drug resistance, among other biological behaviors. Several studies have illustrated the interactive relationship between FOXO3 and β-catenin and the functional role of FOXO3 in the PI3K/AKT signaling axis[22]. In the present study, we found that miR-3682-3p directly targets FOXO3 and suppresses its expression, thereby enhancing P3K/AKT/c-Myc and downstream signaling, including upregulating the expression of known stemness markers, and,consequently, promoting the stemness of HCC cells.
c-Myc is an integral member of the MYC family of transcription factors. It is known to be involved in the progression of various cancers[34,35], including through the regulation of the expression of numerous miRNAs, thereby promoting tumor stemness, metastasis, proliferation, and chemoresistance[36,38]. We have previously shown the existence of three binding sites for c-Myc in the miR-3682-3p promoter using online bioinformatics tools. The results of the current study suggested that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression; c-Myc, in turn, activates miR-3682-3p, thereby forming a positive feedback loop.
Despite the consensus that HBx is closely related to HBV-associated HCC[9,39], the role of miRNAs in HBx-associated HCC is poorly understood[40]. HBx is known to influence the development of HCC by regulating β-catenin entry into the nucleus, which, in turn, affects downstream signaling[39-41]. In the present study, we found that miR-3682-3p expression was positively correlated with HBV infection.We further found that HBx can promote miR-3682-3p expression, thereby downregulating that of FOXO3 and promoting stemness in HCC.
CONCLUSlON
We identified a novel positive feedback regulatory loop involving miR-3682-3p, FOXO3, β-catenin, and c-Myc that plays a pivotal role in the stemness of HCC (Figure 7F). Our findings unveiled a novel mechanism by which miR-3682-3p promotes stem cell maintenance in HCC progression and elucidated for the first time the regulatory relationship between HBV and miR-3682-3p.
ARTlCLE HlGHLlGHTS
Research background
Cancer stem cells (CSCs) have been implicated in tumorigenesis and tumor recurrence and metastasis are key therapeutic targets in cancer treatment. MicroRNAs display therapeutic potential by controlling the properties of CSCs; however, whether an association exists between miR-3682-3p and CSCs is unknown.
Research motivation
However, whether an association exists between miR-3682-3p and CSCs is unknown. Here, we investigated whether miR-3682-3p has a role in hepatocellular carcinoma (HCC).
Research objectives
To investigate the mechanism by which miR-3682-3p promotes stemness maintenance in HCC.
Research methods
MiR-3682-3p expression in HCC cell lines and 34 pairs of normal and HCC specimens was assayed by qPCR. The functional role of miR-3682-3p was investigated in vitro and in vivo. Dual-luciferase reporter and chromatin immunoprecipitation assays were performed for target assessment, and western blotting was utilized to confirm miR-3682-3p/target relationships.
Research results
We found that miR-3682-3p plays a key role in HCC pathogenesis by promoting HCC cell stemness. The upregulation of miR-3682-3p enhanced CSC spheroid-forming ability, side population cell fractions, and the expression of CSC factors in HCC cells in vitro and the tumorigenicity of transplanted HCC cells in vivo. Furthermore, silencing miR-3682-3p prolonged the survival of HCC-bearing mice. Mechanistically,we found that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression through PI3K/AKT; c-Myc, in turn, activates miR-3682-3p, forming a positive feedback loop. Intriguingly, miR-3682-3p expression was induced by hepatitis B virus X protein (HBx)and was involved in HBx-induced tumor stemness-related pathogenesis.
Research conclusions
Our findings reveal a novel mechanism by which miR-3682-3p promotes stemness in HCC stem cells.Silencing miR-3682-3p may represent a novel therapeutic strategy for HCC.
Research perspectives
This study has shed some light on the mechanism of action of miR-3682-3p in promoting stemness maintenance in HCC and provides a potential target for the treatment of HCC.
FOOTNOTES
Author contributions:Zuo S designed the experiments and reviewed the manuscript; Chen Q, Yang SB and Li B performed the experiments and wrote the manuscript; Zhang YW, Han SY, Zhang Y and Jia L performed the statistical analysis; all authors checked the final manuscript.
Supported bythe 12thSpecial Fund for Young Scientists and Technicians in Guizhou Province, No. (2019) 5647; the Science and Technology Fund of Guizhou Provincial Health and Health Commission, No. gzwjkj2020-1-101; the National Natural Science Foundation Training Program of Guizhou Medical University, No. 19NSP055; and Dongguan Science and Technology of Social Development Program, No. 201950715024201.
lnstitutional review board statement:The study was reviewed and approved by the Affiliated Hospital of Guizhou Medical University Institutional Review Board (Approval No. 2021039).
lnstitutional animal care and use committee statement:All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Guizhou Medical University [IACUC protocol number: (Protocol No. 2100555)].
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:The data that support the findings of this study are available from the corresponding author upon reasonable request.
ARRlVE guidelines statement:The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Shi Zuo 0000-0002-8595-5062.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Zhang YL
杂志排行
World Journal of Stem Cells的其它文章
- Therapeutic potential of dental pulp stem cells and their derivatives:lnsights from basic research toward clinical applications
- Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles
- Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges
- Metabolic-epigenetic nexus in regulation of stem cell fate
- Stem cell therapy for insulin-dependent diabetes: Are we still on the road?
- Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles
