Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles
2022-08-01EjlalAbuElRubRamadaKhasawnehFatimahAlmahasneh
Ejlal Abu-El-Rub, Ramada R Khasawneh, Fatimah Almahasneh
Ejlal Abu-El-Rub, Fatimah Almahasneh, Department of Physiology and Pathophysiology,Yarmouk University, Irbid 21163, Jordan
Ramada R Khasawneh, Department of Anatomy and Histology, Yarmouk University, Irbid 21163, Jordan
Abstract Mesenchymal stem cells (MSCs) have gained wide-ranging reputation in the medical research community due to their promising regenerative abilities. MSCs can be isolated from various resources mostly bone marrow, Adipose tissues and Umbilical cord. Huge advances have been achieved in comprehending the possible mechanisms underlying the therapeutic functions of MSCs. Despite the proven role of MSCs in repairing and healing of many disease modalities, many hurdles hinder the transferring of these cells in the clinical settings. Among the most reported problems encountering MSCs therapy in vivo are loss of tracking signal post-transplantation, insufficient migration, homing and engraftment postinfusion, and undesirable differentiation at the site of injury. Magnetic nanoparticles (MNPs) have been used widely for various biomedical applications.MNPs have a metallic core stabilized by an outer coating material and their magnetic properties can be modulated by an external magnetic field. These magnetic properties of MNPs were found to enhance the quality of diagnostic imaging procedures and can be used to create a carrying system for targeted delivery of therapeutic substances mainly drug, genes and stem cells. Several studies highlighted the advantageous outcomes of combining MSCs with MNPs in potentiating their tracking, monitoring, homing, engraftment and differentiation.In this review, we will discuss the role of MNPs in promoting the therapeutic profile of MSCs which may improve the success rate of MSCs transplantation and solve many challenges that delay their clinical applicability.
Key Words: Mesenchymal stem cells; Magnetic nanoparticles; Tracking; Homing;Migration; Differentiation
lNTRODUCTlON
Mesenchymal stem cells (MSCs) are the mostly investigated stem cells due to their enchanting, widerange therapeutic and regenerative potential[1]. Since their discovery by Friedenstein in 1970, MSCs have been thoroughly analyzed and characterized to discover the mechanistic explanations for their therapeutic abilities[2]. MSCs are easily reached stem cells and can be isolated from many sources including bone marrow (BM), adipose tissues and umbilical cord (UC)[3]. These cells are extensively studied compared to other types of stem cells because they are ethically benign and have low teratogenic tendency[3]. In addition, MSCs have an acceptable safety profile and less likely to cause serious side effects[3]. MSCs beneficial effects have been linked primarily to the ability of MSCs to secrete a cocktail of therapeutically active paracrine factors[4]. These paracrine factors secreted by MSCs can attenuate many pathological processes including apoptosis, necrosis, fibrosis, and inflammation and initiate repairing mechanisms in the damaged organs[4]. MSCs immunomodulatory functions also contribute strongly to their curative potential[5]. Moreover, MSCs can exert actual regeneration of the injured tissues by adopting the intrinsic machinery and differentiating to many functional cell types such as osteocytes, chondrocytes, adipocytes, and cardiomyocytes-like cells[6]. Endogenous or exogenous MSCs must migrate and home in the damaged tissues in order to gain their therapeutic benefits[3]. After homing in the damaged tissues, MSCs should endure the harsh microenvironment that may present[7]. Despite the numerous studies that highlighted the therapeutic efficiency of MSCs, many serious obstacles encumber the shift of MSCs from bench to bedside and delay their presence in the treatment guidelines[8]. The most reported post-transplantation challenges that researchers bump into when they use MSCs in clinical studies: (1) The disparities in the differentiation potential betweenin vitroandin vivo[9]; (2) The shift in their immunological characteristics and cytokines secretion profile under different stress microenvironments that may exist at the site of injury mainly Hypoxia and inflammation[5]; (3) The poor homing and migratory abilities of administered MSCs which may vary based on the route of injection and microenvironment status[10]; and (4) The loss of signal emitted from labelled cells due to the leakage of contrast agent after being injected, leads to difficulties in tracking and monitoring of these cells[11].
Magnetic nanoparticles (MNPs) have gained great attention among the medical researchers due to their unique biochemical and physical characteristics, their intrinsic biocompatibility and being biodegradable through normal cellular pathways which make them suitable for wide range of biomedical applications[12]. The intrinsic magnetic field elicited by the MNPs, which can be modulated externally by an applied magnetic field , is the basis for using these MNPs as contrast agents for biomedical imaging[13], biomarkers and biosensors[14], and targeted drug[15], cell and gene delivery[16]. Combining MNPs with stem cells was found to enhance their therapeutic performance and solve many challenges that hamper their regenerative potential and delay their clinical applications[17]. There are many types of MNPs that have been fabricated, but the most non-toxic and non- immunogenic MNPs that have been used with MSCs are iron oxide nanoparticles (IONs) such as magnetite (Fe3O4) or its oxidized form maghemite (γ-Fe2O3)[18-20]. These iron oxides based MNPs can be synthesized with different particles’ diameters such as Superparamagnetic iron oxide (SPIO) nanoparticles (50-200 nm diameter)[21] and ultra-small SPIO (USPIO) nanoparticles (around 35 nm diameter)[22] and different types of stabilizing non-toxic coating substrates such as dextran, polyethylene glycol, and Silica[23]. In general, the uptake of MNPs by MSCs is mediated mostly through endocytosis. MNPs usually are engulfed by MSCs to form endosomes, which then transformed into Mature multivesicular endosomes(MVEs). The MVEs then combined with lysosomes and get digested and decomposed into Fe3+. The free iron released into the cytoplasm of MSCs modified many cellular pathways to induce and promote their survival, migration, homing, anti-apoptosis and anti-inflammatory, and differentiation. These magnetized MSCs can be further modulated and guided to enhance their therapeutic outcomes by external magnetic fields. The internalization of MNPs inside MSCs can be also achieved by passive diffusion if their particle size is small and by using MNPs that bind specific cell surface immune marker found on MSCs. The prodigious power of using MNPs with MSCs to potentiate their tracking,migration and homing, differentiation and regenerative abilities will be the focus of this review.
MNPs AS A CONTRAST AGENT TO TRACK MSCs
The use of MSCs in the clinical settings requires more accurate tracking methods of MSCs after transplantation to determine their destinations, survival and final differentiated fates[24]. To visualize transplanted MSCs using imaging modalities importantly the computed tomography, positron emission tomography, and magnetic resonance imaging (MRI), these cells must be labelled with contrast agents[25-28]. The problem with the traditional contrast agents is the high leakage rate which causes the loss of emitted signal after short time course[29]. The contrast features of MNPs and their high safety profile encouraged many researchers to use them for labeling MSCs prior to injection[18]. MSCs labelled with MNPs have less leaking tendency and do not affect their stemness[30], rate of proliferation and the differentiation potential beside providing higher contrast-to-noise ratio for effective imaging[31,32].
IONs are the most commonly used MNPs for labelling and tracking MSCs due to their non-toxic and non-immunogenic features, high spatial resolution and penetration depth, and the non-ionizing radiation characteristics[33]. Superparamagnetic iron oxide nanoparticles (SPIONs), ultra-small SPIOpoly (acrylic acid) (USPIO-PAA)[34], glucosamine-modified USPIO-PAA (USPIO-PAA-GlcN)[35], and microgel iron oxide (MGIO)[36] are the most studied MNPs for MSCs labelling and tracking by multiple imaging methods. Using SPIONs for stem cell labeling and tracking is a relatively new application.Recently, ferumoxytol (Feraheme®, AMAG Pharmaceuticals), an ultrasmall SPION used clinically as an MRI contrast agent[37]. Ferumoxytol colloidal particle size is less than 50 nm and can be phagocytized efficiently by the MSCs-which have an inherited phagocytosis property- and can then be imaged and tracked by MRI[37]. FeraTrackTM, a dextran coated SPIONs, have a positive surface charge making it cell penetrable through a vesicular endocytosis route[38]. FeraTrackTMhas gained utility as a biocompatible MRI contrast agent for cell tracking purposes due to their high safety profile[38]. Mesentier-Louroet al[39] used FeraTrack to track BM-MSCs at site of injury in a rodent model of optical nerve injury[39].They reported that the after injecting FeraTrackTMlabeled MSCs intravitreously, they migrated to the site of optical nerve injury and remained there for up to 18 wk which is suitable to monitor their integration with the host tissues at the site of injury using MRI. The incorporation of cationic compounds such as poly-l-lysine and protamine onto the surface coating of SPIONs can enhance labeling of MSCs by promoting interactions with the negatively charged cell surface[39]. Guldriset al[35] studied the contrast characteristics of SPIOs and USPIOs coated with PAA, and USPIO-PAA-GlcN as labeling agents for MSCsin vitro[35]. A portion of these MNPs was cultured with MSCs for 24 h at a concentration of 100 μg mL-1. In the second group, the conditions were maintained, but polylysine(PLL) was used to promote particle uptake. The study found that in the absence of PLL, SPIO-PAA showed a very low and non-homogeneous labeling efficiency. USPIO-PAA and USPIO-PAA-GlcN showed little to no internalization by MSCs, while combining USPIO-PAA-GlcN with polylysine enhances their biocompatibility with MSCs and increases the detection sensitivity by MRI in bothin vitroandin vivoexperiments[35]. Studies also reported that using an external pulsed magnetic field opened channels in the cell membrane and increased the uptake of SPIONs by MSCs which intensified the contrast signal[40,41]. Interestingly, Ngen and Artemov[42] developed a dual-contrast agent by combining SPIONs and gadolinium chelate to monitor and track MSCs[42]. This dual contrast agent generates powerful positive contrast and increases the signal gain[42]. Furthermore, this dual-contrast agent was also able to distinguish between dead and live cells at the site of injury. This helps in estimating the percentage of MSCs survival rate, as Gadolinium dependent positive contrast is expunged in the live cells, whereas enhanced contrast level found in dead cells[42].
MGIO particles were studied to track human fetal MSCs through using 1.5T MRI[43]. MGIO particles were found to achieve high detection sensitivity with low cellular toxicity through a simple incubation protocol, which makes them useful for cellular tracking using standard MRI scanners[43]. These results were similar to that reported by Mailänderet al[44] using adult BM-MSCs[44]. The tracking efficacy achieved by MGIO was higher than that achieved with USPIO particles and the larger polystyrene particles[43]. Extracellular vesicles (EVs) are secreted lipid bilayered vesicles containing enzymes,nucleic acids and lipoproteins that are involved in intercellular communication. MSCs can activate various repairing machineries by secreting EVs[45]. SPIONs were also used to facilitate the labelling and imaging of EVs derived from MSCs. Dabrowskaet al[46] labeled these EVs derived from MSCs using the fluorescent lipophilic stain PKH26 and SPION nanoparticles conjugated with rhodamine (Molday ION Rhodamine B™) which was found to be highly biocompatible with EVs to be imaged using MRI[46]. The prospective use of MNPs in MSCs tracking is highly encouraging. MRI and MNPs are complementary and provide integrated information, like tracking and monitoring MSCs transplanting and engulfing overtime, and this will provide more information to guide further therapy.
MNPs TO ENHANCE THE HOMlNG OF TRANSPLANTED MSCs
Most of MSCs curative applications require injecting these cells directly to the injured tissues or delivering them intravenously, which requires their migration and homing in the damaged tissues[47,48]. MSCs homing is one of the major challenges in clinical settings because only a small percentage of delivered MSCs reaches the desired injury site and integrates with host tissues, while the majority of the administrated cells are trapped in the draining organs and get washed.
Recently, MNPs have been used to improve the homing percentage of transplanted MSCs at the site of injury[49]. Among all nanoparticles, SPIONs are the most extensively used nanomaterials to increase MSCs homing tendency without affecting their viability, proliferation and differentiation[31,32]. MSCs labeled with SPIONs exhibit enhanced homing due to magnetic attraction[50]. Several research groups have investigated the homing and tracking of MSCs after being labelled with SPIONs. Menget al[51]used SPIONs and green fluorescent protein (GFP) reporter gene to create a double labelling of Wharton’s Jelly human umbilical cord-derived MSCs (WJ-MSCs)[51]. These cells were injected to a nude mouse with cutaneous tissue injury. In this work, they used 25 μg/mL of SPION, and they divided the nude mice into three groups: The first group treated with WJ-MSCs only, the second group treated with GFP/SPIONs-positive WJ-MSCs, and the third group treated with SPIONs/GFP-positive WJ-MSCs and exposed to an external magnetic field (0.5T)[51]. In all three groups, MSCs were injected subcutaneously. The results showed a remarkable increase the migration abilities of GFP/SPIONs-labeled WJMSCsin vivowithout changing their inherited characteristics. The employment of a non-invasive external magnetic field provides a rapid guided homing of WJ-MSCs to the targeted injury site. Yunet al[48] used 15 μg/mL Rhodamine B, which was added to SPIONs to label MSCs that were injected to mouse model of wounded olfactory bulb. The Rhodamine B/SPIONs-labelled MSCs showed an improved homing by upregulating various homing factors mainly CXCR4 and CXCR4-SDF-1[51]. Yunet al[48] also used a magnetic field of 0.32 T to direct the Rhodamine B/SPIONs-labelled MSCs rapidly to the site of injury. Based on many studies, SPIONs enhance the MSCs homing by stimulating the expression of chemokine receptors mainly CXCR4-SDF-1α signaling[48].
A recent study by Branisteet al[52] in which they created a semiconductor nanoparticle by combining nanometer scale GaN thin layers with a sacrificial zinc ferrite core (ZnFe2O4)[52]. Branisteet al[52]incubated MSCs with (10 mg/mL) semiconductor nanoparticles and applied a remote magnetic field to control the direction of their movement. They found that these semiconductor nanoparticles were effectual to redistribute and rearrange MSCs according to the remote magnetic field intensity, thus enhanced the long term tracking and monitoring of the injected cellsin vivo[52].
Silvaet al[53] fabricated gold and maghemite nanoparticles that were functionalized with 2,3-dimercaptosuccinic acid (DMSA) (Au-DMSA and γ-Fe2O3-DMSA)[53]. These nanoparticles were incubated with human MSCs and these labelled MSCs were inoculated through intranasal route and tracked using standard computed microtomography. Despite the high biocompatibility of these nanoparticles with MSCs, γ-Fe2O3-DMSA and Au-DMSA based contrast was not strong enough for tracking MSCsin vivoby standard computed microtomography[53]. An innovative iron-doped hydroxyapatite nanoparticles (FeHA NPs) were prepared by Panseriet al[54] and were found to be superior to SPIONs in improving the survival of MSCs due to rapid degradation and lower resulting intracellular iron content[54]. The unique magnetic properties of FeHA NPs make them a suitable carrier for delivering MSCs to the injury site and other therapeutically active products such as drugs,growth factors, and miRNA[54].
Moayeriet al[55] used a poly-L-lysine hydrobromide coated SPIONs to label adipose-derived stem cells (ADSC-SPION/PLL)[55]. These labeled ADSCs were injected in the medial forebrain bundle in a rat model of Parkinson’s disease (PD), and simultaneously an external magnetic field were placed on the top of rat skull for 2 wk[55]. The results of this study showed a significant improvement in the migration and homing of these labeled ADSCs in the damaged sites of substantia nigra[55]. These abovementioned studies provided strong evidence about the importance of these non-toxic and biocompatible MNPs in potentiating the homing percentage of transplanted MSCs which may improve the successful rate of MSCs transplantation in different disease models.
MNPs TO lMPROVE THE MlGRATlON AΒlLlTlES OF TRANSPLANTED MSCs
Migration and subsequent engraftment following the infusion of MSCs are essential to enkindle the regenerative power of MSCs. The desultory, undirected movement of MSCs and poor accumulation at the injured site can hinder their therapeutic abilities. It has been found that MNPs can improve the migratory features of MSCs and directed them to the target site[56]. Dextran-coated iron oxide nanoparticles have been reported by Chunget al[57] to boost MSCs migration and the subsequent transdifferentiation into dopaminergic like neurons in a mouse model of PD[57].
Liet al[33] also examined thein vitromigration of rat BM-MSCs to an injury site in the presence or absence of polydopamine (PDA)-capped Fe3O4(Fe3O4@PDA) superparticles[33]. The results showed a significant difference in the number of migrated cells between control MSCs and MSCs labeled with these superparticles[33]. Iron oxide nanoparticles were also found to increase the number of MSCs in the S-phase, their proliferation index, migration ability and secretion of vascular endothelial growth factor[47]. This suggests that labeling with iron oxide nanoparticles increased MSCs migration, while the cell cycle progression was unaffected. It was also demonstrated that labeling MSCs with Fe3O4@PDA NPs increase their migration towards laser burn injury sites in a living rat model, as well as their expression of CXCR4[47]. The latter could explain the increased migration ability of labeled MSCs. Indeed,previous studies had showed that the migration process is heavily dependent on the interaction between SDF-1α and CXCR4, and the internalization of magnetic iron oxide nanoparticles elevates CXCR4 levels in MSCs[58,59]. Furthermore, SPIONs have been found to activate the hepatocyte growth factor/tyrosine-protein kinase Met pathway in MSCs to regulate their migratory and engraftment properties[60].
MNPs TO POTENTlATE THE DlFFERENTlATlON AND SURVlVAL OF TRANSPLANTED MSCs
The superparamagnetic properties of MNPs are not only suitable for improving the homing and migration properties of MSCs, studies found that MNPs can potentiate the MSCs survival and differentiation[61,62]. Several studies demonstrated a substantial enhancement of MSCs differentiation when these cells are combined with magnetic iron oxide nanoparticles, magnetic field and a specialized differentiation medium. MNPs improve the engraftment of MSCs at the injury site which is an essential step to adopt the cellular and molecular machinery required to initiate the differentiation to committed cell type[63-66]. MNPs can be also used to enhance the quality of MSCs cryopreservation and survival after thawing these cells[67]. Naseroleslamiet al[68] transplanted a SPIONs-labelled human-derived MSCs(hAMSCs) in a rat model of isoproterenol-induced myocardial injury[68]. They reported that SPIONslabeled hAMSCs produce a remarkable activation of cardiac repair machinery in the presence of magnetic field through suppressing nuclear factor-kappaB/mitogen-activated protein kinases dependent inflammation[68]. Zhanget al[69] reconstructed a Fe3O4MNPs by adding graphene oxide(GO) to generate Fe3O4@GO magnetic nanocomposites (MNCs) that were loaded with bone morphogenetic protein-2 (BMP2)[69]. This Fe3O4@GO MNCs were able to mitigate the cell damage caused by oxidative stress and through delivering BMP2, they also improved the osteogenic differentiation abilities of MSCs[69]. Wanget al[70] created a magnetic lanthanum-doped HA/CS scaffolds(MLaHA/CS)[70]. They found after placing the MLaHA/CS scaffolds into rats with calvarial defects, it significantly enhances the recruitment of endogenous MSCs and facilitated regeneration of new bone matrix[70]. The dose of internalized MNPs found to have a great influence on the preferential differentiation of MSCs. When less than 10 pgFe/cell was used, the differentiation of MSCs into chondrocytes,adipocytes or osteocytes using citrate-coated maghemite nanoparticles was similar to that of control unlabeled cells[71]. On the other hand, when higher dose of 30 to 60 pgFe/cell was used, the chondrogenesis was significantly turned off while the adipogenesis and osteogenesis were turned on.Intriguingly, the source of MSCs may also govern their response to certain MNPs[72]. Labuscaet al[73]showed some discrepancies in the response of ADSCs and WJ-MSCs to uncoated MNPs, with average size of 20 nm[73]. When external magnetic field was applied, the chondrogenic differentiation was more pronounced in the ADSCs cell culture but not in WJ-MSCs cell culture[73]. The possible explanation for these findings was the presence of an active senescent protective mechanism in WJ-MSCs. Fanet al[74]studied the differences in intracellular iron content, labeling efficiency, cell viability, and Adipogenic and osteogenic differentiation potentials between AD-MSCs and BM-MSCs after labelling them with SPIOs. They found that SPIO-labeled AD-MSCs and SPIO-labelled BM-MSCs were similar in their labeling efficiency, intracellular iron level, survival, proliferation, differentiation potentials, and MRI imaging[74]. Since the presence of an external magnetic field can dictate the differentiation fate of MSCs,the same group of investigators, Labuscaet al[75], also studied the effect of duration, intensity and frequency of magnetic field on the differentiation abilities of ADSCs labeled with MNPs[75]. These scientists revealed that using an intermittent low intensity magnetic field (0.5 MT) for short time (2 d)triggered their differentiation to adipocytes, while applying intermittent high intensity magnetic field(21.6 MT) for short time (2 d) or continuous low intensity magnetic field (0.5 MT) for longer time (7 d)activated the osteogenic machinery[75]. Wanget al[76] injected SPION-labeled ADSCs in a rat model of stress urinary incontinence. These magnetically labeled MSCs found to have a high survival rate posttransplantation and efficiently enhanced the repairing process of the non-functional sphincter[76]. In the similar context, Xuet al[77] showed that UC-MSCs labelled with SPIONs can tolerate the inflammatory microenvironment in mouse model of sepsis by enhancing their immunomodulatory abilities and the expression of heme oxygenase-1 and tumor necrosis factor receptor-associated factor (TRAF1)[77]. These findings highlighted the advantageous outcomes of incorporating MNPs with MSCs therapy which may ultimately potentiate the success rate of MSCs transplantation and increase the chance to shift these cells toward bedside. Future studies should be designed to extensively investigate the long-term efficacy and safety of these MNPs labeled MSCs, and in parallel clinical trials must be conducted to reveal the translational possibilities of these MNPs-labeled MSCs. Table 1 summarizes the different studies thatused MNPs to improve the transplantation characteristics of MSCs. Combining Nanotechnology with MSCs opens new avenues to enhance their therapeutic outcomes and long-term regenerative abilities.The incorporation of MNPs with MSCs has been extensively investigated and it revealed great chances to increase their survival, promote their homing and retention at the site of injury, improve their tolerance to stress microenvironments and enhance their integration with host tissues and trigger their differentiation. The use of MNPs with MSCs still in need for further investigation to answer many concerns surrounding their combination. Some of these concerns are related to assessing the safety profile of MNPs on the long-run, determining the optimal non-toxic dose that can be added to MSCs based on the type of pathology and the ultimate target to be achieved, finding the best coating substrate to be used with MNPs without affecting their therapeutic functions, exploring the possibility of combining more than one MNPs for synergistic effects, finding the exact molecular mechanisms that are exerted by MNPs to alter the cellular pathways in MSCs, and studying the impact of the internal microenvironment which varies based on and the type of disease in influencing the uptake of MNPs by MSCs and their ultimate response. Future studies should also focus on addressing the role of MNPs in solving other MSCs therapy challenges including cellular heterogeneity which highly depends on the source of MSCs and the culturing procedures being used, the undesirable pre-transplantation differentiation, and the switch in their immunological characteristics under stress microenvironments. A Schematic summary depicted the role of MNPs in improving the transplantation and biological characteristics of MSCs can be found in Figure 1.
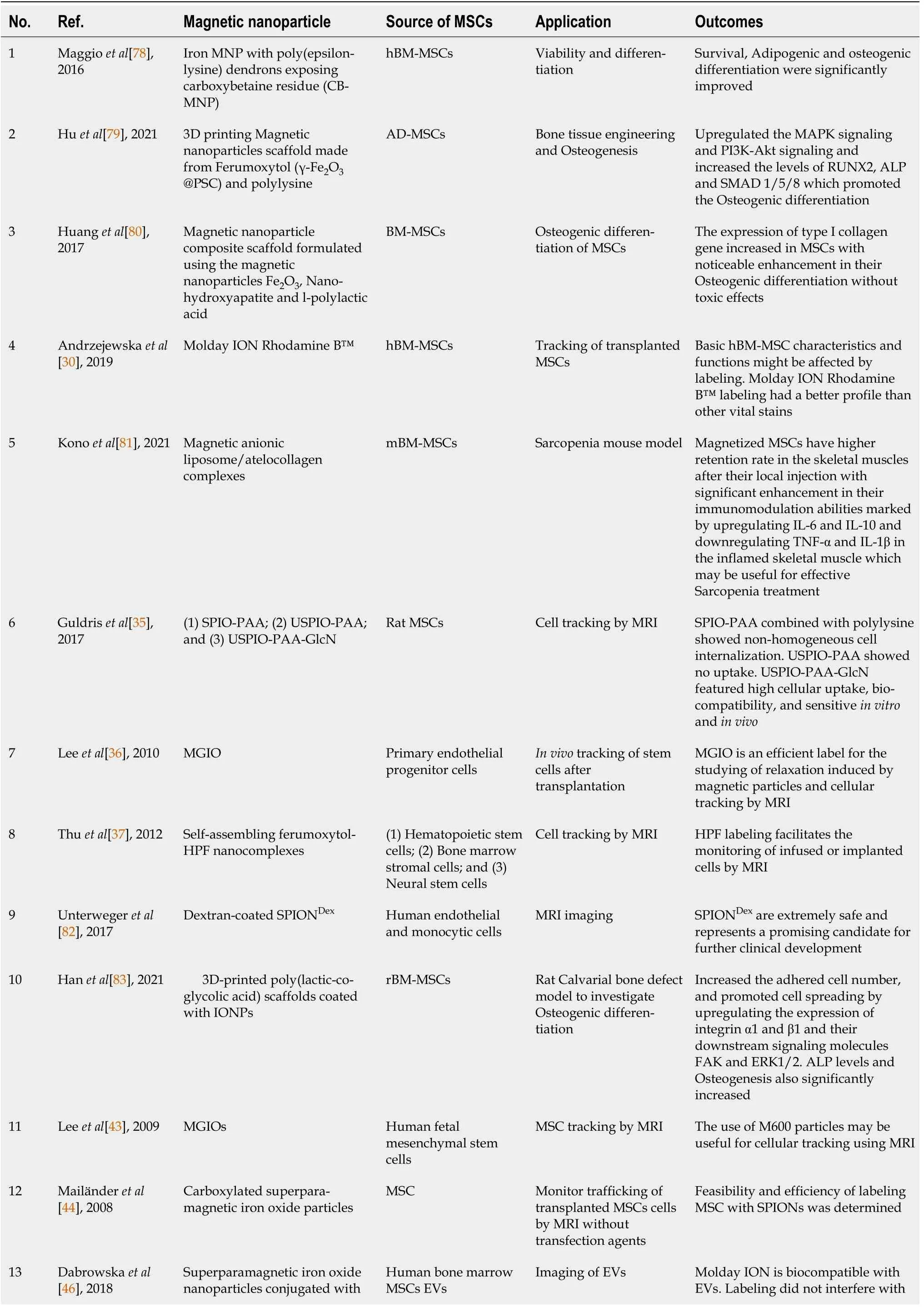
Table 1 Summary of studies that used magnetic nanoparticles to improve the transplantation characteristics of mesenchymal stem cells
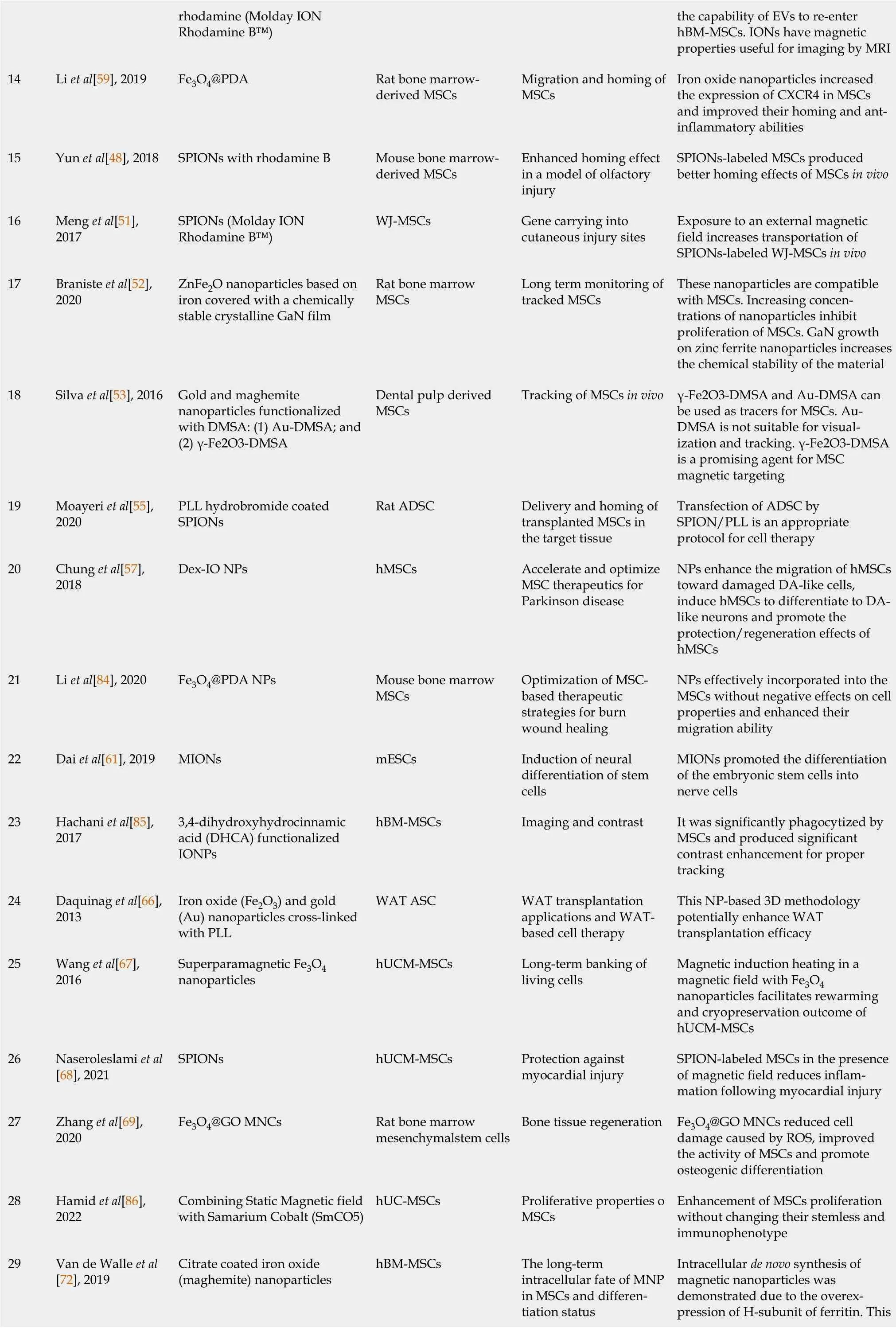
rhodamine (Molday ION Rhodamine B™)the capability of EVs to re-enter hBM-MSCs. IONs have magnetic properties useful for imaging by MRI 14 Li et al[59], 2019 Fe3O4@PDA Rat bone marrowderived MSCs Migration and homing of MSCs Iron oxide nanoparticles increased the expression of CXCR4 in MSCs and improved their homing and antinflammatory abilities 15 Yun et al[48], 2018 SPIONs with rhodamine B Mouse bone marrowderived MSCs Enhanced homing effect in a model of olfactory injury SPIONs-labeled MSCs produced better homing effects of MSCs in vivo 16 Meng et al[51],2017 SPIONs (Molday ION Rhodamine B™)WJ-MSCs Gene carrying into cutaneous injury sites Exposure to an external magnetic field increases transportation of SPIONs-labeled WJ-MSCs in vivo 17 Braniste et al[52],2020 ZnFe2O nanoparticles based on iron covered with a chemically stable crystalline GaN film Rat bone marrow MSCs Long term monitoring of tracked MSCs These nanoparticles are compatible with MSCs. Increasing concentrations of nanoparticles inhibit proliferation of MSCs. GaN growth on zinc ferrite nanoparticles increases the chemical stability of the material 18 Silva et al[53], 2016 Gold and maghemite nanoparticles functionalized with DMSA: (1) Au-DMSA; and(2) γ-Fe2O3-DMSA Dental pulp derived MSCs Tracking of MSCs in vivo γ-Fe2O3-DMSA and Au-DMSA can be used as tracers for MSCs. Au-DMSA is not suitable for visualization and tracking. γ-Fe2O3-DMSA is a promising agent for MSC magnetic targeting 19 Moayeri et al[55],2020 PLL hydrobromide coated SPIONs Rat ADSC Delivery and homing of transplanted MSCs in the target tissue Transfection of ADSC by SPION/PLL is an appropriate protocol for cell therapy 20 Chung et al[57],2018 Dex-IO NPs hMSCs Accelerate and optimize MSC therapeutics for Parkinson disease NPs enhance the migration of hMSCs toward damaged DA-like cells,induce hMSCs to differentiate to DAlike neurons and promote the protection/regeneration effects of hMSCs 21 Li et al[84], 2020 Fe3O4@PDA NPs Mouse bone marrow MSCs Optimization of MSCbased therapeutic strategies for burn wound healing NPs effectively incorporated into the MSCs without negative effects on cell properties and enhanced their migration ability 22 Dai et al[61], 2019 MIONs mESCs Induction of neural differentiation of stem cells MIONs promoted the differentiation of the embryonic stem cells into nerve cells 23 Hachani et al[85],2017 3,4-dihydroxyhydrocinnamic acid (DHCA) functionalized IONPs hBM-MSCs Imaging and contrast It was significantly phagocytized by MSCs and produced significant contrast enhancement for proper tracking 24 Daquinag et al[66],2013 Iron oxide (Fe2O3) and gold(Au) nanoparticles cross-linked with PLL WAT ASC WAT transplantation applications and WATbased cell therapy This NP-based 3D methodology potentially enhance WAT transplantation efficacy 25 Wang et al[67],2016 Superparamagnetic Fe3O4 nanoparticles hUCM-MSCs Long-term banking of living cells Magnetic induction heating in a magnetic field with Fe3O4 nanoparticles facilitates rewarming and cryopreservation outcome of hUCM-MSCs 26 Naseroleslami et al[68], 2021 SPIONs hUCM-MSCs Protection against myocardial injury SPION-labeled MSCs in the presence of magnetic field reduces inflammation following myocardial injury 27 Zhang et al[69],2020 Fe3O4@GO MNCs Rat bone marrow mesenchymalstem cells Bone tissue regeneration Fe3O4@GO MNCs reduced cell damage caused by ROS, improved the activity of MSCs and promote osteogenic differentiation 28 Hamid et al[86],2022 Combining Static Magnetic field with Samarium Cobalt (SmCO5)hUC-MSCs Proliferative properties o MSCs Enhancement of MSCs proliferation without changing their stemless and immunophenotype Intracellular de novo synthesis of magnetic nanoparticles was demonstrated due to the overexpression of H-subunit of ferritin. This 29 Van de Walle et al[72], 2019 Citrate coated iron oxide(maghemite) nanoparticles hBM-MSCs The long-term intracellular fate of MNP in MSCs and differentiation status
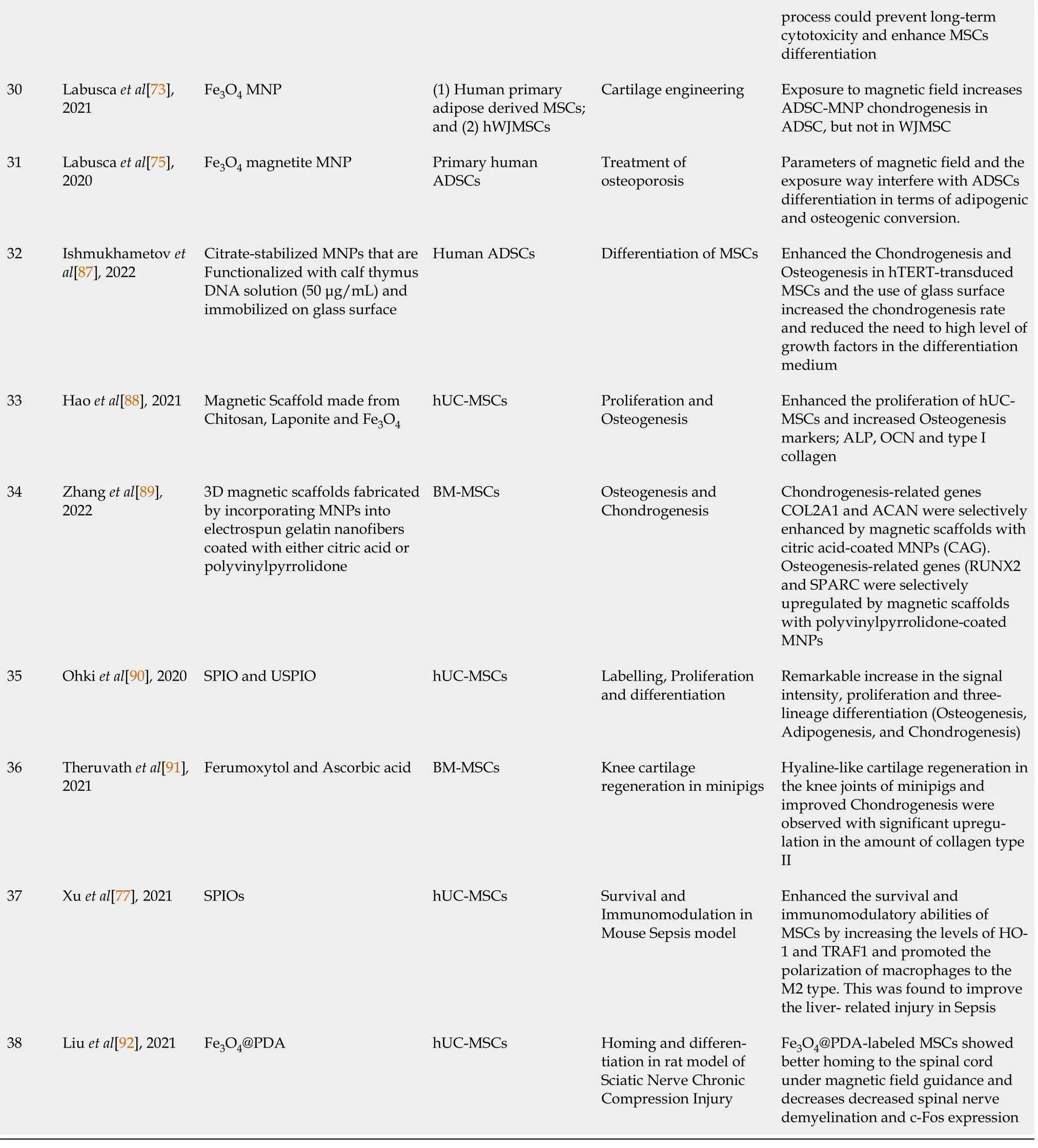
hBM-MSCs: Human bone marrow-derived mesenchymal stem cells; PDA: Polydopamine; SPIOs: Superparamagnetic iron oxide nanoparticles; AD-MSCs:Adipose tissue-derived mesenchymal stem cells; BM-MSCs: Bone marrow derived Mesenchymal stem cells; USPIO: Ultrasmall superparamagnetic iron oxide; MNPs: Magnetic nanoparticles; OCN: Osteocalcin; ROS: Reactive oxygen species; GO: Graphene oxide; WAT: White adipose tissue; ASC: Adipose stromal cells; MIONs: Magnetic iron oxide nanoparticles; Dex-IO NPs: Dextran-coated iron oxide nanoparticles; PLL: Poly-L-lysine; DMSA: 2,3-dimercaptosuccinic acid; WJ-MSCs: Wharton’s Jelly of the human umbilical cord-derived MSCs; EVs: Extracellular vesicles; HPF: Heparin-protamine;MGIO: Microgel iron oxide nanoparticle; MAPK: Mitogen-activated protein kinases; PI3K: Phosphatidylinositol 3-kinase; mESCs: Mouse embryonic stem cells; IL: Interleukin; TNF-α: Tumor necrosis factor-α; MRI: Magnetic resonance imaging; USPIO-PAA-GlcN: Glucosamine-modified iron oxide nanoparticles; MNC: Magnetic nanocomposites; HO-1: Heme oxygenase-1.
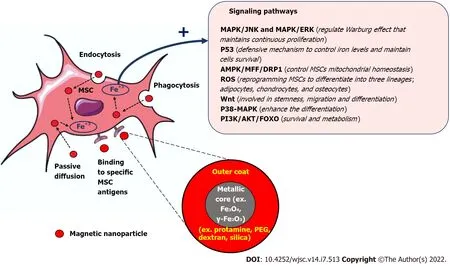
Figure 1 The prodigious therapeutic outcomes of combining mesenchymal stem cells with magnetic nanoparticles. MSC: Mesenchymal stem cell; MAPK: Mitogen-activated protein kinases; JNK: c-Jun NH2-terminal kinase; ERK: extracellular signal-regulated kinase; ROS: Reactive oxygen species; PI3K:Phosphatidylinositol 3-kinase; AKT: Protein kinase B; FOXO: Forkhead box O.
CONCLUSlON
The regenerative abilities of MSCs have been thoroughly investigated and discussed. Despite the great improvement in understanding the curative mechanisms of MSCs, many challenges are still there which slow down the transferring of these cells in the treatment guidelines. Loss of tracking signal, poor migration and homing to the injury site, and undesirable differentiation are the most reported hurdles that thwart the therapeutic outcomes of MSCs in clinical trials. The new strategy of combining MSCs with MNPs has been proven to boost the success rate of MSCs transplantation. MNPs have been employed as an effective contrast agent for long term tracking and monitoring of injected MSCs. MNPs also increase the migration and homing tendency of MSCs and enhance the committed differentiation of these cells. Future studies should be designed to investigate the long term safety profile of these MNPs and determine the suitable formulation and doses based on the specificity of each disease model and the source of MSCs.
FOOTNOTES
Author contributions:Abu-El-Rub E and Khasawneh RR conceptualized the review subtopics; Abu-El-Rub E,Khasawneh RR and Almahasneh F collected the literature used to write the review and drafted the manuscript; Abu-El-Rub E revised and formatted the content of the manuscript and verified spelling, punctuation and grammatical errors; all authors have read and approved the final manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Jordan
ORClD number:Ejlal Abu-El-Rub 0000-0001-9217-2560; Ramada R Khasawneh 0000-0003-3873-925X; Fatimah Almahasneh 0000-0002-8799-2721.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Stem Cells的其它文章
- Therapeutic potential of dental pulp stem cells and their derivatives:lnsights from basic research toward clinical applications
- Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles
- Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges
- Metabolic-epigenetic nexus in regulation of stem cell fate
- Stem cell therapy for insulin-dependent diabetes: Are we still on the road?
- Application of extracellular vesicles from mesenchymal stem cells promotes hair growth by regulating human dermal cells and follicles
