Metabolic-epigenetic nexus in regulation of stem cell fate
2022-08-01YiLiuDiXinCuiYuePanSiHanYuLiWeiZhengMianWan
Yi Liu, Di-Xin Cui, Yue Pan, Si-Han Yu, Li-Wei Zheng, Mian Wan
Yi Liu, Di-Xin Cui, Yue Pan, Si-Han Yu, Li-Wei Zheng, State Key Laboratory of Oral Diseases,National Clinical Research Center for Oral Diseases, Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Mian Wan, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Cariology and Endodontics, West China Hospital of Stomatology,Sichuan University, Chengdu 610041, Sichuan Province, China
Abstract Stem cell fate determination is one of the central questions in stem cell biology,and although its regulation has been studied at genomic and proteomic levels, a variety of biological activities in cells occur at the metabolic level. Metabolomics studies have established the metabolome during stem cell differentiation and have revealed the role of metabolites in stem cell fate determination. While metabolism is considered to play a biological regulatory role as an energy source,recent studies have suggested the nexus between metabolism and epigenetics because several metabolites function as cofactors and substrates in epigenetic mechanisms, including histone modification, DNA methylation, and microRNAs.Additionally, the epigenetic modification is sensitive to the dynamic metabolites and consequently leads to changes in transcription. The nexus between metabolism and epigenetics proposes a novel stem cell-based therapeutic strategy through manipulating metabolites. In the present review, we summarize the possible nexus between metabolic and epigenetic regulation in stem cell fate determination, and discuss the potential preventive and therapeutic strategies via targeting metabolites.
Key Words: Metabolism; Epigenetic regulation; Stem cell fate; Nexus effect
lNTRODUCTlON
Stem cells are specialized cells with a capacity for prolonged self-renewal and production of various lineage cells, which contribute to the development, maintenance and repair of organs, such as teeth, hair follicles, and liver. These long-lived cells produce proliferating progenitors that differentiate into functional cells. Disorder of this procedure results in hyperplasia, hypoplasia or dysfunction of the organs[1]. How such cell fate determination is regulated is one of the central questions in stem cell biology. High-throughput sequencing has been conducted to establish gene expression profiles of both embryonic and adult stem cells, which helps address the crucial genes in stem cell fate regulation[2].Epigenetic mechanisms, including histone modification, DNA methylation, and microRNAs (miRNAs),have uncovered the post-transcriptional regulation associated with stem cell fate[3]. Parallel proteomics studies have expanded our understanding of stem cell biology through constructing protein expression profiles of various stem cell populations. Despite these findings, the molecular network that regulates stem cell fate, maintaining pluripotency or initiating differentiation, is not completely understood due to the expression differences between mRNA and protein, the inconsistency between protein expression and its function, or the discordance between gene expression and cellular phenotype[4].
Although genomics and proteomics discuss the biological events at the gene and protein levels,respectively, several biological activities in cells occur at the metabolic level, including cell signaling,energy transfer, and intercellular communication[5]. To establish the metabolome, the collection of all metabolites at a specific time, metabolomics has been developed as one of the important components in system biology. Metabolomics is considered to be a prospective approach in various areas of researches,such as development, pathology, diagnosis, and environmental science, since it elaborates what happens in cells[6]. To address what occurs during regulation of stem cell fate determination,metabolomic research has been conducted to construct metabolic profiles of embryonic stem cells and differentiated neurons and cardiomyocytes in mice. Stem cells are characterized by highly unsaturated metabolites that regulate cell differentiation through oxidative reactions, suggesting the vital role of metabolism in stem cell fate determination. Metabolism is considered to function as a major energy source during the process[5,7,8]. Recent studies have demonstrated that lipid metabolism provided 90%of acetyl-CoA in histone acetylation. S-adenosylmethionine (SAM), one of the methionine metabolism metabolites, functions as a methyl donor in histone as well as DNA methylation[9]. Additionally, the epigenetic modification could be sensitive to the dynamic change in the metabolites, leading to changes in transcription. These findings provide compelling evidence that establishes the nexus exists between metabolism and epigenetics and propose a novel stem cell-based therapeutic strategy through manipulating metabolites[10-12].
In the present review, we summarize the nexus between metabolic and epigenetic regulation in stem cell fate determination, along with potential preventive and therapeutic strategies targeting metabolites(Figures 1 and 2).
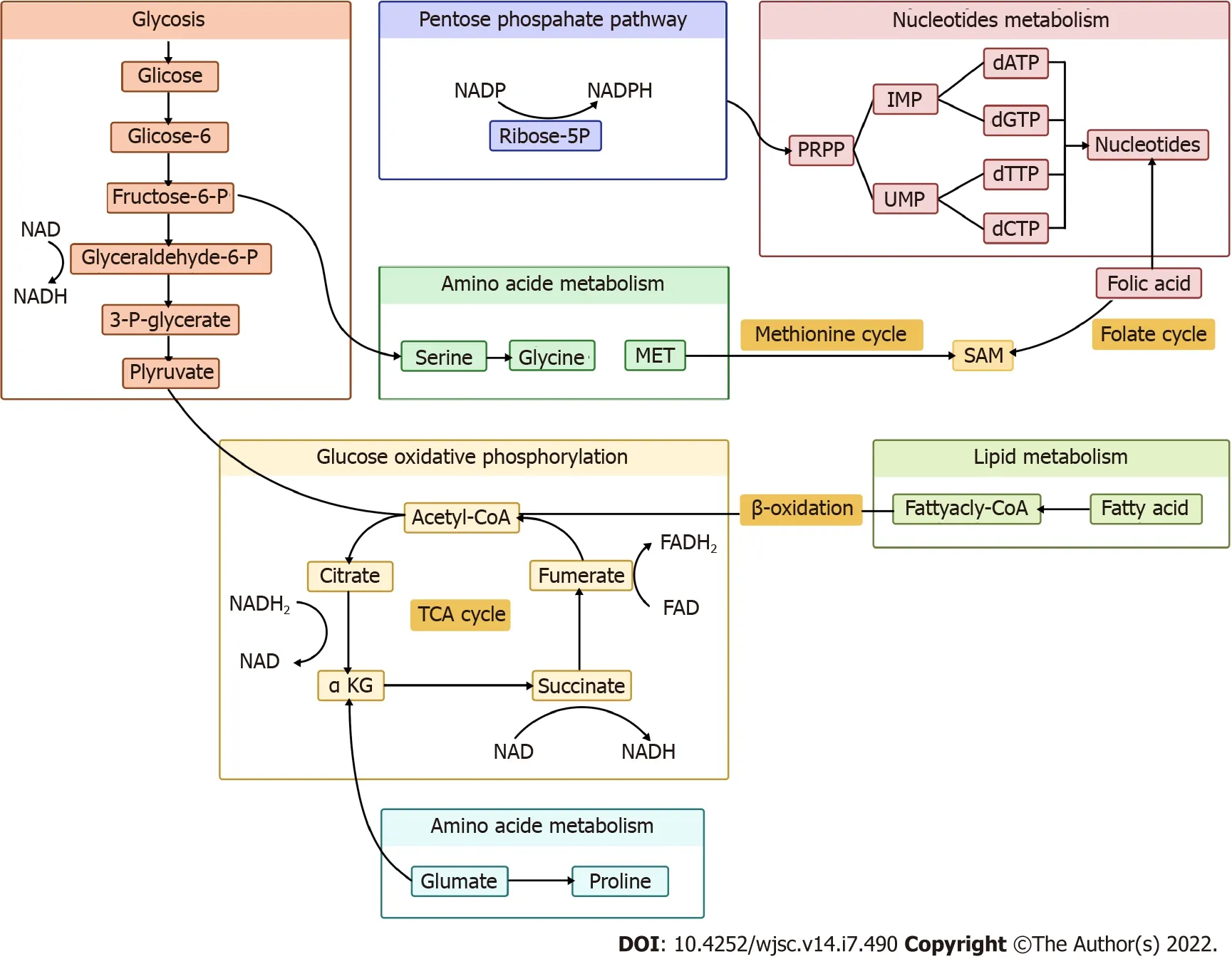
Figure 1 Schematic diagram of metabolic network. SAM: S-adenosylmethionine; TCA: Tricarboxylic acid; α-KG: α-ketoglutarate.
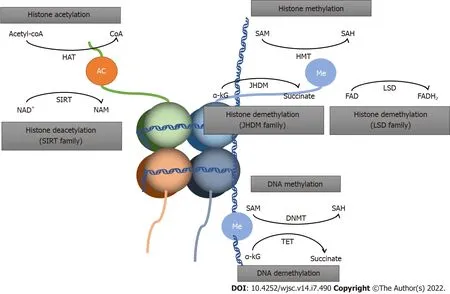
Figure 2 Schematic diagram of epigenetic metabolism. SAM: S-adenosylmethionine; SIRT: Sirtuin; α-KG: α-ketoglutarate; NAM: Nicotinamide; SAH: Sadenosylhomocysteine; HAT: Histone acetyltransferases; HMT: Histone methylases; JHDM: Jumonji-domain histone demethylase; LSD: Lysine-specific demethylase;DNMT: DNA methyltransferases; TET: Ten-eleven translocation.
LlPlD METAΒOLlSM
Lipids, crucial in maintaining cellular homeostasis, is attached to epigenetic reprogramming of homeostasis[13]. Acetyl-CoA from lipid metabolism could promote histone acetylation and drive cellular growth. Hence, acetyl-CoA is a crucial indicator for cell growth and development. Furthermore,acetyl-CoA reduces the production of b-hydroxybutyrate, an inhibitor of histone deacetylases (HDACs),which functions as antiproliferative and prodifferentiative properties[14]. The relation between lipid metabolism and epigenetic modification of gene expression in different stem cells has been reported by several studies[15].
Lipid metabolism contributes 90% of acetyl. Chromatin structure opening occurs when histone acetylation is present, activating stem cell transcription[16]. This suggests that lipid availability regulates the pluripotency of stem cells and promotes cell differentiation[17]. Itokazu group’s research on the importance of gangliosides in neural stem cells (NSCs) found that when the cellular histone deacetylase activity was inhibited by fatty acids, the levels of acetylated histone H3 and H4 on theGM2/GD2synthase gene increased, promoting neuronal differentiation of NSCs[18]. Ardahet al[19] and Boddekeet al[20] also showed that the increased level of saturated fatty acids promoted NSC differentiation into neurons. Murrayet al[21] reported that butyrate promoted myogenic differentiation of satellite cells.
Cornacchiaet al[22] showed that the level of H3K27Ac, H3K9Ac and H4K8Ac was elevated by activation of histone acetylation in human pluripotent stem cells, while histone deacetylases (HDAC),sirtuin 1 (SIRT1) and HDAC1were limited. Similar evidence has also been reported in animal studies[23,24]. The level of H3K27ac decreased in the presence of low fatty acid metabolism in the gonads, leading to male differentiation-specific signal inhibition[25]. Acetyl-CoA production can be regulated by acetyl-CoA carboxylase , a rate-limiting enzyme whose activation limits the production of acetyl-CoA, thus promoting stem cell pluripotency. This is a traditional pathway in human as well as mouse embryonic stem cells (ESCs)[26,27]. These results show that lipid metabolism affects stem cell differentiation through histone acetylation modification.
AMlNO AClD METAΒOLlSM
Amino acids are one of the most fundamental substrates in cells, and are essential for metabolism of proteins, lipids and nucleotides. Previous reports have demonstrated that amino acid metabolism affected maintenance of stem cell pluripotency. In this review, we highlight the amino acids that influence stem cells critically.
Glutamine
Glutamine is the most abundant amino acid in metabolism, and is especially active in synthesis of nucleotides and fatty acids[28,29]. Glutamine changes α-ketoglutarate (α-KG) through deamination[30],which is a critical substrate for modification of proteins and DNA by demethylases. The mechanism is that α-KG acts as a substrate for Jumonji-C and ten-eleven translocation (TET), which regulate demethylase interaction with histone and DNA, respectively. Demethylases are essential for stem cell pluripotency acquisition and maintenance. Many studies have highlighted the role of glutamine metabolism in the maintenance and differentiation of stem cells.
DNA methylation correlates with the repression of expression. α-KG positively regulates demethylation of DNA and promotes stem cell differentiation[31,32], and is key to the determination of stem cells fate as an appropriate balance between H3K9me2 acquisition and H3K27me3 depletion.Tischleret al[33], Xinget al[34] and Zyliczet al[35] came to similar conclusions in experiment on mouse primordial germ cell-like cells (PGCLCs). Okabeet al[36] have reported that histone H3K9me3 demethylation induced by an increase in α-KG activates transcription, leading to steatoblast cellular differentiation. Glutamine also regulates fetal oocyte differentiation through DNA demethylation enzyme TET1[37].
As DNA demethylation can lead to higher levels of 5-hydroxymethylcytosine, several recent studies have reported that α-KG fluctuations influence ESC differentiation[38]. The self-renewal of ESCs decreases with deficiency of glutamine, but can recover with α-KG supplementation[39]. Hepatic stellate cell (HSC) and effector T cell differentiation is also promoted by α-KG. A surprising finding is that α-KG can suppress tumor initiation and influence progression. These effects are inhibited by succinate and fumarate, providing a possible therapy for cancer[40].
Singhet al[41] have suggested that α-KG induced cell death, with degradation of hypoxia-inducible facor-1α and suppression of histone H3 (Lys 27) acetylation. The exact mechanism of histone acetylation regulated by α-KG still needs to be explored. Morriset al[42] has shown that α-KG was an effector ofp53-mediated tumor suppression, whose accumulation inp53-deficient tumors can drive tumor cell differentiation and inhibit malignant progression. Ascorbate has a positive effect on HSC differentiation and suppresses leukemogenesis[43].
All these studies above highlight the importance of glutamine in cell fate determination.
Methionine
Methionine is an essential amino acid that plays an irreplaceable role in the synthesis of SAM.Methionine in the normal diet promotes production of SAM, which serves as a methyl donor for methyltransferases of histones and DNA[44]. The fluctuation of methionine and SAM levels regulates H3K4me3 formation and maintains the undifferentiated state of human ESCs/induced pluripotent stem cells (iPSCs)[45]. Kostiet al[46] have reported that limited methionine level was associated with neuronal differentiation, along with reduction of H3K27me3. Tanget al[47] have also provided evidence that reduced conversion of methionine to SAM lead to reduced ESC pluripotency. Zhanget al[48] have also found similar evidence that SAM played an important role in the differentiation of B cells into plasmablasts, and SAM deficiency was accompanied by induction of H3K27me3. The theory may be an attractive option for improving therapeutic effectiveness in patients with systemic lupus erythematosus.
Fluctuation in the methionine cycle is related to cancer epigenetics. The increase in H3K4me3 and H3K27me3 level in cells treated with methionine in cancer stem cells parallels the increase in SAM to some extent[49,50]. This may provide a new therapy for cancer[51].
Taken together, these findings show that methionine has an important influence on stem cell fates.
Proline
Proline is a nonessential amino acid derived from glutamine metabolism. Pyrroline-5-carboxylate (P5C)is an intermediate product of both proline biosynthesis and catabolism. P5C is converted to proline by P5C reductase (Pycr1). Emerging evidence indicates that L-proline influences the epigenetic landscape of stem cells by regulating histones and DNA methylation[52,53]. L-Proline regulates H3K9 methylation and activates reprogramming of stem cells. Supplementation with L-proline increases DNA 5-methylcytosine and reduces of 5-hydroxy-methylcytosine, which promotes DNA methylation. It has recently emerged that hypermethylation lead to α-KG depletion, limiting the activity of TETs and Jumonji, and resulting in increased DNA and histone methylation .A study on mouse embryonic stem cell has shown that L-proline influenced the balance between self-renewal and differentiation[54]. Proline availability increases DNA and histone methylation, and is an essential procedure in embryonic-stem-tomesenchymal like transition[55].
Proline is one of the most important amino acids in stem cell fate determination because of its epigenetic effects.
Glycine
Glycine takes part in one-carbon metabolism as a methyl group provider through the glycine cleavage system[56]. The glycine cleavage system is a multienzyme complex consisting of four individual components: glycine decarboxylase, amino methyltransferase, glycine cleavage system protein H, and dihydrolipoamide dehydrogenase[57]. It has been revealed that glycine influenced stem cell pluripotency by controlling the synthesis of SAM, thus promoting H3K4me3 modification, and open euchromatin[58]. This process is present in human and mouse PSCs[59].
NUCLEOTlDE METAΒOLlSM
Noncoding RNA (ncRNA) is RNA that does not encode a protein. ncRNA is transcribed from the genome and exerts its effects at the RNA level. Global ncRNA abundance influences cell fate determination and differentiation, and is important in embryonic development and its dysregulation causes cancer[60-62].
There are reports suggesting that long noncoding RNA (lncRNA) lnc13728 positively regulates expression of zinc finger BED-type containing 3 to promote the adipo-genic differentiation of human adipose-derived mesenchymal stem cells[63]. lncRNA has effects on hematopoietic cells in hematopoiesis regulation and the early stage of cell fate determination. Wuet al[64] have reported that,in hematopoietic stem cells and in differentiated lineage progenitors, lncRNA expression is given priority.
Griffiths and his colleagues have demonstrated that miRNA181a inhibition activated the early latent neurogenic gene to restore CA1 neurons, providing a positive clinical outcome in survivors of forebrain ischemia[65]. Zhanget al[66] have shown that miR-124 inhibited pancreatic progenitor cell proliferation to maintain a quiescent state, thus determining the fate of pancreatic progenitor cells. In cancer cells,miRNA might be a preferential pathway in cell reprograming. It has been reported that glucose transporter type 1 (GLUT1), GLUT3 and GLUT4 were overexpressed in most cancers. miR-122 regulates lipid levels in liver. miR-185 and miR-342 inhibit migration and invasion of prostate cancer cells, which could be a therapeutic option for prostate cancer[63]. Heet al[67] have shown that miR-146a from exosomes had an effect on β-cell dedifferentiation, which provide a new therapy for type 2 diabetes[68].High expression of miR-130a can increase osteogenic differentiation of bone marrow mesenchymal stem cells, which could be a potential therapy for age-related bone loss[69,70]. Huanget al[71] have reported that miR-330-5p negatively regulated differentiation of mesenchymal stem cells.
In summary, ncRNA plays an essential role in stem cell fate determination and could act as a breakthrough point in disease therapy. However, we still have a long way to go to understand the whole regulatory network of ncRNA.
GLUCOSE METAΒOLlSM
Glucose and oxygen are important regulatory elements that help direct stem cell fate. In the undifferentiated state, stem cells, and their artificially reprogrammed equivalent iPSCs, are characterized by limited oxidative capacity and active anaerobic glycolysis. The importance of optimizing glucose metabolism during nuclear reprogramming by epigenetic regulation has been demonstrated in several studies.
Glycolysis
Glycolysis is defined as a cytosolic redox reaction that transform a single glucose molecule into two pyruvate molecules accompanied by generation of two net ATP and two reduced NADH molecules.Although glycolysis is not as energetically efficient as complete oxidation, this pathway can occur in the absence of oxygen and enables a fast rate of ATP production, which may also be the reason why some highly proliferating cell types typically utilize glycolysis.
High glycolytic flux could be frequently observed in various stem cell populations and is critical for the acquisition and maintenance of cell pluripotency[72]. Liet al[73] have shown that GLIS family zinc finger 1 could directly bind to and open chromatin structure at glycolysis-related genes to promote glycolysis. Higher glycolytic flux subsequently upregulates cellular acetyl-CoA and lactate levels,leading to increased acetylation of H3K27 and pluripotency gene loci.
Glycolytic flux can be influenced by several factors, including epigenetic regulators and environmental conditions. For example, NAD-dependent histone deacetylase SIRT6 has been proved to act as a key regulator of glucose homeostasis, and its absence favors the metabolic profile of anaerobic glycolysis, which may activate gene reprogramming and pluripotency maintenance[74]. The epigenetic modifications are essential for the cell fate decisions in NSCs as well. High glucose levels increase H3K14 acetylation level, which can lead to premature neurogenetic differentiation of NSCs, providing a promising target for intervention in fetal neurodevelopment deficits[75]. Protein glycosylation is one of the most diverse and complicated co- and post-translational modifications, regulating self-renewal,pluripotency, and differentiation of stem cells through epigenetic mechanisms by histone modification and DNA methylation[76]. Glycolytic flux can also be regulated by oxygen. Glycolysis increases at 5%oxygen and acetylation of H3K9 and H3K27 is elevated, while H3K27 trimethylation is downregulated,leading to a more open chromatin structure and altered fate of human PSCs[77,78].
In summary, glycolysis is the dominant metabolic phenotype that controls stem cell fate.
Glucose oxidative phosphorylation
Glucose oxidative phosphorylation is another critical pathway for maintaining bioenergetic homeostasis as a bridge between the tricarboxylic acid (TCA) cycle and ATP synthesis. Oxidative phosphorylation is a more efficient pathway for ATP production compared to glycolysis, producing 36 ATP molecules per glucose. Oxidative phosphorylation promotes stem cell differentiation. Uittenbogaardet al[79] have provided evidence that enhancing oxidative phosphorylation can trigger neuronal differentiation by generating H3K27ac. Oxidative phosphorylation also mediates hematopoiesis stem cell differentiation toward definitive hematopoiesis through actyl-CoA metabolism[80].
Several TCA-cycle-related metabolic intermediates like NADH, FADH, fumarate and succinate are reported to contribute to epigenetic regulation of transcription and be connected with stem cell fate.
NADH:NAD+is a coenzyme that serves as a co-substrate for sirtuins, an HDAC family, and catalyzes deacetylation of histone lysine; a crucial protein post-translational modification[81-83].
NAD/NADH ratio can dictate the fate and function of different cell types. Increased NAD+production is required for cell differentiation[84]. Bmal1 regulates primary myoblast proliferation and differentiation through increasing cytosolic NAD+. Reduced NAD+level prevents the differentiation of preadipocytes[85]. Okabeet al[36] have confirmed that high NAD+levels upregulated the TCA cycle,increasing α-KG and contributing to histone H3K9 demethylation and transcriptional activation. Zhuet al[85] have demonstrated that increasing cytosolic NAD levels could restore hypoxic cell proliferation and myofiber formation in Bmal1-deficient myoblasts, influencing oxygen-dependent myoblast cell fate.The effect of NAD/NADH ratio on stem cell fate is caused by generation of L-2-hydroxyglutaric acid,an analog of α-KG that regulates histone and DNA methylation by competitive inhibition of Jumonjidomain histone demethylase (JHDM) and TETs. There are reports revealing that increased NAD+levels delay aging-related phenotypes, which may provide new therapeutic option for type 2 diabetes and heart failure[86,87]. Besides, NAD+is a cosubstrate of Sirtuins, potentially regulating T cells, and could provide a therapeutic option for immune-related diseases[88].
In summary, NAD+plays a key role in a diverse array of biological processes.
FADH:FAD, the oxidized form of FADH2, is a cofactor of human lysine-specific demethylase-1 (LSD1),and plays a pivotal role during early embryonic development and differentiation of ESCs and cancer stem cells[89-92]. LSD1 catalyzes the demethylation of mono- and dimethylated K4 or K9 on histone H3viathe FAD-dependent enzymatic oxidation[93]. Recent studies have found that LSD1 inhibition can enhance death in rhabdomyosarcoma cells[94]. Decreased expression of LSD1 is involved in the programmed oocyte death by autophagy in perinatal mice through promotion of H3K4me2 expression[95]. FAD also regulates NSC proliferation through modulation of histone methylation by affecting the action of LSD1. In addition, LSD1 is highly expressed in a few aggressive cancer types and is closely related with differentiation, proliferation, migration and invasion of cancer cells and poor prognosis.
Succinate:Succinate accumulation can decrease α-KG/succinate ratio, leading to inhibition of TET and JHDM enzymes and delayed differentiation of primed human PSCs. This effect can be reversed when the α-KG/succinate ratio increases[32,86]. Accumulation of succinate, resulting in genetic and epigenetic changes like histone hypermethylation, may lead to transformation of normal cells to cancerous cells[96,97] . Wonget al’s study in colorectal cancer cells showed that promoting accumulation of succinate upregulated DNA methylation and stem cell features[98]. AA6 is a novel compound succinic acid,identified as an inhibitor of α-KG dehydrogenase, which can increase the α-KG level in diabetic human cardiac mesenchymal cells and in the heart of high-fat diet, leading to DNA demethylation, and has beneficial effects of cardiac mesenchymal stem cells protection in diabetes[99].
Fumarate:Fumarate is reported to inhibit α-KG-dependent dioxygenases involved in DNA and histone demethylation. Laukkaet al[100] have shown that fumarate downregulates global 5-hydroxymethylcytosine level in neuroblastoma cellsviaTET inhibition. Furthermore, Shardaet al[101] have reported that fumarate promotes monomer-to-dimer transition of malic enzyme 2 to enhance mitobiogenesis,linking metabolism to mitobiogenesis. Aberrant accumulation of fumarate may mediate epigenetic reprogramming. Some studies have reported the link between fumarate accumulation, epigenetic changes, and tumorigenesis. Accumulation of fumarate, inhibiting Tet-mediated demethylation, induces epithelial-to-mesenchymal transition; a phenotypic switch associated with cancer initiation, invasion and metastasis[102]. This implies that fumarate accumulation contributes to the aggressive features tumors[103].
Pentose phosphate pathway
The pentose phosphate pathway (PPP) is another glucose metabolism pathway, divided into oxidative and nonoxidative arms, producing NADH and ribose-5-phosphate and/or xylulose-5-phosphate that influence the regulation of transcription[104]. NADPH production in the pathway is involved in folate metabolism[105]. Previous studies have reported that regulation of the PPP resulted in iPSC reprogramming[106]. The PPP actively provides energy and metabolic intermediates for proliferation and pluripotency in cancer cells, ESCs and iPSCs[107,108]. Intracellular pH increase selectively activates catalysis, enhancing PPP flux, leading to nucleotide upregulation, increased NADPH/NADP+ratio, and cell proliferation[109].
It remains to be elucidated whether PPP is linked to stem cell epigenetic remodeling.
OTHER POTENTlAL lNFLUENTlAL FACTORS
Structure
The structure of scaffolds can affect stem cell metabolism. Three-dimensional graphene foam has better properties than two-dimensional foam for NSC differentiation. However, the possible mechanism needs to be explored[110].
Micronutrients
Vitamin C:Vitamin C is a crucial micronutrient that may be involved in stem cell pluripotency by activating H3K36 and H3K9 demethylases through Jumonji-C function[111]. A study using human PGCLCs also indicated the pathway[112,113]. Micronutrients influence stem cells specification.
Folic acid:Folic acid is first metabolized to dihydrofolate and then to tetrahydrofolate, taking part in DNA synthesis, influencing DNA and histone methylation[105]. Several studies have elucidated the role of folate metabolism in regulating of the epigenetic landscape of stem cells[114,115]. Liet al[116] have shown that folic acid deficiency in NSCs decreased cell proliferative capacity but increased apoptosis.Kasulanatiet al[117] in a study of ESCs have provided more evidence for the effect of folic acid on PSC pluripotency. Peiet al[118] in a study of mouse ESCs have demonstrated that under folate deficiency conditions, H2AK119ub1 increases, and expression of neural tube closure-associated genes decreases.This suggests a possible mechanism for neural tube defects. Xieet al[119] have shown that folate inhibition can activate histone modification of monomethylation at lysine 4 of histone H3 transcription,suggesting that epigenetic regulation varies for different histone modifications.
Crosslinking:Horitaniet al[120] have reported that glucose along with triglyceride increased metabolic stress spikes in mice, resulting in demethylation of H3K27me3, and expression of senescence-like phenotypes in bone marrow stem/progenitor cells. This could provide a therapeutic method for patients with cardiovascular disease and type 2 diabetes.
CONCLUSlON
This review summarizes the recent studies about the metabolic-epigenetic nexus and provides compelling evidence that metabolism regulates stem cell fate determination through epigenetic mechanisms, such as histone acetylation, histone methylation and DNA methylation, in a variety of physical and pathological phenomena. The latest studies have also suggested that potential manipulation of metabolites held great promise in developing novel preventive, diagnostic and therapeutic strategies for a variety of diseases, which still requires further study prior to application in clinic settings.
There are still some essential questions. For example, what is the outcome of the regulation of metabolism on the epigenetic and transcriptional procedures of stem cells. The interplay of metabolism and epigenetics also brings out the complexity in environmental exposures studies, as the method of cell metabolism and potential transgenerational inheritance has been changed[15,121].
Furthermore, there were still some limitations. Most studies have attached importance to the level of enzymatic activity in cells, and we must accept that there is a difference between the measured and actual values[15]. All researches were conducted under experimental and not physiological conditions,and it is not hard to conclude that there might be some variation.
In summary, when it comes to the mechanism of stem cell fate determination, there is indeed interplay between metabolism and epigenetics. We need more accurate data acquisition and more realistic simulation as well as more specific mechanisms. The development of new technologies makes it easier to measure cellular metabolic status, and the accumulation of past studies supports our further exploration in this field.
FOOTNOTES
Author contributions:Liu Y and Wan M conceived and designed the study; Cui DX, Pan Y and Yu SH collected materials; Liu Y wrote the paper; Zheng LW and Wan M revised the manuscript critically; all authors have read and approved the final manuscript.
Supported bythe National Natural Science Foundation of China (General Program), No. 82170921; the Sichuan Science and Technology Program, No. 2022YFS0284; and the Research and Develop Program, West China Hospital of Stomatology Sichuan University, No. LCYJ2019-24.
Conflict-of-interest statement:The authors declare that there is no conflict of interests regarding the publication of this paper.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yi Liu 0000-0003-4038-6051; Di-Xin Cui 0000-0002-8102-9504; Yue Pan 0000-0001-8197-869X; Si-Han Yu 0000-0002-7740-2660; Li-Wei Zheng 0000-0002-0467-1720; Mian Wan 0000-0002-3373-2836.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
杂志排行
World Journal of Stem Cells的其它文章
- Therapeutic potential of dental pulp stem cells and their derivatives:lnsights from basic research toward clinical applications
- Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles
- Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges
- Stem cell therapy for insulin-dependent diabetes: Are we still on the road?
- Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles
- Application of extracellular vesicles from mesenchymal stem cells promotes hair growth by regulating human dermal cells and follicles
