Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles
2022-08-01VictoriaPulidoEscribanorbaraTorrecillasBaenaMartaCamachoCardenosaGabrielDoradoMarngeleslvezMorenoAntonioCasadoaz
Victoria Pulido-Escribano, Bárbara Torrecillas-Baena, Marta Camacho-Cardenosa, Gabriel Dorado, María Ángeles Gálvez-Moreno, Antonio Casado-Díaz
Victoria Pulido-Escribano, Βárbara Torrecillas-Βaena, Marta Camacho-Cardenosa, María Ángeles Gálvez-Moreno, Antonio Casado-Díaz, Unidad de Gestión Clínica de Endocrinología y Nutrición-GC17, Instituto Maimónides de Investigación Biomédica de Córdoba, Hospital Universitario Reina Sofía, Córdoba 14004, Spain
Gabriel Dorado, Dep. Bioquímica y Biología Molecular, Campus Rabanales C6-1-E17, Campus de Excelencia Internacional Agroalimentario (ceiA3), Universidad de Córdoba, CIBERFES,Córdoba 14071, Spain
Abstract The use of mesenchymal stem-cells (MSC) in cell therapy has received considerable attention because of their properties. These properties include high expansion and differentiation in vitro, low immunogenicity, and modulation of biological processes, such as inflammation, angiogenesis and hematopoiesis.Curiously, the regenerative effect of MSC is partly due to their paracrine activity.This has prompted numerous studies, to investigate the therapeutic potential of their secretome in general, and specifically their extracellular vesicles (EV). The latter contain proteins, lipids, nucleic acids, and other metabolites, which can cause physiological changes when released into recipient cells. Interestingly,contents of EV can be modulated by preconditioning MSC under different culture conditions. Among them, exposure to hypoxia stands out; these cells respond by activating hypoxia-inducible factor (HIF) at low O2 concentrations. HIF has direct and indirect pleiotropic effects, modulating expression of hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, angiogenesis, metabolism, and cell apoptosis. Expression of these genes is reflected in the contents of secreted EV. Interestingly, numerous studies show that MSC-derived EV conditioned under hypoxia have a higher regenerative capacity than those obtained under normoxia. In this review, we show the implications of hypoxia responses in relation to tissue regeneration. In addition, hypoxia preconditioning of MSC is being evaluated as a very attractive strategy for isolation of EV, with a high potential for clinical use in regenerative medicine that can be applied to different pathologies.
Key Words: Cell priming; Extracellular vesicles; Hypoxia; Hypoxia-inducible factor; Mesenchymal stemcells; Regenerative medicine
lNTRODUCTlON
Mesenchymal stem-cells or mesenchymal stromal-cells (MSC) derived from adult tissues are characterized by their low immunogenicity, high proliferation capacity, differentiation capabilities, and modulation of physiological processes such as inflammation, hematopoiesis, and angiogenesis[1-3].MSC can be isolated from different tissues for their culture and expansionin vitro. Therefore, they are currently considered an important therapeutic tool in the field of regenerative medicine[4,5]. However,one of the main limitations of their use is the need to obtain and expand MSCin vitro, which cannot always be obtained from the same patient to be treated. Unfortunately, MSC manipulations may cause cell-functionality loss and genetic instability when performed outside their natural niches[6]. Moreover,one risk of the application of cell therapy in regenerative medicine is that MSC may remain undifferentiated and produce tumors[7].
Recently, numerous studies have shown that the regenerative capacity of MSC mainly depends on their paracrine functions. Therefore, an alternative or complement to cell therapy in regenerative medicine is the use of media enriched in cytokines and other factors secreted byin vitrocultures of progenitor cells[8]. The MSC secretome is composed by soluble factors and extracellular vesicles (EV)[9]. The main functions of EV are cell communications and interactions. Their contents depend on their cellular origin and the physiological conditions in which they are produced[10]. Therefore, preconditioning MSC under conditions that increase their regenerative power, like hypoxia, may induce the production of EV with enhanced regenerative potential. In the presence of damage, tissues normally undergo ischemic processes. These reduce the supply of O2and nutrients to damaged areas. This causes cellular responses that induce the release of factors, promoting vessel formation and tissue regeneration[11]. Indeed, MSC preconditioning in hypoxia can induce this response, leading to the production of EV rich in angiogenic factors and inducers of tissue regeneration. In this context, the main aim of this review is to describe the effects that preconditioning in hypoxia may have on MSC, mainly in the contents of their EV, and how this strategy has a great potential for regenerative medicine.
MSC AND REGENERATlVE MEDlClNE
MSC are multipotent cells first discovered by Friedensteinet al[12] in 1970. They have fibroblast-like morphology, behaving as colony-forming units-fibroblasts. These cells originate in the mesoderm,having the ability to differentiate into different cell types including osteoblasts, adipocytes, and chondrocytes[13]. The minimum characteristics that a cell must have to be considered MSC, according to the International Society for Cellular Therapy, are: (1) Adhere to plastic under standard culture conditions; (2) Exhibit several clusters of differentiation (CD): CD-73, CD-90, and CD-105, lacking CD-11b, CD-14, CD-19, CD-34, CD-45, CD-79a, and human leukocyte antigen-D-related isotype; and (3)Differentiation potency into osteoblasts, adipocytes, or chondrocytesin vitro[14]. Isolated MSC may have different origins, such as adipose tissue, placenta, umbilical-cord blood or Wharton’s jelly,synovium, periodontal ligament, menstrual blood, and bone marrow, the latter being one of the essential sources of these cells for research and clinical applications[15-17].
MSC are involved in tissue regeneration, being necessary for maintaining vital functions and delaying aging. The application of MSC in regenerative therapies is gaining great interest due to their advantages. Thus, these cells can be isolated and culturedin vitro, have the capacity to undergo multilineage differentiation, and also possess anti-inflammatory and immunosuppressive properties[5].Indeed, such cells have great potential to treat various pathologies, including those of the nervous system, bone, skin, myocardium, and liver, among others[4,18-20]. In this regard, multiple clinical trials related to these pathologies have demonstrated the potential of MSC in human clinical practice[21-23].Nevertheless, despite the potential and good results obtained in cell therapy, the risks involved when using cells in regenerative medicine should be considered, as indicated above. Therefore, in the last few years, cell-free therapies have gained attention, becoming the preferred options in many instances.
MSC-DERlVED EV AS A NOVEL APPROACH TO CELL-FREE THERAPlES
It is well known that MSC-based cell therapies have beneficial therapeutic effects in different pathologies. Nevertheless, some studies suggest that these benefits may not be due to the cells themselves, but mainly linked to their paracrine effects. For instance, at the site of injury[13,24,25], the EV secreted by these cells are the key players[26]. Thus, the use of MSC-derived EV has been found to be beneficial to improve cartilage repair and regeneration, cardiac repair after myocardial infarction,wound healing, and lung repair, among other clinical applications[27-30].
According to their size, biogenesis, release pathway, function, and content, EV have been classified into microvesicles, exosomes, and apoptotic bodies. Microvesicles range between 100 to 1000 nm in diameter and are formed through outward outgrowth. Exosomes are vesicles generating after fusion of multivesicular bodies with plasma membranes, ranging between 40 to 100 nm[10,31]. They should not be confused with RNA-degrading complexes with the same name found in both archaea and eukaryotes. On the other hand, apoptotic bodies are released during early apoptosis and are larger than 1000 nm[32,33]. However, there is a lack of consensus about classification and biochemical markers characterizing the different EV types. Therefore, the International Society for EV stated the following in the “Minimal Information for Studies of Extracellular Vesicles 2018” (MISEV2018), in relation to the EV nomenclature: “EV is the preferred generic term for the subject of our investigations, and subtypes should be defined by physical and biochemical characteristics and/or conditions/sources. When other terms are used, careful definition is required”[34].
EV may contain proteins, nucleic acids (including coding and non-coding RNA), lipids, and other metabolites. Normally, the content is rich in cytoskeletal proteins (such as TSG10 or CD63 tetraspanins),integrins, and major histocompatibility complex molecules[35]. Depending on cell types and microenvironments in which they are secreted, contents of EV may change. Thus, EV reflect physiological states of cells generating them. For this reason, MSC growth under different conditions, such as hypoxia,presence of trophic and physical factors, or chemical and pharmacological agents, may stimulate secretion of EV enriched in certain cytokines, growth factors, or non-coding RNA like microRNA(miRNA)[36].
In relation to the functionality of EV, at first it was thought that they were a mechanism for cells to get rid of unwanted material. It was later demonstrated that EV play a fundamental role in cellular homeostasis, being key elements in cell-to-cell communications[33,37]. Thus, these vesicles regulate different physiological processes such as cell proliferation, differentiation, and migration[38].
EV can be isolated from various sources, including blood, urine, breast milk, amniotic fluid, and synovial fluid, among others, as well as supernatants from cell cultures such as endothelial, epithelial,cancer, MSC,etc[39]. There are different purification approaches such as differential and densitygradient ultracentrifugation, ultrafiltration, size-exclusion chromatography, precipitation,immunoaffinity, and microfluidic-based methods[33]. Likewise, isolated EV can be characterized by different techniques like electron microscopy, flow cytometry, nanoparticle tracking analysis, dynamic light scattering, tunable-resistive pulse sensing, and atomic-force microscopy, among others[38,40].
Using EV in regenerative medicine has some advantages in comparison with whole-cell therapies[41]including: (1) Can be easily stored, being immediately available for clinical applications; (2) Production of large quantities of cells is not required; (3) Can be evaluated for safety, dosage, and activity in a manner similar to conventional pharmaceutical agents; (4) Are stable, exhibiting a long half-life; indeed,the lipid bilayers of their membranes protect their contents from degradationin vivo; (5) Can be more easily applied for clinical purposes than proliferative cells; for example, they can be intravenously injected, circulate through the smallest capillaries, and cross the blood-brain barrier; (6) Risks of immune rejection, cellular dedifferentiation, or tumor formation are lower than in whole-cell therapies;and (7) EV can be manipulated for more precise effects as therapeutic agents[10,41]. Therefore, the use of EV in therapy has become a great tool for regenerative medicine in recent years.
ROLE OF HYPOXlA-lNDUClΒLE FACTOR lN ADAPTATlON TO HYPOXlA AND TlSSUE REGENERATlON
When oxygen concentrations decrease to less than 5% in tissues, cells have to adapt their metabolism and functions to such hypoxic conditions. Moderate (< 5% to > 2% O2), severe (≤ 2% to ≥ 0.1% O2), and anoxia (< 0.1% O2) are hypoxia levels equal or below 5% oxygen concentration. Depending on O2concentration and hypoxia time, cells show different responses, as observed in human embryonicderived MSC[42]. That occurs mainly through activation of hypoxia-inducible factor (HIF). This is a transcription factor consisting in a heterodimer of two basic helix-loop-helix proteins: Alpha (HIFA) and beta (HIFB)[43,44]. While expression of the gene encoding alpha subunits is induced by hypoxia, the gene encoding HIFB, also known as aryl hydrocarbon-receptor nuclear translocator, is constitutively expressed[45]. There are three alpha subunits (HIF1A, HIF2A, and HIF3A),with the first two wellknown. HIF1A and HIF2A have 48% amino acid sequence identity and similar protein structures.Although they share functions, they can regulate the expression of different genes[46].
HIF2A, which is also known as endothelial PAS (Period, Aryl-hydrocarbon-receptor, Single minded)domain protein-1, was originally associated with endothelial development and regulation. Its encoding gene exhibits a more restricted expression relative to the one ofHIF1A[47]. Furthermore, whereas HIF1A requires very low O2concentrations for stabilization, H2FA can be activated at less severe levels of hypoxia (approximately 5%). Therefore, HIF1A would act in the initial response, whereas HIF2A would regulate the response to long periods of hypoxia[48,49]. On the other hand, HIF3A has three isoforms (HIF3A, neonatal and embryonic PAS, and inhibitory PAS protein). They inhibit the transcriptional activity of HIF1A and HIF2A by preventing their heterodimerization with HIF1B[50,51].
Under normoxia, HIF1A proteins in the cytoplasm are continuously degraded, through the proteasome pathway[52]. However, when the O2concentration decreases, HIF1A proteins are not degraded, but rather they are accumulated and translocated into the nucleus (Figure 1). Regulation of HIF1A levels depends on the presence of an oxygen-dependent degradation domain in the protein. This domain is constituted by Fe2+and two prolyl residues (Pro402 and Pro564). Such residues undergo hydroxylation through prolyl hydroxylases (PHD1, PHD2, and PHD3) in the presence of oxygen and αketoglutarate, allowing HIF1A to be recognized by the von Hippel-Lindau tumor suppressor protein, a component of the E3 ubiquitin-ligase complex. That way, it is degraded by the ubiquitin-proteosome pathway[53,54] (Figure 1).
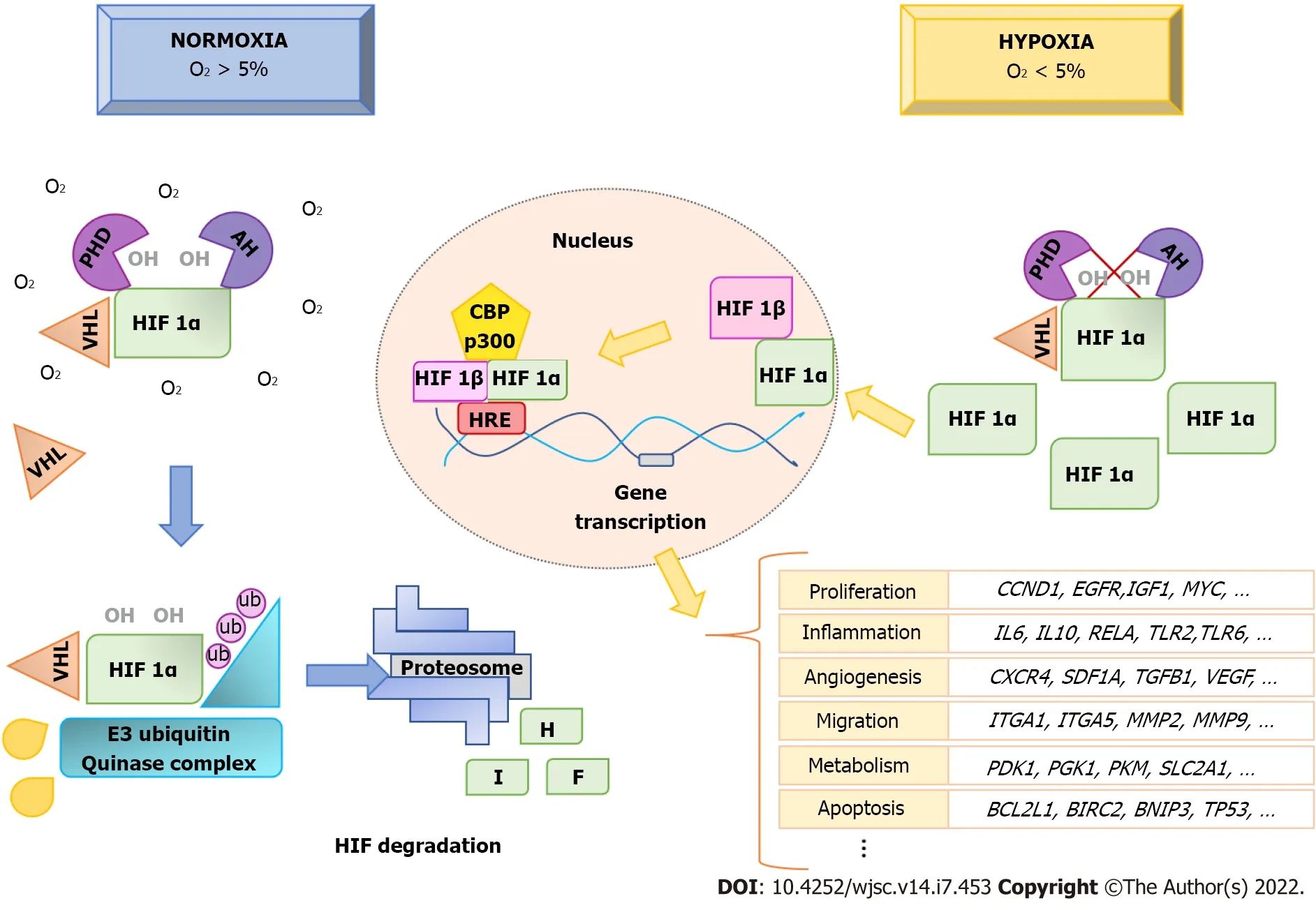
Figure 1 Hypoxia-inducible factor 1A protein regulation in hypoxia. Hypoxia-inducible factor (HIF) 1A is continuously hydroxylated and degraded by the proteosome, when O2 concentrations are greater than 5%. However, in hypoxia (O2 < 5%) HIF1A hydroxylation is inhibited and it accumulates in the cytoplasm. It then translocates to the nucleus, where it forms a heterodimer with HIF1B. This, together with the coactivator CBP/P300, binds to hypoxic-response elements at gene-promoter sites, activating transcription of genes involved in biological processes such as angiogenesis, proliferation, migration, inflammatory response,metabolism, and apoptosis, among others. This produces physiological adaptive responses of cells to hypoxia. HIF: Hypoxia-inducible factor; HRE: Hypoxic-response elements; PHD: Prolyl HyDroxylases; VHL: Von Hippel-Lindau.
In addition to PHD, another enzyme, called factor inhibiting HIF1 (FIH), can inhibit the transcriptional activity of HIF1A. In this case, FIH hydrolyzes residues within the C-terminal transactivation domain of HIF1A, preventing their binding to coactivators to initiate transcription in the nucleus[55].Under hypoxic conditions, prolyl hydroxylation is inhibited, and, thus, the degradation of HIF1A is also inhibited. They accumulate and translocate into the nucleus, where they form heterodimers with HIF1B.That way, they can induce gene transcription through binding to pentanucleotide sequences(A/GCGTG) called hypoxic-response elements in the promoters of target genes. For transcription of target genes to occur, coactivators are recruited, mainly p300/CBP[56] (Figure 1).
It has been described that more than 1000 genes can be directly or indirectly regulated by HIF. These genes are involved in adaptation of cells to hypoxic conditions. They affect different physiological processes including metabolism, angiogenesis, inflammatory response, cell differentiation, migration,and apoptosis[57]. In order to present an overview of the various functions of genes regulated by HIF1α and HIF2α, we have analyzed the information contained in Qiagen Ingenuity Pathway Analysis (Qiagen IPA) web-based software application (https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa)[58]. This platform allows querying information gathered from databases and findings described in the literature for a given gene.Information from 493 references related to genes regulated by HIF1A and 215 for HIF2A were integrated in the description of functions of human HIF at the time of writing. From the information of these references, the application shows 191 genes regulated by HIF1α and 111 by HIF2α. Among them,72 are common to both.
Functional analyses of HIF1A- and HIF2A-regulated genes with IPA show categories and functional annotations in which they are involved. Tables 1 and 2 show these data together withPvalues and the number of genes identified for each of the categories obtained from Qiagen IPA. Regarding the functional annotations, a maximum of the five most significant annotations in each category are shown.The list of genes for HIF1A and HIF2A obtained from such application, as well as genes corresponding to each of the categories presented in Tables 1 and 2, are shown in elsewhere (Supplementary Tables 1-3). Among the functions of genes regulated by HIF1A and HIF2A are those related to glucose metabolism, vessel formation, inflammatory response, cell proliferation, cell migration, and apoptosis.Interestingly, they play relevant roles in tissue regeneration[57].
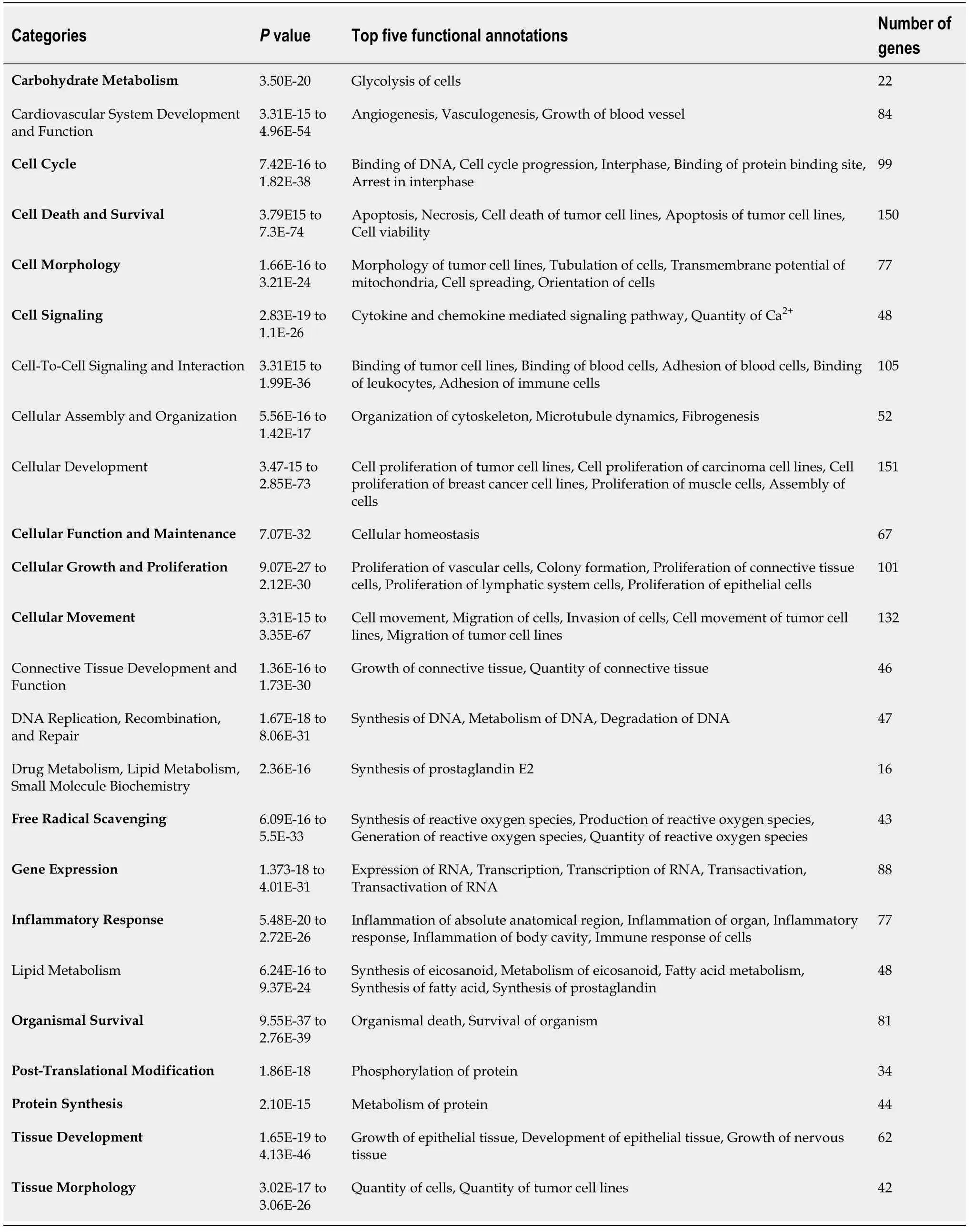
Table 1 Functional categories and annotations in ingenuity pathway analyses of genes regulated by hypoxia-inducible factor 1A
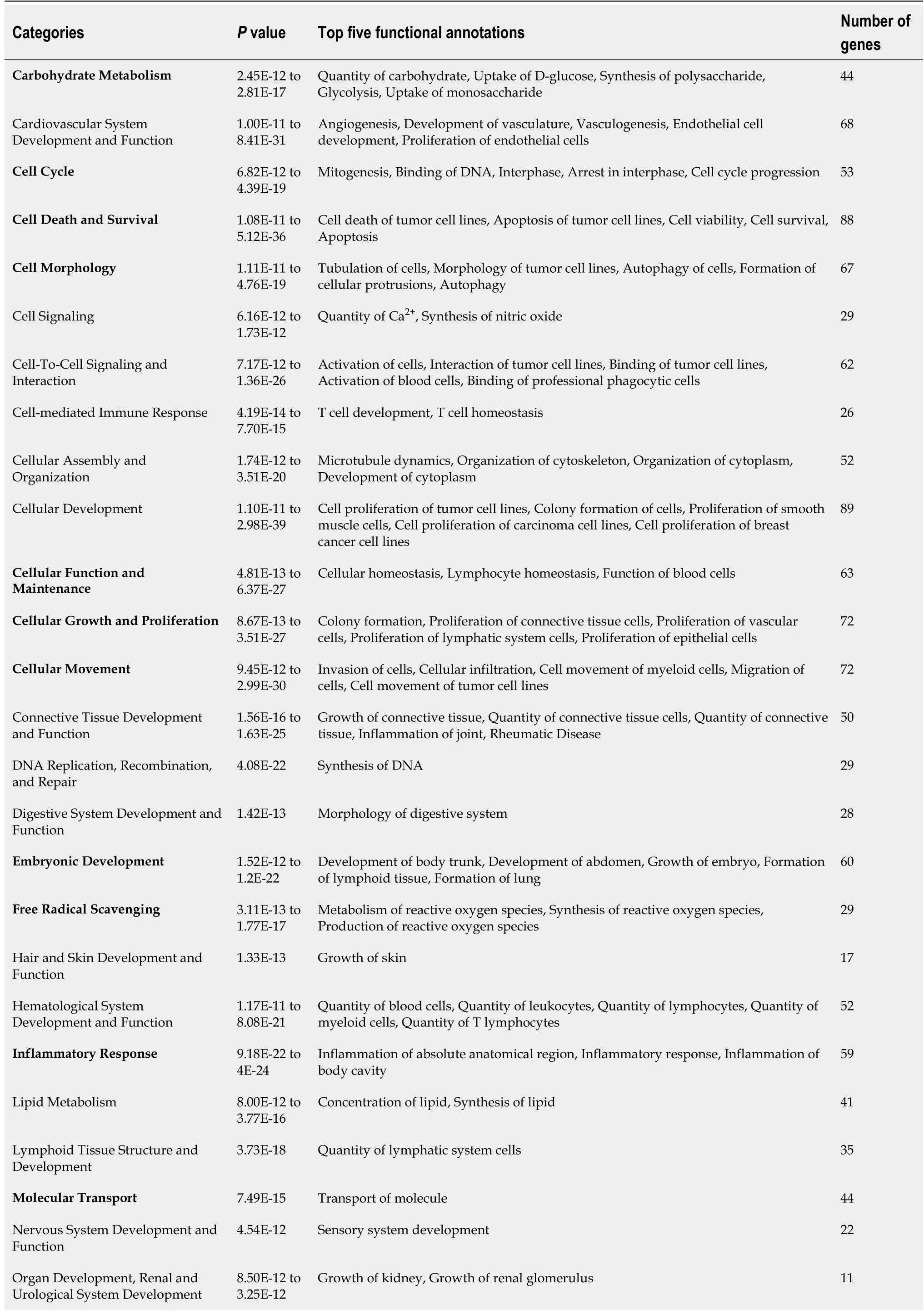
Table 2 Functional categories and annotations in lngenuity Pathway Analyses of genes regulated by hypoxia-inducible factor 1A
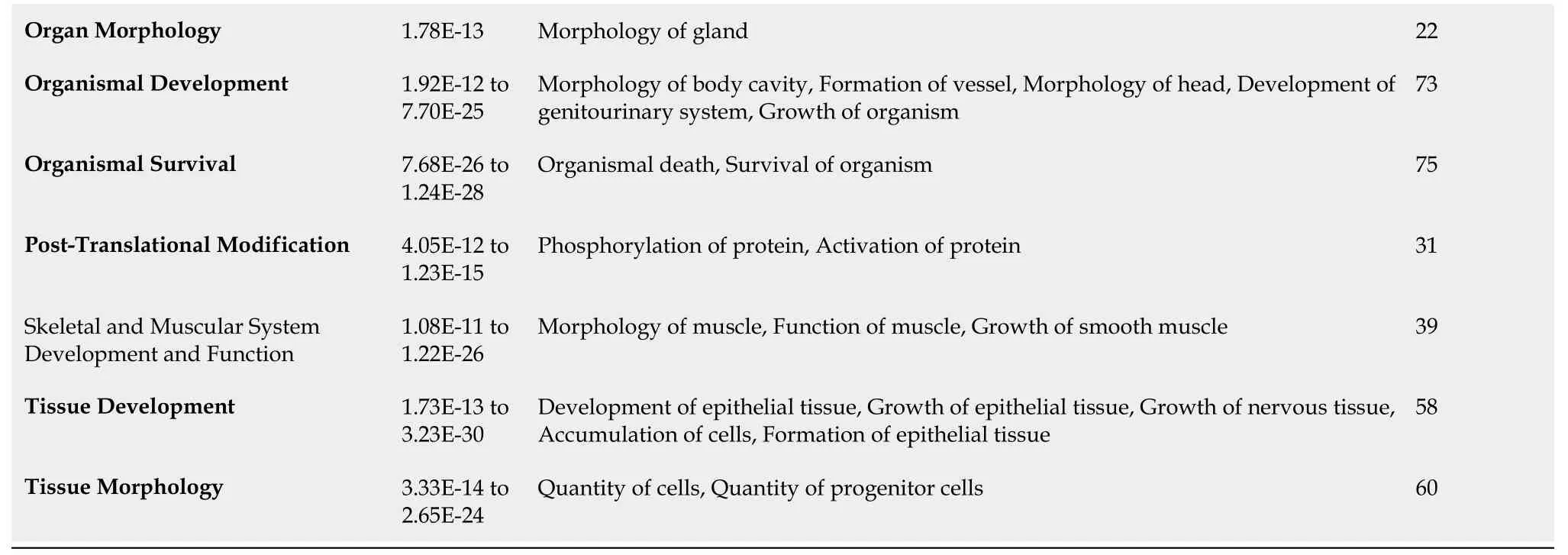
Organ Morphology 1.78E-13 Morphology of gland 22 Organismal Development 1.92E-12 to 7.70E-25 Morphology of body cavity, Formation of vessel, Morphology of head, Development of genitourinary system, Growth of organism 73 Organismal Survival 7.68E-26 to 1.24E-28 Organismal death, Survival of organism 75 Post-Translational Modification 4.05E-12 to 1.23E-15 Phosphorylation of protein, Activation of protein 31 Skeletal and Muscular System Development and Function 1.08E-11 to 1.22E-26 Morphology of muscle, Function of muscle, Growth of smooth muscle 39 Tissue Development 1.73E-13 to 3.23E-30 Development of epithelial tissue, Growth of epithelial tissue, Growth of nervous tissue,Accumulation of cells, Formation of epithelial tissue 58 Tissue Morphology 3.33E-14 to 2.65E-24 Quantity of cells, Quantity of progenitor cells 60
The importance of HIF1A in tissue regeneration has been further demonstrated using a Murphy Roths large mouse model. These animals are characterized by high basal expression ofHIF1Agene which has been associated with the ability of these animals to regenerate significant ear lesions without the appearance of fibrotic areas[59]. Indeed,HIF1Ainduction upregulates genes such as vascularendothelial growth factor (VEGF), stromal cell-derived factor-1 alpha protein (SDF-1A), transforminggrowth factor beta 1 (TGFB1), platelet-derived growth factor (PDGF), and matrix metallopeptidase 9 (MMP9), among others. All of them have important functions in healing processes. Therefore, upregulatingHIF1Acan accelerate wound healing. This has been observed in hyperbaric oxygen therapy(HBOT) treatments of diabetic skin ulcers. Interestingly, HBOT treatments increased HIF1A levels[60],probably due to high reactive oxygen species (ROS) concentrations produced by increased O2in the tissue, which may inhibit PHD and FIH, thus stabilizing HIF1A. In fact, HIF1A activity is decreased in diabetics, being associated with wound healing difficulty in these patients[61].
However, if hypoxia is maintained, wounds may become chronic, and fibrotic processes may appear.This is because, among the genes regulated by the HIF pathway, there are some that encode pro-fibrotic enzymes, producing an excess of extracellular matrix. Some of these genes are related to collagen biosynthesis like collagen type-IV, V, IX and XVIII-Alpha-1 and 2-chains (COL4A1,COL4A2,COL5A1,COL9A1andCOL18A1, accordingly), and the ones encoding enzymes that produce modifications in collagen, such as procollagen PHD and lysyl hydroxylases[62].
Inflammation is the first phase activated by injuries, and hypoxia is related to inflammatory responses. Several protein-encoding genes of nuclear factor kappa-light-chain-enhancer of activated B(NF-κB) cell complex, such as reticuloendotheliosis (REL)-Associated (RELA) proto-oncogene(transcription factor p65, also known as nuclear factor NF-kappa-B p65 subunit p65, involved in NF-κB heterodimer formation, nuclear translocation, and activation) are induced by HIF1A[63]. NF-κB is a family of transcription factors whose activation regulates different physiological processes. They include inflammatory response, as well as cell differentiation, proliferation, and survival[64]. Among the genes that NF-κB regulates isHIF1A, thus producing a reciprocal regulation[65]. The HIF1A proteins also induce expression of genes encoding proteins belonging to the Toll-Like Receptor (TLR) family. Thus, it enhances the activation of NF-κB[66]. This is because TLR have the capacity to recognize pathogenassociated molecules, inducing immune responses through activation of transcription factors, such as NF-κB[67].
Upon injury, the resulting hypoxia promotes macrophage recruitment, through regulation of Sphingosine 1-Phosphate (S1P) levels. It acts as a signal for recruitment, activation, differentiation, and polarization of macrophages[68]. This may be mediated by induction of expression of genes such as sphingosine kinase 1. this gene is involved in the last step of S1P synthesis. It has been described that HIF1A and HIF2A act on M1 and M2 macrophages through different pathways. While HIF1A induces the gene encoding inducible nitric-oxidase synthase, HIF2A acts through arginase-1, maintaining nitric oxide homeostasis during inflammation. In the case of HIF1A, its overexpression induces glycolysis metabolism, resulting in macrophage polarization to M1 (proinflammatory)[69]. However, although HIF2A has also been associated with the M1 phenotype, other studies have shown that it may promote anti-inflammatory and pro-resolving/regenerative M2 macrophages[70]. HIF1A may also produce immunosuppression through induction of Programmed Death-ligand 1 (PD-L1) encoding gene (CD274).Binding of PD-L1 to its programmed cell death protein 1 receptor on activated T cells inhibits immunity by counteracting T cell-activating signals[71].
Adaptation to hypoxia also requires metabolic changes. Cells must reduce mitochondrial oxygen consumption. In this sense, glycolysis is activated as the only way to produce adenosine triphosphate(ATP) under such hypoxic conditions. Not surprisingly, HIF1A upregulates genes related to glucose metabolism. Among them is solute carrier family 2 member A1, encoding glucose transporter-1,necessary for glucose uptake by cells[72]. Also, the genes encoding phosphoglycerate kinase 1 and pyruvate kinase M1/2 are transcriptionally upregulated by HIF1A[73,74]. Additionally, in the adaptation to hypoxia, the tricarboxylic acid (TCA) (or Krebs cycle) must be suppressed to prevent accumulation of ROS in mitochondria. For this purpose, HIF1A induces the gene encoding pyruvate dehydrogenase (PDH) kinase 1. This inactivates PDH, which is responsible for converting pyruvate to acetyl-CoA in the TCA[75].
In response to hypoxia caused by tissue damage, cells produce angiogenic factors to induce generation of vessels, restore oxygen levels, and increase nutrient delivery. HIF1A and HIF2A induce expression of genes encoding these factors. Among them,VEGF,SDF-1A, C-X-C chemokine-Receptor type 4 (CXCR4), angiopoietin-2 (ANG-2),PDGF,andTGFB[76] stand out. These factors favor endothelial-cell proliferation, differentiation, and migration for vessel formation. That also involves the mobilization and recruitment of endothelial progenitor cells (EPC) from bone marrow[61]. Mobilization of EPC is mediated by production of SDF-1 in hypoxic tissues. It acts as a chemoattractant of EPC expressing the CXCR4 receptor[77]. On the other hand, regulation of EPC migration to ischemic tissues through CXCR4/SDF1 axis is specific to HIF2A[78].
Tissue regeneration also induces cell proliferation and migration processes. HIF activation can affect cell-cycle progression due to regulation of genes such as cyclin D1 andcellular myelocytomatosis(c-MYCorMYC)[79,80]. Interestingly, while HIF1A downregulatesc-MYCexpression and results in cell-cycle arrest[79], HIF2A upregulatesc-MYCexpression, promoting cell-cycle progression and proliferation[81]. Regarding cell migration, HIF regulates genes encodingintegrin-alphaandbeta-1, 3 and 5(ITGA1,ITGA5,ITGAV,ITGB3,andITGB5, accordingly) andMMP2,MMP7,andMMP9, which are important in such processes[82]. The induction of cell migration by hypoxia is essential under physiological conditions for tissue regeneration after injury. This favors recruitment and homing of inflammatory and precursor cells, eliminating pathogens and cellular debris, further regenerating damaged tissues[68].
Hypoxia causes important changes in cellular microenvironments that might condition cell viability.Therefore, another set of important genes regulated by hypoxia are related to cell survival and death.Thus, HIF1A regulates genes activators of apoptosis such as the ones encodingtumor protein p53(TP53;antioncogene) andB-Cell Lymphoma 2(BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3[83], as well as anti-apoptotic genes, such asbaculoviral inhibitor of apoptosis protein (IAP) repeat containing 2andBCL2[83,84]. The balance of expression of these genes, and thus cell survival, will depend on the adaptation of cells to hypoxic conditions. Thus, cell survival may predominate under mild hypoxia, but apoptosis is preferentially activated under severe hypoxia[85].
HYPOXlA AND MSC
MSC reside in areas of 3%-9% of oxygen tension, allowing this hypoxic niche it’s capacities for selfrenewal, proliferation, migration, and ultimately, their differentiation[86,87]. Based on this, MSC in culture have been grown at low levels of oxygen to condition or acclimate them before their therapeutic use[88]. These cells exposed to hypoxic conditions activate protein kinase B (also known as Akt, name derived from Ak mouse strain with thymoma transforming tumors) or AKT signaling pathway mediated by HIF-1 activation to improve their survival and proliferation[89]. However, different modes,severity, and duration of hypoxic exposure could provoke different responses on MSC. Indeed, cells can become stressed and even undergo apoptosis under extreme (< 1.5%) oxygen levels[87]. Furthermore, if hypoxic exposures are maintained, internal energy reserves of glucose are rapidly consumed. That is due to glycolysis characteristic of MSC causing poor survival after implantation[90].
Ischemic conditions could be solved by providing glucose supplementation to hypoxic MSC. That allows them to retain their proliferative capacity and differentiation potency[91]. Therefore, survival of MSC could be improved by preconditioning them at 1%-4% O2for 24 to 48 h prior to implantation[88].Hypoxia could also reduce cell viability and proliferation of MSC. Nevertheless, reoxygenation processes might promote recovery of cells, enhancing expression of pro-survival genes, as well as various trophic factors[92], further promoting multipotency of MSC[93,94]. Therefore, maintenance of MSC cultures in hypoxia may influence processes such as proliferation[87,94,95], migration[87], differentiation[93,95], metabolism[87], and apoptosis[88,96], which may affect their regenerative capacity.Interestingly, cyclic hypoxic exposure, defined as periodic exposure to hypoxia interrupted by normoxic exposure or lower levels of hypoxia[97], could have positive effects on proliferation and migration abilities of MSC[98].
THERAPEUTlC POTENTlAL OF EV DERlVED FROM MSC PRECONDlTlONED lN HYPOXlA
Microenvironments in which MSC are cultivated are extremely important for their proliferation, differentiation, and therapeutic potential. Factors, such as time in culture, oxygen levels, medium composition, or cell-material interactions, should be considered[99]. As indicated in previous sections,many factors induced by hypoxia are involved in processes related to tissue regeneration, such as inflammation, angiogenesis, cell proliferation, and migration[100]. Thus, priming MSC in hypoxia favors generation of EV enriched in hypoxia-induced factors. Their functions include alterations of microenvironments for tissue adaptations to low O2concentrations[101-103]. Production and isolation of these EV for use in regenerative medicine is of great interest from a clinical point of view. Therefore,numerous studies have evaluated their potential therapeutic applications. In this scenario, time exposure and degree of hypoxia may represent relevant factors influencing contents and therapeutic properties of EV (Table 3).
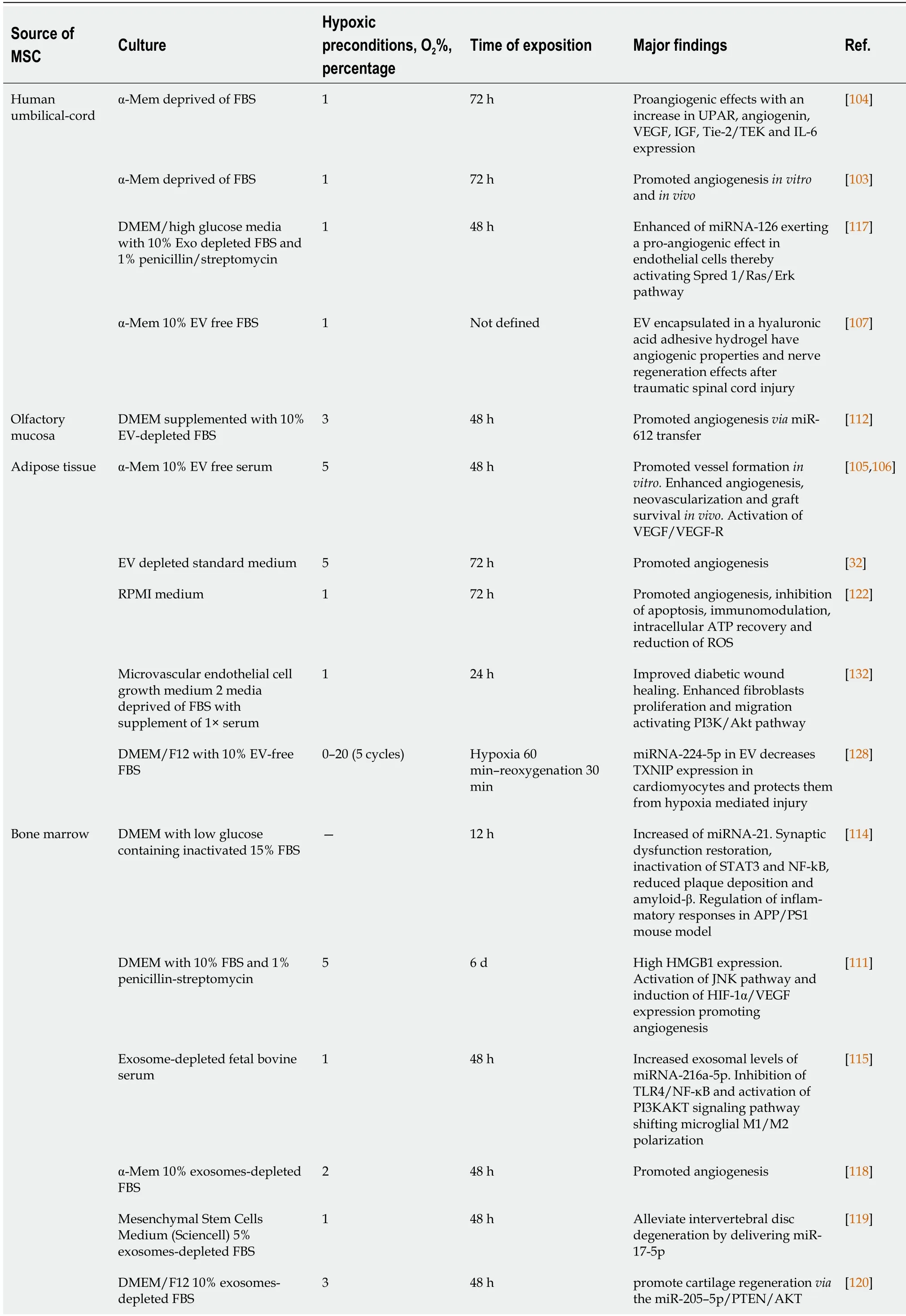
Table 3 Use of extracellular vesicles, derived from hypoxic mesenchymal stem cells, in regenerative medicine
AIFM3: Apoptosis-inducing factor, mitochondria-associated 3; AKT: Protein kinase B (PKB), named derived from kinase encoded by oncogene in transforming retrovirus from thymoma cell line AKT-8 of stock A, strain k, AKR mouse; APP: Amyloid precursor protein; ATP: Adenosine triphosphate;DMEM: Dulbecco’s modified Eagle medium; Erk: Extracellular signal-regulated kinase; Exo: Exosomes; FBS: Fetal bovine serum; GM-CSF: Granulocyte macrophage colony-stimulating factor; HMGB1: High mobility group box 1 protein; IGF: Insulin-like growth factor; IL-6: Interleukin 6; JNK: Jun Nterminal kinase; MAPK: Mitogen-activated protein kinase; NF-B: Nuclear factor kappa B; P53: Tumor protein 53 (antioncogene); PI3K:Phosphatidylinositol 3-kinase; PS1: PreSenilin 1; RKCM: Growth medium stem cell; ROS: Reactive oxygen species; RPMI: Gibco Roswell Park Memorial Institute; Spred 1: Sprouty-related EVH1 domain-containing protein 1; STAT3: Signal transducer and activator of transcription 3; TEK: Tyrosine endothelial kinase; Tie-2: Tyrosine kinase receptor 2; TLR4: Toll-like receptor 4; TXNIP: Thioredoxin-interacting protein.; UPAR: Urokinase-type plasminogen activator receptor; VEGF: Vascular endothelial growth factor.k
Hypoxia is an important inducer of angiogenesis, which plays a key role in tissue regeneration.Therefore, diverse studies have analyzed whether MSC-derived EV exposed to low levels of O2are enriched in angiogenic factors, and likewise, whether this has an impact on their ability to induce vessel formation. One of these studies showed that MSC cultivated for 72 h under hypoxic conditions (1% O2)produced exosomes with proangiogenic effects, through overexpression of genes encoding urokinase receptor [also known as urokinaseplasminogen-activator surface receptor(uPAR)],angiogenin(ANG),VEGF,insulin-like growth Factor(IGF),angiopoietin receptor tyrosine kinase with immunoglobulin-like and epidermal growth factor (EGF)-like domains 2(Tie-2) [also known astyrosine endothelial kinase(TEK)], andinterleukin 6(IL-6)[104]. Additionally, umbilical cord MSC-derived EV have the ability to enhance endothelial-cell angiogenesisin vitroand in a rat hindlimb ischemia model, being able to restore blood flow[103].Preconditioning adipose-derived MSC in moderate hypoxia (5% O2) also produced EV with capacity to increase formation of tubular structures in human umbilical-vein endothelial cells (HUVEC) with respect to EV obtained in normoxia. On the other hand, effects of EV were greater than those produced by media obtained after isolation of microvesicles. This indicates that EV, rather than soluble factors in the media, are responsible for angiogenic induction[32].
In vivostudies have also shown the potential of EV derived from MSC grown under hypoxia on angiogenesis. For example, in a mouse model of fat grafting, co-transplantation of exosomes in subcutaneous fat grafting enhanced angiogenesis, neovascularization, and graft survival[105]. A significant rise in protein synthesis of EGF, fibroblast growth-factors, VEGF/VEGF receptors (VEGF-R),Ang-1, and angiopoietin receptor tyrosine kinase with immunoglobulin-like and EGF-like domains 1(Tie-1) were shown in grafted animals, 30 d after transplantation[106]. Inclusion in hydrogels that allow local release of EV with high angiogenic capacity has also been used for treatment of spinal-cord injuries[107]. One of the proteins that has been found to be over synthesized in MSC-derived EV under hypoxia, compared to those obtained in normoxia, is jagged-1 (JAG1). This is one of the notch ligands.The notch pathway modulates processes such as angiogenesis, embryonic development, and hematopoietic stem-cell (HSC) biology[108,109]. HSC from umbilical-cord blood were treatedin vitrowith EV from MSC preconditioned in 1% O2for 48 h. As expected, their expansion capacity, selfrenewal, and clonogenic potential was increased through JAG1/notch pathway regulation[110].
Treatment of endothelial cells with hypoxia-conditioned MSC-derived EV modulates angiogenesisrelated signaling pathways. For instance, it has recently been described that EV obtained from MSC maintained in 5% O2for 6 d induced angiogenesis in HUVEC. That was accomplished through increased synthesis of high-mobility group box 1. It activates c-Jun N-terminal Kinases pathway (name derived from viral homolog v-jun, discovered in avian sarcoma virus 17 and named ju-nana, the Japanese word for 17), and consequently upregulatedHIF1A/VEGFexpression[111]. The angiogeniceffects of MSC-derived EV exposed to hypoxia are mediated, in part, by their cargos, specifically by certain miRNA. One of these miRNAs is miR-612, which inhibits translation of TP53 mRNA, favoring the activity of HIF-1A-VEGF signaling, and consequently angiogenesis[112].
According to the properties of MSC-derived EV under hypoxia, their applications may be useful in multiple disease treatments (Figure 2). Among them is Alzheimer’s disease, which is characterized by neuronal and synaptic loss caused by deposition of beta-amyloid peptides[113] due to erroneous protein folding. Experiments have been carried out with an Alzheimer’s transgenic mouse model overexpressing mutated forms of human amyloid-precursor protein (APP) and presenilin 1 (PS1).Interestingly, they improved learning and memory functions after treatment with exosomes from MSC preconditioned for 12 h under hypoxia. These improvements could be due to reduced β-amyloid accumulation through increased levels of miR-21 in the brain, synthesis of synaptic proteins, and a decrease of inflammatory factors[114].
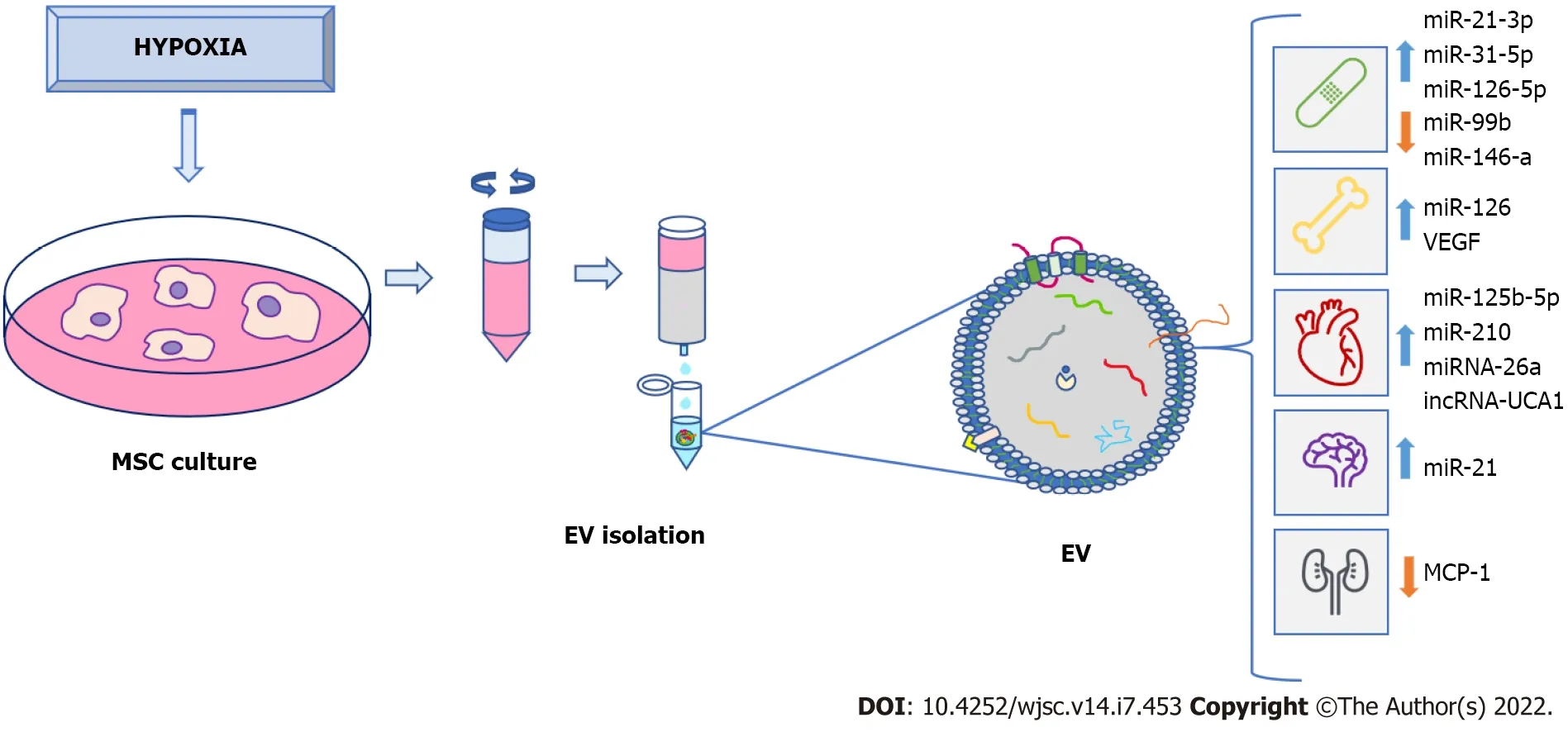
Figure 2 Clinical potential of extracellular vesicles from preconditioned mesenchymal stem-cells under hypoxia. Mesenchymal stem-cell(MSC) exposed to hypoxia secrete extracellular vesicles (EV) that can be isolated and used for clinical purposes, such as treatment of wound healing and bone fractures, as well as cardiovascular, neurodegenerative, and renal diseases, among others. Isolation of EV is made from MSC culture medium, which can be carried out in different ways. In this case, the use of a size-exclusion column is shown. EV secreted under hypoxia were enriched in various proteins, nucleic acids (like microRNA), as well as grow factors that are implicated in modulation and improvement of different biological processes related to tissue regeneration in different pathologies. EV: Extracellular vesicles; MSC: Mesenchymal stem-cell.
Repair of traumatic injuries of spinal cords have also been studied using exosomes released during 48 h under hypoxia (1% O2). An enrichment of miR-216a-5p in exosomes was observed involving TLR4/NF-κB/phosphoinositide 3-kinase (PI3K)/AKT signaling cascades. These miR-216a-5p-enriched exosomes promoted functional behavioral recovery using bothin vitroandin vivomodels carried out by shifting microglial polarization from classically-activated macrophage (M1) to alternatively-activated macrophage (M2) phenotype, effectively switching from pro-inflammatory to non-inflammatory states[115]. Also, application of EV derived from bone-marrow MSC preconditioned in hypoxia (1% O2)reduced neuronal degeneration, brain atrophy, and improved neurological recovery. These effects were due to the EV effects on angiogenesis in a mouse model of cerebral ischemia[116].
In relation to the skeletal system, EV may be used in bone-fracture healing. Thus, exosomes generated by MSC obtained from human umbilical cord were exposed to 1% O2during 48 h. They promoted bone fracture healing in an animal model. These exosomes are enriched in miR-126 by the action of HIF1A exerting proangiogenic effects by means of sprouty-related, N-terminal enabled/vasodilator-stimulated phosphoprotein homology-1 domain-containing protein 1/Ras (name derived from Rat sarcoma-virus protein)/mitogen-activated protein kinase (originally called extracellular signal-regulated kinase or Erk)pathway activation[117].
Also, treatment with EV, released from MSC preconditioned in 2% of oxygen, prevented bone loss,increasing blood-vessel formation in a rat model of steroid-induced osteonecrosis of femoral head[118].Additionally, other studies have showed that MSC-derived EV, grown in hypoxia, protect from intervertebral-disc degeneration through their content in mir-17-5p, which modulates proliferation of nucleus-pulposus cell (NPC) matrix,viathe TLR4/PI3K/AKT pathway[119]. Furthermore, preconditioning in hypoxia also increased the capacity of MSC-derived EV in cartilage regeneration by positively acting on chondrocytes. Thus,in vivoassays have shown that an injectable silk-fibroin hydrogel,containing articular chondrocytes and MSC-derived EV in hypoxia promoted cartilage regeneration[120]. Several miRNA were involved in this process, including miR-18a-3p, miR-181c-5p, miR-205-5p,miR-337-5p, and miR-376a-5p[120,121].
Treatment with EV derived from MSC has also been proposed for kidney injury. Thus, EV from adipose tissue-derived MSC cultured 72 h under hypoxia (1% O2) or normoxia conditions were compared in treatment of kidney injury induced by ischemia in a rat model. Both conditions reduced tissue damage, but renal regeneration was higher under hypoxia conditions, triggering antiapoptotic,angiogenetic, immunomodulatory, and anti-oxidative stress responses. This could be due to differences in proteomic profiles of EV types[122].
On the other hand, EV derived from MSC cultured in hypoxia have been applied, using models of myocardial infarction, in several studies. Generally, protective effects of cardiac tissues from ischemic injury were observed. They were due, at least in part, to the ability of these EV to promote blood-vessel formation[123]. Additionally, exosomes from conditioned bone-marrow MSC cultured in hypoxia (24 h,0.5% O2) or normoxia were used. They were injected intramyocardially into infarcted hearts of C57 Black 6 inbred mice strain. Treatment with hypoxia-derived exosomes produced interesting results: (1)Decrease in fibrotic tissue and apoptotic cardiomyocytes; and (2) Increase in cardiac-progenitor cells.These exosomes, compared to normoxia ones, had a significant increase in expression of miR-210, which had positive effects on endothelial cells and cardiomyocytes[124].
Such miRNA were also abundant in EV secreted by rat bone-marrow MSC, cultured in 1% O2for 72 h. Their antiapoptotic effects in cardiomyocytes have also been demonstrated in a rat model of myocardium infarction[125]. Other EV derived from MSC cultured under hypoxia were also enriched in miRNA, showing antiapoptotic activity in cardiomyocytes. They include miR-125b-5p, which works through repression ofp53and BCL2 Antagonist/Killer 1[126]. It has also been shown that EV obtained from MSC cultured in hypoxia were enriched in miR-26a in relation to EV obtained in normoxia. Such miRNA is involved in upregulating glycogen-synthase kinase 3 beta (GSK3B) expression, which enhanced the beta-catenin pathway, reducing ischemia-reperfusion injury in a rat model[127].
Other miRNA enriched in EV, derived from adipose and bone-marrow MSC preconditioned in hypoxia were miR-224-5p and miR-24. The former decreased synthesis of thioredoxin-interacting protein, which facilitates degradation of HIF1A. EV enriched in miR-224-5p favored adaptation of cardiomyocytes to hypoxia, therefore protecting them against myocardial infarction[128]. On the other hand, miR-24 decreased in infarcted myocardium of rats. Thus, application of EV containing this miRNA protected cardiomyocytes from apoptosis, reducing infarct size, and improving cardiac function[129].
In addition to miRNA, other RNA types have also been identified, showing cardioprotective effects in EV generated by MSC, under hypoxia conditions. This is the case of long non-coding RNA of urothelial carcinoma-associated 1, which is related to the anti-apoptotic miR-873-5p/X-linked inhibitor of apoptosis protein/phosphorylated AMP-activated protein kinase pathway[130].
Exosomes derived from MSC grown under hypoxia may be also useful for treatments of chronic skinulcers. They are associated with pathologies such as diabetes. Their healing is difficult and isa serious problem for patients and public health systems[131]. Recently, a study has evaluated the potential application of EV obtained from adipose-tissue stem cells maintained at 1% O2for 24 h.In vitroassays showed that they promoted fibroblast proliferation and migration. That was accomplished by activating PI3K/AKT pathway in a more effective way than when EV obtained under normoxia were used. Differential expression analyses of miRNA contents between both types of EV showed upregulated miR-21-3p, miR-31-5p, and miR-126-5p and downregulated miR-99b and miR-146a. They may be involved in signaling pathways related to fibroblast proliferation and migration, modulating immune responses.Indeed, treatment with hypoxia-derived EV improved healing in a diabetic nude mice model of wound healing, which was carried outviadownregulation ofIL-6, upregulation ofVEGFand modulation of extracellular matrix[132]. Additionally, EV derived from umbilical cord MSC exposed to 1% O2for 3 to 6 h were used in a full-thickness skin-injury mouse model, improving wound healing with respect to EV obtained in normoxia. In this case, it was demonstrated that EV in hypoxia had anti-apoptotic effects on endothelial cells due to miR-125b, which suppressed expression of TP53-inducible nuclear-protein 1[133].
CONCLUSlON
In recent years, the therapeutic potential of using MSC-derived EV has become apparent. This is because the regenerative effects of MSC are partly due to their paracrine activity. Besides, the contents of EV can be modulated through preconditioning of MSC under different culture conditions. Among they,exposure to hypoxia stands out. HIF activation affects hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, metabolism, and cell apoptosis. That is related to the contents of secreted EV, and thus their therapeutic potential, which is better than the one of EV obtained under normoxic conditions. Therefore, hypoxia preconditioning of MSC is a very attractive strategy for isolation of therapeutic EV. They have a high potential for use in regenerative medicine and can be applied to different pathologies. However, studies published to date show a great variability.That includes sources of MSC, culture media, O2concentrations, and exposure times to hypoxia, as well as methods of EV isolation. Such factors may influence the degree of induction ofHIF1AandHIF2A,and therefore MSC responses and EV cargos. Thus, it would be necessary to perform studies to optimize and standardize conditions for obtaining EV in the future according to their therapeutic applications.Also,in vivostudies carried out so far have been performed mainly in animal models. Only two active MSC-derived EV clinical trials in recruitment phase in which hypoxia is being evaluated are shown in ClinicalTrials (https://clinicaltrials.gov): “Treatment of Severe COVID-19 Patients Using Secretome of Hypoxia-Mesenchymal Stem Cells in Indonesia” (ID: NCT04753476) and “Regeneration of Posterior Cruciate Ligament Injury Using Hypoxic Conditioned Allogenic Adipose Mesenchymal Stem Cell and Condition Medium” (ID: NCT04889963). Therefore, in order to ascertain the greater potential effectiveness of EV obtained from MSC preconditioned in hypoxia, it would be necessary to carry out a greater number of properly designed clinical trials.
Using EV in regenerative medicine is very promising, as shown above. Yet, possible adverse effects associated with the use of the ones derived from MSC in human clinical practice must be taken into account. One of them is that the contents of EV may enhance tumor-cell activity[134]. In any case, that should be significantly lower -if it exists- than using whole stem-cells. Therefore, these risks should be properly evaluated in animal models and potential clinical trials. In this regard, there are several challenges for the use of MSC-derived EV in regenerative medicine that must be properly addressed beforehand. These include: (1) Identification of the most suitable MSC sources for each pathology; (2)Optimization and consensus of culture methods and conditions to obtain EV with greater regenerative capacity; (3) Scaling up of production for clinical use; (4) Control of variability and stability of produced EV; (5) Increase in clinical trials to make them statistically significant; and (6) A better understanding of pharmacokinetics and biodistribution of applied EV[135].
FOOTNOTES
Author contributions:Pulido-Escribano V, Torrecillas-Baena B, and Casado-Díaz A designed the study; Pulido-Escribano V, Torrecillas-Baena B, Camacho-Cardenosa M, and Casado-Díaz A conducted reviews and literature analyses; Dorado G, Gálvez-Moreno MÁ, and Casado-Díaz A drafted and edited; all authors reviewed and approved the final version.
Supported by“Instituto de Salud Carlos III” (ISCIII), “Ministerio de Economía y Competitividad” (MINECO) and European Union (EU), No. PI18/01659 and No. PI21/01935.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Spain
ORClD number:Victoria Pulido-Escribano 0000-0002-1107-1547; Bárbara Torrecillas-Baena 0000-0001-6934-3958; Marta Camacho-Cardenosa 0000-0002-0921-2670; Gabriel Dorado 0000-0002-0648-5278; María Ángeles Gálvez-Moreno 0000-0002-1680-1932; Antonio Casado-Díaz 0000-0002-8520-8278.
Corresponding Author′s Membership in Professional Societies:“Sociedad Española de Investigación Ósea y del Metabolismo Mineral”, No. 547.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
杂志排行
World Journal of Stem Cells的其它文章
- Therapeutic potential of dental pulp stem cells and their derivatives:lnsights from basic research toward clinical applications
- Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges
- Metabolic-epigenetic nexus in regulation of stem cell fate
- Stem cell therapy for insulin-dependent diabetes: Are we still on the road?
- Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles
- Application of extracellular vesicles from mesenchymal stem cells promotes hair growth by regulating human dermal cells and follicles
