Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges
2022-08-01YuanZhongRenShanShanDingYaPingJiangHuiWenTaoLi
Yuan-Zhong Ren, Shan-Shan Ding, Ya-Ping Jiang, Hui Wen, Tao Li
Yuan-Zhong Ren, Hui Wen, Department of Emergency Trauma Surgery, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang 471000, Henan Province, China
Shan-Shan Ding, Department of Geriatrics, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang 471000, Henan Province, China
Ya-Ping Jiang, Department of Oral Implantology, The Affiliated Hospital of Qingdao University, Qingdao 266000, Shandong Province, China
Tao Li, Department of Joint Surgery, The Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province, China
Abstract With advances in the fields of regenerative medicine, cell-free therapy has received increased attention. Exosomes have a variety of endogenous properties that provide stability for molecular transport across biological barriers to cells, as a form of cell-to-cell communication that regulates function and phenotype. In addition, exosomes are an important component of paracrine signaling in stemcell-based therapy and can be used as a stand-alone therapy or as a drug delivery system. The remarkable potential of exosomes has paved the pathway for cell-free treatment in bone regeneration. Exosomes are enriched in distinct noncoding RNAs (ncRNAs), including microRNAs, long ncRNAs and circular RNAs.Different ncRNAs have multiple functions. Altered expression of ncRNA in exosomes is associated with the regenerative potential and development of various diseases, such as femoral head osteonecrosis, myocardial infarction, and cancer. Although there is increasing evidence that exosome-derived ncRNAs (exoncRNAs) have the potential for bone regeneration, the detailed mechanisms are not fully understood. Here, we review the biogenesis of exo-ncRNA and the effects of ncRNAs on angiogenesis and osteoblast- and osteoclast-related pathways in different diseases. However, there are still many unsolved problems and challenges in the clinical application of ncRNA; for instance, production,storage, targeted delivery and therapeutic potency assessment. Advancements in exo-ncRNA methods and design will promote the development of therapeutics,revolutionizing the present landscape.
Key Words: Exosomes; Non-coding RNA; Bone; Osteogenesis; Angiogenesis; Osteoclasts
lNTRODUCTlON
Exosomes
Although extracellular vesicles (EVs) were first mentioned in the late 1960s[1], it was not until the last decade that they were named[2]. According to characteristics and cell sources, EVs can be divided into three types: Apoptotic bodies, microvesicles, and exosomes[3]. Exosomes are small EVs[4] that were first found in reticulocytes[5]. Johnston named these structures as exosomes in 1987[6]. The formation and secretion of exosomes is a complex biological process. Exosomes express biological effects through the paracrine pathway and transport bioactive substances to regulate intercellular communication[7].Therefore, stable and efficient separation and extraction methods are the prerequisites for their clinical application. Although diverse exosome isolation techniques have been developed based on their biophysical and biochemical properties, there is still a lack of standardized and large-scale clinical isolation and purification methods.
Biogenesis and isolation of exosomes
Exosomes are spherical endocytic vesicles with a diameter of 40-150 nm. They are formed in intracellular multivesicular bodies (MVBs) and removed from various cell types[8,9]. Although the biogenesis mechanism of exosomes has not been fully elucidated[10], recent studies have implicated that exosomes originate from the endocytotic-exogenous pathway[11]. The formation process of exosomes mainly includes the following three phases: (1) The constitution of endocytic vesicles by invagination of the plasma membrane; (2) MVBs with intracavitary vesicles are produced in the Golgi complex; and (3) Mature MVBs are fused with the plasma membrane and then released into the extracellular space as exosomes (Figure 1)[2,12-14].
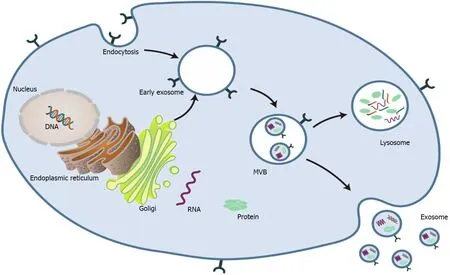
Figure 1 Schematic profile of the biogenesis of exosomes[2]. MVB: Multivesicular body. Citation: Liu Y, Wang Y, Lv Q, Li X. Exosomes: From garbage bins to translational medicine. Int J Pharm 2020; 583: 119333. Copyright© The Authors 2020. Published by Elsevier B.V.
Exosomes can be identified from the extracellular matrix (ECM) in almost all types of eukaryotic cells[15]. Based on the different physiochemical properties of exosomes, various separation and purification techniques have been developed[16,17]. Ultracentrifugation is the most widely used and most basic isolation method[18,19]. However, exosomes obtained by ultracentrifugation are time-consuming and low-yield and contain other vesicles, proteins, or aggregates of proteins and RNAs. Martínez-Greeneet al[20] enhanced the production and purity of exosome preparations by combining polymer-based precipitation and size exclusion chromatography. Recently, the application of microfluidics in exosome isolation has received more attention. Wanget al[21] used a three-dimensional nanostructured microfluidic chip to capture exosomes. Ultrafiltration with size exclusion indicated higher yields with satisfactory purity[22]. Unfortunately, so far, no extraction/separation method is perfect.
Identification of exosomes
As a subclass of EVs, exosomes can be obtained from various cell types and extracellular media[23].Although there is no consensus on the specific markers of EV subtypes[24], exosomes are mostly 30-200 nm in diameter and round or oval in shape[25]. Exosomes are composed of diverse molecules such as RNA, proteins and carbohydrates[26]. The proteins are composed of the transmembrane family and endosomal proteins. Various proteins in exosomes can be used as potential biomarkers, such as annexin,MVB-producing proteins such as ALIX, and tumor susceptibility gene 101 protein[27]. The tetraspanins CD9, CD81 and CD63 are well-established markers of exosomes[28].
Exosomes and noncoding RNA
Exosomes have been demonstrated to contain proteins and nucleic acids, and exosomes can regulate the functional activity of proteins and nucleic acidsviatranscriptional and translational regulation[29].Currently, the genome-wide analysis has demonstrated that a significant portion (> 66%) is actively transcribed into noncoding RNAs (ncRNAs) that have functional roles in regulating the expression of protein-coding genes[30]. NcRNAs include microRNAs (miRNAs), long ncRNAs (lncRNAs) and circular RNAs (circRNAs)[31]. Length is an essential criterion for defining ncRNA. ncRNA with more than 200nt is called lncRNA and miRNA is about 20 nt and is the best-known group of small ncRNA[32]. Mature miRNA sequences are located in introns or exons of ncRNA, many of which are produced by introns (mirtron) of Pri-miRNAs (pre-mRNAs)[33]. CircRNAs range from 100 nt to over 4 kb in length and have remarkable stability due to their lack of exposed ends that are susceptible to nuclear degradation and contain single or multiple exons[34-36]. Numerous publications have indicated that exosomes are closely related to bone regeneration[37-40]. In particular, exosome-derived ncRNAs (exoncRNAs) have obtained extensive attention as an essential component of exosomes[41].
Bone regeneration
Bone is a dynamic tissue that remodels and regenerates itself throughout life activities[42]. Bone regeneration is a complex procedure that demands coordinating multiple cell types and biogenesis, such as osteoblasts, osteoclasts, endothelial cells, chondrocytes and mesenchymal stem cells (MSCs)(Figure 2)[43-46].
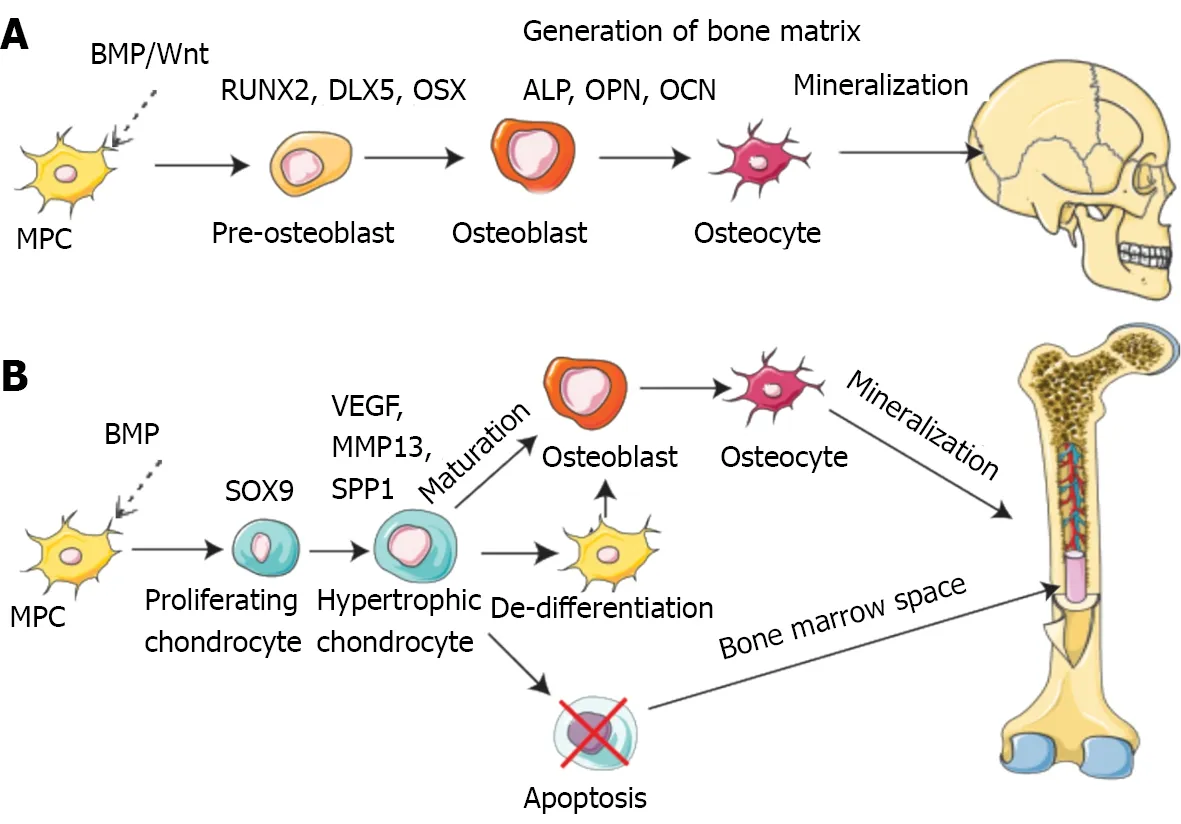
Figure 2 Pathways of bone formation during development[46]. A: Direct (intramembranous); B: Indirect (endochondral). BMP: Bone morphogenetic protein; MPC: Muscle precursor cell; VEGF: Vascular endothelial growth factor; RUNX2: Runt-related transcription factor 2; DLX5: Distal-less homeobox gene 5; ALP:Alkaline phosphatase; OPN: Osteopontin; OCN: Osteocalcin; MMP13: Matrix metallopeptidase-13. Citation: Schott NG, Friend NE, Stegemann JP. Coupling osteogenesis and vasculogenesis in engineered orthopedic tissues. Tissue Eng Part B Rev 2021; 27: 199-214. Copyright© The Authors 2021. Published by Mary Ann Liebert, Inc.
Mechanistically, intramembranous or endochondral ossification is a pathway to bone regeneration[47]. Intramembranous ossification is primarily the differentiation of stem cells into osteoblasts, which in turn deposit a mineralized ECM. The sources of stem cells mainly comprise bone marrow, fat,peripheral blood, and the umbilical cord[48]. In endochondral ossification, osteoblast progenitor cells,osteoclasts, and vascular endothelial cells enter the hypertrophic cartilage. The next step is to differentiate osteoblast progenitor cells into osteoblasts to form trabecular bone, hematopoietic cells, and endothelial cells to form bone marrow so that hypertrophic cartilage is absorbed[49]. Finally, the balance and coordination among various cells complete bone regeneration. During these processes, several signaling pathways are involved in osteogenesis, including bone morphogenetic proteins (BMPs),Notch, Hedgehog, and Wnt/β-catenin[50]. The BMP group is one of three subfamilies of the transforming growth factor (TGF) family[51]. Smad1/5 is regulated by BMP receptor complex[52].Multiple miRNAs regulate osteogenesis by balancing bone morphogenetic protein receptor 2(BMPR2)/Activin receptor type 2b competition for BMPR-triggered phosphorylation of Smads[53].
ROLES OF EXO-NCRNAS lN ΒONE REGENERATlON
Roles of exo-ncRNAs in osteogenic differentiation
Osteocytes are fully mature and differentiated osteoblasts usually derived from mesenchymal cells,including bone-marrow- and adipose-derived MSCs[54]. Growing evidence indicates that ncRNAs influence MSC differentiation[55]. For example, miR-214 impedes the osteogenic differentiation of MSCs by reducing the expression of BMP2[56]. Furthermore, ncRNAs promote osteogenic differentiation in addition to their inhibitory effect. Daiet al[57] remarked that miR-217 improves the expression of Runtrelated transcription factor (RUNX2) to promote proliferation and osteogenic differentiation of bone MSCs (BMSCs) significantly by targeting Dickkopf-1[57]. Nevertheless, little is understood about the regulatory functions of exo-ncRNAs in these procedures, and we summarize their role in osteogenic differentiation (Table 1).
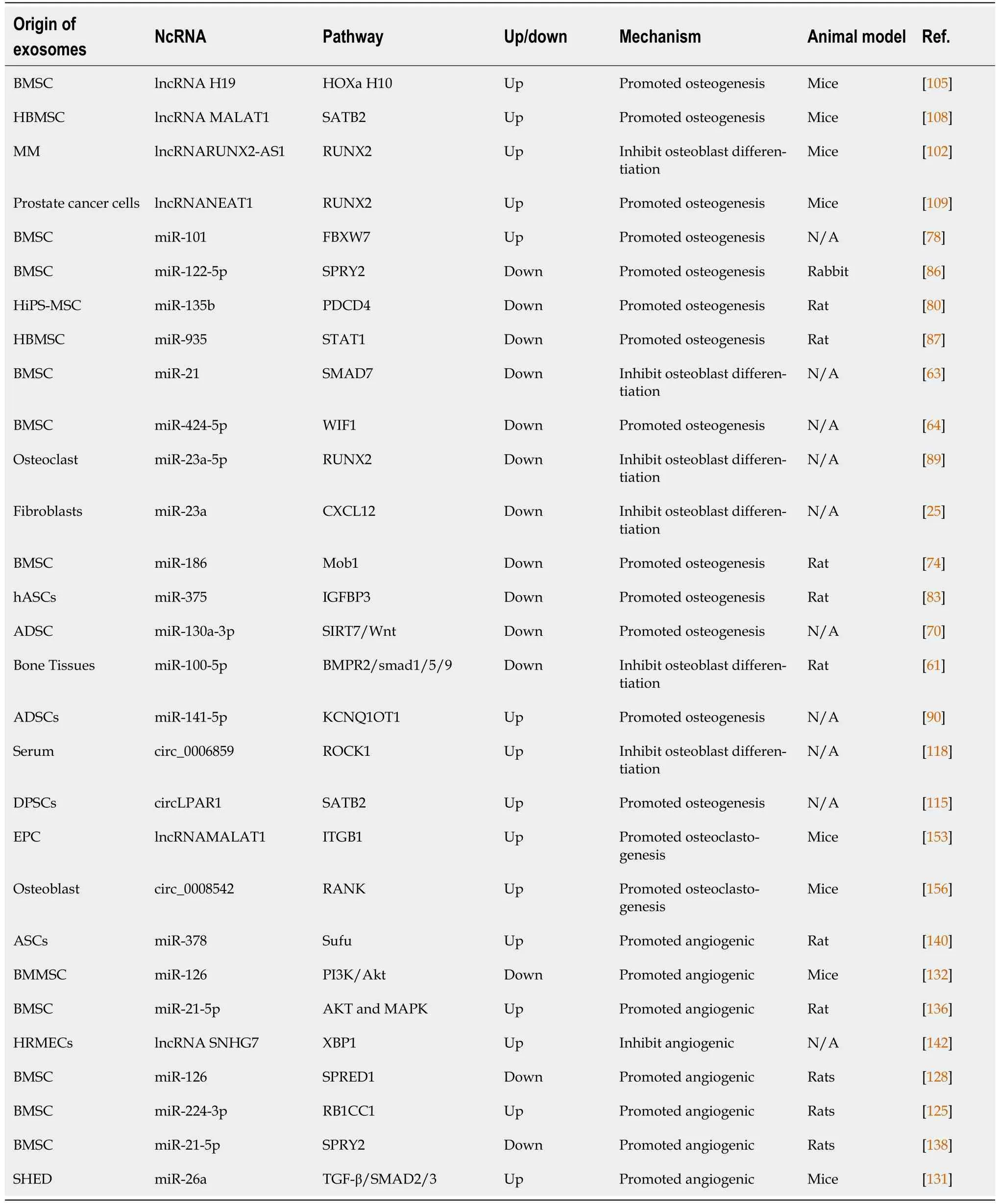
Table 1 The role of exosome-derived ncRNA in bone regeneration
Regulatory mechanisms of exosome-derived miRNAs in osteogenic differentiation
MiRNAs are 19-24 nucleotide ncRNA molecules[58] that can an regulate mRNA transcription and inhibit protein translation[59]. Exosome-derived miRNAs (exo-miRNAs) are important components of exosomes and largely determine the impact of exosomes on target cells[60]. Recent studies implicate that BMPR2 is the target gene of exo-miR-100-5p. Further research has confirmed that miR-100-5p inhibits osteogenesis of human BMSCsviatargeting BMPR2 and inhibiting the BMPR2/smad1/5 pathway[61]. Exo-miRNA-128-3p promotes osteogenic differentiationviatargeting Smad5[62]. Jianget al[63] observed that exo-miRNA-21 inhibits osteogenesis through regulating MSC-derived exosomes pulled from osteoporosis patientsviatargeting Smad7. Additionally, exo-miR-424-5p attenuates osteogenesisviaregulating the WIF1-mediated Wnt/β-catenin axis[64]. The CXCL12/CXCR4 axis regulates osteogenic differentiation by regulating the BMP2/Smad/Osterix axis[65,66]. Exo-miR-23a released from fibroblasts inhibits osteogenic differentiationviasilencing CXCL12[25]. As a highly conserved signaling pathway, Wnt signaling positively affects osteogenic differentiation[67]. Previous studies have confirmed that many sirtuin family members are closely related to the Wnt signaling pathway[68,69]. Yanget al[70] showed that exo-miR-130a-3p promotes the osteogenic differentiation of Adipose-derived stem cellsviainhibiting sirtuin 7[70]. Wnt activates Yes-associated protein(YAP)/transcriptional co-activator with PDZ-binding motif (TAZ), and the Hippo pathway regulates YAP[71,72].
As an essential gene in the Hippo signaling pathway, Mps One binder 1 (MOB1) regulates the expression of downstream genes, including YAP/TAZ[73]. Exo-miR-186 promotes osteogenesis by targeting MOB1 in postmenopausal osteoporosis[74]. A study has indicated that FZD4 explicitly activates the Wnt signaling pathway[75]. In contrast, exo-miR-129-5p from the jaw of diabetic rats targets FZD4 to inhibit the β-catenin signaling pathway[76]. As an E3 ubiquitin ligase, FBXW7 can inhibit osteogenic differentiation by regulating the degradation of substrates[77]. Exo-miR-101 increases osteogenic differentiationviainhibiting FBXW7 to control the HIF1α/FOXP3 axis[78]. As a tumor suppressor gene, PDCD4 wields antitumor activityviafacilitating apoptosis[79]. Zhanget al[80]demonstrated that exo-miR-135b relieves the harshness of Osteonecrosis of femoral head (ONFH)viadiminishing the level of PDCD4-induced apoptosis of osteoblasts. Insulin-like-growth-factor-binding proteins (IGFBPs) are regulators of the functions of IGF[81]. A previous study reported that IGF-2 enhanced BMP9-induced osteogenic differentiation[82]. Recently, it has been reported that exo-miR-375 restricts the expression of IGFBP3 from plying osteogenic effects[83]. Previous studies have shown that SPRY2 inhibits the Ras/MAPK signaling pathway[84,85]. Liaoet al[86] demonstrated that miR-122-5p
promotes osteoblast differentiation by suppressing the SPRY2 declaration and creating receptor tyrosine kinase (RTK) activity through RTK/Ras/MAPK signaling. As noted by Zhanget al[87], exo-miR-935 deters signal transducer and activator of transcription 1 expression and stimulates osteoblast expansion and differentiation potential. Also, upregulated exo-miR-935 alleviates osteoporosis presentation[87].RUNX2 is concerned with the regulation of bone metabolismviamultiple pathways[88]. Yanget al[89]found that osteoclast-derived exosomes including exo-miR-23a-5p inhibit osteogenic differentiationviaabating RUNX2. There is an interaction between exo-lncRNAs and exo-miRNAs. According to one study, exo-lncRNA-KCNQ1OT1 inhibits apoptosis of primary osteoblasts by sponging miR-141-5p[90].
Regulatory mechanisms of exo-lncRNAs and circRNA in osteogenic differentiation
LncRNAs are abundant in the genome, and > 27000 have already been recognized in the human genome[91]. LncRNA can serve not only as a critical molecule in regulating bone and cartilage degeneration,promoting bone metastasis, and repairing spinal cord injury, but also as a new class of potential biomarkers and therapeutic targets for the treatment of cancer[92,93].
More importantly, lncRNAs can action as miRNAs spongesviabinding miRNAs[94]. Furthermore,lncRNAs and miRNAs exert their biological functions by forming a large and complex regulatory network interacting with each other, leading to regulation of gene expression. LncRNA/miRNA interactions allow proper function of the musculoskeletal system, control of bone homeostasis and regeneration, and osteogenic differentiation of stem cells[95,96].
It was reported that lncRNA MEG3 could inhibit the adipogenic and osteogenic differentiation of human adipose-derived stem cells by regulating the expression of miR-140-5p[97]. Previous studies indicated that lncRNA Rmst was induced by BMP9viathe Smad signaling pathway. Further studies found that the lncRNA Rmst-miRNA-Notch regulatory axis could be a key mediator of BMP9-induced osteogenic differentiation of MSCs[98]. Moreover, lncPCAT1 promotes the osteogenic differentiation of PDLSCs by sponging miR-106a-5p and upregulating the expression of the miR-106a-5p-targeted gene BMP2[99]. Jiaet al[100] have found that LINC00707 is involved in the osteogenic differentiation of BMSCs. Mechanistically, LINC00707 can sponge miR-370-3p and upregulate Wnt2B to promote the osteogenic differentiation of HBMSCs[100]. All these data demonstrate that lncRNAs can promote osteogenesis through multiple pathways. Numerous publications have indicated that theRUNX2gene plays a vital role in the osteogenic differentiation process[101]. Liet al[102] identified that myeloma-cellderived exo-lncRNA RUNX2-AS1 could be loaded into exosomes and delivered to MSCs, thereby inhibiting the osteogenesis of MSCs[102]. Mechanistically, RUNX2-AS1 can form RNA duplexes with RUNX2 pre-mRNA, and this duplex transcriptionally suppresses RUNX2 expressionviadecreasing splicing efficiency[101,103]. Previous studies have confirmed that Hoxa10 can participate in regulating osteogenic differentiation[104]. More recently, Wanget al[105] demonstrated that exo-H19 can regulate the expression of Hoxa10 through competitive binding to miR-467 and promote osteogenic differentiation[105]. Moreover, exo-lncRNA-H19 stimulates osteogenesisviamediating Angpt1/Tie2-NO signaling in mice[106]. Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) may act as a prognostic biomarker for lung cancer metastasis[107]. exo-lncRNA MALAT1 improves osteoblast actionviamoderating the miR-34c/SATB2 axis, which may enhance the osteogenic activityviafunctioning as a miR-34c sponge to upregulate SATB2 expression[108]. In addition, exo-lncRNA NEAT1 boosts osteogenic differentiation of human BMSCs. Mechanistically, NEAT1 upregulates RUNX2 expression through competitively binding to miR-205-5p[109].
Exosome-derived circRNAs play essential roles in osteogenic differentiation. circRNAs are endogenous covalently linked RNA molecules that do not have 5'-3'polarity or poly A tails[110].CircRNAs have diverse roles, such as modulating translation and functioning as miRNA sponges[111].CircRNAs are enriched in exosomes, implying the potential of circRNAs as biomarkers for complicated diseases[112]. For example, hsa-miR-31 is considered a miRNA inhibitor of osteogenic differentiation[113,114]. Xieet al[115] demonstrated that exo-circLPAR1 has an osteogenic effectviacompetitively binding to hsa-miR-31[115]. Also, previous studies reported Hsa_circ_0006859 expression in different diseases but did not discuss the related cell signaling[116,117]. Zhiet al[118] reported that exo-Hsa_circ_0006859 inhibits osteogenesis by sponging miR-431-5p to upregulate Rho-associated kinase 1(ROCK1)[118].
Roles of exo-ncRNAs in angiogenic differentiation
Blood vessels serve as channels for transporting nutrients and oxygen[119]. There is a firm connection between the growth of blood vessels in bones and osteogenesis[120]. Bone tissue is a highly vascularized tissue, and the development of the vascular system requires a synergistic interaction between osteoblasts and angioblasts[121]. Endothelial progenitor cells (EPCs) are acknowledged to stimulate bone restorationviafacilitating neovascularization and osteogenesis[122,123]. In line with this,endothelial cells are also implicated in the vascularization of the bone tissue[54].
Regulatory mechanisms of exo-miRNAs and lncRNAs in angiogenic differentiation
Exosomal miRNAs regulate the progression of angiogenic differentiation through diverse mechanisms.ONFH is naturally known to develop at a cellular level, inferring the value of adopting cytotherapy[124]. Liaoet al[86] demonstrated that BMSC-derived exosomes modified through miR-122-5p promote angiogenesis and healing.
However, there is a lack of specifically related mechanisms. According to one study, lower levels miR-224-3p promote angiogenesis of ONFH by upregulating FIP200[125]. In contrast, according to another study, exo-miR-100-5p inhibits angiogenesis of human umbilical vein endothelial cells(HUVECs)viatargeting BMPR2[61]. Vascular endothelial growth factor (VEGF) is a critical factor in blood vessel growth and is also involved in bone development and regeneration[126]. For example,miR-126 promotes angiogenesisviainhibiting negative regulators of the VEGF pathway[127]. Huanget al[128] demonstrated that exo-miR-126 promoted angiogenesis of HUVECs by suppressing SPRED1 and PIK3R2[128]. As a member of the miRNA-26 family, miR-26a has a vital role in bone regeneration by promoting angiogenesis-osteogenesis coupling[129,130]. Wuet al[131] reported that exo-miR-26a stimulates angiogenesis by upregulation of the TGF-β/Smad2/3 pathway[131]. Exo-miR-126 downregulated PIK3R2 to trigger the PI3K/Akt signaling pathway in HUVECs. Further analyses in mice confirmed that exo-miR-126 improved angiogenesis in the wound site[132]. In addition, MSC exomiRNAs promotes diabetic foot repair. exo-miRNA-210-3p can stimulate angiogenesisviapromoting VEGF gene expression and triggering proangiogenic essential proteins[133]. However, exo-miRNA-100 inhibits angiogenesis by regulating the mTOR/HIF-1α/VEGF pathway[134]. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma (MM)[135]. Huanget al[136] demonstrated that exomiRNA-21-5p stimulates angiogenesisviaVEGFR and AKT and MAPK pathway upregulation[136].However, exo-miR150 inhibits HUVEC tube formation by downregulation of VEGF[137]. miR-21-5p is highly expressed in mag-BMSC-Exos and acts as a key mediator of mag-BMSC-Exo-induced regulation;mechanistically, exo-miR-21-5p boosts angiogenesis in HUVECsviaregulating SPRY[138]. ASCs-Exos can stimulate angiogenesis and neovascularization in ischemic disease[139]. Mechanistically, miR-378-ASCs-exos not only promote osteogenic differentiation, but also increase cell migration and angiogenic capacity. miR-378-ASCs-Exos upregulate the Shh signaling pathway by targeting Sufu to enhance osteogenesis and angiogenesis[140].
Beheraet al[106] demonstrated that exo-lnc-H19 acts as a sponge to absorb miR-106 and control the expression of Angpt1. In line with this, exosomes promotion of angiogenesisviaAngpt1 triggers lnc-H19/Tie2-NO signaling in endothelial cells[106]. XBP1, as a primary transcription factor, has been shown to regulate protein homeostasis in cells under endoplasmic reticulum stress[141]. Caoet al[142]reported that exo-lncRNA SNHG7 inhibited tube formation of human retinal microvascular endothelial cellsviaregulating the miR-34a-5p/XBP1 signal pathway[142].
Roles of exo-ncRNAs in osteoclast differentiation
Osteoclasts are generated by monocyte-macrophage precursors of the hematopoietic lineage in the bone marrow[143]. The role of osteoclasts is to remove the organic and inorganic parts of bone, and is crucial to healthy bone function[144]. miR-124 reduces the proliferation of osteoclast precursors and negatively regulates osteoclastogenesis[145]. miR-214 and miR-21 promote osteoclastogenesisviatargeting the PTEN/PI3K/AKT pathway[146]. Although the regulation of osteoclasts by ncRNA is currently comprehended, there have been few studies on the role of exo-ncRNA in osteoclast differentiation.
Regulatory mechanisms of exo-ncRNAs in osteoclast differentiation
Signaling between osteoblasts and osteoclasts is vital for osteoclast maturation[147]. miRNAs play paramount functions in the post-transcriptional control of gene expression[148]. miRNAs induce the translational repression or degradation of their target genesviabinding to the complementary sequences in the 3′-UTRs of their marker mRNAs[149]. Xuet al[150] investigated the effects of osteoclast-secreted exo-lncRNAs on osteogenesis in the process of particle-induced osteolysis. The results showed that miR-214 levels in exosomes were significantly up-regulated in osteoclast-specific miR-214 transgenic mice. Further studies have found that in ovariectomized mice, preventing exosome formation by downregulating Rab27a increased osteoblast activity. Taken together, osteoclast-derived exosomes transferred miR-214 into osteoblasts to suppress their activity[151]. Coculture systems of osteoblasts and osteoclasts to simulate bone regeneration have been reviewed by Borcianiet al[152]. Cuiet al[153] reported that EPC-derived exosomes stimulated osteoclastogenesisviathe lncRNAMALAT1/miR124 pathway. Exo-lncRNA-MALAT1 can negatively control miR-124 activity.Furthermore, there was a negative correlation between miR-124 mRNA and ITGB1. They also indicated that EPC-derived exosomes increased neovascularization in a mouse femoral fracture model. CircRNAs were discovered as ncRNAs with covalently closed structures, and they regulate disease occurrence and development[154]. m6A methylation is an ordinary state of RNA methylation, and it participates in and regulates many vital functions of RNA[155]. circ_0008542 in osteoblast exosomes enables osteoclastinduced bone resorptionviam6A methylation[156].
NcRNA includes not only several types that have been introduced above but also other types. For example, tRNA-derived small RNAs (tsRNAs) are a recently discovered form of ncRNA[157,158].TsRNAs participate in translation inhibition and exert control in various physiological phenomena[159]. Fanget al[160] reported that tsRNA-10277-loaded BMSC exosomes improved osteogenic differentiation capacity of dexamethasone-induced BMSCs[160]. In addition, the ECM plays a significant role in bone repair and regeneration[161]. Hyaluronic acid (HA) exists naturally as a critical component of the ECM. Zhaiet al[162] focused on the recent applications of HA in bone regeneration. Recently, the emergence of decellularized ECM scaffolds have been studied in bone regeneration[163]. Decellularized ECM scaffolds promote osteogenic differentiation of stem cells and maintain cytokines that regulate bone regeneration[164,165].
CONCLUSlON
Opportunities
Over the past few decades, due to the development of genetic engineering, the surface of exosomes has been packed with inhibitors of ncRNA and marker molecules by modifying the isolation and purification of exosomes. Researchers have delivered targeted ncRNAs to designated tissues or organs through exosome carriers.
Different research methods can have multiple effects. The most common delivery of miRNAs to target cells isviaexosomes or liposomes[166]. Tahmasebiet al[167] proposed novel tissue-engineering methods premised upon miRNA-incorporated polycaprolactone nanofibers in treating bone lesions and defects. It is well established that ECs and MSCs are critical performers in orthopedic tissue regeneration and vascularization. Coculture studies have demonstrated that ECs and MSCs have synergistic effects on tissue regeneration[46,168].
Hypoxic preconditioning of MSCs can enhance their biological functions[169]. In fact, hypoxic MSCs are close to thein vivoenvironment[170]. Liuet al[171] showed that hypoxia enhanced the production of exo-miR-126, which further promoted fracture healing[171]. Liuet al[172] explored biomaterialmediated chemical signaling through a model lithium-binding bioactive glass-ceramic (Li-BGC).Mechanistically, Li-BGC-exo transfers proangiogenic miR-130a and in turn, promotes the angiogenesis of ECsviaactivating the AKT pathway[172]. Liuet al[173] previously reported that knee loading protects against osteonecrosis of the femoral headviaenhancing vessel remodeling[173]. Knee loading stimulates type H vessel formation and promotes angiogenesisviadownregulating exo-miR-214-3p[174].
In diagnostics, numerous studies have found differential expression of exo-ncRNAs in various diseases[175,176]. It implies that exo-ncRNAs have advantages as biomarkers over non-exo-ncRNAs.Meanwhile, exo-ncRNAs are concerned with bone regeneration processes like cell proliferation,migration, and angiogenesis. Exo-ncRNAs promote intercellular and intertissue crosstalk in a paracrine and autocrine manner, leading to multiple applications in diseases like femoral head necrosis, bone defects, and osteoporosis.
In therapeutics, upregulation or downregulation of exo-ncRNAs may have different clinical consequences. For instance, Lvet al[177] utilized electroporation for packaging miR-21-5p mimic into exosomes. The study indicated that the miR-21-5p promotes angiogenesis and vessel maturation[177].Additionally, overexpressed exo-miR-122-5p weakens ONFH aggravation[87]. There may also be therapeutic effects by decreasing the number of harmful ncRNAs in exosomes. In addition, the use of exo-ncRNAs has multiple potential benefits. Exosomes holding a characteristic cargo can function as a drug delivery system. The use of exosomes as endogenous vehicles can evade the immune response.Despite the great potential of exo-ncRNAs as biomarkers and therapeutics for bone regeneration, there are still many obstacles before their clinical application.
Challenges
The investigations and clinical transformation of exo-ncRNAs in bone regeneration have exposed several challenges. First, we need to explore more efficient exosome purification methods to exclude exogenous exosomes and RNA interference. Exosomes, microvesicles, and smaller vesicles are different but still disorganized[178,179]. In addition, according to the existing technology, there is still a lack of efficient and fast methods for extracting and isolating exosomes. Second, further study is needed on the pharmacokinetics and toxicity of potential exo-ncRNAs. Expansion of exosome production by increasing intracellular calcium concentration and serum starvation or transfer of the oncogene c-myc may alter exosome content (including ncRNA) and increase tumorigenic potential[180,181]. Third, an extensive study is needed to comprehend fully the mechanism by which exo-ncRNAs exert their physiological roles. Fourth, further investigations are demanded in the future to characterize whether miRNAs, circRNAs, lncRNAs, and other ncRNAs may form competing endogenous RNA networks.Hence, if we better understand the bioactive molecules’ exact mechanism, we can improve bone tissue regeneration. Fifth, the expression profile of miRNAs is altered with age. For example, miR-183-5p increases with aging, suppresses osteogenic differentiation in BMSCs, and reduces Hmox1 levels[182].Sixth, most of the current research focuses on cellular experiments and a small number of animal models. It is urgent to use large-scale animal and clinical models to conduct investigations to determine whether exo-ncRNAs can play a role in regulating homeostasis. Overall, the transition of exo-ncRNAs from basic laboratory studies to clinical application remains challenging. Nonetheless, these studies provide renewed approaches for potential clinical diagnosis and therapeutic direction for exosomemediated human diseases.
FOOTNOTES
Author contributions:Ren YZ and Ding SS drafted and wrote the review; Wen H made critical revisions related to the necessary intellectual content of the manuscript; Jiang YP and Li T contributed study design and supervision; all authors read and approved the final version of the manuscript.
Supported byQingdao Traditional Chinese Medicine Science and Technology Project, No. 2021-zyym28; and Science and technology Development Project of Shandong Geriatric Society, No. LKJGG2021W082.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yuan-Zhong Ren 0000-0003-0020-4006; Shan-Shan Ding 0000-0003-4870-283X; Ya-Ping Jiang 0000-0002-6614-0079; Hui Wen 0000-0003-0306-1557; Tao Li 0000-0002-0698-2960.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Stem Cells的其它文章
- Therapeutic potential of dental pulp stem cells and their derivatives:lnsights from basic research toward clinical applications
- Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles
- Metabolic-epigenetic nexus in regulation of stem cell fate
- Stem cell therapy for insulin-dependent diabetes: Are we still on the road?
- Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles
- Application of extracellular vesicles from mesenchymal stem cells promotes hair growth by regulating human dermal cells and follicles
