金属有机配合物Mg(Salen)和Pb(Salen)对HMX 的催化分解作用和机理
2022-07-13马文喆杨燕京赵凤起刘兴利付东晓贾玉馨徐抗震
马文喆,杨燕京,赵凤起,刘兴利,付东晓,贾玉馨,徐抗震
(1. 陕西应用物理化学研究所,陕西 西安 710061;2. 西北大学化工学院,陕西 西安 710069;3. 西安近代化学研究所,陕西 西安 710065)
1 Introduction
The thermal decomposition characteristics of energetic components in solid rocket propellants have a profound effect on the combustion properties of the propellants[1-4]. HMX,a widely used energetic component,can improve the energetic performance of solid propellants[2]. However,large quantities of HMX in solid propellants lead to slow burning rates and high burning rate pressure indices,which are harmful to the performance reliability of solid motors[5-8]. Many studies have proven that one of the effective approaches to modulate the combustion performance of solid propellants is to add combustion catalysts[9-11]. Besides,the thermal decomposition properties of energetic compounds are closely related to their combustion properties in propellants[12-16].According to the mechanism analysis,the reduction of the decomposition temperature of energetic materials is one of the most obvious characteristics of the combustion catalysis process of solid propellants[17-19]. Moreover, the development of compounds could adjust the thermal decomposition behavior of HMX and further optimize the combustion properties of solid propellants[2,9,20-23].
Compounds based on Schiff bases have various structures and possess many interesting properties.Specifically,they have been proved to have high catalytic activities in hydrogenation of cyclohexene reactions,carbene reactions,and nitrogen cycle reactions[24-26]. It is noticed that nitrogen cycle reactions are involved in the thermal decomposition of HMX and the combustion processes of propellants containing HMX. Therefore,the metal Schiff base compounds are proposed to be potential catalysts for enhancing the decomposition and combustion of HMX[26]. N,N'-Bis(salicylidene)ethylenediamine,also called Salen ligand(Fig.1),is one of the widely used Schiff bases with an inner cavity between two salicylaldehyde moieties,which could accommodate suitable metal ions to form stable Salen metal complexes[28-29].
Fig.1 The structure of Salen ligand
Herein, N,N'-Bis(salicylidene)ethylenediamine-Mg and -Pd complexes were synthesized and presented as potential candidates for combustion catalysts of solid propellants,and their catalytic effects and mechanisms on the thermal decomposition of HMX were investigated.
2 Experimental
2.1 Materials
All chemicals used were commercially available. Salicylaldehyde (99.5%), ethylenediamine(99.9%),magnesium chloride(99.5%),lead(Ⅱ)nitrate(99.5%),and NaOH were purchased from Aladdin Inc. HMX was obtained from Xi'an Modern Chemistry Research Institute,and its purity was over 99%. Mg(Salen)/Pb(Salen)and HMX were mixed in a mass ratio of 1∶10 for the DSC tests.
2.2 Synthesis of Mg(Salen) and Pb(Salen)
N,N'-Bis (salicylidene) ethylenediamino(Salen)was prepared according to Reference[30].Briefly,Salen and NaOH generate a sodium salt in ethanol solution. Then MgCl2·6H2O or Pb(NO3)2was added in the ethanol solution,and Mg(Salen)or Pb(Salen)complexes were obtained after stirring for 2 h. Mg(Salen):yield,71.5%;elem. anal.(%),calcd for C16H14N2MgO2:C,66.13;H,4.86;N,9.64;found:C,66.53;H,4.72;N,9.19. Pb(Salen):yield,63.2%;elem. anal.(%),calcd for C16H14N2PbO2: C, 40.72; H, 2.75; N, 5.89;found:C,40.52;H,4.79;N,8.85.
2.3 Characterization
Elemental analysis(C,H,N)was performed on a Flash EA 1112 full-automatic trace element analyzer. X-ray diffraction(XRD)patterns were determined using a Rigaku Mini Flex 600 X-ray diffractometer with Cu Kα radiation(2θfrom 5° to 60°).The vibrational characteristics of the chemical bonds were determined using a Bruker Tensor 27 Fourier transform infrared (FTIR) spectrometer. The morphologies of complexes were studied by scanning electron microscope(SEM). The differential scanning calorimetry (DSC) experiments were performed using a DSC200 F3 apparatus(NETZSCH,Germany)under nitrogen atmosphere at a flow rate of 80 mL·min-1,and the heating rates were 5.0,10.0,15.0 ℃·min-1and 20.0 ℃·min-1from ambient temperature to 350 ℃,respectively. The TG/DTG experiments were performed using a SDT-Q600 apparatus(TA,USA)under nitrogen atmosphere at a flow rate of 100 mL·min-1,and the heating rate was 10.0 ℃·min-1from ambient temperature to 500 ℃.
3 Results and discussion
3.1 Structure and morphology
Salen is one of the most widely used organic Schiff base ligands. Fig.2a shows the XRD patterns of the as-prepared Salen and Salen complexes. It can be seen that the XRD pattern of Salen has low-density diffraction peaks with high intensity and narrow half-width,which is due to its great crystallinity and large size. Only a few diffraction peaks located at approximately 5.80°,11.56°,17.34°,23.28°and 29.22° are present on the XRD pattern of Salen.However,there is no diffraction peaks of Salen in the XRD patterns of Mg(Salen)and Pb(Salen),indicating the absence of free Salen in complexes,which confirms that these compounds are successfully synthesized. In addition,the high signal-to-noise ratio of the XRD patterns suggests the good crystallinity of both complexes.
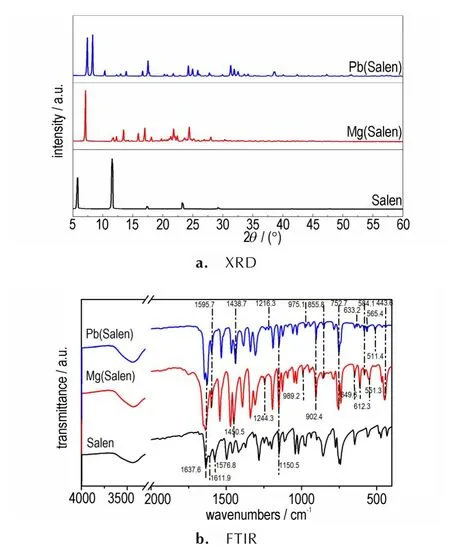
Fig.2 XRD patterns and FTIR spectra of the Salen ligand,Mg(Salen)and Pb(Salen)
The structural characteristics of Salen, Mg(Salen)and Pb(Salen)were further comfirmed by using FTIR spectroscopy. As shown in Fig.2b,two stretching vibrational absorption bands of the bridging carbon-nitrogen double bond(1150.5 cm-1)and the carbon-nitrogen single bond(1637.6 cm-1)are detected on the spectra of Salen,Mg(Salen)and Pb(Salen),indicating that the bridging ethyl group does not participate in the coordination. Additionally,the two absorption peaks at 1576.8 cm-1and 1611.9 cm-1merge into one(1595.7 cm-1)in Mg(Salen)and Pb(Salen),which can be attributed to the coordination of —OH with either the Mg ion or Pb ion. During the formation process of complexes,the bands at 1450.5, 1244.3, 989.2, 649.6,612.3 cm-1and 551.3 cm-1indicate the presence of Mg—N coordination bonds in Mg(Salen). And the bands at 1438.7,1216.3,975.1,633.2,565.4 cm-1and 511.4 cm-1are attributed to the Pb-N bond in Pb(Salen). Besides,the absorption peaks appearing at 902.4,752.7,584.1 cm-1and 443.6 cm-1can be assigned to either the Mg—O or Pb—O coordination bonds,suggesting that the transition metal in the complexes is coordinated to the phenolic hydroxyl group.
As can be seen in Fig.3,the Salen sample is composed of irregularly sheet particles with an average diameter of 0.3-1 μm,which are clearly agglomerated. However,many sheets with lengths of dozens of micrometers and widths of several micrometers are observed in the image of Mg(Salen),which reveals that it has a distinct 2D layered structure. The sheet surface of Mg(Salen)appears to be relatively smooth and the layered crystal structure is closely related to its morphology. However,the microscopic morphology of Pb(Salen)differs from that of Mg(Salen),showing numerous fibrous filaments. The filaments are between 100 nm and 500 nm in diameter,which provides the possibility of a high contact activity of the catalyst due to its high surface area.

Fig.3 SEM images of the Salen ligand,Mg(Salen)and Pb(Salen)
3.2 Thermal decomposition behavior
DSC curves of HMX/Mg(Salen)and HMX/Pb(Salen)are shown in Fig.4,and the HMX is measured for comparison. It can be seen that two peaks are detected for HMX from 100℃ to 350 ℃ at a heating rate of 10.0 ℃·min-1,where an endothermic peak can be observed at 199 ℃due to the transition of HMX from the low-temperature orthorhombic crystalline form to the high-temperature cubic form. HMX melts at 282.3 ℃with the thermal decomposition. However, we can see a distinct change of the decomposition characteristics with the addition of Mg(Salen)or Pb(Salen). The exothermic decomposition peak temperature,Tp,of HMX/Mg(Salen)is 279.3 ℃,which is 3.0 ℃lower than that of HMX. Surprisingly,theTpof HMX/Pb(Salen)is reduced to 248.3 ℃,which presents a reduction of 34.0 ℃compared to that of HMX. The results suggest that the decomposition of HMX is promoted by the addition of Mg(Salen)and Pb(Salen).
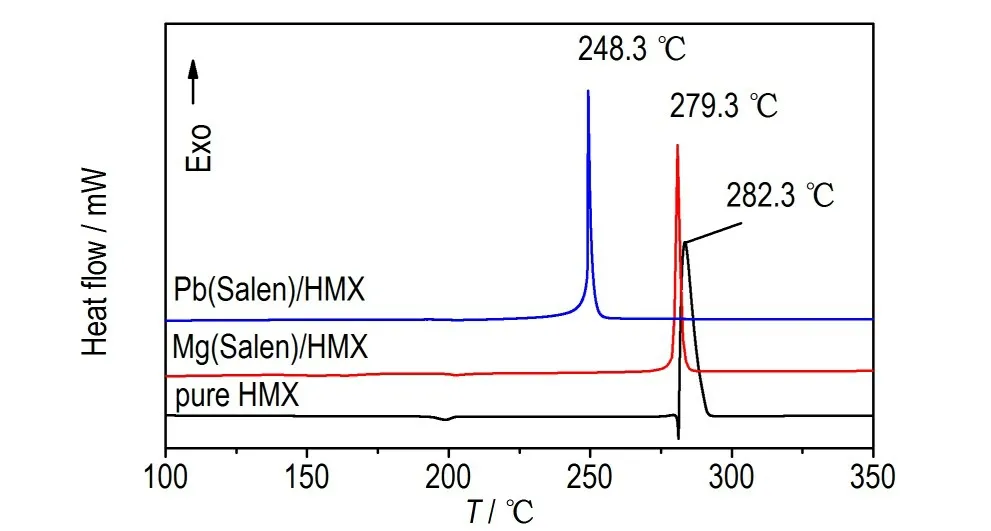
Fig.4 DSC curves of HMX,HMX/Mg(Salen)and HMX/Pb(Salen)
The TG/DTG curves of HMX, HMX/Mg(Salen),and HMX/Pb(Salen)were recorded at a heating rate of 10 ℃·min-1and are shown in Fig.5.We can observe only one stage of mass loss for the three samples. The total mass loss of HMX is 97.4%,which is due to the fact that only the C residue remains in the decomposition products. However,the mass loss for HMX/Mg(Salen)and HMX/Pb(Salen)change from 97.4% to 69.1% and 76.3%,respectively,which may be due to the addition of the complexes that makes the decomposition products of HMX include some C residues and corresponding metal oxides. The corresponding peak temperatures of DTG for HMX/Mg(Salen)and HMX/Pb(Salen)are advanced by 3.1 ℃and 34.6 ℃,respectively,which agrees well with the DSC results.
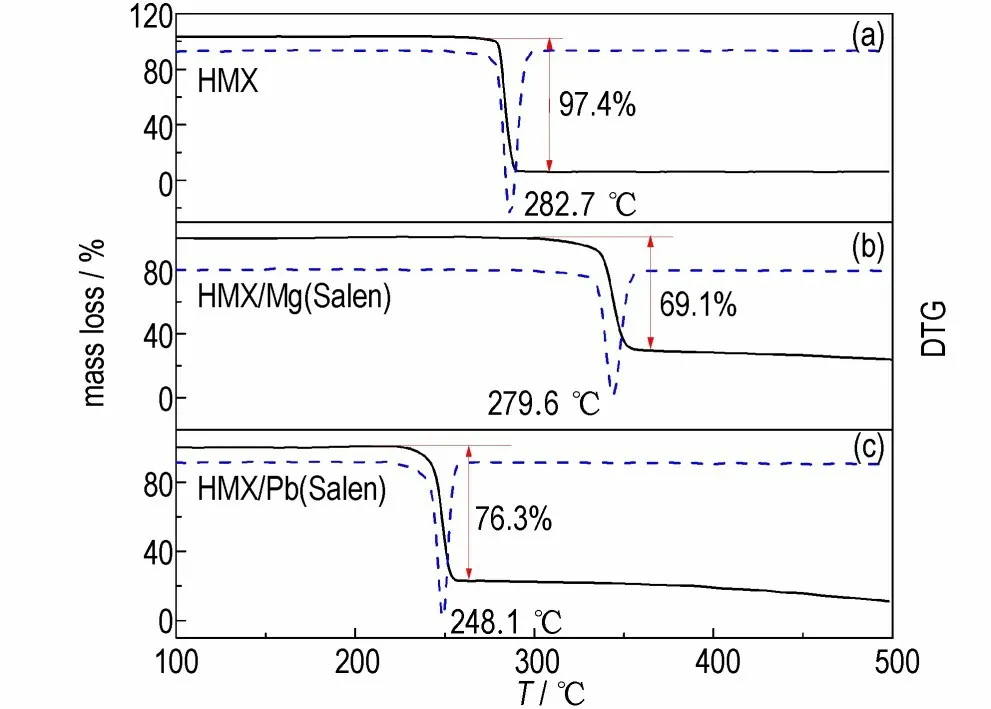
Fig.5 TG/DTG curves of HMX,HMX/Mg(Salen)and HMX/Pb(Salen)
The FTIR characteristic patterns of the gaseous pyrolysis products of HMX,HMX/Mg(Salen)and HMX/Pb(Salen)at the peak temperature are shown in Fig.6. Although the positions of the absorption peaks of HMX/Mg(Salen)and HMX/Pb(Salen)are consistent with that of HMX,the absorption peak intensity of HMX/Mg(Salen)is lower. The phenomenon may be related to the sample amounts used in the test. Additionally,the strongest absorption double peaks at 2208 cm-1and 2241 cm-1can be attributed to N2O (υas),the peaks at 1263 cm-1and 1762 cm-1are attributed to NO,and the peak around 2360 cm-1belongs to CO2. The sharp peak at 715 cm-1may be formed by HCN,and the peaks at 1746 cm-1and 2841 cm-1can be assigned to HCHO. Meanwhile,the broad band near 2841 cm-1is assigned to the rupture of the eight-membered heterocycle,indicating the cleavage of the C—N bond.The band around 3505 cm-1can be attributed to the presence of H2O,and the peaks at 1635 cm-1is attributed to the generation of NO2. Therefore,the pyrolysis gaseous products of HMX/Mg(Salen) and HMX/Pb(Salen)at the peak temperature are consistent with those of HMX,proving that the addition of the complexes does not change the nature of the decomposition products during the decomposition process. Based on this,the addition of Pb(Salen)or Mg(Salen) has an effect on the activation energy of HMX decomposition,but does not involve changes of the decomposition mechanism.
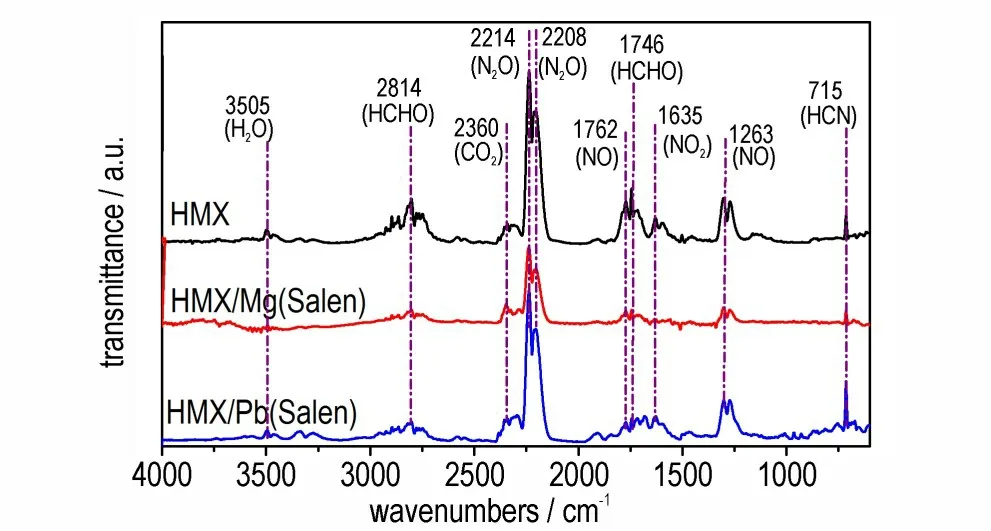
Fig.6 FTIR spectra of the gaseous products
MS spectra show that all gaseous products are consistent during the decomposition of HMX and HMX/Salen(Fig.7). The intense peaks atm/z=18 andm/z=44 are attributed to H2O and CO2in HMX/Mg(Salen) and HMX/Pb(Salen). The existence of H2O and CO2from the ambient environment is an external disruptive factor that must be taken into account throughout the test. Therefore,all gaseous products of HMX,HMX/Mg(Salen),and HMX/Pb(Salen) can be considered as consistent.The results indicate that the pyrolysis of HMX follows similar pathways under the three conditions.
Fig.7 MS spectra of HMX,HMX/Mg(Salen)and HMX/Pb(Salen)
3.3 Apparent activation energy
To further investigate the catalytic properties of Mg(Salen)and Pb(Salen)for the thermal decomposition of HMX,the DSC curves of the samples were recorded at different heating rates,and the results are shown in Fig.8. The peak temperature(Tp),the apparent activation energy(E),and linear correlation coefficient(r)were calculated using Kissinger method and Ozawa method[7,18]and are listed in Table 1.
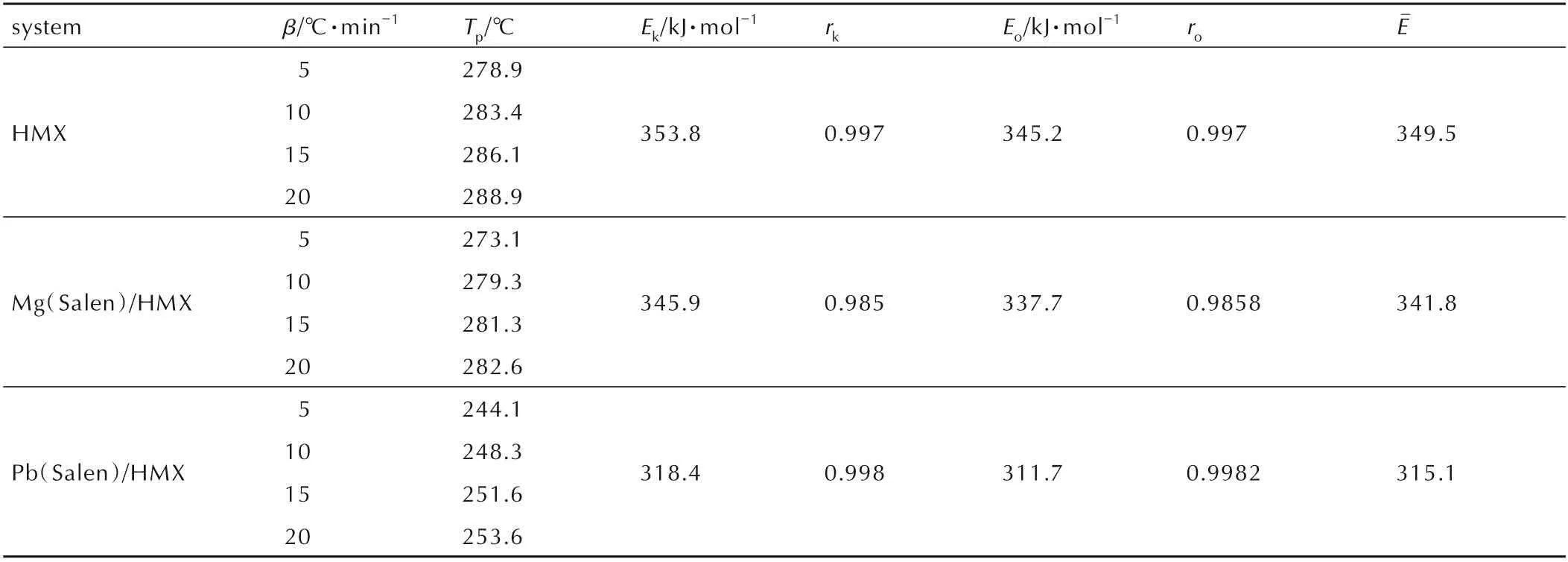
Table 1 Peak temperatures and kinetic parameters of the thermal decomposition of HMX,HMX/Mg(Salen)and HMX/Pb(Salen)
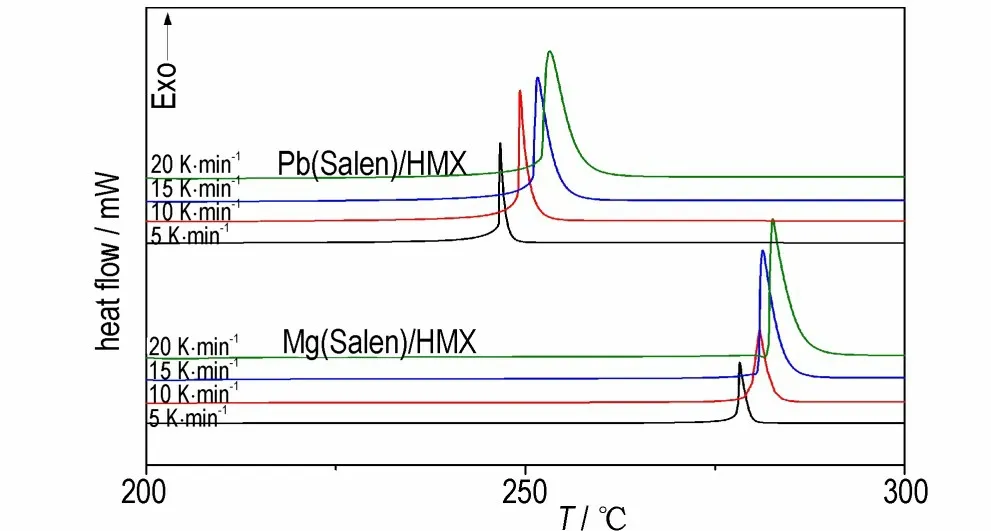
Fig.8 DSC curves of HMX/Mg(Salen)and HMX/Pb(Salen)at different heating rates
From Table 1,we can see that the addition of Mg(Salen)and Pb(Salen)decreases the apparent activation energy of the decomposition of HMX from 349.5 kJ·mol-1to 341.8 kJ·mol-1and 315.1 kJ·mol-1,respectively. Meanwhile,Pb(Salen)/HMX has much lower apparent activation energy than Mg(Salen)/HMX,further indicating that Pb(Salen)is more effective than Mg(Salen)in catalyzing the thermal decomposition of HMX.
The values ofEawere obtained from DSC data at the different heating rates by Ozawa method,and the relationship betweenEaand conversion(α)is shown in Fig.9. It can be seen that the activation energy slightly changes in the range of 0.35-0.95(α),and this range was selected to calculate the non-isothermal reaction kinetic parameters and the most probable kinetic model functions.
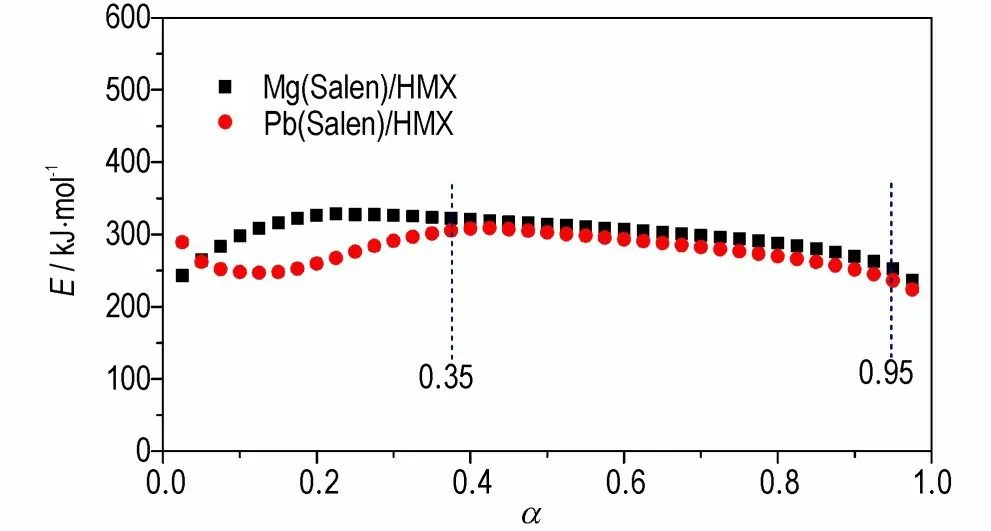
Fig.9 Curves of Ea versus α of HMX/Mg(Salen)and HMX/Pb(Salen)obtained by Ozawa method
Five integral methods(Agrawal,Satava-Sestak,MacCallum-Tanner,Universal integral,and General integral)were employed to calculate the kinetic equations. Forty-one kinetic model functions and the basic data were put into the integral and differential equations for the calculation[18,31-33]. The kinetic parameters and the probable kinetic model function were selected by the logical choice method and satisfying the ordinary range of the thermal decomposition kinetic parameters for energetic materials. These data together with their appropriate values of linear correlation coefficient(r)are presented in Table 2.The values ofEaand logAthat obtained from each single DSC curve are in good agreement with the values calculated by Kissinger method and Ozawa method. Therefore,it seems reasonable to conclude that the reaction mechanism for the intense exothermic decomposition process of HMX/Mg(Salen)and HMX/Pb(Salen) follows the Mampel power law equationG(α)=α1/4. Thef(α)variable in Eq.(1)can be substituted by 4α3/4,whileEacan be replaced by 332.46 kJ·mol-1or 316.34 kJ·mol-1,andAby 1017.49s-1or 1018.7s-1.
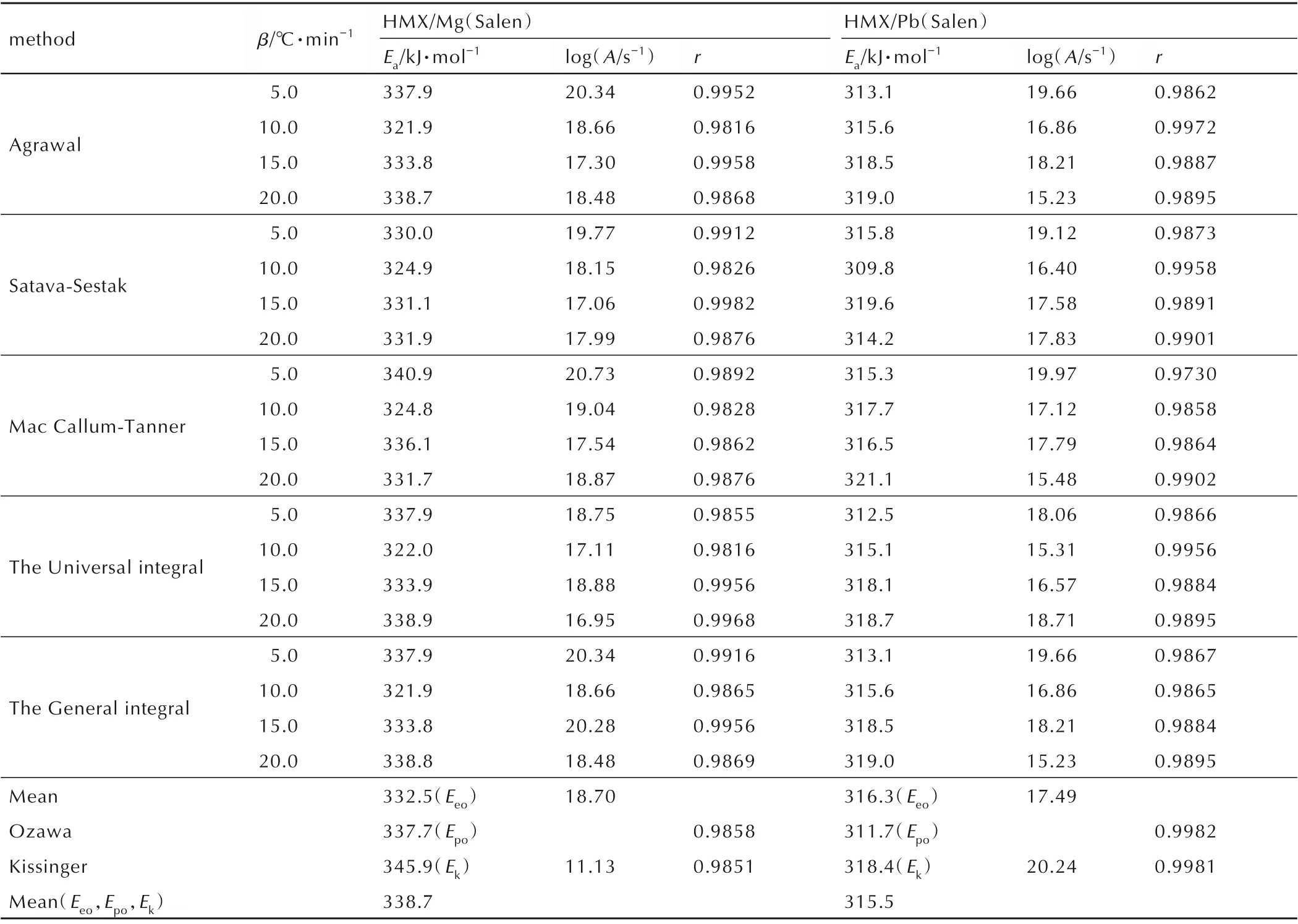
Table 2 Calculated values for the kinetic parameters of the decomposition reaction

wheref(α) and dα/dTare the differential model function and the rate of conversion,respectively.
The kinetic equations of the exothermic decomposition reaction for HMX/Mg(Salen)and HMX/Pb(Salen)may be described as follows:
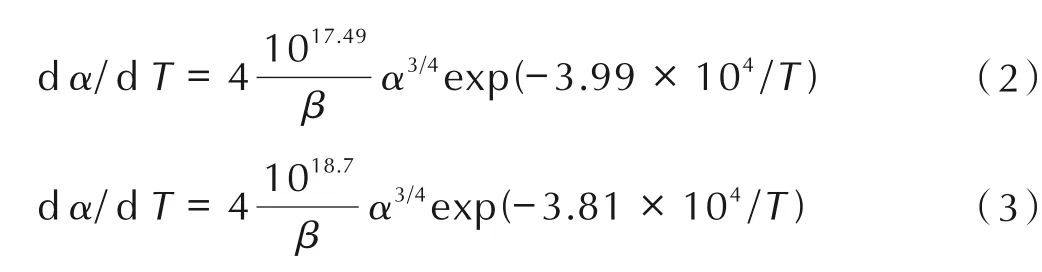
The thermal behavior of HMX was also analyzed using the same method. The results show that the reaction mechanism of the intense exothermic decomposition process can be described asf(α)=4(1-α)3/4,G(α)=[1-(1-α)]1/4,which differs from the decomposition mechanism function of HMX/Mg(Salen)and HMX/Pb(Salen). Thus,the addition of Mg(Salen)or Pb(Salen)obviously reduces the apparent activation energy and changes the kinetic model function of decomposition for HMX. Mg(Salen)and Pb(Salen) can accelerate the decomposition of HMX.
4 Conclusion
Mg(Salen)and Pb(Salen)were prepared and their structures were confirmed. Thermal analysis results show that these complexes can facilitate the decomposition of HMX. Mg(Salen)and Pb(Salen)can reduce the thermal decomposition peak temperature and apparent activation energy of HMX by 3 ℃and 34 ℃,and 7.7 kJ·mol-1and 34.4 kJ·mol-1,respectively. The addition of Mg(Salen)or Pb(Salen)also changes the kinetic model function of the decomposition of HMX.The addition of Mg(Salen)and Pb(Salen)can significantly improve the thermal decomposition characteristics of HMX,which is beneficial for improving the combustion performance of HMX-containing propellants. They may be considered as combustion catalysts for solid propellants.
