大豆异黄酮对牦牛卵巢颗粒细胞增殖和凋亡的影响
2022-05-16阿依木古丽阿不都热依木阿尔祖古丽阿依丁王家敏石嘉琛马芳芳蔡勇乔自林
阿依木古丽·阿不都热依木,阿尔祖古丽·阿依丁,王家敏,石嘉琛,马芳芳,蔡勇,乔自林*
大豆异黄酮对牦牛卵巢颗粒细胞增殖和凋亡的影响
阿依木古丽·阿不都热依木1,2,阿尔祖古丽·阿依丁1,2,王家敏1,3,石嘉琛1,2,马芳芳1,2,蔡勇4,乔自林1,3*
1西北民族大学生物医学研究中心甘肃省动物细胞技术创新中心,兰州 730030;2西北民族大学生命科学与工程学院,兰州 730030;3西北民族大学生物医学研究中心生物工程与技术国家民委重点实验室,兰州 730030;4西北民族大学实验教学部,兰州 730030
【背景】大豆异黄酮主要包括染料木素和大豆苷元,发挥类雌激素样作用,能够清除自由基,促进细胞增殖。颗粒细胞是卵泡内重要的细胞群体,其生理状态与卵巢功能直接相关。颗粒细胞凋亡常引起卵泡闭锁。牦牛是青藏高原特有物种之一,但其繁殖率低,其具体机制尚不明确。卵巢颗粒细胞是研究雌性动物生殖调控机制的理想细胞模型。【目的】探讨大豆异黄酮对牦牛卵巢颗粒细胞增殖和凋亡的影响,为大豆异黄酮的作用机制研究提供依据。【方法】分离培养牦牛卵巢颗粒细胞,FSHR免疫细胞化学染色鉴定,MTT法测定细胞增殖情况,绘制生长曲线;细胞传代后添加不同浓度染料木素或大豆苷元(0,1 000,2 000,3 000,4 000和5 000 pg·mL-1),选择染料木素或大豆苷元最佳作用浓度分别处理二代颗粒细胞48 h,收集细胞培养液,ELISA法检测培养液中雌二醇(E2)和孕酮(P4)浓度;同时收集细胞,提取总RNA,实时荧光定量PCR法检测细胞增殖相关基因和细胞凋亡相关基因、、、、、和的表达水平。【结果】牦牛卵巢颗粒细胞呈典型的上皮样细胞生长特性。接种后2 h开始贴壁,12 h出现聚集生长;24 h细胞体积较大,呈长梭形、星形或多边形;48 h细胞呈“铺路石”样单层排布;卵巢颗粒细胞特异标志蛋白FSHR免疫细胞化学染色阳性,表明,分离培养的是卵巢颗粒细胞;MTT法检测细胞增殖情况,牦牛卵巢颗粒细胞呈“S”形曲线生长:培养24 h内生长缓慢,处于潜伏生长期;24—48 h细胞增殖较快,进入指数生长期;48—120 h细胞平稳增殖,处于平顶期;120 h后活细胞密度开始下降,细胞进入退化衰亡期;第二代细胞生长较快,24 h进入指数增殖期,故试验选择第二代颗粒细胞进行。MTT法检测不同浓度染料木素或大豆苷元对细胞增殖的影响,结果,添加3 000 pg·mL-1染料木素或大豆苷元作用48 h后,细胞活力均极显著增强(<0.01);染料木素和大豆苷元均促进颗粒细胞分泌雌二醇,大豆苷元促进效果显著(<0.05);染料木素显著抑制颗粒细胞分泌孕酮(<0.05);qRT-PCR结果显示,染料木素和大豆苷元均显著上调颗粒细胞增殖调控相关基因和凋亡相关基因、和的表达(<0.01),显著下调凋亡相关基因和的表达(<0.01),并显著上调比值(<0.01)抑制细胞凋亡。此外,染料木素抑制表达(<0.01)而大豆苷元促进表达(<0.01)。【结论】分离培养了牦牛卵巢颗粒细胞,为牦牛雌性生殖调控机制研究提供了有效的细胞模型。试验结果表明,染料木素和大豆苷元抑制牦牛颗粒细胞分泌孕酮,促进细胞增殖,且主要通过上调和,以及下调和的表达,保护卵巢颗粒细胞免于凋亡。
染料木素;大豆苷元;牦牛;卵巢颗粒细胞;增殖;凋亡
0 引言
【研究意义】牦牛(bos grunniens)是分布在高寒低氧牧区的珍稀高原动物,是世界上罕见的物种资源之一[1]。受高海拔、寒冷等因素的影响,牦牛的繁殖率较低,大多是两三年产一胎,故牦牛数量不断减少[2]。因此,利用人工授精[3]、体外受精[4]等辅助繁殖技术来提高牦牛繁殖性能广受关注。颗粒细胞是卵巢中最大的细胞群,与卵母细胞间存在信息交流,对卵母细胞发育和成熟起重要作用,故卵巢颗粒细胞成为研究雌性生殖调控的重要细胞模型[5-6]。卵巢颗粒细胞作为主要的功能细胞参与原始卵泡发育、卵泡成熟、排卵及黄体形成等过程[7]。研究表明,颗粒细胞中蛋白表达水平影响卵母细胞的生长发育[8],颗粒细胞凋亡直接影响卵巢功能[9-10]。【前人研究进展】大豆异黄酮属于植物雌激素,主要存在于大豆、鹰嘴豆和扁豆等豆类中,主要成分为染料木素和大豆苷元[11]。大豆异黄酮在抗肿瘤[12]、清除自由基[13]、降低胆固醇[14]及神经保护[15]等方面具有广泛的生物活性,同时能增强机体抗应激能力,并对肠绒毛有一定保护作用[16]。【本研究切入点】雌激素能够促进体外培养的卵巢颗粒细胞合成和分泌孕酮(P4),P4可以显著促进颗粒细胞合成和分泌雌激素,而雌激素可抑制体外培养的颗粒细胞自发凋亡,并具有剂量依赖性[17]。大豆异黄酮具有类雌激素样作用[18]。【拟解决的关键问题】为了研究大豆异黄酮(染料木素和大豆苷元)对牦牛卵巢颗粒细胞增殖和凋亡的影响,本研究分离培养了牦牛卵巢颗粒细胞,添加不同浓度染料木素和大豆苷元,检测细胞增殖和凋亡相关基因,为体外培养牦牛颗粒细胞增殖和凋亡机制的研究奠定理论基础,为大豆异黄酮的作用机制研究提供基础数据。
1 材料与方法
1.1 试验动物
2018年11月,在甘肃省临夏州八坊清和园屠宰场选取12头4岁左右健康母牦牛。屠宰,检查无肉眼可视病变,迅速采集卵巢,75%酒精冲洗2次,灭菌PBS冲洗3次,放入含1%青霉素和链霉素的37℃ PBS缓冲液中快速带回实验室。
1.2 卵巢颗粒细胞原代培养
从含双抗的PBS缓冲液中取出卵巢,75%酒精浸泡20 s,移入无菌培养皿中,无菌PBS缓冲液冲洗5—6次。注射器抽吸直径为5—10 mm卵泡的卵泡液,加入等体积DMEM/F12细胞培养液,1 000 r/min离心5 min,弃上清,细胞培养液吹打沉淀的细胞,1 000 r/min离心5 min,弃上清,细胞培养液重悬细胞,按5×105个/mL接种至T25细胞培养瓶,用含10%胎牛血清和1%双抗的DMEM/F12培养液置37℃、5% CO2培养箱中培养,细胞贴壁后每48 h换液一次。细胞汇合度达90%以上,传代并冻存。复苏的第二代细胞用于后续试验。
1.3 卵巢颗粒细胞生长曲线绘制
细胞长满后进行消化,以2×104个/mL接种于24孔板,置37℃、5% CO2培养箱中培养,台盼蓝计数法每24 h计数1次,设3个重复,连续7 d,未计数组每48 h换液一次,绘制细胞生长曲线。
1.4 卵巢颗粒细胞FSHR免疫细胞化学染色
参照文献[19]介绍的方法,采用免疫细胞化学SP法检测促卵泡激素受体(follicle-stimulating hormone receptor, FSHR)表达情况。
1.5 染料木素和大豆苷元对卵巢颗粒细胞增殖的影响
颗粒细胞长满瓶底时,消化、计数,按3.5×103个/mL密度接种于96孔板,置37℃、5% CO2培养箱中培养48 h,细胞贴壁后弃培养液,分别加入含不同浓度染料木素(GEN组)(0,1 000,2 000,3 000,4 000和5 000 pg·mL-1)或大豆苷元(DAI组)(0,1 000,2 000,3 000,4 000和5 000 pg·mL-1)的新培养液,其中浓度为0记为对照组(CON组),继续培养48 h,MTT法测定细胞增殖。每组设6个重复孔,试验重复3次。
1.6 染料木素和大豆苷元对卵巢颗粒细胞分泌雌二醇和孕酮的影响
根据MTT法检测结果,筛选出染料木素和大豆苷元对颗粒细胞增殖影响最佳浓度,该浓度作用颗粒细胞48 h后,收集细胞培养液,ELISA法检测培养液中雌二醇(E2)和孕酮含量。
1.7 RNA提取及增殖和凋亡相关基因的检测
根据MTT法检测结果,筛选出染料木素和大豆苷元对颗粒细胞增殖影响最佳浓度,该浓度作用颗粒细胞48 h后,收集细胞,提取总RNA,用TSINGKE逆转录试剂盒进行反转录,采用qRT-PCR法分别检测细胞增殖相关基因和细胞凋亡相关基因、、、、、和的表达情况,以为内参基因,引物信息详见表1。
1.8 数据处理
所有试验均设置3次重复,数据结果使用Excel进行初步整理,再通过GraphPad Prism 5进行t检验。>0.05表示差异不显著;<0.05表示差异显著,用不同小写字母表示;<0.01表示差异极显著,用不同大写字母表示。
2 结果
2.1 卵巢颗粒细胞形态观察
倒置显微镜下观察,牦牛卵巢颗粒细胞呈典型的上皮样细胞生长特性。接种后2 h开始贴壁,12 h出现聚集生长;24 h细胞体积较大,形态完整,呈长梭形、星形或多边形;48 h细胞呈“铺路石”样单层排布(图1- A、B)。颗粒细胞第一代生长速度较缓慢,潜伏生长期较长;第二代细胞生长较快,24 h进入指数增殖期,故试验选择第二代颗粒细胞进行。经免疫细胞化学SP染色,细胞呈FSHR阳性,表明分离培养的细胞为卵巢颗粒细胞(图1-C)。牦牛卵巢颗粒细胞呈“S”形曲线生长:培养24 h内生长缓慢,处于潜伏生长期;24—48 h细胞增殖较快,进入指数生长期;48—120 h细胞平稳增殖,处于平顶期;120 h后活细胞密度开始下降,细胞进入退化衰亡期(图1-D)。
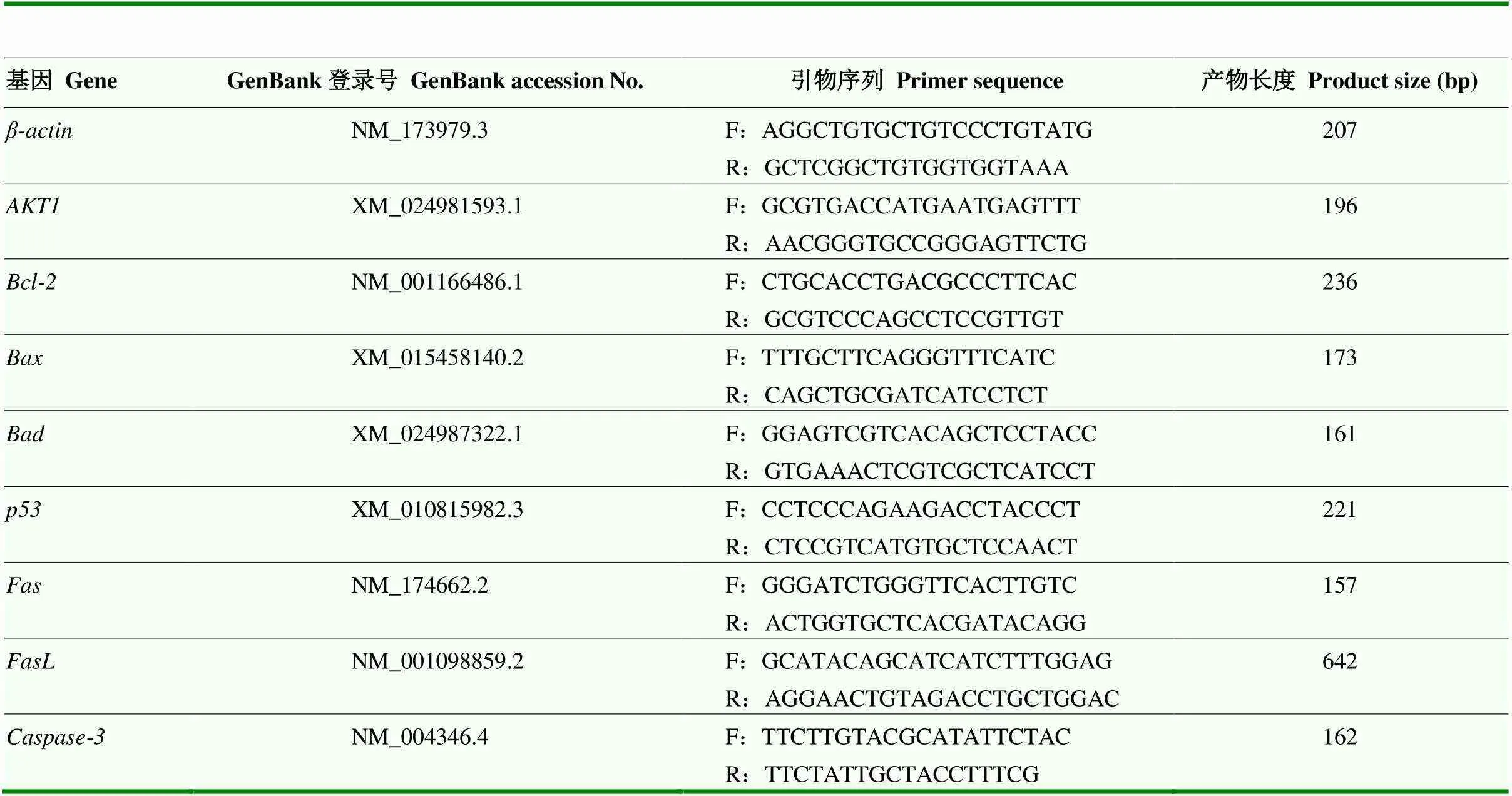
表1 Real-time PCR引物信息
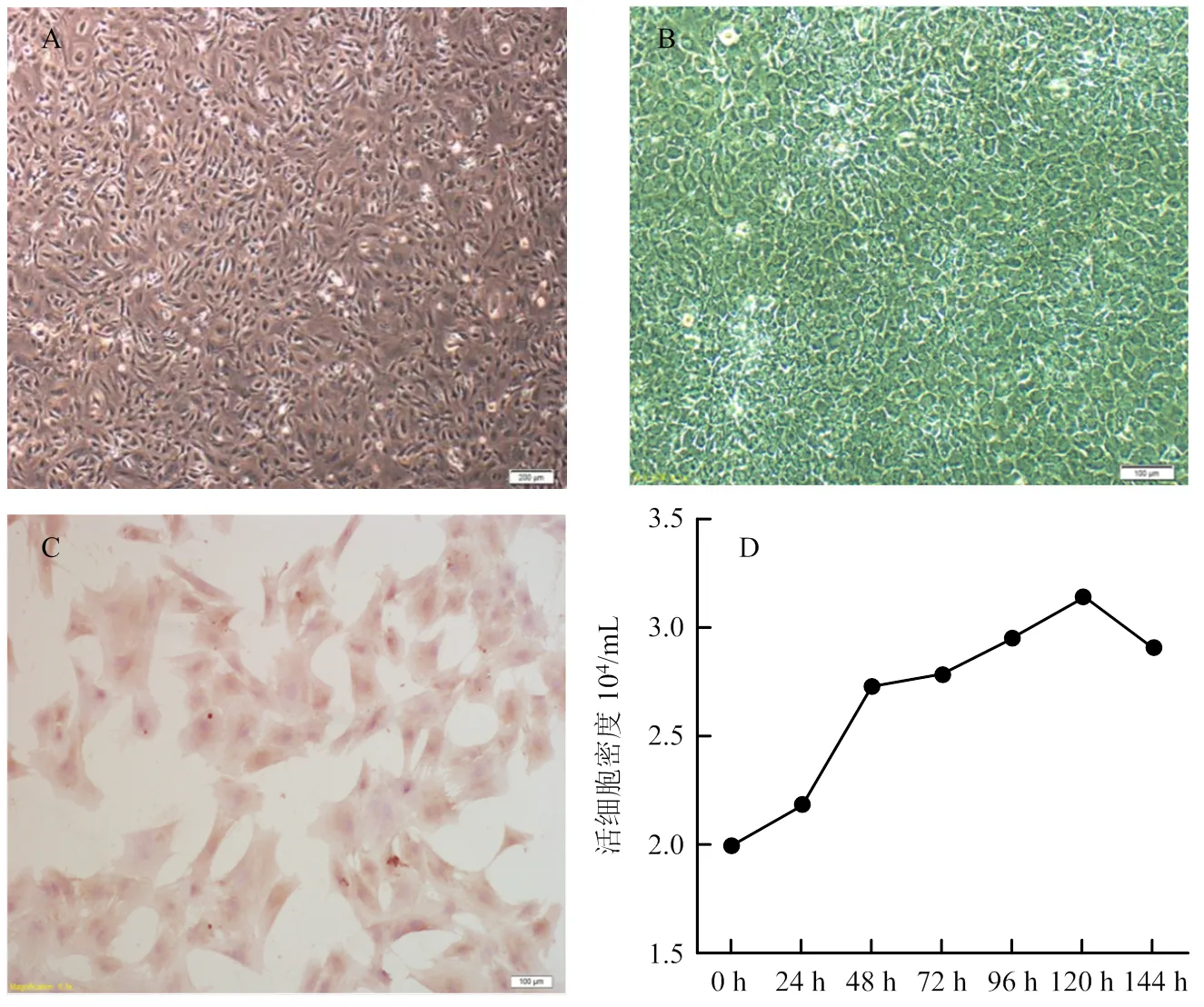
A:分离培养24 h ×40;B:分离培养48 h ×100;C:FSHR免疫细胞化学SP染色 ×400;D:生长曲线
2.2 染料木素和大豆苷元可提高卵巢颗粒细胞活力
牦牛卵巢颗粒细胞分离培养48 h长满瓶底,弃去细胞培养液,加入含有不同浓度染料木素或大豆苷元的培养液,继续培养48 h,MTT法检测细胞活力,结果如图2所示:与对照组相比,添加不同浓度染料木素或大豆苷元,均能显著(<0.05)或极显著(<0.01)提高细胞活力,因此,选择3 000 pg·mL-1为最佳作用浓度,进行后续试验。
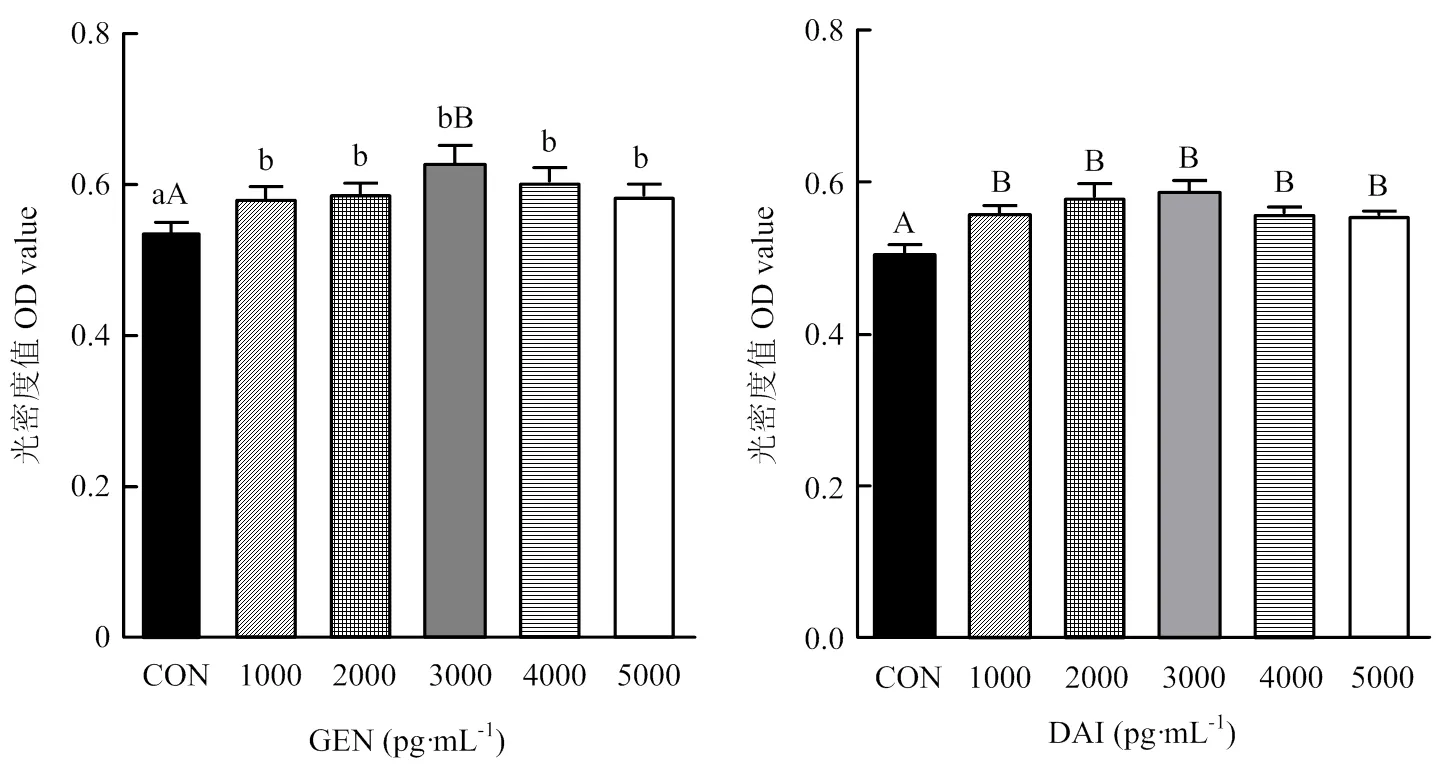
不同小写字母代表差异显著(P<0.05);不同大写字母代表差异极显著(P<0.01)。下同
2.3 染料木素和大豆苷元对卵巢颗粒细胞分泌功能的影响
以添加3 000 pg·mL-1染料木素或大豆苷元的细胞培养液继续培养卵巢颗粒细胞48 h,ELISA法检测培养液中E2和P4的含量,结果如图3所示:与对照组相比,大豆苷元显著促进颗粒细胞分泌E2(<0.05);染料木素和大豆苷元均抑制颗粒细胞P4分泌,且染料木素抑制效果显著(<0.05)。
2.4 染料木素和大豆苷元对卵巢颗粒细胞增殖和凋亡相关基因的影响
添加3 000 pg·mL-1染料木素或大豆苷元继续培养48 h,qRT-PCR检测细胞增殖相关基因和细胞凋亡相关基因、、、、、和的表达情况,结果如图4所示。与对照组相比,染料木素和大豆苷元均显著上调增殖相关基因的表达;都显著上调、、和基因表达及比值,且大豆苷元上调更明显;染料木素和大豆苷元都显著抑制的表达;染料木素显著下调表达,而大豆苷元显著上调;对于,大豆苷元显著下调,而添加染料木素组多次检测均未检出基因,故无法统计。
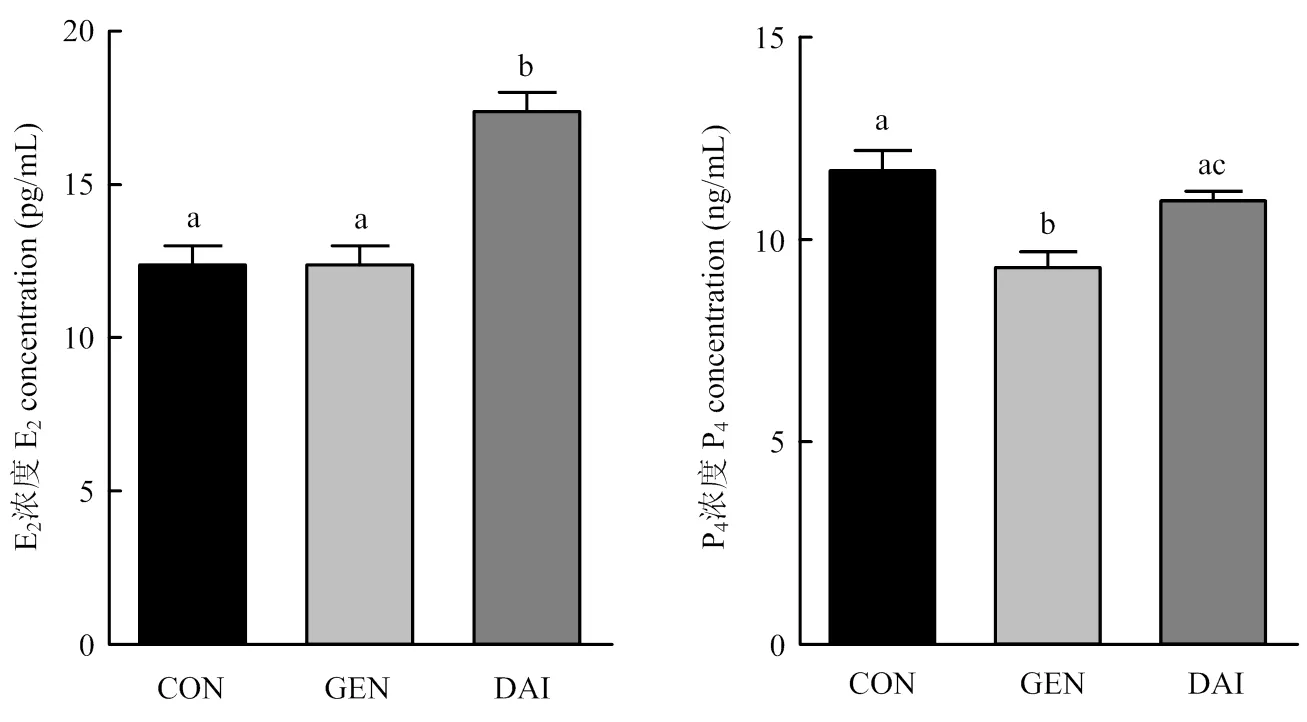
图3 染料木素和大豆苷元对牦牛卵巢颗粒细胞E2和P4分泌的影响
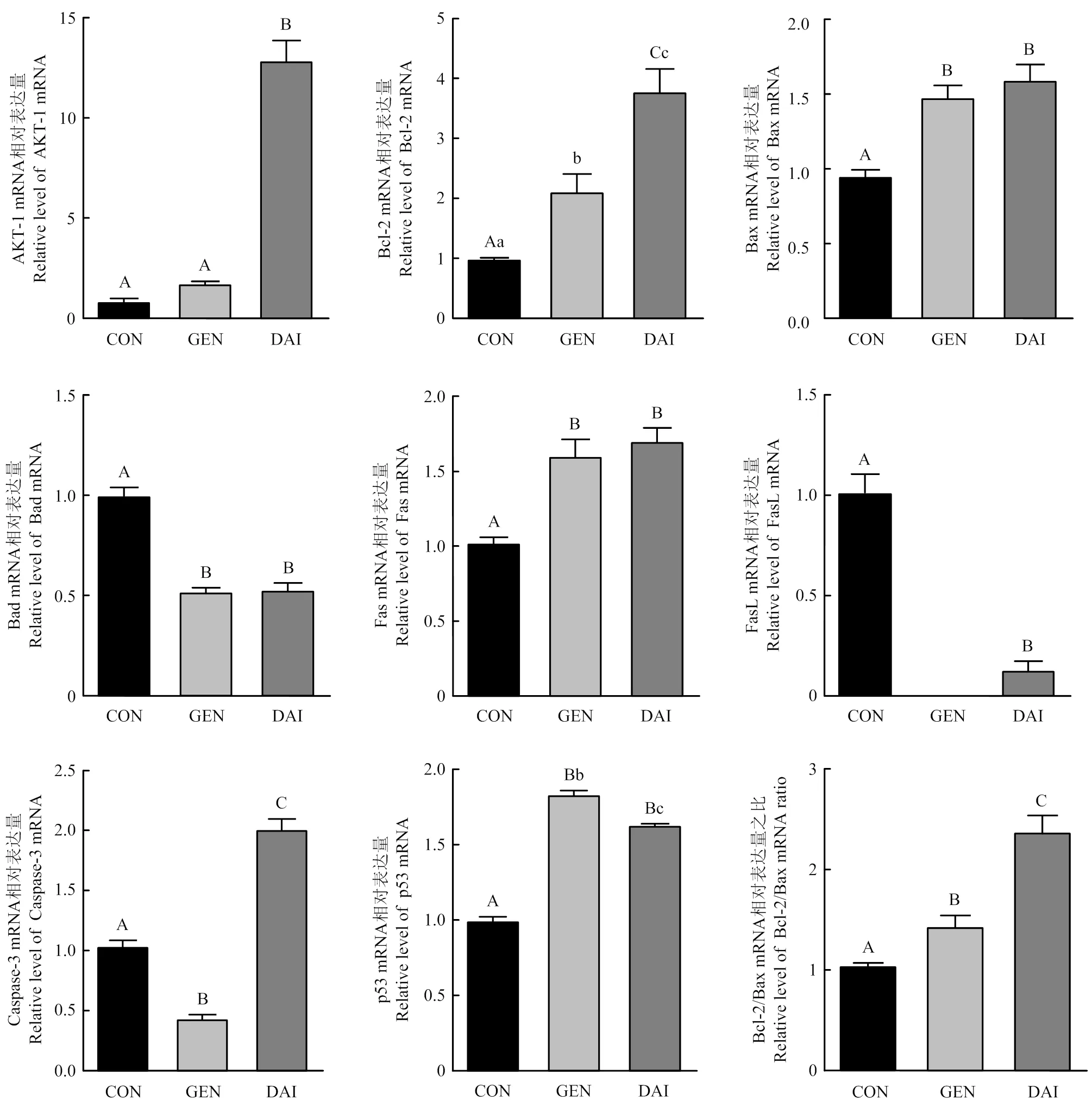
图4 染料木素和大豆苷元对牦牛卵巢颗粒细胞增殖和凋亡相关基因的影响
3 讨论
大豆异黄酮结构与雌激素相似,在体内能与雌激素受体α(estrogen receptor α, ERα)和雌激素受体β(estrogen receptor β, ERβ)结合,发挥雌激素样作用[20],临床上用于治疗更年期综合征。研究发现,染料木素促进大鼠[21]和牛[22]卵巢颗粒细胞增殖,试验结果显示,3 000 pg·mL-1染料木素和大豆苷元都显著提高了牦牛卵巢颗粒细胞增殖活力;但也有学者报道染料木素抑制猪卵巢颗粒细胞增殖活力[23],推测染料木素和大豆苷元对颗粒细胞增殖活力的调节可能与其浓度和作用时间有一定联系,其具体机制仍需进一步研究。
试验发现,染料木素显著抑制颗粒细胞P4分泌,大豆苷元促进牦牛卵巢颗粒细胞分泌E2,与Nynca等[24-25]研究结果一致。推测,大豆异黄酮对颗粒细胞增殖活力的影响可能是通过增殖和凋亡相关基因调控而实现。
又称为PKBα,为蛋白激酶B(protein kinase B, PKB)家族成员之一,活化的主要通过磷酸化底物蛋白引起下游信号级联反应,进而参与调控细胞生长、增殖、迁移及细胞周期等多种细胞活动[26]。试验发现染料木素和大豆苷元均显著上调牦牛颗粒细胞的表达,从而促进牦牛颗粒细胞增殖。细胞凋亡存在两种调控途径:死亡受体介导的“外源性凋亡通路”和线粒体介导的“内源性凋亡通路”。其中,在卵巢颗粒细胞凋亡过程中由蛋白家族介导的线粒体内源凋亡通路占主导[27]。和的比值[28]是细胞的“凋亡开关”,当表达占优势时,细胞发生凋亡,反之细胞凋亡活动受到抑制。二者的平衡决定了卵泡发育或是闭锁[29]。为促凋亡基因,与或等结合发挥作用。大豆异黄酮下调L02细胞中的比值,抑制的表达,从而抑制细胞凋亡[30]。本研究也发现,染料木素和大豆苷元都能上调和表达,并显著下调表达,上调比值,从而抑制颗粒细胞凋亡。
是重要的抑癌基因之一,细胞受到DNA 损伤、氧化应激等信号刺激时,转录活性增强,作为一种高效的转录因子可通过调控凋亡途径中的途径来介导细胞凋亡[31]。本研究发现,染料木素和大豆苷元都显著上调牦牛卵巢颗粒细胞中的表达,从而参与细胞凋亡调控。
是肿瘤坏死因子受体家族的膜蛋白分子,是的天然配体,与结合活化相关死亡结构域及下游的家族,从而启动外源性凋亡途径诱导细胞凋亡[32]。本研究发现,牦牛颗粒细胞中和都有表达,染料木素和大豆苷元都显著上调mRNA的表达,但下调mRNA的表达,且染料木素作用后没有检测到的mRNA,促凋亡作用主要通过与其配体结合后发挥。因此,大豆异黄酮对死亡受体家族蛋白的调控机制仍需进一步研究。是介导细胞凋亡的关键效应酶,多种细胞凋亡信号通路均需通过激活来进一步激活下游信号调节因子促进细胞凋亡[33]。本研究发现,染料木素显著降低的表达,而大豆苷元极显著上调的表达,这一不同是否和染料木素与大豆苷元结构差异有关,仍需进一步研究。
4 结论
通过分离培养牦牛卵巢颗粒细胞,发现染料木素和大豆苷元可促进牦牛卵巢颗粒细胞增殖,抑制P4分泌,并上调的表达及比值,下调表达,从而起到保护卵巢颗粒细胞免受凋亡的作用。
[1] WANG K, YANG Y, WANG L, MA T, SHANG H, DING L, HAN J, QIU Q.Different gene expressions between cattle and yak provide insights into high-altitude adaptation.Animal Genetics, 2016, 47(1): 28-35.
[2] YU S J, HAUANG Y M, CHEN B X.Reproductive patterns of the yak.I.Reproductive phenomena of the female yak.British Veterinary Journal, 1993, 149(6): 579-583.
[3] OOSTHUIZEN N, FONTES P, SANFIORD C D, CIRIACO F M, HENRY D D, CANAL L B, DILORENZO N, LAMB G C.Estrus synchronization and fixed-time artificial insemination alter calving distribution in Bos indicus influenced beef heifers.Theriogenology, 2018, 15(106): 210-213.
[4] 潘阳阳, 王萌, 芮弦, 王立斌, 何翃闳, 王靖雷, 马睿, 徐庚全, 崔燕, 樊江峰, 余四九.IGF-1调控RBM3表达抑制低温应激诱导牦牛卵丘细胞凋亡.中国农业科学, 2020, 53(11): 2285-2296.
PAN Y Y, WANG M, RUI X, WANG L B, HE H H, WANG J L, MA R, XU G Q, CUI Y, FAN J F, YU S J.RNA-binding motif protein 3(RBM3) expression is regulated by insulin-like growth factor (IGF-1) for protecting yak (grunniens) cumulus cells from apoptosis during hypothermia stress.Scientia Agricultura Sinica, 2020, 53(11): 2285-2296.(in Chinese)
[5] 毕锡麟, 王锴, 李鹏飞, 景炅婕, 韩琦, 吕丽华.牛卵泡颗粒细胞PRSS35表达模式及功能研究.畜牧兽医学报, 2018, 49(11): 2394-2401.
BI X L, WANG K, LI P F, JING J J, HAN Q, LÜ L H.Expression profile and function analysis of PRSS35 in bovine follicle granulosa cells.Acta Veterinaria et Zootechnica Sinica, 2018, 49(11): 2394-2401.(in Chinese)
[6] MATSUDA F, INOUE N, MANABE N, OHKURA S.Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells.The Journal of Reproduction and Development, 2012, 58(1): 44-50.
[7] HATZIRODOS N, HUMMITZSCH K, IRVING-RODGERS H F, HARLAND M L, MORRIS S E, RODGERS R J.Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia.BMC Genomics, 2014, 15: 40.
[8] 赵园园, 李鹏飞, 许勤智, 安清明, 孟金柱.山羊卵泡发育相关基因的筛选及分析.中国农业科学, 2020, 53(17): 3597-3605.
ZHAO Y Y, LI P F, XU Q Z, AN Q M, MENG J Z.Screening and analysis of follicular development related genes in goat.Scientia Agricultura Sinica, 2020, 53(17): 3597-3605.(in Chinese)
[9] REGAN S L P, KNIGHT P G, YOVICH J L, LEUNG Y, ARFUSO F, DHARMARAJAN A.Granulosa cell apoptosis in the ovarian follicle- A changing view.Frontiers in Endocrinology, 2018, 9: 61.
[10] ZEUNER A, MÜLLER K, REGUSZYNSKI K, JEWGENOW K.Apoptosis within bovine follicular cells and its effect on oocyte development duringmaturation.Theriogenology, 2003, 59(5/6): 1421-1433.
[11] NURMI T, MAZUR W, HEINONEN S, KOKKONEN J, ADLERCREUTZ H.Isoflavone content of the soy based supplements.Journal of Pharmaceutical and Biomedical Analysis, 2002, 28(1): 1-11.
[12] SATHYAPALAN T, MO A, RIGBY A S, FRASER W D, THATCHER N J, KILPATRICK E S, ATKIN S L.Soy reduces bone turnover markers in women during early menopause: a randomized controlled trial.Journal of Bone and Mineral Research, 2017, 32(1): 157-164.
[13] KIM H J, JUNG C L, JEONG Y S, KIM J S.Soybean-derived glyceollins induce apoptosis through ROS generation.Food & Function, 2014, 5(4): 688-695.
[14] LUO T, MIRANDA-GARCIA O, SASAKI G, WANG J L, SHAY N F.Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signalingand.Food & Function, 2018, 9(12): 6257-6267.
[15] QIAN Y S, GUAN T, HUANG M H, CAO L X, LI Y M, CHENG H, JIN H X, YU D Y.Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model.Neurochemistry International, 2012, 60(8): 759-767.
[16] 林厦菁, 陈芳, 蒋守群, 蒋宗勇.大豆异黄酮对早期断奶仔猪生长性能、抗氧化功能及肠粘膜形态结构的影响.中国农业科学, 2020, 53(10): 2101-2111.
LIN X J, CHEN F, JIANG S Q, JIANG Z Y.Effects of soybean isoflavones on growth performance, antioxidant performance and intestinal morphology of early-weaned piglets.Scientia Agricultura Sinica, 2020, 53(10): 2101-2111.(in Chinese)
[17] MOTTA-MENA N V, PUTS D A.Endocrinology of human female sexuality, mating, and reproductive behavior.Hormones and Behavior, 2017, 91: 19-35.
[18] ANDRES S, HANSEN U, NIEMANN B, PALAVINSKAS R, LAMPEN A.Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover.Food & Function, 2015, 6(6): 2017-2025.
[19] SHARMA G T, DUBEY P K, KUMAR G S.Localization and expression of follicle-stimulating hormone receptor gene in buffalo () pre-antral follicles.Reproduction in Domestic Animals, 2011, 46(1): 114-120.
[20] 董晓莉, 谢俊霞.雌激素和植物雌激素对大鼠杏仁核多巴胺能系统的调节.生理学报, 2003, 55(5): 589-593.
DONG X L, XIE J X.Regulation of estrogen and phytoestrogen on the dopaminergic systems of amygdala in rats.Acta Physiologica Sinica, 2003, 55(5): 589-593.(in Chinese)
[21] WHITEHEAD S A, LACEY M.Protein tyrosine kinase activity of lavendustin A and the phytoestrogen genistein on progesterone synthesis in cultured rat ovarian cells.Fertil Steril, 2000, 73(3): 613-619.
[22] MAKAREVICH A, SIROTKIN A, TARADAJNIK T, CHRENEK P.Effects of genistein and lavendustin on reproductive processes in domestic animals.Journal of Steroid Biochemistry And Molecular Biology, 1997, 63(4-6): 329-337.
[23] TIEMANN U, SCHNEIDER F, VANSELOW J, TOMEK W.In vitro exposure of porcine granulosa cells to the phytoestrogens genistein and daidzein: effects on the biosynthesis of reproductive steroid hormones.Reprod Toxicology, 2007, 24(3-4): 317-325.
[24] NYNCA A, JABLONSKA O, SLOMCZYNSKA M, PETROFF B K, CIERESZKO R E.Effects of phytoestrogen daidzein and estradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles.Journal of Physiology and Pharmacology, 2009, 60(2): 95-105.
[25] SWART A C, JOHANNES I D, SATHYAPALAN T, ATKIN S L.The effect of soy isoflavones on steroid metabolism.Frontiers in Endocrinology, 2019, 10: 229-239.
[26] KIM M Y, PARK J Y, PARK H S.Akt1-mediated phosphorylation of RBP-jk controls Notch1 signaling.Biochemistry (Moscow), 2019, 84(12): 1537-1546.
[27] ZHU C C, ZHANG Y E, DUAN X, HAN J, SUN S C.Toxic effects of HT-2 toxin on mouse oocytes and its possible mechanisms.Archives of Toxicology, 2016, 90(6): 1495-1505.
[28] CHOI J Y, JO M W, LEE E Y, YOON B K, CHOI D S.The role of autophagy in follicular development and atresia in rat granulosa cells.Fertility and Sterility, 2010, 93(8): 2532-2537.
[29] FIGUEROA F, MOTTA A, ACOSTA M, MOHAMED F, OLIVEROS L, FORNERIS M.Role of macrophage secretions on rat polycystic ovary: its effect on apoptosis.Reproduction (Cambridge, England), 2015, 150(5): 437-448.
[30] 郑峰, 金梅花, 金明, 刘思彤, 张天, 尹学哲, 全吉淑.大豆异黄酮对过氧化氢致L02细胞凋亡的抑制作用.大豆科学, 2020(4): 621-625.
ZHENG F, JIN M H, JIN M, LIU S T, ZHANG T, YIN X Z, QUAN J S.Inhibitory effect of soy isoflavones on apoptosis of L02 cells induced by hydrogen peroxide.Soybean Science, 2020(4): 621-625.(in Chinese)
[31] VOUSDEN K H, PRIVES C.Blinded by the light: the growing complexity of p53.Cell, 2009, 137(3): 413-431.
[32] MYONG N H.Tissue microarray analysis of Fas and FasL expressions in human non-small cell lung carcinomas; with reference to the p53 and bcl-2 overexpressions.Journal of Korean Medical Science, 2005, 20(5): 770-776.
[33] LI X Y, HE Z C, CHENG B Q, FANG Q, MA D, LU T T, WEI D N, KUANG X Y, TANG S S, XIONG J E, WANG J S.Effect of BCLAF1on HDAC inhibitor LMK-235-mediated apoptosis of diffuse large B cell lymphoma cells and its mechanism.Cancer Biology & Therapy, 2018, 19(9): 825-834.
Effects of Soy Isoflavones on the Proliferation and Apoptosis of Yak Ovarian Granulosa Cells
AYIMUGULI·Abudureyimu1,2, AERZUGULI·Ayiding1,2, WANG JiaMin1,3, SHI JiaChen1,2, MA FangFang1,2, CAI Yong4, QIAO ZiLin1,3*
1Gansu Tech Innovation Center of Animal Cell, Biomedical Research Center, Northwest Minzu University, Lanzhou 730030;2College of Life Science and Engineering, Northwest Minzu University, Lanzhou 730030;3Key Laboratory of Biotechnology and Bioengineering of State Ethnic Affairs Commission, Biomedical Research Center, Northwest Minzu University, Lanzhou 730030;4Department of Experiment & Teaching, Northwest Minzu University, Lanzhou 730030
【Background】Soybean isoflavones, mainly including genistein and daidzein, could exert estrogen-like effects, scavenge free radicals and promote cell proliferation.Granulosa cells are an important cell population in follicles and its physiological state is directly related to ovarian function, and the apoptosis of granulosa cells causes oocyte atresia.Yak (Bos grunniens) is one of the endemic species in Qinghai-Tibet plateau, but its reproductive rate is so low, however, the mechanism is still unclear.Ovary granulosa cell is an ideal model to study the regulating mechanism of female animal reproduction.【Objective】The aim this study was to investigate the effects of soybean isoflavones on the proliferation and apoptosis of granulosa cells of yak ovary, and to provide evidence for the mechanism of soybean isoflavones.【Method】The yak ovarian granulosa cells were isolated and cultured.Immunohistochemistry staining was used to check FSHR for ovarian granulosa cell authenticating.MTT assay was used to detect cell proliferation, then the growth curve was drawn.Ovarian granulosa cells treated with different concentration of genistein and daidzein (0, 1 000, 2 000, 3 000, 4 000 and 5 000 pg·mL-1), the optimal concentration of genistein or daidzein for was selected to treat the second-generation granulosa cells for 48 h, then, the cell culture medium was collected and used to detect the concentration of estradiol and progesterone secreted by granulosa cells by ELISA.At the same time, the cells were collected to extract total RNA and to research the expression of proliferation-related geneand apoptosis-related genesandby qRT-PCR.【Result】The yak ovarian granulosa cells were typical epithelial-like cells.After inoculated for 2 h, the granulosa cells began to adhere to the wall.After 12 h, the cells appeared aggregation and growth.After 24 h, the cells were larger and showed long fusiform, star-shaped or polygonal.After cultured for 48 h, the cells looked like paving appearance.Immunohistochemistry staining showed FSHR positive indicated that the cultured cells were ovarian granulosa cells.MTT assay showed that the growth curve of the yak ovarian granulosa cells was S-shape: the growth was slow in 24 h and the cells were in the latent growth stage, and then, which was rapidly proliferated at 24-48 h and entered the exponential growth phase.The granulosa cells, in the plateau phase, steadily proliferated during 48-120 h.After 120 h, the density of living cells began to decline, and the cells entered the phase of degeneration.The second-generation of granulosa cells grew faster and the exponential proliferation phase at 24 h, so the second-generation of granulosa cells was selected for this experiment.MTT assay showed that treated with 3 000 pg·mL-1genistein or daidzein for 48h, the cell viability were significantly promoted(<0.01), the secretion of estradiol were induced treated with daidzein, but the progesterone secretion were markedly inhibited treated with genistein.The results of qRT-PCR showed that 3 000 pg·mL-1genistein or daidzein significantly up-regulated the expression of,,,,and/(<0.01), down-regulated the expression ofand.In addition, genistein significantly down-regulated the expression ofand daidzein significantly up-regulated it (<0.01).【Conclusion】The yak ovarian granulosa cells were isolated and cultured to provide an effective cell model for further study on the yak female reproduction regulating mechanism.The results indicated that genistein and daidzein inhibited the progesterone secretion of yak ovarian granulosa cells, promoted cell proliferation, and protected cells from apoptosis by up-regulating,,andand down-regulatingand.
genistein; daidzein; yak; ovarian granulosa cells; proliferation; apoptosis
甘肃省自然科学基金(20JR10RA122)、甘肃省科技重点研发计划(21YF1FA222)、中央高校基本业务费专项资金(31920210138)、教育部动物医学生物工程创新团队(IRT-17R88)
阿依木古丽·阿不都热依木,E-mail:87032164@qq.com。通信作者乔自林,E-mail:670267497@qq.com
2021-02-19;
2021-07-27
(责任编辑 林鉴非)
