circ-13267通过let-7-19/ERBB4通路调控蛋鸭卵泡颗粒细胞凋亡
2022-05-16吴艳张昊梁振华潘爱銮申杰蒲跃进黄涛皮劲松杜金平
吴艳,张昊,梁振华,潘爱銮,申杰,蒲跃进,黄涛,皮劲松*,杜金平
circ-13267通过let-7-19/ERBB4通路调控蛋鸭卵泡颗粒细胞凋亡
吴艳1,2,张昊1,梁振华1,潘爱銮1,申杰1,蒲跃进1,黄涛1,皮劲松1*,杜金平1
1湖北省农业科学院畜牧兽医研究所/湖北省农业科技创新中心,武汉 430064;2动物胚胎工程及分子育种湖北省重点实验室,武汉 430064
【背景】蛋鸭卵泡发育是决定其产蛋性能的关键因素。已有研究表明,禽类卵泡发育是极为复杂的生物学过程,目前人们已对家禽的卵泡发育模式有了一定的了解,但作为决定产蛋量的重要因素,卵泡发育的具体调控机理仍需进一步深入研究。颗粒细胞是卵泡中主要的功能细胞,可调控卵泡膜细胞和卵母细胞的生长、分化和成熟,同时也精确的调控卵泡的生长发育,维持卵巢的正常功能(诱导排卵、维持成熟分裂的阻断、为卵母细胞提供底物等)。circRNA是一类新型的内源性非编码RNA,在卵泡发育中发挥重要的调控作用。【目的】通过构建circRNA过表达载体上调circ-13267的表达,研究circ-13267在蛋鸭卵泡颗粒细胞中的作用及其调控机制,为蛋鸭卵泡发育的调控机制解析提供依据。【方法】首先,利用Q-PCR检测蛋鸭卵泡颗粒细胞的细胞质与细胞核中circ-13267的表达水平;构建circ-13267的过表达载体circ-13267-pLCDH,在蛋鸭颗粒细胞中过表达circ-13267后,利用Q-PCR法检测circ-13267、let-7-19、ERBB4、FAS和BCL2的表达水平;分别转染circ-13267-pLCDH和pLCDH-ciR于蛋鸭卵泡颗粒细胞,24 h后利用EdU法检测蛋鸭卵泡颗粒细胞的增殖能力;将circ-13267的线性序列或ERBB4的3′UTR克隆到pmirGLo载体中,同时对上述野生型序列中的let-7-19结合位点进行突变,得到表达突变型序列的载体,利用双荧光素酶报告基因验证circ-13267与let-7-19及let-7-19与靶基因ERBB4的结合关系;在蛋鸭卵泡颗粒细胞中转染circ-13267-pLCDH和pLCDH-ciR,利用流式细胞术和Annexin V-FITC检测蛋鸭卵泡颗粒细胞凋亡情况。【结果】环状RNA circ-13267在细胞质和细胞核中均有表达;双荧光素酶报告基因试验结果显示,let-7-19能与ERBB4结合,进而下调荧光素酶的活性,当ERBB4序列中let-7-19的结合位点突变后,let-7-19则无法抑制荧光素酶的表达,说明ERBB4是let-7-19的一个靶基因;荧光定量检测结果显示,过表达环状RNAcirc-13267后BCL2基因的表达显著降低(<0.05),而FAS和ERBB4基因的表达量显著升高(<0.05),当过表达let-7-19后,ERBB4基因的表达量显著升高(<0.05),而抑制let-7-19后ERBB4基因的表达量显著降低(<0.05);EdU试验结果显示,过表达circ-13267后蛋鸭卵泡颗粒细胞数量显著减少,说明其促进了蛋鸭卵泡颗粒细胞的凋亡;然而,在蛋鸭卵泡颗粒细胞中共转染circ-13267和let-7-19后,与对照组相比共转染组中BCL2和FAS的表达量均无显著变化(>0.05),而与过表达circ-13267组相比,共转组中BCL2基因的表达量极显著降低(<0.01)、FAS的表达量极显著升高(<0.01),说明circ-13267促进蛋鸭卵泡颗粒细胞凋亡的作用被抑制;利用流式细胞仪检测转染后的蛋鸭卵泡颗粒细胞发现,与共转染circ-13267和let-7-19组相比,仅过表达circ-13267后,晚期凋亡细胞数和总凋亡细胞数均显著增加(<0.05),而活细胞数显著降低(<0.05)。【结论】蛋鸭circ-13267在蛋鸭卵泡颗粒细胞的细胞质和细胞核中均有表达,circ-13267可以吸附let-7-19并靶向ERBB4基因,从而促进了蛋鸭卵泡颗粒细胞的凋亡,为解析蛋鸭卵泡发育的调控机制提供理论依据。
circRNA;miRNA;蛋鸭;卵泡发育;颗粒细胞
0 引言
【研究意义】蛋鸭卵泡发育是决定其产蛋性能的关键因素。已有研究表明,禽类卵泡发育是极为复杂的生物学过程,目前人们已对家禽的卵泡发育模式有了一定的了解,但作为决定产蛋量的重要因素,卵泡发育的具体调控机理仍需进一步深入研究。【前人研究进展】Circular RNA(circRNA)是一类新型的内源性非编码RNA,它是在mRNA前体剪切过程中通过首尾相连形成的共价闭合环状结构,其形成主要依赖于经典的剪接性位点和剪接性机制[1-3],受特定顺式作用元件和反式作用因子调节[4-5],稳定性显著高于线性RNA,可通过海绵吸附微小RNA (microRNA,miRNA) 或其他分子,在转录和转录后水平发生调控作用[6-7]。已有研究表明,circRNA在卵泡发育过程和子宫内膜细胞中发挥重要的调控作用,如ZHANG等[8]研究发现circRNA在卵巢激素生成等过程中发挥作用;JIA等[9]研究发现CircEGFR可能通过与miR-125a-3p竞争性结合调节Fyn,从而在小鼠卵巢GCs中发挥重要作用;TAO等[10]研究发现circNRA在山羊排卵前卵泡颗粒细胞中发挥重要作用;ZHANG等[11-12]在奶山羊中发现circRNA-9119通过吸附miR-26a调控奶山羊子宫内膜上皮细胞PTGS2基因的表达,circRNA-miR182通过调控睾丸素抑制了山羊受孕前期子宫内膜上皮细胞凋亡。XU等[13]鉴定获得了卵巢中与猪产仔数相关的circRNA。有关circRNA在家禽卵泡发育中的研究相对较少,仅见SHEN等[14]分析了鸡卵泡颗粒细胞circRNA 的动态表达及功能;ZHANG等[15]研究发现circRNA通过PPAR和脂肪酸代谢相关途径调节miRNAs,从而潜在地影响脂肪的形成。【本研究切入点】关于蛋鸭circRNA在卵泡发育中的研究仅见本团队前期研究报道,其中circ-13267在白卵泡和黄卵泡组织中表达量存在显著差异[16]。颗粒细胞是卵泡中主要的功能细胞,其可调控卵泡膜细胞和卵母细胞的生长、分化和成熟,同时也精确的调控卵泡的生长发育[17],维持卵巢的正常功能(诱导排卵、维持成熟分裂的阻断、为卵母细胞提供底物等)[18]。【拟解决的关键问题】本研究拟通过构建circRNA过表达载体上调circ-13267的表达,研究circ-13267调控蛋鸭卵泡颗粒细胞的机制,为蛋鸭卵泡发育的调控提供依据。
1 材料与方法
1.1 试验材料
蛋鸭卵泡颗粒细胞:采集湖北农业科学院畜牧兽医研究所家禽试验场选育的蛋鸭卵泡组织,迅速置于含有2%双抗(青霉素和链霉素)的PBS中,尽快返回实验室,并分离卵泡颗粒细胞。
主要试剂:DMEM 基础培养基、含EDTA 的胰酶稀释液、PBS 缓冲液、胎牛血清、青霉素和链霉素购自Hyclone(美国);Lipofectamine® 3000 转染试剂盒、Opti-MEM®试剂购自Invitrogen(美国);双荧光素酶检测试剂盒购自Promega公司;SYBR Green qPCR Mix 购自Thermo(美国);HⅠ、RⅠ限制性内切酶和T4 DNA连接酶购自Thermo公司;KOD FX酶购自TOYOBO,公司;凝胶DNA小量回收试剂盒和无内毒素质粒抽提试剂盒购自OMEGA公司(美国);PrimeScriptTMRT reagent Kit反转录试剂盒购自宝生物工程(大连)有限公司;荧光定量PCR酶购自BIO-RAD公司;环状RNA过表达载体PLCDH-ciR,购自吉赛生物科技有限公司;其余有机试剂均为国产分析纯。
1.2 试验方法
1.2.1 蛋鸭卵泡颗粒细胞分离培养与转染 选择产蛋期蛋鸭颈静脉放血处死后取整个卵巢组织,置于装有预冷PBS的无菌培养皿中迅速带回实验室,用加有双抗的PBS 清洗数次。将漂洗干净的卵泡移入装有预冷PBS缓冲液的平皿中,剥净卵泡外膜、结缔组织及血管网,划破卵泡后释放卵黄,将漂洗干净的卵泡膜尽量剪碎,置于15 mL离心管中,加4 mL培养基反复吹打1 min,4 ℃ 1 000 r/min离心后弃上清;加入4 mL 0.2%Ⅱ型胶原酶,重悬沉淀,置于37℃恒温摇床80 r/min消化30 min,加入4 mL M199完全培养基(含10%血清)终止消化;用200目不锈钢筛过滤,收集滤液,4℃ 1 000 r/min离心10 min后收集细胞,加入M199完全培养基(含10% FBS及1 %双抗)重悬后,测定细胞密度后接种于6孔培养皿,置于37℃、5 % CO2培养箱内静置培养,培养24 h后可用后续相关试验。使用转染试剂lip 3000按照转染说明书对细胞进行转染,转染24 h后收获细胞。
1.2.2 蛋鸭卵泡颗粒细胞核质分离与RNA提取 根据PARIS™ Kit(Invitrogen,ThermoFisher Scientific)试剂盒说明书进行蛋鸭卵泡颗粒细胞并提取细胞中的总RNA。
1.2.3 载体构建 环状RNA过表达载体pLCDH-ciR购自广州吉赛生物技术有限公司。根据环状RNA测序分析获得的circ-13267基因序列,设计并合成circ-13267的全长序列引物(表1)。扩增circ-13267序列全长,切胶回收后,用RI和HI于37 ℃双酶切PCR产物和pLCDH-ciR空载体。酶切后回收目的片段和空载体,利用T4连接酶将目的片段与pLCDH-ciR载体连接,将其命名为circ-13267-pLCDH。
1.2.4 RNA反转录与实时荧光定量PCR 使用TRIzol提取细胞和组织总RNA,利用RNase R处理RNA后按照TaKaRa反转录试剂盒说明书进行cDNA的合成,qPCR使用Bio-Rad公司的实时荧光定量PCR仪完成。circ-13267、β-actin、let-7-19、U6及ERBB4基因的定量引物见表1。反应条件为: 95 ℃预变性10 min;95℃10 s,60℃ 30 s,72℃30s,重复40个循环,用2–ΔΔCt法计算基因表达。

表1 试验所需引物序列
1.2.5 circRNA与miRNA及靶基因结合位点的预测 利用在线预测软件BiBiServ中的rnahybrid 进行circ-13267与miRNA let-7-19及let-7-19与靶基因ERBB4之间的结合位点,详细预测网址如下:https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid。
1.2.6 双荧光素酶报告实验检测荧光素酶活性 将circ-13267的线性序列或ERBB4的3′UTR克隆到pmirGLo载体中,同时对上述野生型序列中的let-7-19结合位点进行突变,得到表达突变型序列的载体,分别命名为circ-13267-pmirGLO-W、circ-13267-pmirGLO- M、pmirGLo- ERBB4-W和pmirGLo- ERBB4-M。将上述载体分别与let-7-19模拟物共转染蛋鸭卵泡颗粒细胞,按照荧光素酶活性检测试剂盒说明书进行,测定萤火虫荧光素酶(firefly luciferase,FL)和海肾荧光素酶(ranilla luciferase,RL)强度,根据FL/RL比值来判断相对荧光素酶活性。
1.2.7 EdU试验检测细胞增殖能力 将分离的蛋鸭卵泡颗粒细胞接种到24孔板, 使细胞汇合度达到80%,分别转染circ-13267-pLCDH和pLCDH-ciR于蛋鸭卵泡颗粒细胞,24 h后进行EdU处理。每孔加300 μL稀释EdU溶液孵育2 h。然后按照EdU试剂盒说明书(广东锐博)进行细胞固定、Apollo染色和DNA染色。最后在荧光显微镜下(Olympus SZX16)拍照。
1.2.8 颗粒细胞凋亡检测 参照杭州联科生物技术股份有限公司的Annexin V-FITC/PI 双染细胞凋亡检测试剂盒说明书进行蛋鸭颗粒细胞凋亡检测。取细胞接种于6 孔板,当细胞汇合度达到80%左右,转染circ-13267-pLCDH和pLCDH-ciR于颗粒细胞,培养24 h后消化并收集细胞。然后按照Annexin V-FITC/PI试剂盒说明书进行细胞处理,最后用流式细胞仪检测分析(每个试验组设3个重复)。
1.2.9 数据统计分析 每个试验设3个重复,采用SPSS 19.0进行结果统计分析,两组样本均数比较采用t检验,利用GraphPad Prism 6进行作图。
2 结果
2.1 circ-13267对蛋鸭卵泡颗粒细胞增殖、凋亡的影响
转染circ-13267- pLCDH和pLCDH-ciR后的蛋鸭卵泡颗粒细胞后,利用荧光定量PCR法检测细胞增殖凋亡关键基因BCL2、FAS的表达情况,结果见图1-a,由图1-a可以看出,过表达circ-13267后细胞增殖标志基因BCL2的表达量极显著降低(<0.01),细胞凋亡标志基因FAS的表达量显著升高(<0.01),说明circ-13267促进了蛋鸭卵泡颗粒细胞的凋亡;EdU法检测细胞凋亡情况,结果表明过表达circ-13267后,蛋鸭卵泡颗粒细胞数量显著减少(图1-b)。

A:过表达circ-13267后荧光定量检测;B:过表达circ-13267后EdU检测
2.2 circ-13267抑制let-7-19在蛋鸭颗粒细胞中的表达
为探讨circ-13267在蛋鸭卵泡颗粒细胞中的功能,分别提取细胞质和细胞核中的RNA进行逆转录,荧光定量PCR检测circ-13267的表达情况,结果表明circ-13267在细胞质和细胞核中均有表达(图2),说明circ-13267可能具备竞争性结合miRNA的作用。分析circ-13267中miRNA的结合位点,结果发现circ-13267中存在let-7-19的结合位点(图3),并在前期研究中得到证明[16]。荧光定量检测结果发现let-7-19在白卵泡中的表达显著高于黄卵泡(<0.05)(图4)。

图2 circ-13267在细胞质与细胞核中的表达量

图3 circ-13267与let-7-19的结合位点
2.3 circ-13267通过let-7-19上调ERBB4基因表达
分析let-7-19的下游靶基因,发现let-7-19在ERBB4基因3′UTR区域存在结合位点(图5-a)。利用双荧光素酶报告基因s试验表明,let-7-19能与ERBB4结合,进而下调荧光素酶的活性,当ERBB4序列中let-7-19的结合位点突变后,let-7-19则无法抑制荧光素酶的表达(图5-b)。荧光定量结果显示,ERBB4基因在白卵泡中的表达显著低于黄卵泡(<0.05)(图5-c);当过表达let-7-19后,ERBB4基因的表达量显著升高(<0.05),而抑制let-7-19后ERBB4基因的表达量显著降低(<0.05)(图5-d)。综上可知,circ-13267可通过let-7-19上调ERBB4基因的表达。
** P<0.01
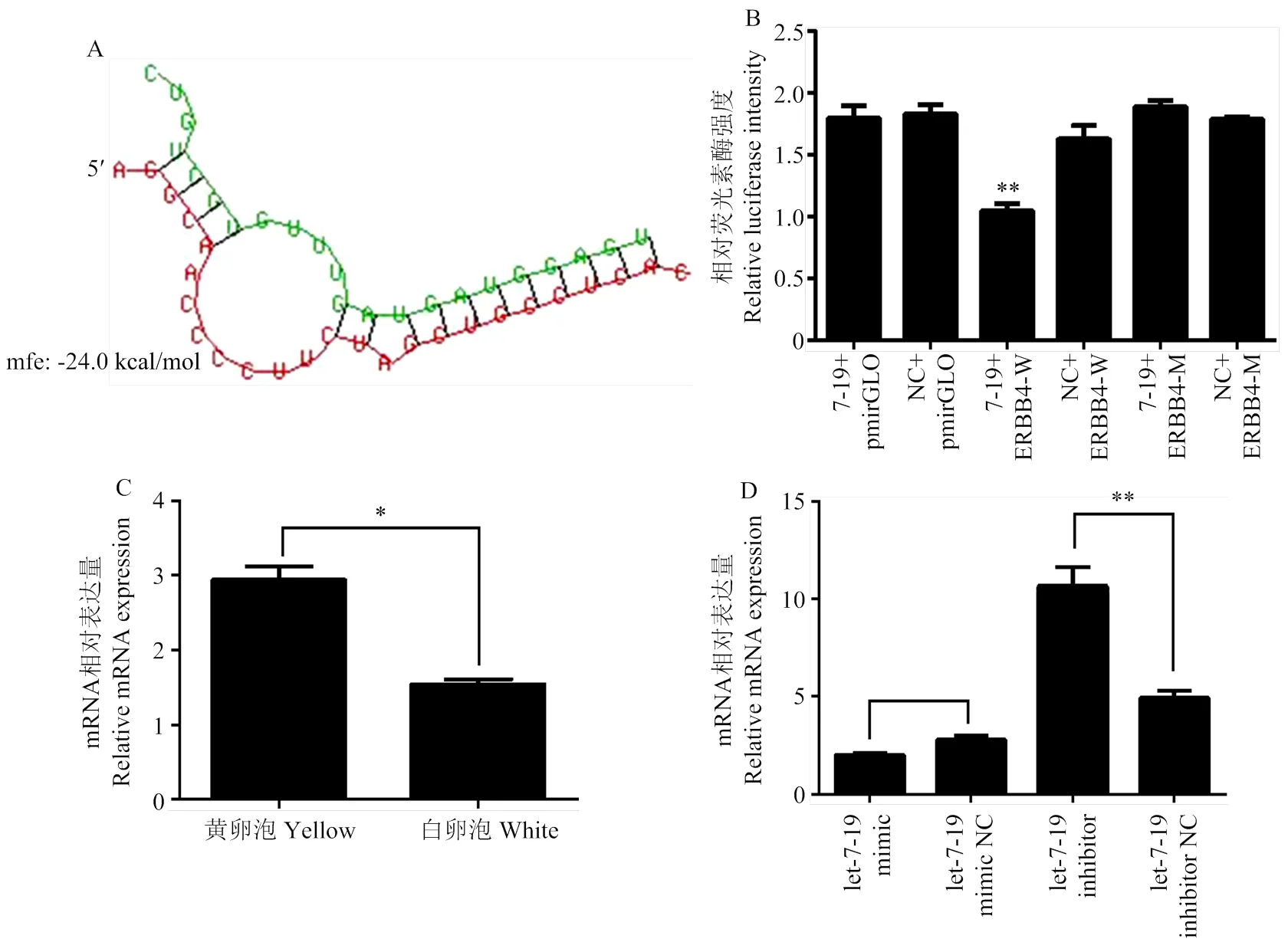
A:let-7-19在ERBB4基因3′非编码区(3′ untranslated region,3′UTR)中的结合位点;B:双荧光素酶报告基因实验检测let-7-19与ERBB4的结合能力;C:ERBB4在黄卵泡和白卵泡中的表达情况;D:过表达(抑制表达)let-7-19后ERBB4的表达变化情况。* P<0.05; ** P<0.01
2.4 circ-13267通过let-7-19/ERBB4途径促进蛋鸭卵泡颗粒细胞的凋亡
进一步探讨circ-13267是否通过let-7-19/ERBB4通路在蛋鸭卵泡颗粒细胞中发挥作用。在蛋鸭卵泡颗粒细胞中共转染circ-13267和let-7-19后,与对照组相比共转染组中BCL2和FAS的表达量均无显著变化(>0.05),而与过表达circ-13267组相比,共转组中BCL2基因的表达量极显著降低(<0.01)、FAS的表达量极显著升高(<0.01)(图6-a),circ-13267促进蛋鸭卵泡颗粒细胞凋亡的作用被抑制;利用流式细胞仪检测转染后的蛋鸭卵泡颗粒细胞发现,与共转染circ-13267和let-7-19组相比,仅过表达circ-13267后,晚期凋亡细胞数和总凋亡细胞数均显著增加(<0.05),而活细胞数显著降低(<0.05)(图6-b)。此外,荧光定量结果表明,过表达circ-13267后,ERBB4基因的表达量显著升高(<0.05),而同时过表达circ-13267和let-7-19后,ERBB4基因的表达量无显著变化(>0.05)(图6-C)。综上,说明circ-13267通过let-7-19/ERBB4途径促进了蛋鸭卵泡颗粒细胞的凋亡。
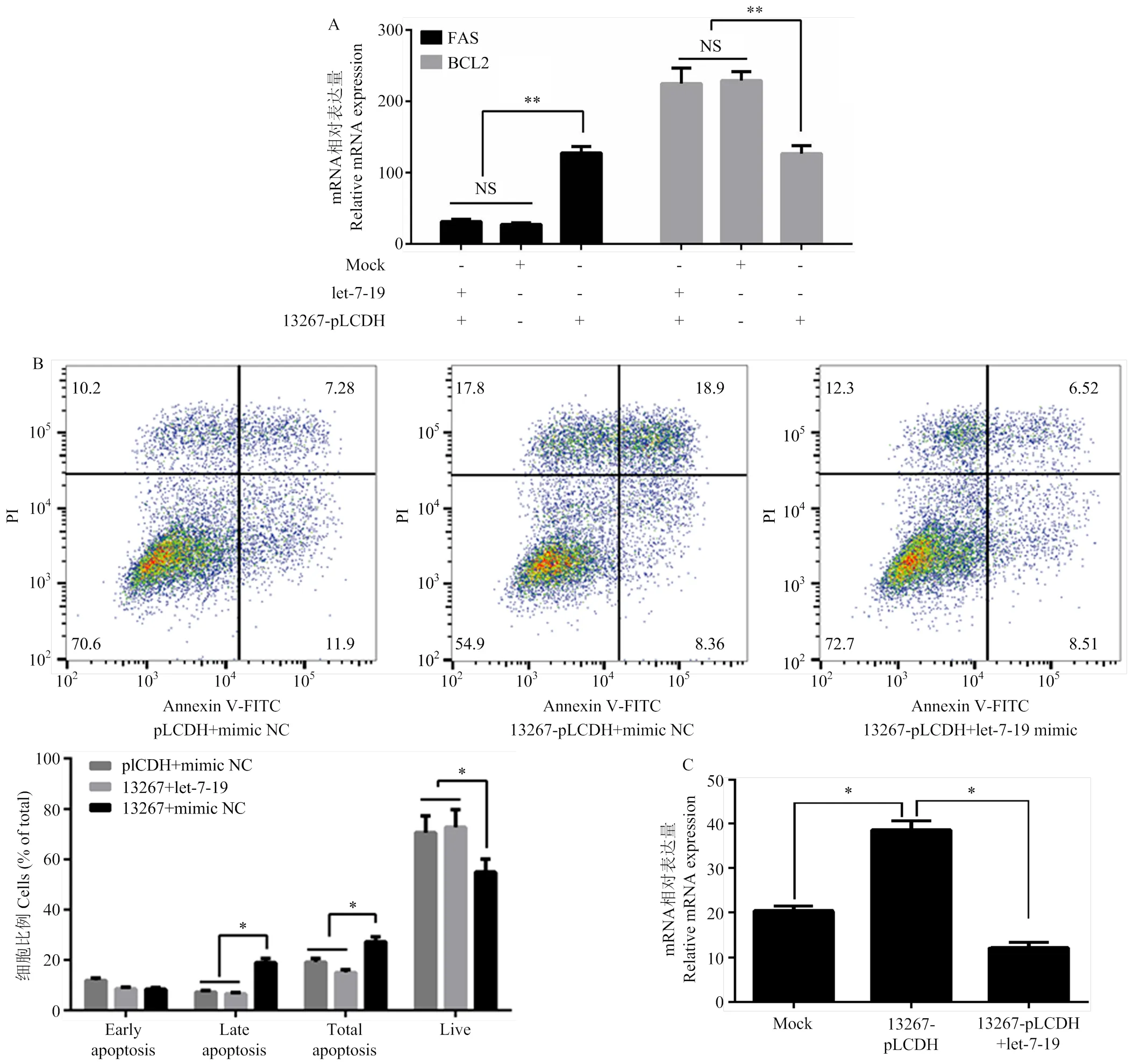
A:过表达circ-13267及let-7-19对颗粒细胞增殖凋亡的Q-PCR检测;B:过表达circ-13267及let-7-19对颗粒细胞增殖凋亡的流式细胞检测;C:过表达circ-13267和let-7-19后ERBB4基因表达变化情况
3 讨论
卵巢中卵泡的发育对蛋鸭的产蛋量具有直接影响,但作为决定产蛋量的重要因素,蛋鸭卵泡发育受多种因素的影响、调控机制复杂。因此,探讨明确蛋鸭卵泡发育的具体调控机制具有重要的实践意义。
家禽的卵泡发育按照其直径的大小分为等级前卵泡(包括小白卵泡、大白卵泡和小黄卵泡)和等级卵泡(又称为排卵前卵泡)[19-20]。鸡卵泡发育的现有理论认为,每当一次排卵活动发生后,就会有一个等级前卵泡进入卵泡选择,即有一个小黄卵泡被选择进入等级发育阶段[21-22]。由此可见,小黄卵泡的发育是决定等级卵泡的关键。前期研究发现circ-13267在白卵泡组织中的表达量显著高于小黄卵泡组织[16],提示circ-13267可能在蛋鸭的卵泡发育过程中发挥重要的作用。颗粒细胞的发育早于卵母细胞的发育[23],由此可见颗粒细胞的生长分化是原始卵泡生长的关键因素,而颗粒细胞的凋亡是卵泡闭锁的主要标志[24],因此本研究以蛋鸭卵泡颗粒细胞作为研究卵泡发育的细胞模型。本研究发现,过表达circ-13267后促进了蛋鸭卵泡颗粒细胞凋亡,而已有研究表明颗粒细胞的凋亡会导致生长卵泡和排卵前卵泡的闭锁[25],因此推测circ-13267对蛋鸭卵泡发育具有一定的抑制作用。
已有研究表明,circRNAs通常通过吸附miRNA从而调控下游靶基因的表达,且在鸡的卵泡发育中发挥重要的作用[14]。已有研究表明ERBB4基因是表皮生长因子受体(EGFR)家族的成员之一,与EGFR基因具有高度的同源性[26];调节细胞的增殖、迁移和存活等过程[27]。WU等[28]研究表明EGFR对鹌鹑卵泡颗粒细胞的增值具有促进作用,因此推测ERBB4基因与卵泡发育相关。且VEIKKOLAINEN等[29]最新研究发现ERBB4的确在卵泡发生过程中起着重要作用。此外,有多项研究表明ERBB4基因表达受到多个miRNA的调控,包括miR146a[30]、miR-551b[31]、miR-302b[32]和miR-193a-3p[33]。已有研究表明miRNA let-7家族let-7家族的miRNAs在多种癌症中都有很好的抑癌作用[34-36];过表达let-7 miRNAs后在某些白血病环境中抑制增殖和促进分化[37];let-7家族在视网膜和玻璃体发育过程中具有重要作用,可能会调节透明质酸的含量[38];SPINT1-AS1通过调控miR-let-7a/b/ i-5p促进乳腺癌细胞增殖和迁移[39];let-7b抑制t(8;21) AML细胞系的增殖[40]。综上所述,miRNA let-7家族在细胞凋亡中具有重要的作用。本研究结果表明,circ-13267和靶基因ERBB4的3′UTR中均存在miRNA let-7-19的结合位点,且circ-13267可通过吸附let-7-19上调ERBB4基因的表达;且circ-13267通过let-7-19/ERBB4途径促进了蛋鸭卵泡颗粒细胞的凋亡,但circ-13267调控蛋鸭卵泡发育的是否还通过其他途径发挥作用尚需进一步研究证实。
4 结论
对前期筛选获得的环状RNA circ-13267的功能及其调控蛋鸭卵泡发育的机制进行研究,构建了circ- 13267-miRNA调控网络,初步证实circ-13267可通过let-7-19/ERBB4途径促进蛋鸭卵泡颗粒细胞的凋亡,对蛋鸭卵泡发育的调控机制研究具有借鉴作用。
[1] MEMCZAK S, JENS M, ELEFSINIOTI A, TORTI F, KRUEGER J, RYBAK A, MAIER L, MACKOWIAK S D , GREGERSEN L H, MUNSCHAUER M, LOEWER A, ZIEBOLD U, LANDTHALER M, KOCKS C, LE NOBLE F, RAJEWSKY N.Circular RNAs are a large class of animal RNAs with regulatory potency.Nature, 2013, 495(7441): 333-338.doi:10.1038/nature11928.
[2] ASHWAL-FLUSS R, MEYER M, PAMUDURTI N R, IVANOV A, BARTOK O, HANAN M, EVANTAL N, MEMCZAK S, RAJEWSKY N, KADENER S.circRNA biogenesis competes with pre-mRNA splicing.Molecular Cell, 2014, 56(1): 55-66.doi:10.1016/j.molcel.2014.08.019.
[3] STARKE S, JOST I, ROSSBACH O, SCHNEIDER T, SCHREINER S, HUNG L H, BINDEREIF A.Exon circularization requires canonical splice signals.Cell Reports, 2015, 10(1): 103-111.doi:10.1016/j.celrep.2014.12.002.
[4] LI X, YANG L, CHEN L L.The biogenesis, functions, and challenges of circular RNAs.Molecular Cell, 2018, 71(3): 428-442.doi:10.1016/ j.molcel.2018.06.034.
[5] KRISTENSEN L S, ANDERSEN M S, STAGSTED L V W, EBBESEN K K, HANSEN T B, KJEMS J.The biogenesis, biology and characterization of circular RNAs.Nature Reviews Genetics, 2019, 20(11): 675-691.doi:10.1038/ s41576-019-0158-7.
[6] ZHANG H D, JIANG L H, SUN D W, HOU J C, JI Z L.CircRNA: a novel type of biomarker for cancer.Breast Cancer (Tokyo, Japan), 2018, 25(1): 1-7.doi:10.1007/s12282-017-0793-9.
[7] YAN Y, SU M, QIN B L.CircHIPK3promotes colorectal cancer cells proliferation and metastasis via modulating of miR-1207-5p/FMNL2 signal.Biochemical and Biophysical Research Communications, 2020, 524(4): 839-846.doi:10.1016/j.bbrc.2020.01.055.
[8] ZHANG C R, LIU J Q, LAI M H, LI J, ZHAN J H, WEN Q D, MA H X.Circular RNA expression profiling of granulosa cells in women of reproductive age with polycystic ovary syndrome.Archives of Gynecology and Obstetrics, 2019, 300(2): 431-440.doi:10.1007/ s00404-019-05129-5.
[9] JIA W C, XU B, WU J.Circular RNA expression profiles of mouse ovaries during postnatal development and the function of circular RNA epidermal growth factor receptor in granulosa cells.Metabolism, 2018, 85: 192-204.doi:10.1016/j.metabol.2018.04.002.
[10] TAO H, XIONG Q, ZHANG F, ZHANG N, LIU Y, SUO X J, LI X F, YANG Q P, CHEN M X.Circular RNA profiling reveals chi_circ_0008219 function as microRNA sponges in pre-ovulatory ovarian follicles of goats ().Genomics, 2018, 110(4): 257-266.doi:10.1016/j.ygeno.2017.10.005.
[11] ZHANG L, LIU X R, CHE S C, CUI J Z, LIU Y X, AN X P, CAO B Y, SONG Y X.CircRNA-9119 regulates the expression of prostaglandin- endoperoxide synthase 2 (PTGS2) by sponging miR-26a in the endometrial epithelial cells of dairy goat.Reproduction, Fertility, and Development, 2018, 30(12): 1759-1769.doi:10.1071/RD18074.
[12] ZHANG L, LIU X R, MA X N, LIU Y X, CHE S C, CUI J Z, AN X P, CAO B Y, SONG Y X.Testin was regulated by circRNA3175-miR182 and inhibited endometrial epithelial cell apoptosis in pre-receptive endometrium of dairy goats.Journal of Cellular Physiology, 2018, 233(10): 6965-6974.doi:10.1002/jcp.26614.
[13] XU G X, ZHANG H F, LI X, HU J H, YANG G S, SUN S D.Genome-wide differential expression profiling of ovarian circRNAs associated with litter size in pigs.Frontiers in Genetics, 2019, 10: 1010.doi:10.3389/fgene.2019.01010.
[14] SHEN M M, LI T T, ZHANG G X, WU P F, CHEN F X, LOU Q H, CHEN L, YIN X M, ZHANG T, WANG J Y.Dynamic expression and functional analysis of circRNA in granulosa cells during follicular development in chicken.BMC Genomics, 2019, 20(1): 96.doi:10.1186/s12864-019-5462-2.
[15] ZHANG M, HAN Y, ZHAI Y H, MA X F, AN X L, ZHANG S, LI Z Y.Integrative analysis of circRNAs, miRNAs, and mRNAs profiles to reveal ceRNAs networks in chicken intramuscular and abdominal adipogenesis.BMC Genomics, 2020, 21(1): 594.doi:10.1186/s12864- 020-07000-3.
[16] WU Y, XIAO H W, PI J S, ZHANG H, PAN A L, PU Y J, LIANG Z H, SHEN J, DU J P.The circular RNA aplacirc_13267 upregulates duck granulosa cell apoptosis by the apla-miR-1-13/THBS1signaling pathway.Journal of Cellular Physiology, 2020, 235(7/8): 5750-5763.doi:10.1002/jcp.29509.
[17] MATSUDA F, INOUE N, MANABE N, OHKURA S.Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells.The Journal of Reproduction and Development, 2012, 58(1): 44-50.doi:10.1262/jrd.2011-012.
[18] FAN H Y, LIU Z L, SHIMADA M, STERNECK E, JOHNSON P F, HEDRICK S M, RICHARDS J S.MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility.Science, 2009, 324(5929): 938-941.doi:10.1126/science.1171396.
[19] ETCHES R J, PETITTE J N.Reptilian and avian follicular hierarchies: models for the study of ovarian development.The Journal of Experimental Zoology Supplement: Published Under Auspices of the American Society of Zoologists and the Division of Comparative Physiology and Biochemistry, 1990, 4: 112-122.doi:10.1002/jez.1402560419.
[20] 林金杏.局部性促生长因子对鸡卵泡发育的调控及其机理的研究[D].杭州: 浙江大学, 2011.
LIN J X.Regulation of local growth-promoting factors on follicular development in the laying chickens[D].Hangzhou: Zhejiang University, 2011.(in Chinese)
[21] JOHNSON P.Follicle selection in the avian ovary.Reproduction in Domestic Animals, 2012, 47: 283-287.doi:10.1111/j.1439-0531.2012.02087.x.
[22] WANG Y Y, CHEN Q Y, LIU Z M, GUO X L, DU Y Z, YUAN Z J, GUO M, KANG L, SUN Y, JIANG Y L.Transcriptome analysis on single small yellow follicles reveals that Wnt4 is involved in chicken follicle selection.Frontiers in Endocrinology, 2017, 8: 317.doi:10.3389/fendo.2017.00317.
[23] 魏泽辉, 贾存灵.家禽卵泡选择过程中颗粒细胞的分子调控机制.中国家禽, 2017, 39(21): 1-5.doi:10.16372/j.issn.1004-6364.2017.21.001.
WEI Z H, JIA C L.The molecular regulation mechanism of granulosa cells in the process of poultry follicle selection.China Poultry, 2017, 39(21): 1-5.doi:10.16372/j.issn.1004-6364.2017.21.001.(in Chinese)
[24] 陆思羽, 何颖婷, 周小枫, 辛晓萍, 张爱玲, 袁晓龙, 张哲, 李加琪.干扰KISS1基因对猪卵巢颗粒细胞功能的影响.中国农业科学, 2020, 53(23): 4940-4949.doi:10.3864/j.issn.0578-1752.2020.23.018.
LU S Y, HE Y T, ZHOU X F, XIN X P, ZHANG A L, YUAN X L, ZHANG Z, LI J Q.Effect of KISS1interference on the function of porcine granulosa cells in porcine ovary.Scientia Agricultura Sinica, 2020, 53(23): 4940-4949.doi:10.3864/j.issn.0578-1752.2020.23.018.(in Chinese)
[25] DEPALO R, NAPPI L, LOVERRO G, BETTOCCHI S, CARUSO M L, VALENTINI A M, SELVAGGI L.Evidence of apoptosis in human primordial and primary follicles.Human Reproduction, 2003, 18(12): 2678-2682.doi:10.1093/humrep/deg507.
[26] ROSKOSKI R J.The ErbB/HER receptor protein-tyrosine kinases and cancer.Biochemical and Biophysical Research Communications, 2004, 319(1): 1-11.doi:10.1016/j.bbrc.2004.04.150.
[27] YARDEN Y, PINES G.The ERBB network: at last, cancer therapy meets systems biology.Nature Reviews Cancer, 2012, 12 (8): 553-563.doi:10.1038/nrc3309.
[28] WU Y, XIAO H W, PI J S, ZHANG H, PAN A L, PU Y J, LIANG Z H, SHEN J, DU J P.EGFR promotes the proliferation of quail follicular granulosa cells through the MAPK/extracellular signal-regulated kinase (ERK) signaling pathway.Cell Cycle, 2019, 18(20): 2742-2756.doi:10.1080/15384101.2019.1656952.
[29] VEIKKOLAINEN V, ALI N, DOROSZKO M, KIVINIEMI A, MIINALAINEN I, OHLSSON C, POUTANEN M, RAHMAN N, ELENIUS K, VAINIO S J, NAILLAT F.Erbb4 regulates the oocyte microenvironment during folliculogenesis.Human Molecular Genetics, 2020, 29(17): 2813-2830.doi:10.1093/hmg/ddaa161.
[30] AN R, FENG J X, XI C, XU J, SUN L J.miR-146a attenuates-induced myocardial dysfunction by suppressing IRAK1 and TRAF6via targeting ErbB4 expression.Oxidative Medicine and Cellular Longevity, 2018, 2018: 7163057.doi:10.1155/2018/7163057.
[31] SONG G Y, ZHANG H C, CHEN C L, GONG L J, CHEN B, ZHAO S Y, SHI J, XU J, YE Z Y.miR-551b regulates epithelial- mesenchymal transition and metastasis of gastric cancer by inhibiting ERBB4expression.Oncotarget, 2017, 8(28): 45725-45735.doi:10.18632/oncotarget.17392.
[32] ZHANG M X, ZHANG L M, CUI M L, YE W G, ZHANG P J, ZHOU S N, WANG J J.miR-302b inhibits cancer-related inflammation by targeting ERBB4, IRF2and CXCR4 in esophageal cancer.Oncotarget, 2017, 8(30): 49053-49063.doi:10.18632/oncotarget.17041.
[33] LIANG H W, LIU M H, YAN X, ZHOU Y, WANG W G, WANG X L, FU Z, WANG N, ZHANG S Y, WANG Y B, ZEN K, ZHANG C Y, HOU D X, LI J, CHEN X.miR-193a-3p functions as a tumor suppressor in lung cancer by down-regulating ERBB4.Journal of Biological Chemistry, 2015, 290(2): 926-940.doi:10.1074/jbc.M114.621409.
[34] NISHI M, EGUCHI-ISHIMAE M, WU Z, GAO W, IWABUKI H, KAWAKAMI S, TAUCHI H, INUKAI T, SUGITA K, HAMASAKI Y, ISHII E, EGUCHI M.Suppression of the let-7b microRNA pathway by DNA hypermethylation in infant acute lymphoblastic leukemia withgene rearrangements.Leukemia, 2013, 27 (2): 389-397.doi:10.1038/leu.2012.242.
[35] 许文前,黄源茂,肖慧芳.microRNA let-7b在急性淋巴细胞白血病的表达分析与表观遗传学研究.中国试验血液学杂志, 2015, 23(6): 1535-1541.doi:10.7534/j.issn.1009-2137.2015.06.001.
XU W Q, HUANG Y M, XIAO H F.Expression analysis and epigenetics of microRNA let-7b in acute lymphoblastic leukemia.Journal of Experimental Hematology, 2015, 23(6): 1535-1541.doi:10.7534/j.issn.1009-2137.2015.06.001.(in Chinese)
[36] BALZEAU J, MENEZES M R, CAO S Y, HAGAN J P.The LIN28/let-7 pathway in cancer.Frontiers in Genetics, 2017, 8: 31.doi:10.3389/fgene.2017.00031.
[37] PELOSI A, CARECCIA S, LULLI V, ROMANIA P, MARZIALI G, TESTA U, LAVORGNA S, LO-COCO F, PETTI M C, CALABRETTA B, LEVRERO M, PIAGGIO G, RIZZO M G.miRNA let-7c promotes granulocytic differentiation in acute myeloid leukemia.Oncogene, 2013, 32(31): 3648-3654.doi:10.1038/onc.2012.398.
[38] AKAMINE P S, LIMA C R, LUSTOZA-COSTA G J, FUZIWARA C S, DEL DEBBIO C B, KIMURA E T, SANTOS M F, HAMASSAKI D E.Age-related increase of let-7 family microRNA in rat retina and vitreous.Experimental Eye Research, 2021, 204: 108434.doi:10.1016/j.exer.2020.108434.
[39] ZHOU T Z, LIN K, NIE J J, PAN B, HE B S, PAN Y Q, SUN H L, XU T, WANG S K.LncRNA SPINT1-AS1 promotes breast cancer proliferation and metastasis by sponging let-7 a/b/i-5p.Pathology - Research and Practice, 2021, 217: 153268.doi:10.1016/j.prp.2020.153268.
[40] JOHNSON D T, DAVIS A G, ZHOU J H, BALL E D, ZHANG D E.microRNA let-7b downregulates-oncogene expression in t(8;21) AML by targeting its 3'UTR.Experimental Hematology & Oncology, 2021, 10(1): 8.doi:10.1186/s40164-021-00204-7.
circ-13267 Regulates Egg Duck Granulosa Cells Apoptosis Through Let-7-19/ERBB4 Pathway
WU Yan1,2, ZHANG Hao1, LIANG ZhenHua1, PAN AiLuan1, SHEN Jie1, PU YueJin1, Huang Tao1, PI JinSong1*, DU JinPing1
1Institute of Animal Husbandry and Vetervinary, Hubei Academy of Agricultural Science; Hubei Innovation Center of Agricultural Science and Technology, Wuhan 430064;2Hubei Key Lab of Animal Embryo Technology and Molecular Breeding, Wuhan 430064
【Background】 Follicle development is a key factor for laying performance of egg ducks.Previous studies have shown that follicular development is a very complex biological process in poultry.At present, the pattern of follicular development in poultry has been understood.However, as an important factor determining egg production, the specific regulation mechanism of follicular development still needs further study.Granulosa cells are the main functional cells in follicles.They can regulate the growth, differentiation and maturation of theca cells and oocytes.They also regulate the growth and development of follicles, maintain normal ovarian function, such as induce ovulation, maintain maturation division, and provide substrates for oocytes.Circular RNAs (circRNAs) are a new type of endogenous specific non-coding RNA, which plays an important role in follicular development.【Objective】The objective of this study was to explore the effects and regulatory mechanism of circ-13267 on apoptosis in egg duck granulosa cells, through regulating the expression of circ-13267 by constructing the overexpression vector, so as to provide the evidence for analysis the regulatory mechanism of egg duck follicular development.【Method】Firstly, the expression levels of circ-13267 in cytoplasm and nucleus of granulosa cells was detected by Q-PCR.The overexpression vector circ-13267-pLCDH was constructed.After transfection of circ-13267 in egg duck granulosa cells, the expression levels of circ-13267, let-7-19, ERBB4, FAS and BCL2 were detected by Q-PCR.The proliferation of egg duck granulosa cells was detected by EdU method after transfection circ-13267-pLCDH and pLCDH-ciR.The linear sequence of circ-13267 or the 3'UTR of ErbB4 was cloned into pmirGLo vector.At the same time, let-7-19 binding site in the wild-type sequence was mutated to obtain the vector expressing the mutant sequence.The targeting relationships between circ-13267 and let-7-19, let-7-19 and ERBB4 were verified by dual luciferase reporter assay, respectively.Then, after transfection of circ-13267-pLCDH and pLCDH-ciR into egg duck follicular granulosa cells, the flow cytometry and Annexin V-FITC were utilized to explore the effects of circ-13267 on duck granulosa cells.【Result】 In duck granulosa cells, circ-13267 was expressed in both cytoplasm and nucleus.The dual luciferase reporter gene assay confirmed that let-7-19 could bind to ERBB4 and down regulate the activity of luciferase; when the binding site of let-7-19 in ErbB4 sequence was mutated, let-7-19 could not inhibit the expression of luciferase, indicating that ERBB4 was a target gene of let-7-19.The results of Q-PCR showed that, after overexpression of circ-13267, the expression of BCL2 gene decreased significantly (<0.05), while the expression of FAS and ERBB4 gene increased significantly (<0.05); after overexpression of let-7-19, the expression of ERBB4 gene increased significantly (<0.05), while after inhibition of let-7-19, the expression of ERBB4 gene decreased significantly (<0.05).EdU test results showed that the number of follicular granulosa cells in egg ducks decreased significantly after overexpression of circ-13267, it was shown that circ-13267 promoted the apoptosis of follicular granulosa cells in egg ducks.However, after co-transfection of circ-13267 and let-7-19 into egg duck follicular granulosa cells, compared with the control group, there was no significant change in the expression of BCL2 and FAS (>0.05); however, compared with overexpression of circ-13267, the expression of BCL2 gene decreased significantly (<0.01) and FAS increased significantly (<0.01).It was shown that circ-13267 could inhibit the apoptosis of egg duck follicular granulosa cells.In addition, flow cytometry was used to detect the transfected egg duck follicular granulosa cells.Compared with the co-transfection groups of circ-13267 and let-7-19, the number of late apoptotic cells and total apoptotic cells increased significantly (<0.05), while the number of living cells decreased significantly (<0.05).【Conclusion】 circ-13267 was expressed in the cytoplasm and nucleus of egg duck follicular granulosa cells.circ-13267 could sponge let-7-19 and target ERBB4 gene, which promoted the apoptosis of egg duck follicular granulosa cells.This results provided a theoretical basis for analysis of the regulatory mechanism of egg duck follicular development.
circRNA; miRNA; egg duck; follicular development; granulosa cells
2021-02-08;
2021-11-17
国家自然科学基金(32072709)、湖北省自然科学基金(2020CFB655)、湖北省技术创新专项(2019ABA084)、国家水禽现代农业产业技术体系(CARS-42-47)、湖北省农业科学院领军人才项目(L2018017)、湖北省农业科技创新中心项目(2016-620-000-001-023)、湖北省重点研发计划(2020BBA034)
吴艳,E-mail:wuyanwh@163.com。通信作者皮劲松,E-mail:pijinsong@sina.com
(责任编辑 林鉴非)
