Ionomic Profiling of Rice Genotypes and Identification of Varieties with Elemental Covariation Effects
2022-01-20ZhangChengmingNobuhiroTanakaMariaStefanieDwiyantiMatthewShentonHayatoMaruyamaTakuroShinanoChuQingnanXieJunToshihiroWatanabe
Zhang Chengming, Nobuhiro Tanaka, Maria Stefanie Dwiyanti, Matthew Shenton, Hayato Maruyama, Takuro Shinano, Chu Qingnan, Xie Jun, Toshihiro Watanabe
Research Paper
Ionomic Profiling of Rice Genotypes and Identification of Varieties with Elemental Covariation Effects
Zhang Chengming1, Nobuhiro Tanaka2, Maria Stefanie Dwiyanti1, Matthew Shenton2, Hayato Maruyama1, Takuro Shinano1, Chu Qingnan3, Xie Jun4, Toshihiro Watanabe1
(Research Faculty of Agriculture, Hokkaido University, Sapporo 060-8589, Japan; Institute of Crop Science, National Agriculture and Food Research Organization, Tsukuba 305-8518, Japan; Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo 113-8657, Japan; College of Resources and Environment, Southwest University, Chongqing 400715, China)
Ionomic profiles are primarily influenced by genetic and environmental factors. Identifying ionomic responses to varietal effects is necessary to understand the ionomic variations among species or subspecies and to potentially understand genetic effects on ionomic profiles. We cultivated 120 rice () varieties to seedling stage in identical hydroponic conditions and determined the concentrations of 26 elements (including 3 anions) in the shoots and roots of rice. Although the subspecies effects were limited by the genuspre-framework and its elemental chemical properties, we found significant differences in ionomic variations in most elements among the,andsubspecies. Principal component analysis of the correlations indicated that variations in the root-to-shoot ionomic transport mechanisms were the main causes of ionomic differences among the subspecies. Furthermore, the correlations were primarily associated with the screening of varieties for elemental covariation effects that can facilitate breeding biofortified rice varieties with safe concentrations of otherwise toxic elements. Thesubspecies exhibited the strongest elemental correlations and elemental covariation effects, therefore, they showed greater advantages for biofortification than theandsubspecies, whereasandsubspecies were likely safer in metal(loid) polluted soils. We also found that geographical and historical distribution significantly defined the ionomic profiles. Overall, the results of this study provided a reference for further association studies to improve the nutritional status and minimize toxicity risks in rice production.
ionomic profile; rice genotype; elemental covariation; correlation; principal component analysis
At least 25 elements are required for human health, however, it is unfortunate that over 4 billion people suffer from the public health problem of ‘hidden hunger’ due to insufficient intake of essential elements from their daily diets, especially in developing countries (White and Brown, 2010). The biofortification of staple foods has been accepted as a practical and cost-efficient way to alleviate malnutrition by increasing the concentrations of essential nutrients in crops and ultimately in humans (White and Broadley, 2009; Zhang et al, 2018). Paddy rice (), one of the most widely planted staple crops and the source of 80% of the daily caloric and micronutrients for over half of the world population, is the most suitable candidate for crop biofortification strategies (Khush, 2005; Muthayya et al, 2014). Compared with medical supplementation and dietary diversification, it is easier to directly benefit the malnourished conditions of people living in poor rural regions via agronomic or genetically biofortified rice (Zhang et al, 2018; Qiao et al, 2019). Rice is adapted to diverse local edaphic and climatic conditions, resulting in the development of thousands of varieties and genotypes by selective breeding (Singh et al, 2017), however, yield and environmental adaptation are likely the most important factors for varietal breeding to enrich micronutrient content (Tan et al, 2020). For example, superfluous soil iron (Fe) is one of the most significant conditions decreasing rice production in Southeast Asia and South America (Becker and Asch, 2005). Farmers likely prefer rice with a lower Fe uptake to ensure optimal yield. Therefore, it is important to screen for the elemental concentration status of rice varieties.
Moreover, plants take up and translocate nonessential elements through sharing the same pathways as essential elements due to similar chemical properties. For instance, zinc (Zn) and cadmium (Cd) share the same zinc-iron transport protein (ZIP) influx transporter into plant roots (Spielmann et al, 2020); and plant roots can absorb arsenic (As) and selenium (Se) via the phosphate transporter (Zhang et al, 2014; Cao et al, 2017). Nonessential elements in irrigation water can accumulate in the soil and be absorbed by plants (Li et al, 2014; El Azhari et al, 2017). These nonessential elements can be enriched through the food chain from crops to humans, and the consumption of contaminated crops can pose a significant health risk. Rice is easy to accumulate toxic metal(loids) under flooded conditions due to high affinity (Zhou et al, 2018; Gu et al, 2019). Many previous studies have reported the public concern and health risks of Cd and As (Chen et al, 2018; Suriyagoda et al, 2018), and the radioactive contaminant elements cesium (Cs) and strontium (Sr) in crop grains harvested from contaminated areas, such as Fukushima in Japan and Chernobyl in Ukraine (Balonov et al, 2007; Kubo et al, 2020). Hence, increasing the essential nutrient uptake and reducing nonessential elemental contamination in rice is a pressing issue for human health. Elements with similar chemical properties sharing the same transportation pathways can result in competitive uptake between them.
Ionomics, the study of the elemental profiles of all essential and nonessential elements in organisms, tissues and cells, provides a rapid screening strategy for elemental interactions and varietal identification of plant species using high-throughput element analytic methods, such as inductively coupled plasma-mass spectrometry (ICP-MS) and ICP-atomic emission spectrometry (Salt et al, 2008; Huang and Salt, 2016). Numerous ionomic studies for the rapid identification of elemental interactions among plant varieties have been conducted. Chen et al (2009) performed ICP-MS to quickly characterizemutants with altered ionomic profiles. Chu et al (2015) screened extreme Cs accumulation amongspecies to find the ionomic basis for low Cs varietal breeding. Watanabe et al (2016) used ionomic methods to screen vegetable varieties to determine the characteristics of mineral accumulation and nutritional values. Therefore, it is feasible to screen rice varieties for biofortification and safety by ionomic-based research methods.
Consequently, we cultivated 120 rice varieties at the early seedling stage in identical hydroponic systems to simulate the plants’ sensitivity to the external environment. The concentrations of nutrients, trace elements and anions of the rice species were analyzed by ICP-MS and capillary electrophoresis (CE) to screen for rice varieties with high concentrations of nutrients or low concentrations of toxic minerals or both. The ionomic interactions among genotypes and the ionomic variations corresponding to phylogenetic relationships were also characterized.
RESULTS
Comparison of elemental concentrations among rice subspecies
The concentrations of 26 elements (including 3 anions) in the rice shoots and roots were displayed in boxplots (Fig. 1 for essential elements and Fig. 2 for nonessentialelements). Among,and, significant differences were detected in all the elements except barium (Ba), while the magnitudes of the element concentrations were generally similar. The comparison of element concentrations between shoots and roots showed the same pattern in each subspecies, i.e., the concentrations of macronutrients in the shoots were higher than those in the roots, whereas the concentrations of microelements [except for manganese (Mn) and boron (B)] and anions in the shoots were significantly lower than those in the roots (Figs. 1 and 2). The concentrations of potassium (K) in shoots and roots, as well as those of sulfur (S) and calcium (Ca) in roots of, were markedly the lowest, and the concentrations of aluminum (Al), As, Cd, cobalt (Co) and chromium (Cr) inshowed the highest among the three subspecies (Figs. 1 and 2).
Variations in concentrations of elements among rice genotypes
The analysis of variance (ANOVA) descriptive statistical analysis was presented in Table 1. Highly significant(< 0.001) or significant (< 0.01) differences among the rice genotypes were observed for most elements except for Fe, nickel (Ni),Cl‒and Al in the shoots, and P, Ca, Zn, B, Cl‒, NO3‒, Al and lithium (Li) in the roots. Correspondingly, to further quantify the linkage between element and rice genotype, variance contribution of the rice varieties to the total variance was calculated as a range from 30.21% for Al to 81.61% for Cd in the shoots and 29.09% for B to 86.03% for magnesium (Mg) in the roots (Table 1). Contributions for element less than 50% were detected in P, Ca, Fe, Zn, B, Ni, Cl‒, NO3‒, Al, Cr and Li, in which P, Ca and B were only in the roots. Among the elements with a rice variety contribution greater than 50%, only Sr and Cd were mainly contributed by rice variety in the shoots than that in the roots.
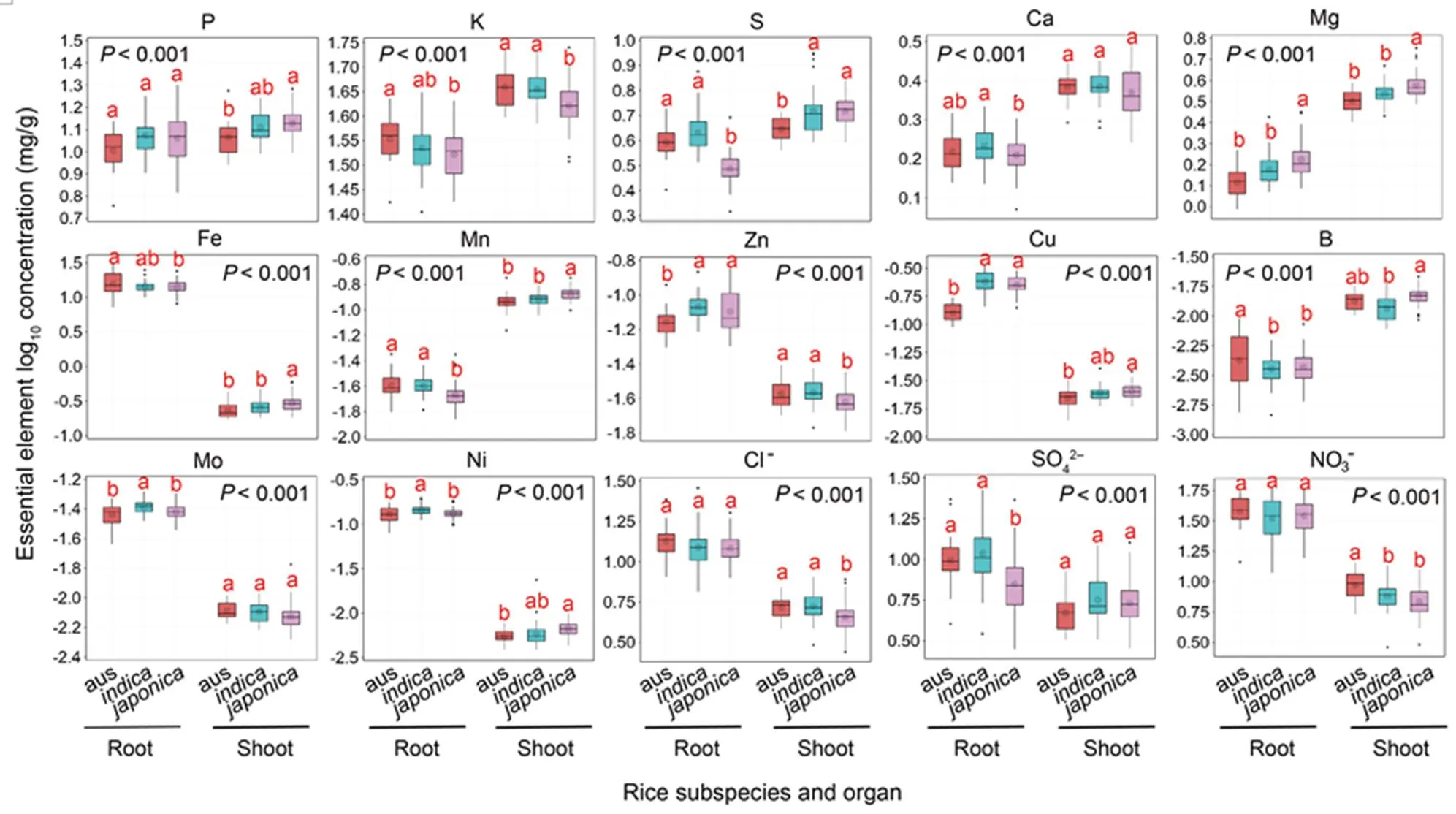
Fig. 1. Boxplot showing log10concentrations of 15 essential elements (P, K, S, Ca, Mg, Fe, Mn, Zn, Cu, B, Mo, Ni, Cl‒, SO42‒and NO3‒) in rice shoots and roots.
Analysis of variance with the Tukey method was performed. Different lowercase letters above the bars represent significant differences at the 0.05 probability level between subspecies and< 0.001 means a significant difference between organs.
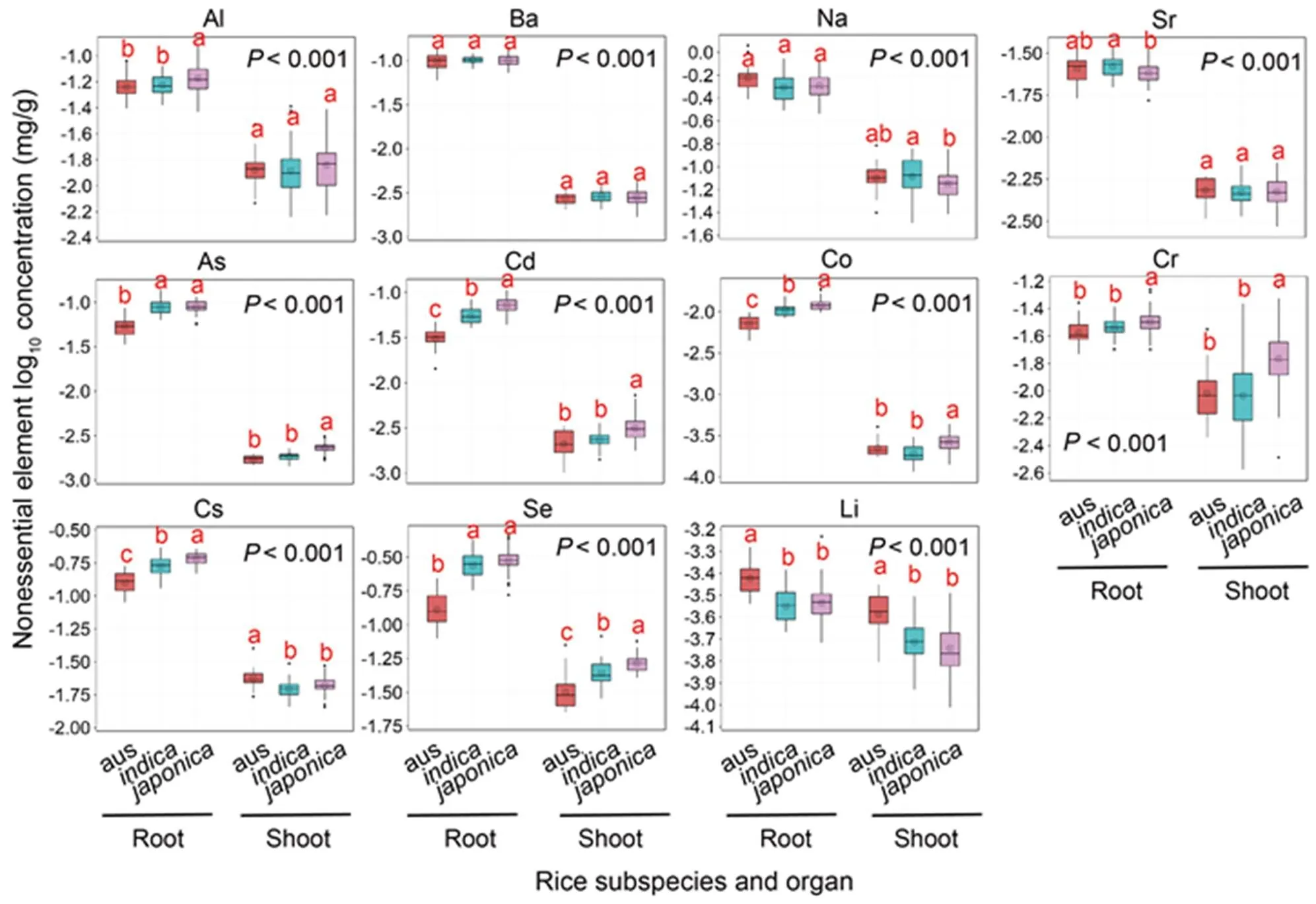
Fig. 2. Boxplot showing mineral log10concentrations of 11 nonessential elements (Al, Na, Ba, Sr, As, Cd, Co, Cr, Cs, Se and Li) in rice shoots and roots.
Analysis of variance with the Tukey method was performed. Different lowercase letters above the bars represent significant differences at the 0.05 probability level between subspecies and< 0.001 means a significant difference between organs.
The coefficient of variation (CV), which reflected the ionomic variations in the subspecies, ranged from 0.07 to 0.68 in the shoots and 0.10 to 0.47 in the roots (Table 1). A similar CV trend was observed between essential and nonessential elements. The CV patterns were significantly different between the shoots and roots and similar among the,andsubspecies. For the macronutrients, the CVs in all the subspecies were less than 20% except for P in the roots. In addition, the CVs of most nonessential elements, such as As, Cd, Co, Cs and Se in theandroots varied more widely than in.
Ionomic interactions among rice genotypes
Pearson’s correlation analysis and visualization were performed to determine the interactions between the pairwise individual elements in the shoots and roots, respectively, of,andsubspecies (Fig. 3). Meanwhile, a principal component analysis (PCA) using correlation coefficients conducted to compare the correlations among subspecies, indicated a 34.0% of the variance was partitioned in the first PCA axis, and root and shoot subgroup samples were separated on that axis (Fig. S1).Root samples also showed a greater degree of separation on the 2nd PCA axis, indicating that the correlations among subspecies showed a differentiation between the shoots and roots. In addition, the PCA correlation scores in the roots among the subspecies were located in the same first quadrant, while the location in the shoots, showing a highly significant difference among subspecies, was in a different quadrant.
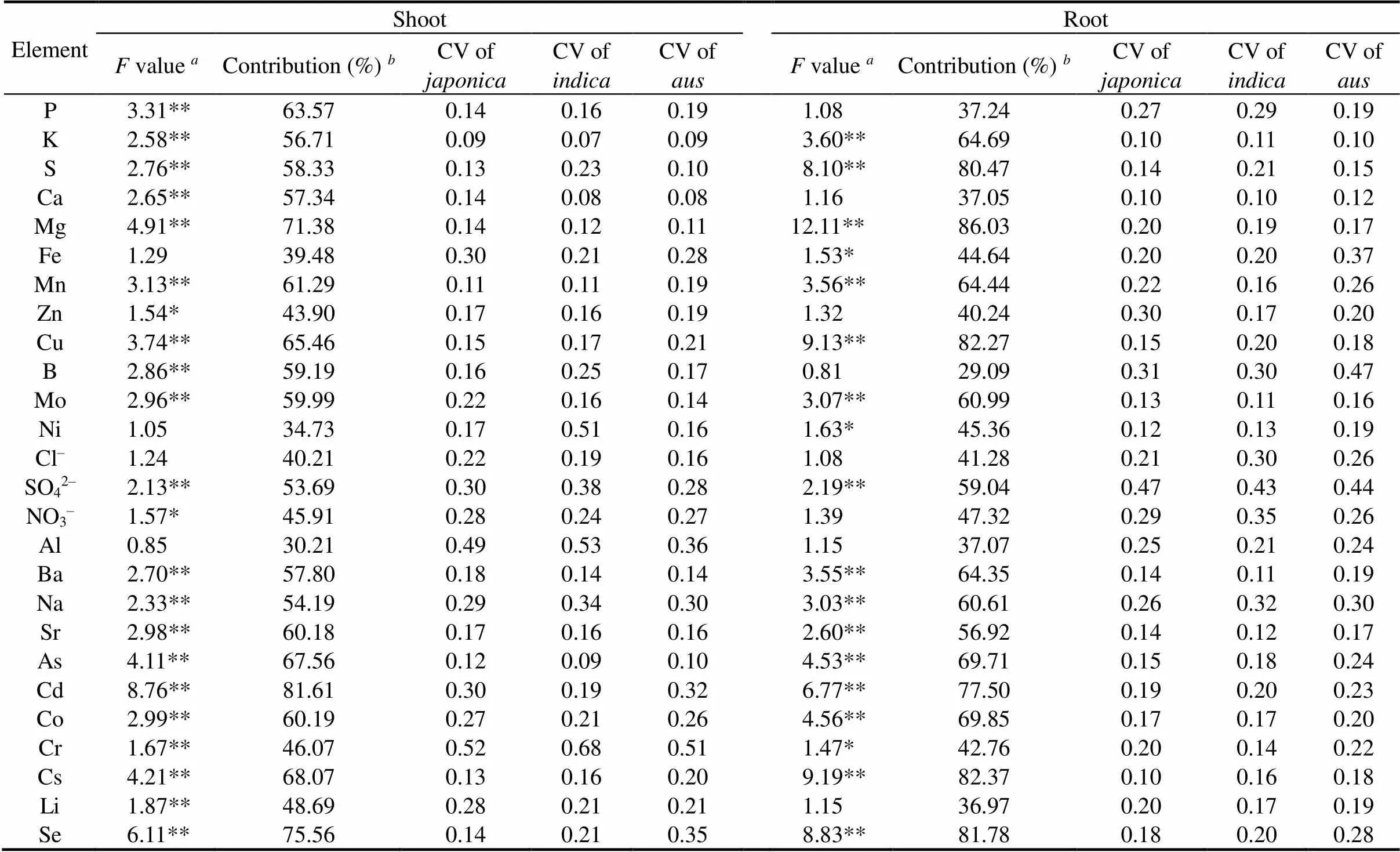
Table 1. Variations in elemental concentrations in shoots and roots among 120 rice varieties.
Analysis of variance with the Tukey test was conducted forvalues of mineral element concentrations attributable to rice varieties (= 360). * and ** represent< 0.01 and< 0.001, respectively.bContribution is the partition of variance for the rice variety difference (percentage of variance between variety to total variance). Due to extremely small contribution of subspecies, data are not displayed. CV, Coefficient of variation.
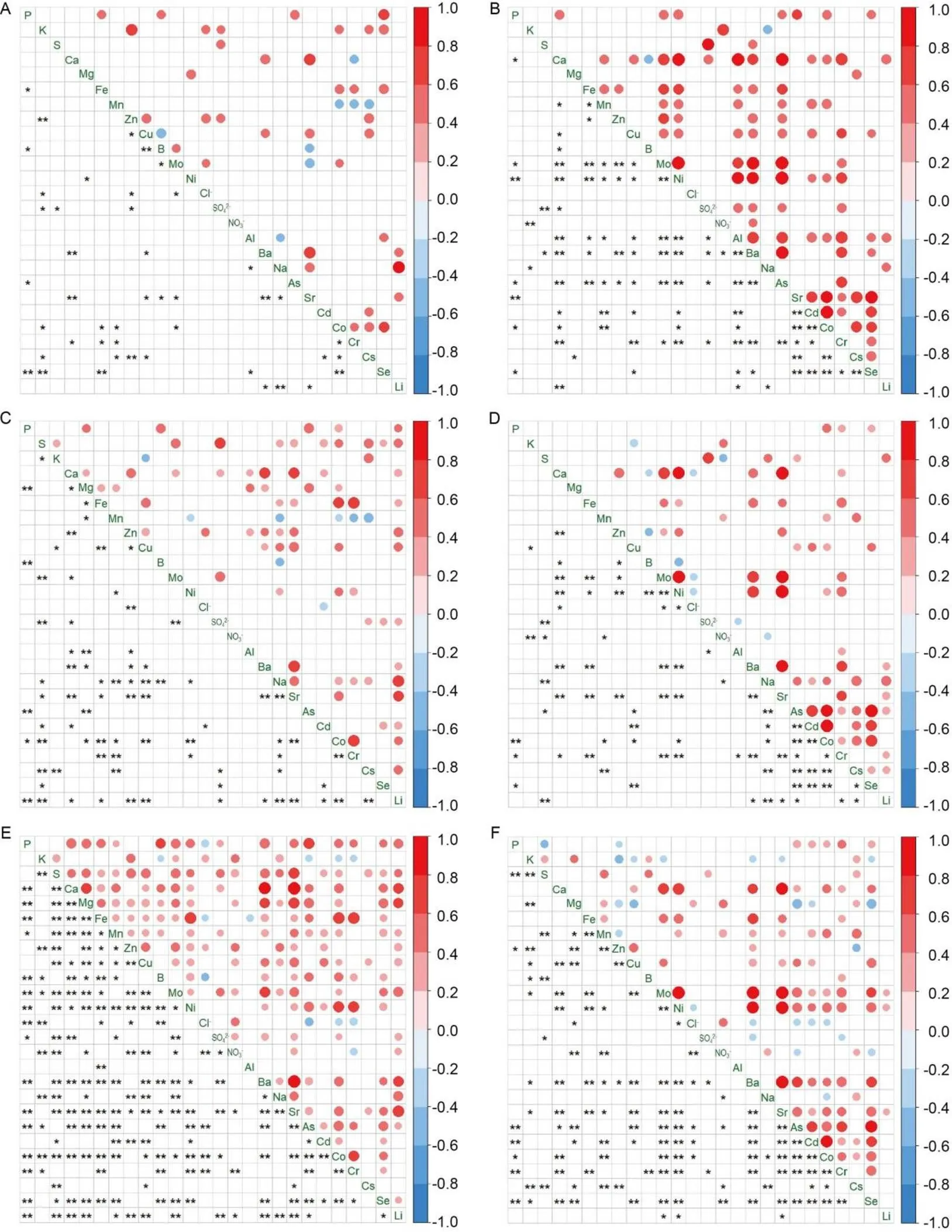
Fig. 3. Correlation heatmaps forshoots (A),roots (B),shoots (C),roots (D),shoots (E) androots (F). Only significant (< 0.05) correlations are displayed in the Pearson’s correlation analysis. The circle in upper triangle matrix represents significant correlation. A larger circle indicates a stronger correlation between the row and the column variables. The red circle indicates a positive correlation coefficient, and the blue indicates a negative one. * and ** in lower triangle matrix represent< 0.05 and< 0.01, respectively.
Consistent to PCA, the elemental correlation patterns of roots were similar among subspecies, while that of shoots were largely different which the strongest correlation pattern was in, and the less was in(Fig. 3). K and Mg correlated with only a few elements inand, and P and Al only correlated with more elements in the shoots ofand roots of, respectively. In contrast, Ca, Cu, Zn and Sr were detected significantly related with many elements in all plots. Mg in the roots ofcorrelated significantly negatively with As, but positive relations showed in the shoots ofand. The same situations were also detected in Mn and Co, Mn and Cs, Zn and Cs. Although different rice subspecies and organs showed different elemental interactions, many still remained the same. Obviously, correlations between S and SO42‒were always significantly positive. Ca interacted significantlyand positively with Ba and Sr, and Ba also significantly and positively correlated to Sr in all rice organs.
Ionomic differences among rice genotypes from different origins
To identify the ionomic differences between organs of the subspecies and to observe the geographic factors, we compared the element concentrations in all samples (Fig. 4-A), shoots (Fig. 4-B) and roots (Fig. 4-C) using PCA, and displayed the loading plots. As shown in Fig. 4-A, there was a significant separation between shoots and roots in all the rice genotypes. Meanwhile, all the macronutrients along with Mn and B loaded on the negative-axis to explain the shoot ionomes, while the root ionomes were mainly determined by the microelements and anions. The PCA results in Fig. 4-B and -C showed that the clusters were largely determined by the different origins of the varieties, whereas the rice subspecies from the same origins were not significantly separated in shoots or roots. For example, theshoots from Japan were separated fromshoots from South Asia and Southeast Asia, but not from theshoots from Japan in the PCA results (Fig. 4-B). According to the loading plots, the differences in shoots and roots from Japan were mainly explained by most nonessential and toxic elements such as As and Cd, whereas K, Na, Li and anions mainly contributed to differences in rice varieties from South Asia.
Identification of multielement accumulation in rice varieties
To identify the potential candidate varieties with accumulation effects of high or low multielement, the rice varieties with more than two elements in the top or bottom 5% concentrations in the shoots were displayed in Table 2. Most varieties, including JRC06 (Kaneko B), JRC12 (Oiran), JRC13 (Bouzu Mochi), WRC47 (Jaguary), WRC48 (Khau Mac Kho), WRC49 (Padi Perak), WRC50 (Rexmont), WRC51 (Urasan 1), WRC67 (Phulba) and WRC68 (Khao Nam Jen), of thesubspecies and varieties WRC26 (Jhona 2) and WRC30 (Anjana Dhan) inshowed high accumulations of multi-metallic elements, including Ca, Mg, Fe, Mn, Zn, Cu and molybdenum (Mo). However, JRC06 (Kaneko B) accumulated the highest concentrations of Mn and B and the nonessential elements Cd, Co, As, Se and Cr, but lower concentration of K, possibly due to the negative correlations of K with B, As, Co and Cr inshoots (Fig. 3). Varieties JRC12 (Oiran) and WRC67 (Phulba) accumulated the highest concentrations of essential elements, including Ca, Mn and Mg, but not toxic metals, such as Cd, As, Cr and Al, indicating that the accumulation of essential elements is antagonistic to the uptake of nonessential toxic elements in these varieties. Moreover, as an element beneficial to human health in the prevention of cancer, Se was also accumulated in the variety JRC12 (Oiran). In thesubspecies, varieties WRC26 (Jhona 2) and WRC30 (Anjana Dhan) showed higher K and Cs (Table 2), and a positive correlation between K and Cs(Fig. 3). Interestingly, thevariety WRC11 (Jinguoyin) showed high Zn and low Cd, and thevariety WRC26 (Jhona 2) showed high Zn and low Cr, indicating a potential for high nutrient and low toxic element rice breeding.
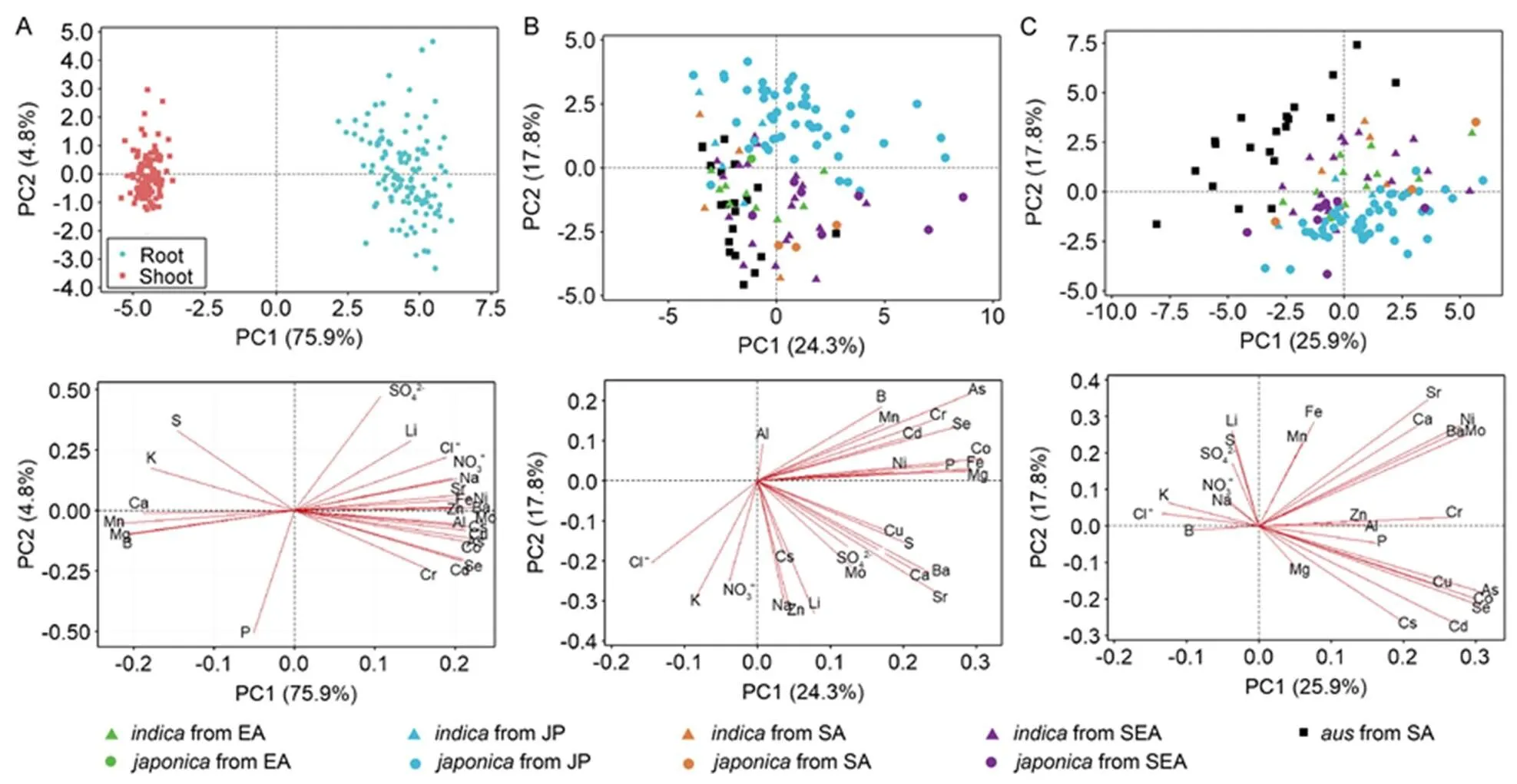
Fig. 4. Combination of principal component analysis (PCA) score plots and loading plots for all samples (A), shoots (B) and roots (C).
EA, JP, SA and SEA represent East Asia (except Japan), Japan, South Asia and Southeast Asia, respectively.
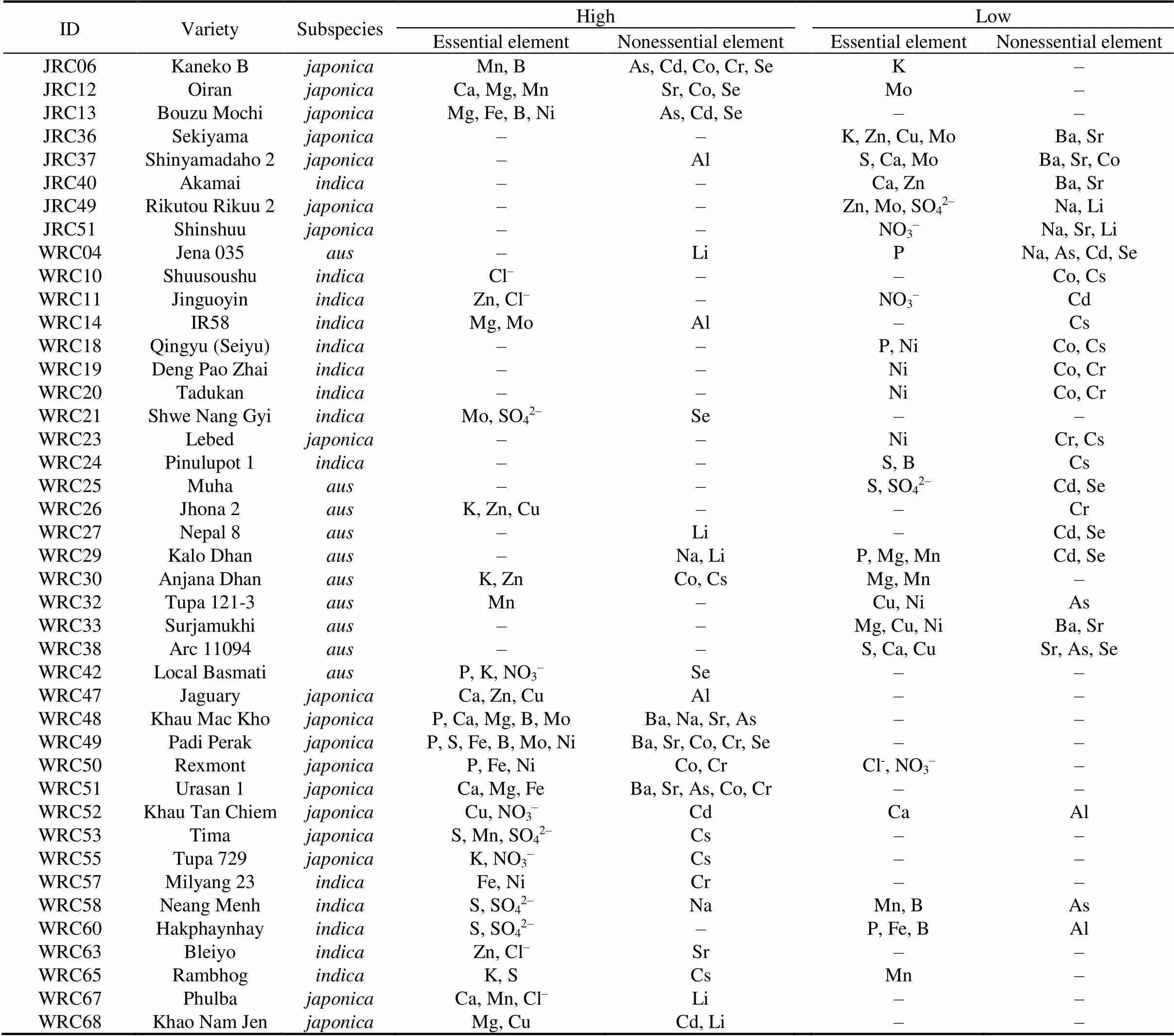
Table 2. Highest and lowest multi-element accumulation varieties for elements in shoots.
The highest or lowest were defined by 5% of each element concentrations in the present study, and only the varieties with multielement highest or lowest accumulated effects were displayed. ‘’ indicates no elemental accumulation effect detected.
DISCUSSION
There were significant differences in the concentrations of all elements except Ba among,and, but the magnitudes of element concentrations generally displayed similar, indicating that the ionomic variations were within the pre-framework of the phylogenetic factors of the genus, although the genomic differences among subspecies have been widely confirmed (Civáň et al, 2015; Stein et al, 2018; Tanaka et al, 2020a, b).As shown in Figs. 1 and 2, all rice subspecies follow the rule that trace elements and anions were concentrated higher in the roots than in the shoots, and it was confirmed by the separation between shoots and roots in all the rice varieties, as well as the loading of microelements on roots in PCA (Fig. 4-A). Rice root is the main barrier to limit translocations of heavy metal(loids) and toxic element to shoots by chelation and compartmentalization (Xu et al, 2017). However, micronutrients for the goal of biofortification, such as Fe and Zn, are also indiscriminately fixed in roots (Kabir et al, 2016). Interestingly, opposite to S, the SO42‒concentration in the roots was higher than that in the shoots, primarily due to most of the inorganic S being fixed in root vacuoles or converted to organic S (cysteine) in leaf for protein synthesis (Rennenberg, 1984). Thus, it would be a meaningful challenge to identify absorptionand translocation mechanisms for specific microelements in roots. As a response to the element concentrations, dry biomass weights among subspecies were showed in boxplot (Fig. S2). The concentrations of essential elements in the shoots or roots ofwere markedly the lowest, while that of harmful elements inshowed the highest (Figs. 1 and 2). Correspondingly, the biomass weights of both shoots and roots inwere the lowest among subspecies. The results showed that improving nutrients and reducing toxic elements also showed a crucial correlation on improvement of the biomass and yield of rice.
Significant differences among diverse rice genotypes detected in most elements in both shoots and roots showed that the phylogenetically-changed elements were more marked in the shoots than in the roots, and further indicated that the variations in elements among the rice varieties were mainly attributed to their translocation from roots to shoots, consistent with previous studies (Yang et al, 2018; Chen et al, 2019). However, Al in the shoots exhibited no significant difference among rice varieties and the lowest contribution of varietal effect, likely due to the low phytoavailability of Al colloidal precipitations in the slightly acidic hydroponic system, indicated that environmental factors also showed a marked influence on the rice ionomes. In contrast, the variance contributions of varietal effect to ionomic variations in Mg, Mn and Cu were even more than 60% in both shoots and roots (Table 1). With large contributions of genotype effects in the shoots and roots, Mg, Mn and Cu were robust to environmental perturbations (White et al, 2012). In the present study, variations of Cr concentration in the shoots among all the subspecies were the largest, which was consistent with a previous study (Chu et al, 2015). Although the transport of Cr has been demonstrated to be associated with S transporters (Schiavon et al, 2008), such a significant difference among the rice genotypes indicated a complex underlying transport mechanism. Compared with S, the CV of SO42‒was higher in all the subspecies, possibly related to the different organic S conversion rates among the varieties. The CVs of macronutrients excluding P in the roots in all rice subspecies were less than that of trace elements and heavy metals, indicating that the variations in macroelements were stable among the rice varieties. Meanwhile, the lower CVs of nonessential elements indemonstrating that microelement and heavy metal uptake in the roots showed greater genetic diversity inandthan in, which was consistent with previous reports thatsubspecies exhibits less genetic and transcriptional diversity thanand(Huang et al, 2010; Campbell et al, 2020). The ionomic variation in rice varieties was mainly dependent on specific chemical element properties and genotype effects, as well as limitedly on subspecies.
One of the most important values of ionomics is in determining the interactions between elements (Baxter and Dilkes, 2012; Feng et al, 2017). The occurrence of antagonism and synergism between elements on uptake and translocation in plants has been reported in many studies (Watanabe et al, 2014, 2016; Chu et al, 2015; El Mehdawi et al, 2018; Affholder et al, 2019). Correlation analysis showed that the rice subspecies and organs exhibited diverse strategies in establishing connections between elements, but many interactions were similar (Fig. 3). The significant correlations between S and SO42‒in all plots provided evidence that the correlation results in this study were reliable, although positive correlations might not always infer the same pathway (Baxter et al, 2008; Du et al, 2020). Ca was always significantly and positively correlated with Ba and Sr, and there was also positive correlation between Ba and Sr (Fig. 3), indicating a significant positive correlation among divalent cations, possibly explained by their sharing the non-selective cation channel transporter-protein superfamilies, such as ZIP, heavy metal ATPases and yellow stripe-like in the xylem due to their similar chemical properties and identical ionic valences (Ozaki et al, 2005; Curie et al, 2009; Pinto and Ferreira, 2015). Significant positive correlations between P-As, P-Se and As-Se were detected in both shoots and roots among the subspecies. It has been reported that P and Se share the phosphate transportergene (Zhang et al, 2014). Moreover, Cao et al (2017) reported reduced As uptake in rice via a P transporter,gene knockout. In general, P application can activate the expression ofandgenes to improve As and Se uptakes in rice. These elemental interactions can provide a strategy to screen multielement accumulation rice genotypes to breed rice varieties with abundant nutrients and that are safe to consume. Moreover, combined with the results of PCA on correlation coefficient, it was clear that the significantly positive correlations between minerals intheshoots ofwere more obviously than those inand, while the patterns of ionomic correlations in the roots among the subspecies were similar. The elemental correlations in rice shoots mainly derive from ionomic transport, whereas in roots, it is due to element uptake, confirming that the ionomic differenceswere primarily caused by different transport mechanisms among the subspecies, consistent with previous studies (Yang et al, 2018; Chen et al, 2019).
In rice domestication and breeding history, farmers have preferred to plant rice varieties adapted to the local agro-climatic conditions, with higher yield and better taste, usually not considering the microelement content (Tan et al, 2020), therefore the separation of elements in PCA mainly determined by different origins rather than subspecies (Fig. 4-B and -C). This finding further indicated that the genetic differences involving in ionomes in subspecies can vary with environmental changes. For example, the elemental differences in rice varieties from Japan mainly explained by most nonessential and toxic elements such as As and Cd, that can be related to the history of wide-ranging incidence of agricultural soil contamination in Japan (Arao et al, 2010). Therefore, considering the geographical and historical distributions of rice varieties associated with the subspecies effects might be informative. Thus, due to the large genetic variation in different rice vareties, it is worthwhile to screen for rice varieties with higher nutrient concentrations, lower levels of toxic elements and healthier food valuesfor use in biofortification strategies.The transportation of elements by the root-to-shoot process has been considered a rate-limitation factor in the shoot-to- grain system (Palmgren et al, 2008). Consequently, identification of element accumulation in shoots is positively correlated with that in grains and determines the distribution of elements in grains. Additionally, owing to the numerous correlations between elements, the elemental covariation effects in shoots should be identified to determine the nutritional values and safety of rice varieties.
In the present study, we identified many varieties accumulated a high concentration of multi-metallic elements (Table 2). Higher concentrations of essential metal or metalloid elements in rice are important in biofortification to promote the synthesis of the coenzymes or proteins required for human health (Clemens, 2014). Meanwhile, consistenting with the results of ionomic correlations in the shoots (Fig. 3), multi-metal accumulation is likely involved in the non-selective metal cation channel transporter-protein superfamilies (Curie et al, 2009; Pinto and Ferreira, 2015). However, nonessential and toxic metals can be also indiscriminately accumulated in these varieties due to similar chemical properties, and potentially resulting in health risks to humans. JRC06 (Kaneko B) accumulated high concentrations of B, As, Co and Cr, while a lower concentration of K (Table 2). As a macronutrient, K plays a highly significant role in alleviating abiotic stress in plants (Amtmann et al, 2008; Römheld and Kirkby, 2010), explaining why with low K concentration, plants’ resistance to heavy metal uptake is likely suppressed, leading to a higher concentration of heavy metals (Wu et al, 2020). Both high K and Cs concentrations in the shoots of some rice varieties can be explained by the positive correlation between K and Cs (Fig. 3), but it seemed contrary to previous studies. Ishikawa et al (2018) reported that the Cs concentration in rice is reduced by applying K, and Rai et al (2017) found that the expression of K transporter OsHAK1 in rice roots is the main route for Cs to accumulate in rice plants under a low K status. The plants in this study with a higher K concentration likely had a stronger affinity for Cs due to their similar chemical properties. However, the multielement accumulation in almost all theandsubspecies was lower than that in thesubspecies (Table 2), indicating that the elemental covariations in the shoots showed subspecies differences and that the correlation of elements was stronger inthan inand, which was consistent with the results shown in Fig. 3. Thevariety WRC11 (Jinguoyin) showed high Zn and low Cd concentrations, while thevariety WRC26 (Jhona 2) showed high Zn and low Cr concentrations. It has been reported that the OsHMA2 transporter in rice is associated with the co-transportation of Zn and Cd from roots to shoots, and that Zn competes with Cd by sharing the same ZIP transporter (Takahashi et al, 2012).Understanding and screening for rice varieties with significant correlations and potential for high nutritional value and safety are essential for rice breeding.
In conclusion, this study revealed the ionomic responses to the genetic effects of rice varieties and subspecies under a strictly controlled environment. The ionomic differences among the subspecies were within the pre-framework of the genus, i.e., the concentrations of macronutrients were greater than micronutrients, and the micronutrients and anions were mainly restricted to the roots. The variations in the rice ionomes primarily depended on genetic factors and specific element chemical properties, and to a lesser extent, on subspecies factors and the geographical and historical distributions of the varieties. Moreover, the rice varieties screened for higher nutrient concentrations and beneficial elements in rice showed a higher value in variety breeding for human health. The potential health risks can be reduced by growing the varieties screened for low heavy metal accumulation even on heavy metal- contaminated soils. Therefore, the identification of rice varieties for biofortification and safe breeding should be closely related to the local edaphic conditions. Furthermore, the ionomic datasets of the rice genotypes in this study can provide a theoretical basis for transcriptional expression analyses of element- related proteins and transporters.
METHODS
Rice materials and hydroponic system
One hundred and twenty rice genotypes, including,andsubspecies, were collected from the World Rice Core Collection (WRC) and the Rice Core Collection of Japanese Landraces (JRC) at the National Agriculture and Food Research Organization GenBank, Ibaraki, Japan (Table S1). Each seed was surface sterilized for 10 min with sodium hypochlorite (NaClO, 0.5%–1.0% available chlorine), germinated on a nylon screen floated on 8 L half-strength modified nutrient solution in a polypropylene container with aeration for 7 d, and then transplanted to a full-strength nutrient solution for an additional 7 d. The modified complete nutrient solution was composed of following elemental compounds: 2.14 mmol/L N (NH4NO3), 0.32 mmol/L P (NaH2PO4·4H2O), 0.77 mmol/L K (K2SO4: KCl = 1 : 1), 1.2 mmol/L Ca (CaCl2·2H2O), 0.82 mmol/L Mg (MgSO4·7H2O), 35 μmol/L Fe (Fe-EDTA), 9.1 μmol/L Mn (MnSO4·5H2O), 45 μmol/L B (H3BO4), 1 μmol/L Zn (ZnSO4·7H2O), 0.3 μmol/L Cu (CuSO4·5H2O), 0.5 μmol/L Mo [(NH4)6Mo7O24·4H2O] and 0.2 μmol/L Co (CoCl2·6H2O). The solution’s pH was adjusted to 5.5 ± 0.5 using 1 mmol/L HCl or 1 mmol/L NaOH. The experiment was conducted in a greenhouse at the Hokkaido University, Sapporo, Japan, under a 14 h light / 10 h dark photoperiod and day/night temperatures of 25–28 ºC / 18–22 ºC, respectively, with 3 repetitions.
After 14 d, the half-strength modified solution in the hydroponic system was replaced by the full-strength complete nutrient solution plus several beneficial and nonessential elements, including: 1 μmol/L Al (AlCl3), 0.5 μmol/L Cr (CrCl2·6H2O), 2 μmol/L Cs (CsCl), 2 μmol/L Sr (SrCl2·6H2O), 1 μmol/L Ba (BaCl2·2H2O), 1 μmol/L Se (Na2SeO3), 1 μmol/L As (NaAsO2), 0.2 μmol/L Cd (CdCl2), 1 μmol/L Ni (NiCl2) and 1 μmol/L Li (LiCl). In a pre-experiment, the growth of rice seedlings was verified not to be significantly affected by the nonessential elemental concentrations (Table S2 and Fig. S3). In the first 7 d, the above supplemental nutrient solution was used, and in the last 7 d, it was replaced by full-strength complete nutrient solution. After cultivation, the seedlings were sampled, washed with deionized water, and cut to separate the roots from the shoots. Then, fresh samples were rapidly frozen in liquid nitrogen, lyophilized, weighed and ground for further measurements.
Digestion and element analysis
Approximately 50 mg aliquot of the powdered rice plant sample was added to 2 mL of 61% HNO3(EL grade; Kanto Chemical, Tokyo, Japan) in a test tube and heated at 110 ºC in a DigiPREP MS apparatus (SCP Science, Quebec, Canada) until the powder had almost disappeared. Then, 0.5 mL of H2O2(semiconductor grade; Santoku Chemical, Tokyo, Japan) was added twice and continually heated until the solution became limpid. After cooling, the test tube was filled to 10 mL with 2% HNO3and analyzed by ICP-MS (Elan, DRC-e; PerkinElmer, Waltham, MA, USA) for the following 23 elements: P, K, S, Ca, Mg, Fe, Mn, Zn, Cu, B, Mo, Ni, Al, Ba, Na, Sr, As, Cd, Co, Cr, Cs, Se and Li. The compound multielement standard solution IV (Merck, Tokyo, Japan) was used for ionomic determination.
Anion analysis by CE
Approximately 10 mg aliquot of the powdered rice plant sample was added to a centrifugal tube with 1.5 mL deionized water, shaken for 30 min, and centrifuged at 12 000 r/min for 10 min. The supernatant was filtered through a 45 μm membrane filter (Advantec, Tokyo, Japan) into a 1.5 mL centrifugal tube and diluted 10× to determine the Cl‒, SO42‒and NO3‒anion concentrations by CE (Quanta 4000 CE; Waters, Milford, MA, USA).
Analysis of statistical data
All the descriptive statistics of ANOVA with Bonferroni test and correlation coefficients were performed using Minitab 19 (Minitab Inc., State College, PA, USA). The correlation coefficients were determined using the Pearson’s correlation analysis, and the correlation visualizations were performed using R (v.3.6.3) with the ‘corrplot’ package.
ACKNOWLEDGEMENTS
This study was partly financially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant No. 20K05762) and China Scholarship Council (Grant No. 201806990031).The authors acknowledge the National Agriculture and Food Research Organization GenBank in the national germplasm collection in Tsukuba, Japan for providing the WRC and JRC seed materials.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Score plot of principal component analysis (PCA) using correlation coefficients.
Fig. S2. Boxplot of dry biomass of rice genotypes.
Fig. S3. Biomasses of pre-experiment for effects of different concentrations of nonessential elements on rice seedling growth.
Table S1. Rice varieties used.
Table S2. Treatments of concentrations of nonessential elements for pre-experiment.
Affholder M C, Flöhr A, Kirchmann H. 2019. Can Cd content in crops be controlled by Se fertilization? A meta-analysis and outline of Cd sequestration mechanisms., 440(1/2): 369‒380.
Amtmann A, Troufflard S, Armengaud P. 2008. The effect of potassium nutrition on pest and disease resistance in plants., 133(4): 682‒691.
Arao T, Ishikawa S, Murakami M, Abe K, Maejima Y, Makino T. 2010. Heavy metal contamination of agricultural soil and countermeasures in Japan., 8(3): 247‒257.
Balonov M I, Bruk G Y, Golikov V Y, Barkovsky A N, Kravtsova E M, Kravtosova O S, Mubasarov A A, Shutov V N, Travnikova I G, Howard B J, Brown J E, Strand P. 2007. Assessment of current exposure of the population living in the Techa River Basin from radioactive releases of the Mayak facility., 92(2): 134‒147.
Baxter I, Dilkes B P. 2012. Elemental profiles reflect plant adaptations to the environment., 336: 1661‒1663.
Baxter I R, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Guerinot M L, Salt D E. 2008. The leaf ionome as a multivariable system to detect a plant’s physiological status., 105(33): 12081‒12086.
Becker M, Asch F. 2005. Iron toxicity in rice: Conditions and management concepts., 168(4): 558‒573.
Campbell M T, Du Q, Liu K, Sharma S, Zhang C, Walia H. 2020. Characterization of the transcriptional divergence between the subspecies of cultivated rice ()., 21(1): 394.
Cao Y, Sun D, Ai H, Mei H Y, Liu X, Sun S B, Xu G H, Liu Y G, Chen Y S, Ma L Q. 2017. Knocking outgene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains., 51(21): 12131‒12138.
Chen H P, Tang Z, Wang P, Zhao F J. 2018. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice., 238: 482‒490.
Chen H R, Yang Y, Ye Y F, Tao L Z, Fu X D, Liu B M, Wu Y J. 2019. Differences in cadmium accumulation betweenandrice cultivars in the reproductive stage., 186: 109795.
Chen Z, Watanabe T, Shinano T, Okazaki K, Osaki M. 2009. Rapid characterization of plant mutants with an altered ion-profile: A case study using., 181: 795‒801.
Chu Q N, Watanabe T, Sha Z M, Osaki M, Shinano T. 2015. Interactions between Cs, Sr, and other nutrients and trace element accumulation inshoot in response to variety effect., 63(8): 2355‒2363.
Civáň P, Craig H, Cox C J, Brown T A. 2015. Three geographically separate domestications of Asian rice., 1: 15164.
Clemens S. 2014. Zn and Fe biofortification: The right chemical environment for human bioavailability., 225: 52‒57.
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. 2009. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters., 103(1): 1‒11.
Du F, Liu P, Wang K, Yang Z G, Wang L. 2020. Ionomic responses of rice plants to the stresses of different arsenic species in hydroponics., 243: 125398.
El Azhari A, Rhoujjati A, El Hachimi M L, Ambrosi J. 2017. Pollution and ecological risk assessment of heavy metals in the soil-plant system and the sediment-water column around a former Pb/Zn-mining area in NE Morocco., 144: 464‒474.
El Mehdawi A F, Jiang Y, Guignardi Z S, Esmat A, Pilon M, Pilon-Smits E A H, Schiavon M. 2018. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/ selenate transporters in selenium hyperaccumulator and nonhyperaccumulator., 217(1): 194‒205.
Feng X M, Han L, Chao D Y, Liu Y, Zhang Y J, Wang R G, Guo J K, Feng R W, Xu Y M, Ding Y Z, Huang B Y, Zhang G L. 2017. Ionomic and transcriptomic analysis provides new insight into the distribution and transport of cadmium and arsenic in rice., 331: 246‒256.
Gu J F, Zhou H, Tang H L, Yang W T, Zeng M, Liu Z M, Peng P Q, Liao B H. 2019. Cadmium and arsenic accumulation during the rice growth period underremediation., 171: 451‒459.
Huang X H, Wei X H, Sang T, Zhao Q, Feng Q, Zhao Y, Li C Y, Zhu C R, Lu T T, Zhang Z W, Li M, Fan D L, Guo Y L, Wang A H, Wang L, Deng L W, Li W J, Lu Y Q, Weng Q J, Liu K Y, Huang T, Zhou T Y, Jing Y F, Li W, Lin Z, Buckler E S, Qian Q, Zhang Q F, Li J Y, Han B. 2010. Genome-wide association studies of 14 agronomic traits in rice landraces., 42(11): 961‒967.
Huang X Y, Salt D E. 2016. Plant ionomics: From elemental profiling to environmental adaptation., 9(6): 787‒797.
Ishikawa J, Fujimura S, Kondo M, Murai-Hatano M, Goto A, Shinano T. 2018. Dynamic changes in the Cs distribution throughout rice plants during the ripening period, and effects of the soil-K level., 429(1/2): 503‒518.
Kabir A H, Begum M C, Haque A, Amin R, Swaraz A M, Haider S A, Paul N K, Hossain M M. 2016. Genetic variation in Fe toxicity tolerance is associated with the regulation of translocation and chelation of iron along with antioxidant defence in shoots of rice., 43(11): 1070‒1081.
Khush G S. 2005. What it will take to feed 5.0 billion rice consumers in 2030., 59(1): 1‒6.
Kubo K, Kobayashi H, Nitta M, Takenaka S, Nasuda S, Fujimura S, Takagi K, Nagata O, Ota T, Shinano T. 2020. Variations in radioactive cesium accumulation in wheat germplasm from fields affected by the 2011 Fukushima nuclear power plant accident., 10: 3744.
Li Z Y, Ma Z W, van der Kuijp T J, Yuan Z W, Huang L. 2014. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment., 468‒469: 843‒853.
Muthayya S, Sugimoto J D, Montgomery S, Maberly G F. 2014. An overview of global rice production, supply, trade, and consumption., 1324(1): 7‒14.
Ozaki T, Ambe S, Abe T, Francis A J. 2005. Competitive inhibition and selectivity enhancement by Ca in the uptake of inorganic elements (Be, Na, Mg, K, Ca, Sc, Mn, Co, Zn, Se, Rb, Sr, Y, Zr, Ce, Pm, Gd, Hf) by carrot (cv. U. S. harumakigosun)., 103(1): 69‒82.
Palmgren M G, Clemens S, Williams L E, Krämer U, Borg S, Schjørring J K, Sanders D. 2008. Zinc biofortification of cereals: Problems and solutions., 13(9): 464‒473.
Pinto E, Ferreira I M P L V O. 2015. Cation transporters/channels in plants: Tools for nutrient biofortification., 179: 64‒82.
Qiao K, Wang F H, Liang S, Wang H, Hu Z L, Chai T Y. 2019. New biofortification tool: Wheat TaCNR5 enhances zinc and manganese tolerance and increases zinc and manganese accumulation in rice grains., 67(35): 9877‒9884.
Rai H, Yokoyama S, Satoh-Nagasawa N, Furukawa J, Nomi T, Ito Y, Fujimura S, Takahashi H, Suzuki R, Yousra E, Goto A, Fuji S, Nakamura S I, Shinano T, Nagasawa N, Wabiko H, Hattori H. 2017. Cesium uptake by rice roots largely depends upon a single gene,, which encodes a potassium transporter., 58(9): 1486‒1493.
Rennenberg H. 1984. The fate of excess sulfur in higher plants., 35(1): 121‒153.
Römheld V, Kirkby E A. 2010. Research on potassium in agriculture: Needs and prospects., 335(1): 155‒180.
Salt D E, Baxter I, Lahner B. 2008. Ionomics and the study of the plant ionome., 59(1): 709‒733.
Schiavon M, Pilon-Smits E A H, Wirtz M, Hell R, Malagoli M. 2008. Interactions between chromium and sulfur metabolism in., 37(4): 1536‒1545.
Singh A, Septiningsih E M, Balyan H S, Singh N K, Rai V. 2017. Genetics, physiological mechanisms and breeding of flood- tolerant rice (L.)., 58(2): 185‒197.
Spielmann J, Ahmadi H, Scheepers M, Weber M, Nitsche S, Carnol M, Bosman B, Kroymann J, Motte P, Clemens S, Hanikenne M. 2020. The two copies of the zinc and cadmium ZIP6 transporter ofhave distinct effects on cadmium tolerance., 43(9): 2143‒2157.
Stein J C, Yu Y, Copetti D, Zwickl D J, Zhang L, Zhang C J, Chougule K, Gao D Y, Iwata A, Goicoechea J L, Wei S, Wang J, Liao Y, Wang M H, Jacquemin J, Becker C, Kudrna D, Zhang J W, Londono C E M, Song X, Lee S, Sanchez P, Zuccolo A, Ammiraju J S S, Talag J, Danowitz A, Rivera L F, Gschwend A R, Noutsos C, Wu C C, Kao S M, Zeng J W, Wei F J, Zhao Q, Feng Q, Baidouri M E, Carpentier M C, Lasserre E, Cooke R, da Rosa Farias D, da Maia L C, dos Santos R S, Nyberg K G, McNally K L, Mauleon R, Alexandrov N, Schmutz J, Flowers D, Fan C Z, Weigel D, Jena K K, Wicker T, Chen M S, Han B, Henry R, Hsing Y I C, Kurata N, de Oliveira A C, Panaud O, Jackson S A, Machado C A, Sanderson M J, Long M Y, Ware D, Wing R A. 2018. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus., 50(2): 285‒296.
Suriyagoda L D B, Dittert K, Lambers H. 2018. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains., 253: 23‒37.
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa N K, Nakanishi H. 2012. Thetransporter is involved in root-to-shoot translocation of Zn and Cd in rice., 35(11): 1948‒1957.
Tan Y J, Sun L, Song Q N, Mao D H, Zhou J Q, Jiang Y R, Wang J R, Fan T, Zhu Q H, Huang D Y, Xiao H, Chen C Y. 2020. Genetic architecture of subspecies divergence in trace mineral accumulation and elemental correlations in the rice grain., 133(2): 529‒545.
Tanaka N, Shenton M, Kawahara Y, Kumagai M, Sakai H, Kanamori H, Yonemaru J, Fukuoka S, Sugimoto K, Ishimoto M, Wu J, Ebana K. 2020a. Whole-genome sequencing of the NARO World Rice Core Collection (WRC) as the basis for diversity and association studies., 61(5): 922‒932.
Tanaka N, Shenton M, Kawahara Y, Kumagai M, Sakai H, Kanamori H, Yonemaru J I, Fukuoka S, Sugimoto K, Ishimoto M, Wu J Z, Ebana K. 2020b. Investigation of the genetic diversity of a core collection of Japanese landraces using whole- genome sequencing., 61(12): 2087‒2096.
Watanabe T, Kouho R, Katayose T, Kitajima N, Sakamoto N, Yamaguchi N, Shinano T, Yurimoto H, Osaki M. 2014. Arsenic alters uptake and distribution of sulphur in., 37(1): 45‒53.
Watanabe T, Maejima E, Yoshimura T, Urayama M, Yamauchi A, Owadano M, Okada R, Osaki M, Kanayama Y, Shinano T. 2016. The ionomic study of vegetable crops., 11(8): e0160273.
White P J, Broadley M R. 2009. Biofortification of crops with seven mineral elements often lacking in human diets: Iron, zinc, copper, calcium, magnesium, selenium and iodine., 182: 49‒84.
White P J, Brown P H. 2010. Plant nutrition for sustainable development and global health., 105(7): 1073‒1080.
White P J, Broadley M R, Thompson J A, McNicol J W, Crawley M J, Poulton P R, Johnston A E. 2012. Testing the distinctness of shoot ionomes of angiosperm families using the Rothamsted Park Grass Continuous Hay Experiment., 196(1): 101‒109.
Wu Q, Zhu X F, Zhao X S, Shen R F. 2020. Potassium affects cadmium resistance inthrough facilitating root cell wall Cd retention in a nitric oxide dependent manner., 178: 104175.
Xu Q, Wang C Q, Li S G, Li B, Li Q Q, Chen G D, Chen W L, WangF. 2017. Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (L.)., 24(12): 11319‒11330.
Yang M, Lu K, Zhao F J, Xie W B, Ramakrishna P, Wang G Y, Du Q Q, Liang L M, Sun C J, Zhao H, Zhang Z Y, Liu Z H, Tian J J, Huang X Y, Wang W S, Dong H X, Hu J T, Ming L C, Xing Y Z, Wang G W, Xiao J H, Salt D E, Lian X M. 2018. Genome-wide association studies reveal the genetic basis of ionomic variation in rice., 30(11): 2720‒2740.
Zhang C M, Zhao W Y, Gao A X, Su T T, Wang Y K, Zhang Y Q, Zhou X B, He X H. 2018. How could agronomic biofortification of rice be an alternative strategy with higher cost-effectiveness for human iron and zinc deficiency in China?, 39(2): 246‒259.
Zhang L H, Hu B, Li W, Che R H, Deng K, Li H, Yu F Y, Ling H Q, Li Y J, Chu C C. 2014. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice., 201(4): 1183‒1191.
Zhou H, Zhu W, Yang W T, Gu J F, Gao Z X, Chen L W, Du W Q, Zhang P, Peng P Q, Liao B H. 2018. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages., 152: 91‒97.
1 January 2021;
13 May2021
Toshihiro Watanabe (nabe@chem.agr.hokudai.ac.jp)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.12.007
(Managing Editor: Wu Yawen)
杂志排行
Rice Science的其它文章
- Rice Drying, Storage and Processing: Effects of Post-Harvest Operations on Grain Quality
- UvWhi2 Is Required for Stress Response and Pathogenicity in Ustilaginoidea virens
- Fine Mapping of QTLs for Stigma Exsertion Rate from Oryza glaberrima by Chromosome Segment Substitution
- Simple Bioassay for PAMP-Triggered Immunity in Rice Seedlings Based on Lateral Root Growth Inhibition
- Cold Plasma: A Potential Alternative for Rice Grain Postharvest Treatment Management in Malaysia
- Diversity of Sodium Transporter HKT1;5 in Genus Oryza
