UvWhi2 Is Required for Stress Response and Pathogenicity in Ustilaginoidea virens
2022-01-20MengShuaiQiuJiehuaXiongMengLiuZhiquanJaneSadhnaJagernathLinFuchengShiHuanbinKouYanjun
Meng Shuai, Qiu Jiehua,, Xiong Meng,, Liu ZhiquanJane Sadhna JagernathLin Fucheng, Shi HuanbinKou Yanjun
Research Paper
Is Required for Stress Response and Pathogenicity in
Meng Shuai1, 2,#, Qiu Jiehua1,#, Xiong Meng1,#, Liu Zhiquan1, Jane Sadhna Jagernath1, Lin Fucheng3, 4, Shi Huanbin1, Kou Yanjun1
(State Key Laboratory of Rice Biology, China National Rice Research Institute, Hangzhou 311400, China; Key Laboratory of Horticultural Plant Biology, Ministry of Education / College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-Products, Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China; State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-Products, Institute of Biotechnology, Zhejiang University, Hangzhou 310058, China; #)
Some stress response-related genes have been identified in, but it is not clear whether and how defects of stress responses affect the pathogenesis processes ofIn this study, we identified a general stress response factor UvWHI2 as a homolog ofWhi2 in. The relative expression level ofwas significantly up-regulated during infection, suggesting that UvWHI2 may be involved in pathogenesis. Furthermore, knockout ofshowed decreased mycelial growth, increased conidiation in the potato sucrose medium and a defect in pathogenicity. In addition, the RNA-Seq and phenotypic analysis showed that UvWHI2was involved in response to oxidative, hyperosmotic, cell wall stress and nutrient limitation. Further studies revealed that the defects of stress responses of the ∆mutant affected the formation of secondary spores on the nutrient limited surface and the rice surface, resulting in a significant reduction of pathogenicity of. Our results suggest that UvWHI2 is necessary for fungal growth, stress responses and the formation of secondary spores in. In addition, the defects of stress responses can affect the formation of secondary spores on the rice surface, and then compromise the pathogenicity of
Whi2;rice false smut;;pathogenesis;secondaryspore
Rice false smut disease is caused by the ascomycete filamentous fungus, which is one of the most devastating rice fungal diseases in the rice-cultivated areas of the world (Fan et al, 2016; Guo et al, 2019; Qiu et al, 2019; Sun et al, 2020). The occurrence of rice false smut seriously affects yield and quality of rice (Lu et al, 2015; Zheng et al, 2017; Lin et al, 2018). During the booting period, the rice spikelets are infected by the, which hinders the nutrition transportation and the normal development of grains, resulting in the increase of empty grain rate and the decrease of 1000-grain weight (Yu et al, 2015; Tang et al, 2020). False smut balls contain various toxins, such as ustilaginoidins and ustiloxins, which can inhibit the assembly of microtubules in eukaryotic cells, disrupt cell mitosis and cause pathological changes in animal organs and plants (Lu et al, 2015; Zheng et al, 2016; Sun et al, 2017; Wang et al, 2017).
Thus far, only a few pathogenesis-related proteins required for development and pathogenicity have been identified and characterized inby forward or reverse genetics, including the hypothetical protein UvPro1 (Lü et al, 2016), the low-affinity iron transporter Uvt3277 (Zheng et al, 2017), the Bax inhibitor UvBI-1 (Xie et al, 2019), the effector Scre2 (Uv_1261) (Fang et al, 2019), core components of signaling pathway such as two protein kinases UvPmk1, phosphodiesterase UvPdeH and adenylate cyclase UvAc1 (Guo et al, 2019; Tang et al, 2020), two transcriptional factors UvHox2 and UvCom1 (Yu et al, 2019; Chen et al, 2020), the phosphataseUvPsr1 (Xiong et al, 2020),and the autophagy-related protein UvAtg8 (Meng et al, 2020).Among these reported proteins, pathogenesis-related proteins UvPmk1, UvCdc2, UvPro1, UvBI-1, UvAc1, UvPdeH, UvPsr1, UvCom1, UvHox2 and UvAtg8are involved in various stress responses in. However,it is not clear whether and how defects ofstress responses affect the pathogenesis processes of
WHI2 (Whiskey2) is a general stress response factor. In yeast, ScWHI2 has been characterized to be involved in general stress response and coordinate nutrient status with cell cycle (Muller and Reichert, 2011; Sadeh et al, 2011; Teng et al, 2018). Deletion ofcauses decreased expression levels of stress-associated genes, increased sensitivity to sodium ions,and disruption of the normal coordination of cell proliferation with nutrient availability (Kaida et al, 2002; Chen et al, 2018; Marsikova et al, 2020). In, theWHI2 homolog controls nutrient sensingand the entrance of cells into the stationary phase and mycelial development (Timpano et al, 2016). In,plays key role in regulation of transition from biotrophic infection to necrotrophic infection via regulation of TOR (target of rapamycin) signaling, which is involved in the coordination of cell growth and proliferation with the availability of growth factors and nutrients (Harata et al, 2016). Until now, the function of Whi2 remains largly unknown in various organisms.
In this study,, the homologous ofin, was disrupted to characterize its function. RNA-Seq, qRT- PCR (quantitative real-time PCR) and phenotypic analyses showed that UvWHI2 was involved in the growth, conidiation, pathogenicity and various stress responsesin. Furthermore, Δshowed defects in the formation of secondary sporesduring germination on thenutrient limited rice surface, which highlights vital roles of UvWHI2 in stress response and pathogenicity in.
Results
Identification of UvWHI2 in U. virens
To identify the homolog of WHI2 in, we used ScWHI2 (accession number NP_014686) sequence as a query to do a BLASTP search in GeneBank to obtain the most closely match protein, which is named as UvWHI2 (accession number KDB15335.1) (Fig. S1-A). Sequence analysis with motif scan revealed that UvWHI2 contained a signal peptide and GSDH (Glucose/Sorbosone dehydrogenase, with a beta-propeller fold) domain. The phylogenetic analysis of the amino acid sequences of WHI2 from,,,,,,andshowed that WHI2 was conserved in various fungi, and UvWHI2 was highly similar to MrWHI2 (Fig. S1-B). In addition, the qRT-PCR analysis showed that the transcription levels ofat 3, 5 and 9 dpi (days post inoculation) were significantly higher than that in mycelia (Fig. S1-C), suggesting that UvWHI2 may have important roles in the infection process of.
Knockout and complementation of UvWhi2 in U. virens
To analyze the function of UvWHI2, theknockout mutants (Δ) were generated by replacing the targeted gene within the wild type (WT) strain HWD-2 (Fig. S2-A). Then, qRT-PCR and Southern blot assays were performed to confirm the targeted gene deletion events and exclude ectopic integrations (Fig. S2-B). Six Δtransformants were obtained with similar phenotypes, and two mutants (Δand Δ) were chosen for the further experiments (Fig. S2-B). To determine whether the altered phenotypes in the Δmutants were caused by deletion of the, the full-length gene copy ofwith its native promoter was inserted into the vector pFGL823 and transformed into the ∆strain. The resultant ∆strains were confirmed by PCR and qRT-PCR analyses, which showed that the abundance oftranscript was comparable to that of the WT strain (Fig. S2-C and -D).
Knockout of UvWhi2 compromised fungal growth but enhanced conidiationunder nutrient-rich condition
To explore the function of UvWHI2in vegetative growth and colony morphology of, the growths of WT, ∆/and ∆strains were determined on the PSA (potato sucrose agar) medium cultured for 14 d. The ∆mutants showed smaller colonies than the WT and complementation strains (Fig. 1-A and -B). The dry weight of mycelium was measured after cultured in the liquid PS (potato sucrose) medium for 7 d to determine whether there was a difference in biomass between the WT and ∆strains. The results showed that mycelial dry weights of the ∆strains were lower than that of the WT strain (Fig. 1-C). In contrast, the colony morphology and mycelium dry weight were rescued in the ∆strain (Fig. 1-B and -C), suggesting that UvWHI2 plays important roles in the vegetative growth in.
The conidia play an important role during the infection processes of. To analysis the function ofin conidiation, the WT, ∆/and ∆strains were cultured in the liquid PS medium for 7 d. Although the vegetative growth of ∆strain was reduced, the conidial production of the ∆(10.67 ± 1.15) and ∆mutants (10.87 ± 0.51) were more than those of the WT (3.60 ± 0.53) and ∆strains (3.70 ± 0.56) (Fig. 1-D and -E). These results showed that UvWHI2 acts as a negative regulator in the sporulation production process in nutrient rich medium.
UvWHI2 is required for pathogenesis in U. virens
To evaluate whether the deletion ofcan affect the virulence of rice false smut, the WT, ∆/and ∆strains were inoculated into panicles of the susceptible rice cultivar Wanxian 98 (L. subsp.). At three weeks after the inoculations, the number of smut balls formed in panicles inoculated with the ∆and ∆strains were significantly less than those of the WT and ∆-strains (Fig. 2). Therefore, knockout ofremarkably attenuatedvirulence, suggesting that UvWHI2 is a key regulator factor of the pathogenicity in.
UvWHI2 is involved in various stress responses
To further explore the function ofin, the RNA-Seq analysis was performed with the ∆and WT strains. RNA-Seq analysis showed that the number of DEGs [differentially expressed genes, FDR (false discovery rate) adjusted≤ 0.05 and absolute log2fold ≥ 2)] was 802, of which 515 were up-regulated and 287 were down-regulated in the ∆mutant when compared with the WT strain (Table S1). Using Gene Ontology (GO) analysis (Fig. S3), some chitin deposition genes and peroxidase activity genes were found to be present in DEGs.
Syntheses of the laccase, peroxidase, chitin synthase and hyperosmotic genes play an important role in stress response (Egan et al, 2007; Song et al, 2010; Li et al, 2016; Zheng et al, 2016). The expression levels of chitin deposition genes (,,and) of ∆strains were significantly lower than those of the WT strain (Fig. S4-A), suggesting that UvWHI2 may be involved in the cell wall stress response. Then, the WT and ∆strains were cultured on the PSA with 0.03% sodium dodecyl sulfate (SDS), 120 μg/mL calcofluor white (CFW) and 120 μg/mL Congo red (CR), which are cell wall disruptors, to determine the sensitivity of indicated strains to these agents. The results showed that the ∆strains were more sensitive to the SDS, CFW and CR than the WT strain (Fig. S4-B and -C). In addition, the expression levels of laccase and peroxidase activity genes (,,,and) were significantly reduced in the ∆strain (Fig. S4-D). Moreover, the growth of both ∆strains was more strongly inhibited by the presence of 0.03% H2O2(oxidative stress reagent) than that of the WT and ∆strains (Fig. S4-B and -C). In contrast, the expression levels of hyperosmotic activity genes (and) were notably increased compared with the WT strain (Fig. S4-E). Consistently, in the presence of 0.4 mol/L NaCl or 0.7 mol/L sorbitol, the growth of the WT strain was reduced by 70%, but that of the ∆mutants was reduced by approximately 35%, indicating ∆was less sensitive to osmotic stress than the WT strain (Fig. S4-B and -C). In conclusion, these results suggested that UvWHI2 contributes to the responses to cell wall, oxidative and osmotic stresses in.
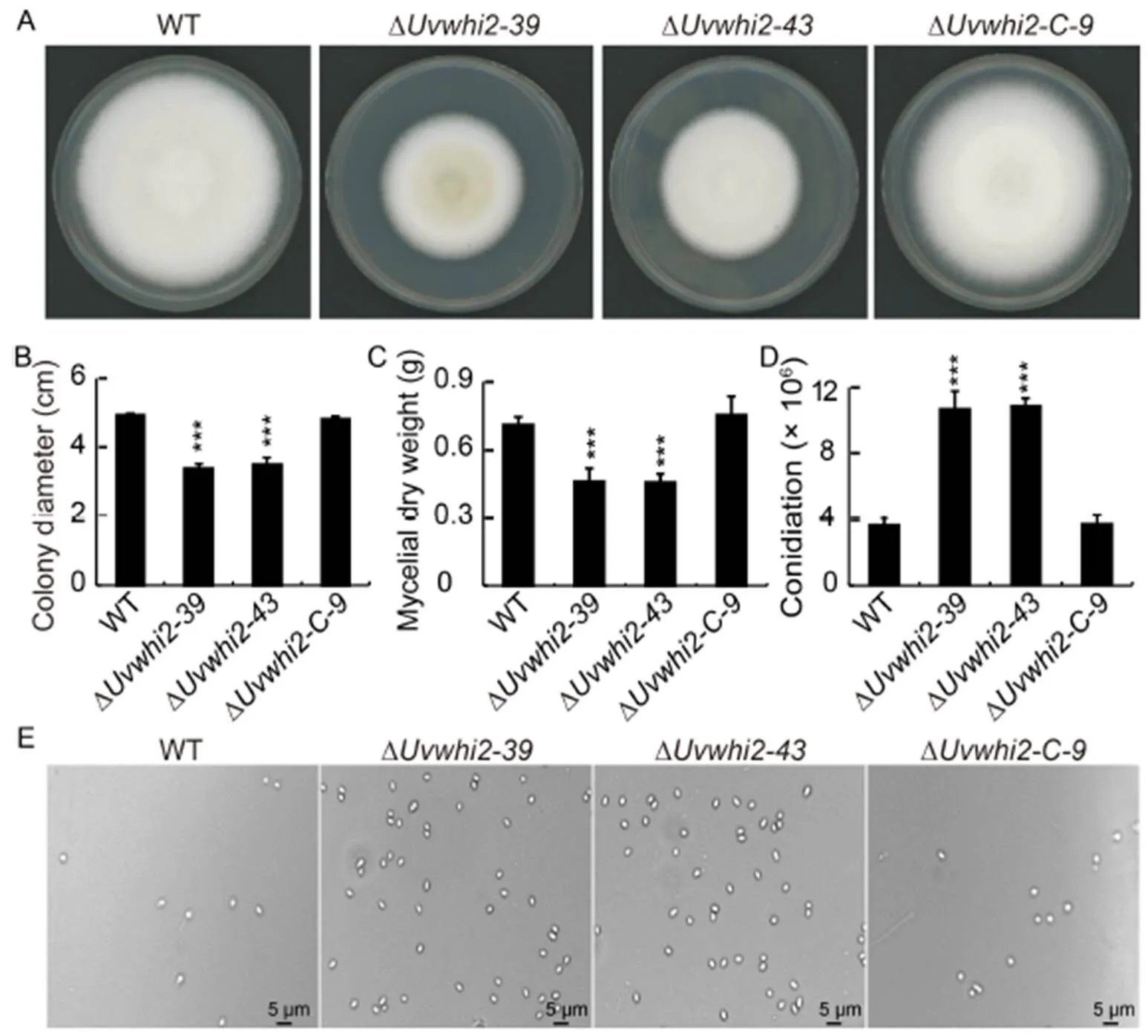
Fig. 1. Deletion ofresults in decreased vegetative growth and increased conidiation in.
A and B, Mycelium growth (A) and colony morphology (B) of the wild type (WT),gene deletionmutants(∆and ∆) and complementation strain (∆) on the potato sucrose agar medium in dark at 28 ºC for 15 d.
C, Dry weight of mycelia was measured in the liquid potato sucrose medium after 7 d culture.
Dand E, Knockout ofenhanced conidiation under nutrient-rich condition.
Data in B–D areMean ± SD (= 3). ***, Significant difference at the 0.001 levelby the Duncan’s test.
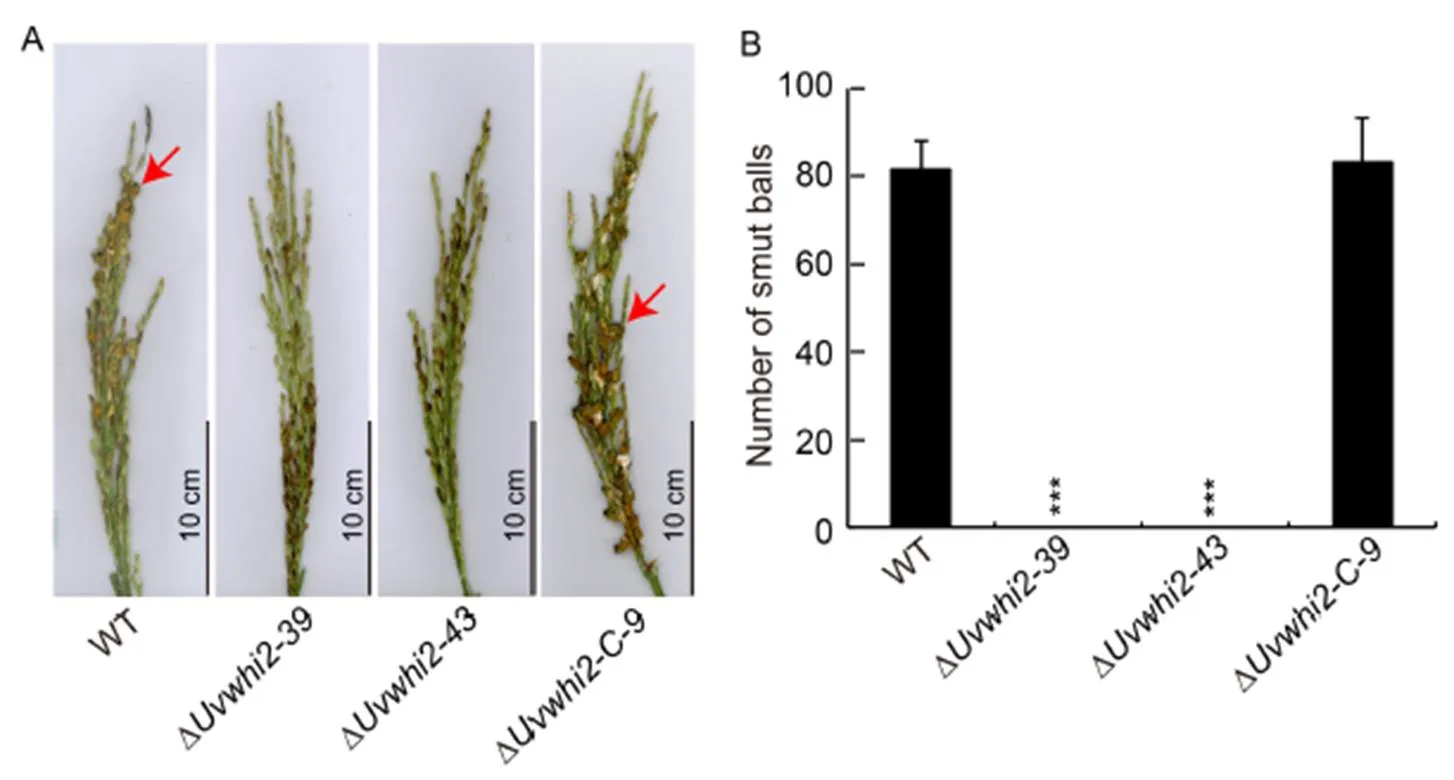
Fig. 2. UvWHI2 is required for pathogenesis in.
A,Disease symptoms of wild type (WT),deletionmutants(∆and ∆) and complementation (∆) strainson the rice panicles of Wanxian 98 at 21 d post inoculation. The red arrows show the false smut balls.
B, Statistical analysis of the average number of false smut balls on the inoculated panicles. Data are Mean ± SD (= 3). ***, Significant difference at the 0.001 levelby the Duncan’s test.
In addition, RNA-Seq and qRT-PCR results also showed that the expression levels of sugar-related genes (and) in the ∆mutant strain were lower than those in WT (Fig. S5-A). We assumed that UvWHI2 may be involved in the regulation of cell growth and proliferation in response to nutrient stress as WHI2 in other fungi (Kaida et al, 2002; Leadsham et al, 2009; Chen et al, 2018). To confirm this hypothesis, the WT, ∆/and Δstrains were cultured on the SD (synthetic dropout medium), SD-G (synthetic dropout medium without glucose) and SD-N (synthetic dropout medium without nitrogen) plates. After 15 d of incubation, compared with the WT strain on SD, the ∆strains were significantly reduced in mycelium growth on both the SD-G and SD-N plates (Fig. S5-B and -C). In contrast, the complementation strain Δ-showed similar phenotypes as the WT strain under all observed stress conditions. All these results indicated that UvWHI2 is involved in the regulation of nutrient stress responses in.
Defects in stress response compromised secondary spore formation on rice surface in ∆Uvwhi2 mutant
In order to further determine whether the defects in stress response in the ∆mutants affect pathogenesis, we carefully observed the germination processes. We found that the ∆mutants had an increase in the formation of secondary spores on the PSA plate (Fig. 3-A). In contrast, when the conidia were germinated on the agarose plate without other nutrient supplementation, the formation of secondary spores was decreased (Fig. 3-B). Moreover, secondary spore formation of the ∆mutant was even rarer on the rice surface (Fig. 3-C). These results suggested that the secondary spore formation of the ∆mutant was compromised under the nutrient limited condition and rice surface. Thus, we inferred that the highly decreased secondary spore formationon the rice surface is one of the main reasons for the lost pathogenicity in the ∆mutants.
Discussion
Rice false smut has posed a serious threat to the yield and quality of rice in recent years, so it is important to study the pathogenesis ofHere, we identified a general stress response factor WHI2in. According to the results, UvWHI2 plays important roles in the hyphal growth, conidiation, various stress responses and pathogenicity.
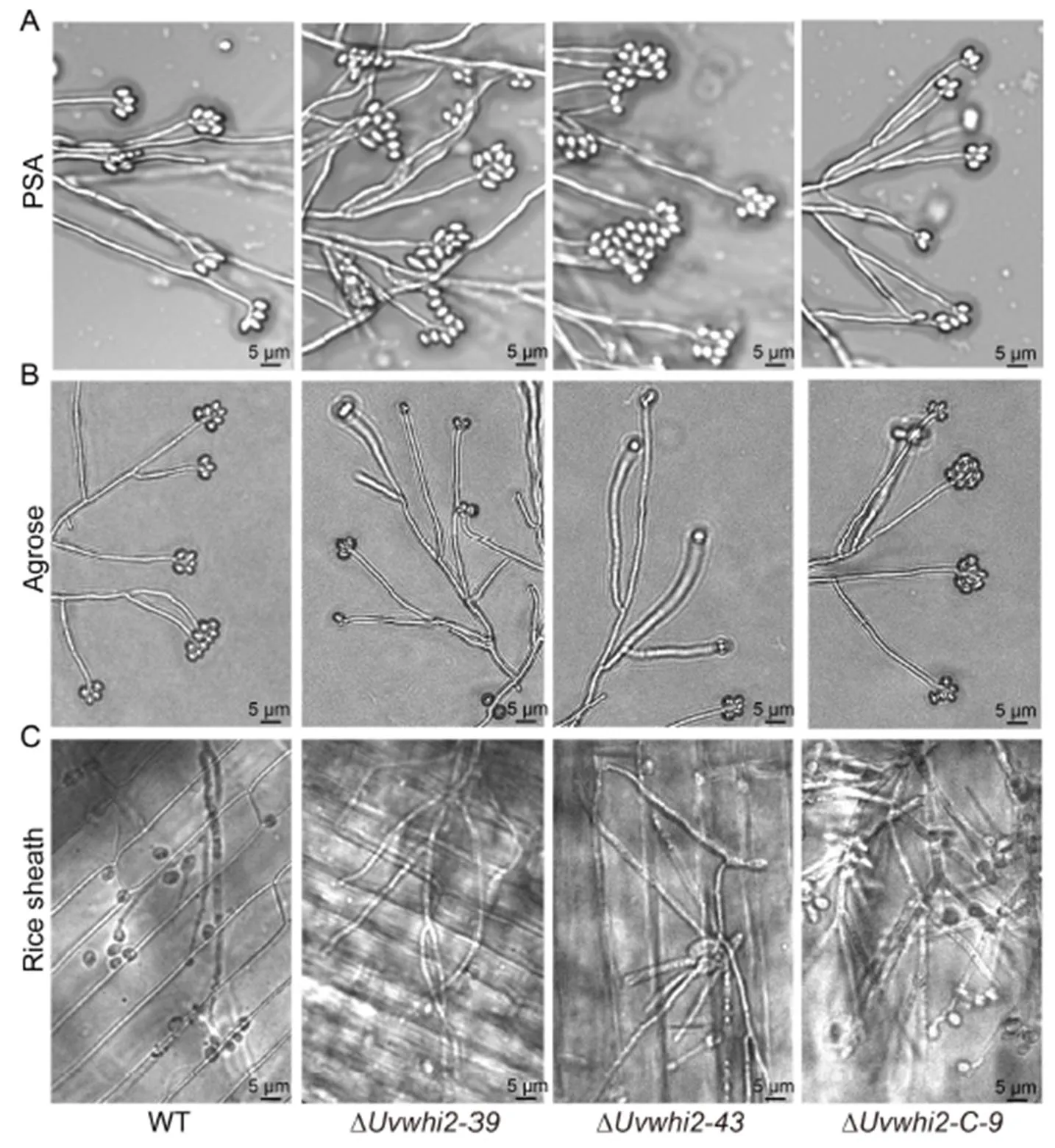
Fig.3. Conidial germination ofinwild type (WT), ∆mutants (∆and ∆) and complementation strain (∆) on potato sucrose agar (PSA, A), agarose plates (B) and rice sheath (C) at 28 ºC for 3 d.
In, a series of genes related to pathogenicity have been identified, but the pathogenic mechanism of this fungus is still largely unknown(Lü et al, 2016; Zheng et al, 2016; Yu et al, 2019; Xiong et al, 2020). In this study, deletion ofhighly reduced the pathogenicity ofto rice. Through a series of analysis, we found that, in addition to the compromised fungal growth, the reduced pathogenicity in the ∆mutants was likely caused by highly reduced formation of secondary spores on the rice surface. During pathogenic process of, the formation of secondary spores tends to greatly increase the amount of inoculums available to infect rice plants (Fan et al, 2014). In addition, the deletion mutant of, which encodes an ubiquitin-like protein required for autophagy-independent function, has defects in formation of secondary spores and shows the loss of pathogenicity, suggesting that the formation of secondary spores play an important role in the infection of(Meng et al, 2020). In the ∆mutants, secondary spore formation was rarer on the rice surface, which might have limited nutrient and oxidative stress (Fan et al, 2014; Wang et al,2019). Thus, we inferred that the main reason for the high reduction in pathogenicity of the ∆mutants may be the decrease of secondary spore formation.
In, UvPmk1, UvCdc2, UvPro1, UvBI-1, UvAc1, UvPdeh, UvPsr1, UvCom1, UvHox2 and UvAtg8 are involved in both various stress responses and pathogenesis (Guo et al, 2019; Yu et al, 2019; Meng et al, 2020; Tang et al, 2020; Xiong et al, 2020). However,it is still unclear whether and how defects of stress responses affect the pathogenesis processes ofThis study analyzed the role of a general stress response factor WHI2 to reveal the relationship between the stress response and pathogenicity inIn yeast, ScWHI2 interacts with the protein ScPsr1 to activate stress response element-mediated genes, possibly via the dephosphorylation of the Msn2 transcription factor (Kaida et al, 2002; Boeckstaens et al, 2014), and then regulates the downstream stress response. In, PaWHI2 is also likely affected in nutrient sensing and regulates the vegetative growth (Timpano et al, 2016). In this study, the results of comparative RNA-Seq analysis showed that some stress response genes were differentially expressed in the WT and ∆mutant strains. Consistent with the RNA-Seq results, knockout ofreduced the sensitivity to salinity stress, increased the sensitivity to oxidative stress and cell wall stress, and decreased the hyphal growth under nutrient limited conditions, indicating that UvWHI2 is also involved in various stress responses in. Meanwhile, the expression level of, which is a member of the gene cluster responsible for ustiloxin synthesis, was lower in ∆than that of the WT strain (Fig. S3), indicating a potential role in mycotoxin production. Furthermore, we noted that deletion ofenhanced secondary spore formation on the PSA plate, and on the contrary, reduced secondary spore formation on the nutrient limited surface, including the agarose plate without other nutrient supplementation and the rice surface. These results suggested that the defects of stress responses in the ∆mutants affected the formation of secondary spores on the nutrient limited rice surface, leading to a severe reduction in pathogenicity of.
In conclusion, our results suggest that UvWHI2 is necessary for fungal growth, stress response, conidiation, secondary spore formation and pathogenicity in. In addition, the defects of stress responses can affect the formation of secondary spores on the rice surface, and then affect the pathogenicity of
methods
Sequence analysis
The sequences of the genes and proteins used in this study were downloaded from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The motif scan was performed online (https://myhits.isb-sib.ch/cgi-bin/ motif_scan). The protein sequence alignments were processed using EPSript 3.0. The following phylogenetic analyses were performed using MEGA 7.0 with a neighbor-joining algorithm method (Robert and Gouet, 2014).
Fungal growth
TheWT strain HWD-2 was a kind gift from the Huazhong Agricultural University (Wuhan, China). Thestrain was culturedat a constant temperature of 28 ºC on the PSA (potato 200 g/L, sucrose 20 g/L and agar 20 g/L). For liquid culture, the mycelia were shaken in the PS (200 g/L potato and 20.0 g/L sucrose) medium at 170 r/min and 28 ºC for 7 d. The mycelial growth, mycelial dry weight and conidia concentration were measured as Meng et al (2020). The conidial germination experiments were performed on the PSA, agarose plates and the rice sheath, respectively. Three independent biological experiments were performed with three replicates each time.
Construction of ΔUvwhi2 strains and complementation analyses
To construct thedeletion mutant strain using the gene replacement strategy, based on the genome sequence (Uv-8b, KDB15335.1), the 1 026 bp upstream and 989 bp downstream flanking sequences ofwere amplified from the genomic DNA of the HWD-2 strain using the primer pairs Uvwhi2-5F/R and Uvwhi2-3F/R (Table S2). Then, these flanking fragments ofwere cloned into a vector pFGL821(Addgene: 58224, www.addgene.org) (Xiong et al, 2020). For the complementation, thefragment, including coding region, promoter and 3′-UTR regions of(Table S2), was inserted into a vector pFGL823, which was derived from the replacement ofgene cassettein pFGL821 by.-mediated transformation was applied for genetic transformation infollowing the protocol (Yu et al, 2015). The correct transformants of Δand complementation assay were ascertained using Southern blot and qRT-PCR analyses.
Southern blotting, RNA isolationand qRT-PCR analyses
For Southern blot analysis, the genomic DNA of the WT strain and mutants was extracted and digested withI. Then, the digested products of genomic DNA were size fractionated through 0.8% agarose gel and mounted onto positively charged nylon membrane (GE Healthcare, Buckinghamshire, UK). The purified probe UvWHI2-probe-F/R (Table S2) was DIG-labeled with Labeling Reagents (GE Healthcare, Buckinghamshire, UK) to hybridize with the digested products of the WT and Δstrains. All the hybridization processes were executed following the manufacturing instruction of AmershamTMAlkPhos Direct Labeling Reagents (GE Healthcare, Buckinghamshire, UK). Then, ChemiDoc XRS+ System (Bio-Rad, Hercules, USA) was used to detect the signals of Southern blotting.
The total RNA of the fungal mycelia was extracted using the Fungal RNA Kit 200 (OMEGA Biotek, Georgia, America). The first-stranded cDNA was synthesized with a reverse transcription kit (TaKaRa, Shiga, Japan), and then TB GreenTMPremix ExTM(Tli RnaseH Plus, TaKaRa, shiga, Japan) was used for qRT-PCR analysis. Thegene was used as the endogenous reference gene (Table S2). To determine the transcription level ofduring the infection stage, the RNA was extracted from infected rice spikelets collected at 3, 5 and 9 dpi.The relative expression levels ofgene were calculated using the 2-∆∆CTmethod. Three biological replicates were performed to calculate the mean and standard deviation.
Pathogenicity assay
For the pathogenicity assay, liquid culture of mycelia and conidia (1×106conidia/mL) were mixed together, and broken down in a juice blender. Then, the conidial suspension was injected into the panicles of selected rice cultivar Wanxian 98 (a susceptible rice cultivar) at the booting stage. The inoculated plants were cultivated under 12 h light/12 h dark conditions at 25 ºC and 95% relative humidity. Then, the phenotype of smut balls was counted and scanned at 21 dpi. This experiment was repeated three times in each test.
RNA-Seq library preparation and illumina sequencing
Total RNA was extracted from seven-day-old mycelia and conidia of WT and ∆strains using the Fungal RNA Kit 200 (OMEGA Biotek, Georgia, America). A total of 3 μg RNA and the NEBNext®UltraTMRNA Library Prep Kit(New England Biolabs,California,America) was used to generate RNA-Seq transcriptome libraries for sequencing on Illumina®(NEB, California, USA) following manufacturer’s recommendations.
For the RNA-Seq data analysis, the reads containing adapter and ploy-N and low-quality reads were removed to obtain clean reads based on their error rate, Q20, Q30 and GC contents. Reference genome and gene model annotation files were downloaded from genome website directly (https://ftp.ncbi. nlm.nih.gov/genomes/all/GCA/000/687/475/GCA_000687475.1_ Assembly_for_version_1_of_the_Villosiclava_virens_genome/). Then, the featureCounts v1.5.0-p3 was used to map the clean reads to reference genome. Each gene expression level was based on the FPKM (Fragments Per Kilobase per Million). Differential expression genes of two samples were obtained using the DESeq2 software with FDR adjusted≤ 0.05 and absolute log2fold ≥ 2. GO enrichment analysis was performed under the Bonferroni-corrected≤ 0.05 compared with the whole-transcriptome background on the website (https://www. omicshare. com/tools/).
Abiotic stress response analysis
To test the sensitivity to various abiotic stresses, the WT, Δ/and Δstrains were cultured at 28 ºC for 15 d on thePSA and PSA with 0.03% H2O2, 0.4 mol/LNaCl, 0.7 mol/L sorbitol, 0.03% SDS, 120 μg/mL CFW and 120 μg/mL CR to measure the colony diameters. The formula of inhibition rate was calculated as follows: Inhibition rate = (Average of strain colony diameters on the PSA – Average of strain colony diameters on the PSA with different chemicals)/ Average of the strain colony diameters on the PSA × 100%. For the nutrient starvation assay, the SD (1.7 g/L yeast nitrogen base without amino acids, 5 g/L ammonium sulfate, 20 g/L glucose and 20 g/L agar) medium, SD-G (1.7 g/L yeast nitrogen base without amino acids, 5 g/L ammonium sulfate and 20 g/L agar), and SD-N (1.7 g/L yeast nitrogen base without amino acids, 20 g/L glucose and20 g/L agar) plates were used. All the experiments were performed three times with three replicates.
ACKNOWLEDGEMENTS
This study was funded by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ19C140004), the Key Research and Development Project of Zhejiang Province, China (Grant No. 2019C02018), and Key Research and Development Project of China National Rice Research Institute (Grant No. CNRRI-2020-04). We thank Prof. Huang Junbin for providing the HWD-2 strain.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Identification of UvWHI2 in.
Fig. S2. Targeted gene deletion ofand complementation assay in.
Fig. S3. Comparative transcriptomic analysis of wild type and ∆strains.
Fig. S4. UvWHI2 contributes to the stress responses to cell wall, oxidative and osmotic agents in.
Fig. S5. UvWHI2 is involved in regulation of nutrient stress responses in.
Table S1. Differently expression genes and Gene Ontology enrichment analysis between wild type and ∆strains.
Table S2. Primers used in this study.
Boeckstaens M, Llinares E, van Vooren P, Marini A M. 2014. The TORC1 effector kinase Npr1 fine tunes the inherent activity of the Mep2 ammonium transport protein., 5(1): 3101.
Chen X H, Wang G Q, Zhang Y, Dayhoff-Brannigan M, Diny N L, Zhao M J, He G, Sing C N, Metz K A, Stolp Z D, Aouacheria A, Cheng W C, Hardwick J M, Teng X C. 2018. Whi2 is a conserved negative regulator of TORC1 in response to low amino acids., 14(8): e1007592.
Chen X Y, Hai D, Tang J T, Liu H, Huang J B, Luo C X, Hsiang T, Zheng L. 2020. UvCom1 is an important regulator required for development and infection in the rice false smut fungus., 110(2): 483–493.
Egan M J, Wang Z Y, Jones M A, Smirnoff N, Talbot NJ. 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease.,104(28): 11772–11777.
Fan J, Guo X Y, Huang F, Li Y, Liu Y F, Li L, Xu Y J, Zhao J Q, Xiong H, Yu J J, Wang W. 2014. Epiphytic colonization ofon biotic and abiotic surfaces implies the widespread presence of primary inoculum for rice false smut disease., 63(4): 937–945.
Fan J, Yang J, Wang Y Q, Li G B, Li Y, Huang F, Wang W M. 2016. Current understanding on, a unique flower-infecting fungus causing rice false smut disease., 17(9): 1321–1330.
Fang A F, Gao H, Zhang N, Zheng X H, Qiu S S, Li Y J, Zhou S, Cui F H, Sun W X. 2019. A novel effector genecontributes to full virulence ofto rice., 10: 845.
Guo W W, Gao Y X, Yu Z M, Xiao Y H, Zhang Z G, Zhang H F. 2019. The adenylate cyclase UvAc1 and phosphodiesterase UvPdeH control the intracellular cAMP level, development, and pathogenicity of the rice false smut fungus., 129: 65–73.
Harata K, Nishiuchi T, Kubo Y. 2016.WHI2, a yeast stress-response regulator homolog, controls the biotrophic stage of hemibiotrophic infection through TOR signaling., 29(6): 468–483.
Kaida D, Yashiroda H, Toh-E A, Kikuchi Y. 2002. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression., 7(6): 543–552.
Leadsham J E, Miller K, Ayscough K R, Colombo S, Martegani E, Sudbery P, Gourlay C W. 2009. Whi2p links nutritional sensing to actin-dependent Ras-cAMP-PKA regulation and apoptosis in yeast.,122(5): 706–715.
Li M Y, Liu X Y, Liu Z X, Sun Y, Liu M X, Wang X L, Zhang H F, Zheng X B, Zhang Z G. 2016. Glycoside hydrolase MoGls2 controls asexual/sexual development, cell wall integrity and infectious growth in the rice blast fungus., 11(9): e0162243.
Lin X Y, Bian Y F, Mou R X, Cao Z Y, Cao Z Z, Zhu Z W, Chen M X. 2018. Isolation, identification, and characterization offrom rice false smut balls with high ustilotoxin production potential., 58(8): 670–678.
Lu S Q, Sun W B, Meng J J, Wang A L, Wang X H, Tian J, Fu X X, Dai J G, Liu Y, Lai D W, Zhou L G. 2015. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen., 63(13): 3501–3508.
Lv B, Zheng L, Liu H, Tang J T, Hsiang T, Huang J B. 2016. Use of random T-DNA mutagenesis in identification of gene, a regulator of conidiation, stress response, and virulence in., 7: 2086.
Maršíková J, Pavlíčková M, Wilkinson D, Váchová L, Hlaváček O, Hatáková L, Palková Z. 2020. The Whi2p-Psr1p/Psr2p complex regulates interference competition and expansion of cells with competitive advantage in yeast colonies., 117(26): 15123–15131.
Mendl N, Occhipinti A, Müller M, Wild P, Dikic I, Reichert A S. 2011. Mitophagy in yeast is independent of mitochondrial fission and requires the stress response gene., 124(8): 1339–1350.
Meng S, Xiong M, Jagernath J S, Wang C C, Qiu J H, Shi H B, Kou Y J. 2020. UvAtg8-mediated autophagy regulates fungal growth, stress responses, conidiation, and pathogenesis in., 13(1): 56.
Müller M, Reichert A S. 2011. Mitophagy, mitochondrial dynamics and the general stress response in yeast., 39(5): 1514–1519.
Qiu J H, Meng S, Deng Y Z, Huang S W, Kou Y J. 2019.: A fungus infects rice flower and threats world rice production., 26(4): 199–206.
Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server.,42: 320–324.
Sadeh A, Movshovich N, Volokh M, Gheber L, Aharoni A. 2011. Fine-tuning of the Msn2/4-mediated yeast stress responses as revealed by systematic deletion of Msn2/4 partners., 22(17): 3127–3138.
Song W W, Dou X Y, Qi Z Q, Wang Q, Zhang X, Zhang H F, Guo M, Dong S M, Zhang Z G, Wang P, Zheng X B. 2010. R-SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of., 5(10): e13193.
Sun W B, Wang A L, Xu D, Wang W X, Meng J J, Dai J G, Liu Y, Lai D W, Zhou L G. 2017. New ustilaginoidins from rice false smut balls caused byand their phytotoxic and cytotoxic activities., 65(25): 5151–5160.
Sun W X, Fan J, Fang A F, Li Y J, Tariqjaveed M, Li D Y, Hu D W, Wang W M. 2020.: Insights into an emerging rice pathogen., 58(1): 363–385.
Tang J T, Bai J, Chen X Y, Zheng L, Liu H, Huang J B. 2020. Two protein kinases UvPmk1 and UvCdc2 with significant functions in conidiation, stress response and pathogenicity of rice false smut fungus.,66(2): 409–420.
Teng X C, Yau E, Sing C, Hardwick J M. 2018. Whi2 signals low leucine availability to halt yeast growth and cell death., 18(8): foy095.
Timpano H, Tong L C H, Gautier V, Lalucque H, Silar P. 2016. Theandgenes are members of the regulatory network that connect stationary phase to mycelium differentiation and reproduction in., 94: 1–10.
Wang X H, Wang J, Lai D W, Wang W X, Dai J G, Zhou L G, Liu Y. 2017. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls., 9(2): 54.
Wang Y F, Wang F, Xie S L, Liu Y, Qu J S, Huang J B, Yin W X, Luo C X. 2019. Development of rice conidiation media for., 14(10): e0217667.
Xie S L, Wang Y F, Wei W, Li C Y, Liu Y, Qu J S, Meng Q H, Lin Y, Yin W X, Yang Y N, Luo C X. 2019. The Bax inhibitor UvBI-1, a negative regulator of mycelial growth and conidiation, mediates stress response and is critical for pathogenicity of the rice false smut fungus., 65(5): 1185–1197.
Xiong M, Meng S, Qiu J H, Shi H B, Shen X L, Kou Y J. 2020. Putative phosphatase UvPsr1 is required for mycelial growth, conidiation, stress response and pathogenicity in., 27(6): 529–536.
Yu J J, Yu M N, Song T Q, Cao H J, Pan X Y, Yong M L, Qi Z Q, Du Y, Zhang R S, Yin X L, Liu Y F. 2019. A homeobox transcription factor UvHOX2 regulates chlamydospore formation, conidiogenesis, and pathogenicity in., 10: 1071.
Yu M N, Yu J J, Hu J K, Huang L, Wang Y H, Yin X L, Nie Y F, Meng X K, Wang W D, Liu Y F. 2015. Identification of pathogenicity-related genes in the rice pathogenthrough random insertional mutagenesis., 76: 10–19.
Zheng D W, Wang Y, Han Y, Xu J R, Wang C F. 2016.is important for hyphal growth and stress responses in the rice false smut fungus., 6: 24824.
Zheng M T, Ding H, Huang L, Wang Y H, Yu M N, Zheng R, Yu J J, Liu Y F. 2017. Low-affinity iron transport protein Uvt3277 is important for pathogenesis in the rice false smut fungus., 63(1): 131–144.
13 November 2020;
2 March 2021
Kou Yanjun (kouyanjun@caas.cn); Shi Huanbin (shihuanbin@caas.cn)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.12.004
(Managing Editor: Wang Caihong)
杂志排行
Rice Science的其它文章
- Rice Drying, Storage and Processing: Effects of Post-Harvest Operations on Grain Quality
- Fine Mapping of QTLs for Stigma Exsertion Rate from Oryza glaberrima by Chromosome Segment Substitution
- Simple Bioassay for PAMP-Triggered Immunity in Rice Seedlings Based on Lateral Root Growth Inhibition
- Ionomic Profiling of Rice Genotypes and Identification of Varieties with Elemental Covariation Effects
- Cold Plasma: A Potential Alternative for Rice Grain Postharvest Treatment Management in Malaysia
- Diversity of Sodium Transporter HKT1;5 in Genus Oryza
