Simple Bioassay for PAMP-Triggered Immunity in Rice Seedlings Based on Lateral Root Growth Inhibition
2022-01-20WangRuiZhangDandanLiShengnanGaoJinlanHanLiebaoQiuJinlong
Wang Rui, Zhang Dandan, Li Shengnan, Gao Jinlan, Han Liebao, Qiu Jinlong, 3
Research Paper
Simple Bioassay for PAMP-Triggered Immunity in Rice Seedlings Based on Lateral Root Growth Inhibition
Wang Rui1, 2, #, Zhang Dandan2, #, Li Shengnan2, #, Gao Jinlan2, Han Liebao1, Qiu Jinlong2, 3
(College of Grassland Science, Beijing Forestry University, Beijing 100083, China; State Key Laboratory of Plant Genomics, Institute of Microbiology, Chinese Academy of Sciences (CAS), Beijing 100101, China; CAS Center for Excellence in Biotic Interactions, University of Chinese Academy of Sciences, Beijing 100049, China; These authors contributed equally to this work)
Pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) is an essential layer of plant disease resistance. Robust bioassays for PTI are pre-required to dissect its molecular mechanism. In this study, we established that lateral root growth inhibition as a simple and robust measurement of PTI in rice seedlings. Specifically, flg22, a well-characterized PAMP from bacterial flagellin, was used to induce PTI in rice seedlings. While flg22 treatment induced PR gene expression and mitogen-activated protein kinase activation in the roots of rice seedlings to support the PTI triggered, this treatment substantially repressed lateral root growth, but it did not alter primary root growth. Moreover, treatments with chitin (i.e., a fungal PAMP) and oligogalacturonides (i.e., classical damage-associated molecular pattern) clearly inhibited the lateral root growth, although a priming step involving ulvan was required for the chitin treatment. The bioassay developed was applicable to various rice cultivars and wild species. Thus, lateral root growth inhibition represents a simple and reliable assay for studying PTI in rice plants.
pathogen-associated molecular pattern (PAMP); PAMP-triggered immunity; lateral root growth; mitogen-activated protein kinase; rice; flg22
Being sessile organisms, plants are constantly attacked by various microbial pathogens. To survive, plants have evolved innate immunity, enabling them to recognize and eliminate pathogenic invaders (Ausubel, 2005). Plant immunity is generally divided into two layers, namely pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) andeffector- triggered immunity(Jones and Dangl, 2006;Sanabria et al, 2008). More specifically,PTI reflects the basal defense system of plants, in which plasma membrane- localized pattern recognition receptors (PRRs) detect PAMPs of microbial pathogens and initiate defense responses to prevent the microbes from colonizing plant tissues.
PAMPs, which were previously designated as elicitors, are conserved microbial features associated with pathogenic as well as nonpathogenic microbes. Therefore, they are also called microbe-associated molecular patterns. The most well-known PAMP is flg22, which is a highly conserved bacterial flagellin comprising 22 amino acids. flg22 can induce a spectrum of PTI-related responses in a broad variety of plant species (Robatzek et al, 2007; Huang et al, 2014). The flg22 peptide is recognized by the PRR FLS2, a leucine-rich repeat receptor-like kinase in(Gómez-Gómez and Boller, 2000), and its orthologues in rice () and other plants (Hann and Rathjen, 2007; Robatzek et al, 2007; Takai et al, 2008).
The most studied fungal PAMP is the chitin from fungal cell walls. Chitin oligosaccharides trigger defense responses in both monocots and dicots (Shibuya and Minami, 2001), and are the most active when the degree of polymerization is between 3 and 8 (Liu et al, 2012). A receptor complex containing LYK5 and CERK1 in(Cao et al, 2014) and a receptor complex consisting of CEBiP and CERK1 in rice (Shimizu et al, 2010) recognize chitin, respectively. PRRs also perceive damage-associated molecular patterns (DAMPs) to trigger PTI (Howard, 1997). For example, oligogalacturonides (OGs) derived from pectin of plant cell walls are recognized by PRR WAK1 in(Brutus et al, 2010).
Perception of PAMPs induces a suite of defense responses in host plants, including ion fluxes, production of reactive oxygen species (ROS), activation of mitogen-activated protein kinases (MAPKs), expression of defense genes and accumulation of phytoalexins (Gómez-Gómez et al, 1999; Boller and Felix, 2009). ROS production, also called oxidative burst, is an early PTI response, occurring within minutes of a PAMP treatment (Zhang et al, 2007; Ranf et al, 2011). A luminol-based bioassay has been established to efficiently quantify the ROS production in plant cells, especially in the model plant. Additionally, seedling growth inhibition upon a prolonged PAMP exposure is often used to measure PTI in(Nekrasov et al, 2009; Laluk et al, 2011;Albrecht et al, 2012). These relatively simple and reliable bioassays have greatly facilitated molecular mechanism dissection of plant innate immunity.
Rice, an important staple food crop, is a model monocotyledonous plant species (Bajaj and Mohanty, 2005). Despite the progress, understanding of the molecular details of immunity in rice is still lagging behind. This is partially due to absence of efficient bioassays for immunity in rice plants. MAPK activation and defense gene expression have been used to monitor PTI in rice seedlings (Takai et al, 2007; Ao et al, 2014; Wang et al, 2015). However, analyses of MAPK activation and gene expression require the extraction of proteins and RNA for either a western blot or a quantitative real-time PCR assay, thereby necessitating tedious sample handling and preparation, which is inefficient for genetic screening. Although the ROS burst assay is rapid and efficient for examining PTI in rice suspension cells (Wang et al, 2015; Yang et al, 2019), generating the cell culture for each rice line tested for ROS production is quite time-consuming and impractical. Detection of ROS production in rice leaves upon PAMP treatment has been reported (Ding et al, 2012; Liu et al, 2015), but this is not robust and has to be optimized in different labs. Consequently, alternative robust bioassays are needed for studying PTI in rice plants. We herein describe a simple method for examining PTI in rice seedlings based on lateral root growth inhibition. This simple and robust PTI assay provides an efficient approach to investigate defense mechanisms in rice.
RESULTS
flg22 inhibits lateral root growth of rice seedlings
In, flg22 treatment represses primary root growth (Gómez-Gómez and Boller, 2000), which has been widely used as a measurement of PTI inseedlings (Anderson et al, 2011; Laluk et al, 2011; Stegmann et al, 2012). To investigate the effects of flg22 on rice root growth, we transferred rice seedlings to half-strength Murashige and Skoog(MS) media containing flg22 and cultivated them vertically for 3 d. In contrast to, rice primary root growth was not affected by the flg22 treatment (Fig. 1-A and -B). However, the lengths of lateral roots in these rice seedlings decreased significantly upon the flg22 treatment (Fig. 1-C and -D), while the lateral root density was unaffected (Fig. 1-E). More importantly, the flg22-induced lateral root growth inhibition was dose dependent, with increasing inhibition as the PAMP concentration increased (Fig. 1-F).
To confirm that the lateral root growth inhibition was a specific response triggered by flg22, we generated a knockout mutant of, the rice orthologue of(Takai et al, 2008) using the CRISPR/Cas9 system (Fig. S1). The lateral root length of themutant treated with flg22 was similar to that of the untreated mutant seedlings (Fig. 1-G), implying that the flg22-induced lateral root growth inhibition was mediated by.
PAMP-triggered immunity responses in rice seedling roots
To validate that flg22 triggers PTI in the lateral roots of rice seedling, we further examined MAPK activationand defense-related gene expression, two well-described PTI responses (Wang et al, 2015; Yang et al, 2019). Roots of 7-day-old rice seedlings were treated with flg22 for 15 and 30 min before MAPK activation were analyzed. As shown in Fig. 2-A, the MAPKs were clearly activated in the rice roots by the flg22 treatment.,andare useful marker genes for PTI in rice (Akamatsu et al, 2013; Chen et al, 2014; Wang et al, 2015; Li et al, 2018). Interestingly, the expression levels of,andin the roots were increased upon the flg22 treatment, withexpression peaking at 1 h, whereasandwere most highly expressed at 6 h (Fig. 2-B to -D). These findings further confirmed that flg22 induced various PTI responses in rice seedling roots.

Fig. 1. Pathogen-associated molecular pattern (PAMP) flg22 inhibits lateral root growth of rice seedlings.
A, Primary root growth following flg22 treatment.ssp.cultivar Nipponbare seedlings with a primary root length of 1.5‒2.0 cm were transferred to half-strength Murashige and Skoog(MS) medium supplied with 10 μmol/L flg22 or without (mock) and grown vertically for 3 d before the data were collected. Scale bars, 1 cm.
B, Primary root length of rice seedlings treated without (mock) or with 10 μmol/L flg22.
C, Lateral root growth of rice seedlings treated without (mock) or with 10 μmol/L flg22. The primary root (1.5–4.5 cm) used for analyzing lateral root length and density is outlined in red. Scale bars, 1 cm.
D and E, Lateral root length (D) and density (E) of rice seedlings treated without (mock) or with 10 μmol/L flg22.
F, Effect of flg22 on lateral root growth inhibition is dose dependence. Lateral root length of rice seedlings was measured 3 d after grown vertically on half-strength MS medium with indicated flg22 concentrations.
G, Lateral root length of themutant seedlings treated without (mock) or with 10 μmol/L flg22.
Bars indicate Mean ± SD of> 8 seedlings per treatment. The significance of the differences was determined by a one-way ANOVA with the Tukey’s multiple comparisons test. Different lowercase letters above the bars indicate significant differences at< 0.01.

Fig. 2. flg22-induced responses in lateral roots of rice seedlings.
A, Activation of mitogen-activated protein kinases (MAPKs) upon the 5 μmol/L flg22 treatment. MAPK activity was analyzed by immunoblotting with Phospho-p44/42 MAPK (Erk1/2) antibody (top panel), and ACTIN was used as a protein loading control (bottom panel). The intensity of themutant before the flg22 treatment was identified as ‘1.0’. WT, Wild type.
B‒D, Defense gene expression induced upon the flg22 treatment. The relative expression levels of(B),(C) and(D) in the roots of rice seedlings treated with 5 μmol/L flg22 were determined by quantitative real-time PCR.was used as an internal reference. Data are presented as Mean ± SD of three biological replicates. Different lowercase letters above the bars indicate significant differences at< 0.01.

Fig. 3. Oligogalacturonides (OGs) inhibit lateral root growth of rice seedlings.
A, Primary root length of rice seedlings following the OG treatment.
B, Lateral root length of rice seedlings after the OG treatment.
C, Lateral root density of rice seedlings after the OG treatment.
Bars indicate Mean ± SD of> 8 seedlings per treatment. The significance of the differences was determined by a one-way ANOVA with the Tukey’s multiple comparisons test. Different lowercase letters above the bars indicate significant differences at< 0.01.
OGs inhibit lateral root growth of rice seedlings
To test whether the lateral root growth inhibition is limited to flg22 and can be triggered by other elicitors, we examined the effect of OGs on the roots of rice seedlings. OGs are well-characterized plant DAMPs and elicit a wide range of defense responses in several plant species (Ferrari et al, 2013). We analyzed the lateral root inhibition on the half-strength MS media containing different concentrations of OGs. The OG treatment significantly inhibited the lateral root growth of rice seedlings, while the primary root growth and lateral root density were unaffected (Fig. 3). Similar to the effects of flg22, the OG-induced inhibition of lateral root growth increased as the OG concentration increased. These results suggested that lateral root growth inhibition can be triggered by different elicitors in rice.
Chitin inhibits lateral root growth of rice seedlings
We used chitin, which is the most studied fungal PAMP, to further test lateral root growth inhibition in rice seedlings. The chitin treatment had no effects on the lateral root growth even at a high concentration (Fig. 4-A). We speculated that the inhibition effect of chitin might be too minor to be detected. It has been previously reported that an exposure to a primary biotic stimulus leads to increased activation of defense responses to subsequent stimuli (Conrath et al, 2015). Ulvan, which is a sulfated polysaccharide from the green alga, reportedly has a priming effect on rice cells (Paulert et al, 2010). Accordingly, to test the priming effect of ulvan on the lateral root growth inhibition, we grew rice seedlings on half- strength MS media containing 1 mg/mL chitin and various concentrations of ulvan. After a 3-day growth period, there was no difference in the lateral root lengths of the untreated seedlings and the seedlings treated with ulvan alone. Interestingly, the rice seedlings grown on the media containing both ulvan and chitin exhibited decreased lateral root growth, suggesting ulvan is able to prime chitin-induced lateral root growth inhibition. The priming effect increased as more ulvan was added, with 100 μg/mL ulvan having the most significant effect (Fig. 4-B).
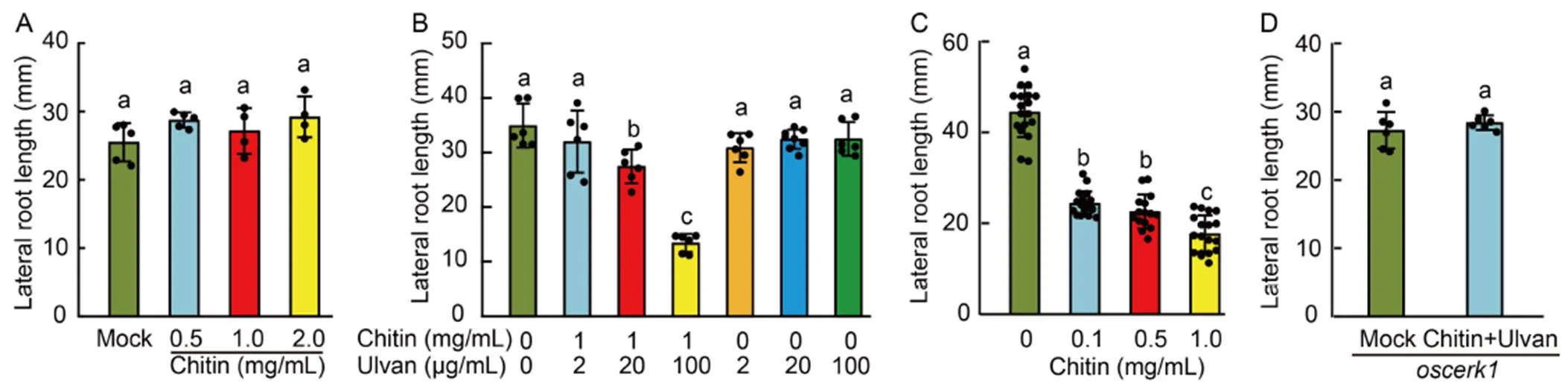
Fig. 4. Ulvan priming of rice seedlings is required for inhibition of lateral root growth by chitin.
A, Lateral root length of rice seedlings following the chitin treatment.
B, Lateral root length of rice seedlings primed with ulvan and treated with chitin.
C, Lateral root length of rice seedlings treated with indicated concentrations of chitin and primed with 100 μg/mL ulvan.
D, Lateral root length ofmutant seedlings treated without (mock) or with 0.5 mg/mL chitin and 100 μg/mL ulvan.
Bars indicate Mean ± SD of≥ 4 seedlings per treatment. The significance of the differences was determined by a one-way ANOVA with the Tukey’s multiple comparison test. Different lowercase letters above the bars indicate significant differences at< 0.01.
We then checked whether the lateral root growth inhibition in the ulvan-treated seedling is chitin dose dependent, and found the inhibition became greater as more chitin was added (Fig. 4-C). To further confirm that chitin specifically inhibits the lateral root growth, we generated a knockout mutant ofthat encodes a co-receptor for chitin in rice cells (Akamatsu, 2013) using the CRISPR/Cas9 system (Fig. S1). The lateral root growth of themutant was not inhibited by chitin in the presence of ulvan (Fig. 4-D), supporting the importance of the receptor for the chitin-induced lateral root growth inhibition. Together, it appeared that the lateral root growth inhibition is a general feature of PTI in rice seedlings.
Lateral root growth inhibition induced by PAMPs in different rice cultivars and wild species
Because the above-mentioned analyses only involved therice cultivar Nipponbare, we next examined the lateral root growth inhibition in another two rice cultivars, Zhonghua 11 () and Huanghuazhan (). The lateral root growth of the Zhonghua 11 and Huanghuazhan seedlings were inhibited by flg22 (Fig. 5-A and -B).
We then evaluated whether this lateral root growth- based assay is applicable for wild rice species. Specifically, we placedGriff. seedlings with a root length of about 1.5‒2.0 cm on half-strength MS media supplemented with or without flg22 and cultivated vertically in the growth chamber for 3 d. The subsequent examination indicated that the lateral root growth ofwas inhibited by PAMP (Fig. 5-C). These results indicated that lateral root growth inhibition is a robust bioassay for investigating PTI in various rice cultivars and wild species.
DISCUSSION
Efficient bioassays are much needed for studying immunity in rice plants. In this study, we developed a simple and reliable bioassay to monitor the PTI response in rice seedlings by simply measuring the lateral root growth following PAMP and DAMP treatments.

Fig. 5. Pathogen-associated molecular pattern (PAMP)-induced lateral root growth inhibition in various rice cultivars and wild species.
A, Lateral root length ofrice Zhonghua 11 seedlings treated without (mock) or with 5 μmol/L flg22.
B, Lateral root length ofrice Huanghuazhan seedlings treated without (mock) or with 5 μmol/L flg22.
C, Lateral root length ofGriff. seedlings treated without (mock) or with 5 μmol/L flg22.
Bars indicate Mean ± SD of> 8 seedlings per treatment. The significance of the differences was determined by a one-way ANOVA with the Tukey’s multiple comparison test. Different lowercase letters above the bars indicate significant differences at< 0.01.
Roots are vital for plant survival and represent an important opportunistic entry site for soil pathogens. Therefore, plant roots must be able to detect pathogensand initiate defense responses to limit pathogen infections and spread (Chuberre et al, 2018). In, treatments with elicitors (PAMPs and DAMPs) inhibit primary root growth (Gómez-Gómez et al, 1999; Laluk et al, 2011; Jiang et al, 2017). However, in rice, we observed that primary root growth was not affected by elicitors in our experimental setting, in contrast to the clearly inhibited lateral root growth in response to an elicitor treatment. This difference in the effects of elicitors may be due to the diversity in the root systems of dicots and monocots. The dicotyledonousplants have a dominant primary root, whereas monocotyledonous rice plants have a typical fibrous root system, with a short-lived primary root (Sasaki et al, 1981; Liu et al, 2005; Xu and Hong, 2013). In addition, the lateral roots of rice appear to have a much simpler internal structure in comparison to the primary roots (Rebouillat et al, 2009; Robin and Saha, 2015). As thus we speculated that PAMPs might be easier to get access to cells within the lateral roots than those in the primary roots.
Previous studies showed thatcan restore flg22 responsiveness inmutants and transient overexpression ofcan enhance flg22 responsiveness in rice cells (Takai et al, 2008). The flg22 peptide is well recognized by OsFLS2 (Wang et al, 2015). There might exist other PAMPs derived from bacterial flagellin and their corresponding receptors that have been shown in tomato (Roberts et al, 2020). The function of OsFLS2 needs to be further investigated. However, in our study, we provided solid data showing lateral root inhibition of rice seedling upon flg22 treatment, and the lateral root growth inhibition was compromised in themutant. Therefore, our work clearly demonstrated thatis involved in the perception of flg22 in rice.
Ulvan is a seaweed-derived sulfated heteropoly- saccharide that primes the ROS burst in wheat and rice suspension cells and enhances the powdery mildew resistance of wheat and barley plants (Paulert et al, 2010). Priming confers a ‘defense memory’ to plants, resulting in faster and stronger defense responses to a subsequent attack. In our analysis of the rice defense response to a chitin treatment, we found that the lateral root length was similar to that of untreated seedlings. However, the chitin treatment substantially inhibited lateral root growth when the priming agent ulvan was added. In addition to ulvan, there are many chemical compounds that can function as priming agents. The use of a lateral root growth inhibition assay might be an efficient and cost- effective way to screen and functionally characterize priming compounds.
Compared with the most analyzed defense responses, including the ROS burst, MAPK activation, and defense-related gene expression, the lateral root growth inhibition assay developed in this study is less costly. Moreover, it does not require specific equipment and is technically simple. Furthermore, this assay may be completed using intact and undamaged whole plants or tissues, enabling the recovery of the tested samples. This method will be useful for screening PTI suppression mutants, and it might also be used to screen PTI activation mutant based on more serious lateral root growth inhibition. For example,mutant exhibits greater inhibition of root length compared with the wild type upon PAMP treatment (Anderson et al, 2011). The apparent limitation with this method is that it will take several days to get the result, which might prohibit its application in fast test.
In summary, we established a simple and robust bioassay for PTI in rice plants. This bioassay will enable the analysis of multiple samples in parallel, implying it will be especially useful for screening mutants or verifying the functions of plant defense- related candidate genes/proteins identified in multi- omics studies.
METHODS
Rice materials
subsp.cultivars Nipponbare and Zhonghua 11 as well assubsp.cultivar Huanghuazhan were preserved in our laboratory.Griff. was provided by Dr. Chu Chengcai (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) and Dr. Lu Tiegang (Chinese Academy of Agricultural Sciences). Theandknockout mutants were generated using a CRISPR/Cas9 system as previously described (Shan et al, 2014). The following primers were designed and synthesized to generate the guide RNA constructs in pHUE411 (Xing et al, 2014):, forward primer (5′-GGCGCTAGCTGCTCGAG CTCGCCG-3′) and reverse primer (5′-AAACCGGCGAGCT CGAGCAGCTAG-3′);, forward primer (5′-GGCGAA CTTTCTAATGCTACACAG-3′) and reverse primer (5′-AAAC CTGTGTAGCATTAGAAAGTT-3′).
Elicitors and priming agents
The flg22 peptide (QRLSTGSRINSAKDDAAGLQIA) was synthesized by GenScript Corporation, China. Chitin was kindly provided by Dr. Morten Petersen(Department of Biology, University of Copenhagen, Denmark). OGs (degree of polymerization: mainly 4‒9) were kindly provided from Dr. Du Yuguang (Institute of Process Engineering, Chinese Academy of Sciences). Ulvan, which was used as a priming agent, was purchased from Elicityl (Catalog no. ULV010, France). All compounds were dissolved in distilled water to prepare stock solution. Appropriate aliquots of the stock solution were added to distilled water or half-strength MS medium to obtain the working concentrations.
Root growth assay
Rice seeds were surface-sterilized with 75% ethanol for 1 min followed by 2.5% sodium hypochlorite for 20 min. After washing with sterile water, the seeds were moved to half- strength MS media in plates and incubated vertically for 2‒3 d in a growth chamber with 85% relative humidity and a light intensity of 100‒150 μmol/(m2∙s) under a cycle of 13 h light (28 ºC) / 11 h darkness (26 ºC). Uniformly germinated seedlings with a primary root length of about 1.5‒2.0 cm were transferred to plates containing fresh half-strength MS supplemented with different compounds and cultivated vertically in the growth chamber for 3 more days. Then, the pictures of the roots were taken, and the lengths of primary and lateral roots were measured with the ImageJ software. Only the lateral roots within 1.5‒4.5 cm from the root base were analyzed.
Protein extraction and MAPK activation analysis
To analyze the PAMP- and DAMP-induced MAPK activation, 7-day-old rice seedlings were incubated in water overnight before being treated with 5 μmol/L flg22 for specific periods, then roots from the treated seedling were collected, and total protein was extracted using protein extraction buffer [50 mmol/L Tris-HCl (pH 7.5), 100 mmol/L NaCl, 10 mmol/L MgCl2, 15 mmol/L EGTA (ethylene glycol tetraacetic acid), 30 mmol/L β-glycerol phosphate, 0.1% NP-40, 2 mmol/L DTT (dithiothreitol), protease inhibitor cocktail (Roche, Swiss) and PhosSTOP (Roche, Swiss)]. Protein concentration was determined using the Bradford assay. The extracted proteins were separated on 12% SDS-PAGE, and were then transferred to nitrocellulose filter membrane according to the manufacturer’s instruction (Bio-Rad, USA). The MAPK activities were detected by immunoblotting with the Phospho-p44/42 MAPK (Erk1/2) antibody (Catalog no. 4370L, Cell Signaling Technology, USA) as previously described (Li et al, 2010).
RNA isolation and quantitative real-time PCR
The roots of rice seedling were collected, and total RNA was extracted using the TRNzol reagent (Tiangen, China). One microgram total RNA from each sample was treated with DNase I (Invitrogen, USA) and reverse transcribed using M-MLV Reverse Transcriptase (Promega, USA) following the manufacturer’s instructions. A quantitative real-time PCR analysis was performed using the CFX96 instrument (Bio-Rad Laboratories, USA) and SYBR Premix Ex(TaKaRa, Japan).was used as an internal reference gene for normalizing mRNA levels. Three replicates were analyzed for each sample. The following primers were designed and synthesized to analyze the gene expression:, forward primer (5′-GGTGTGGGAAGCACATACAA-3′) and reverse primer (5′-GTCTCCGTCGAGTGTGACTTG-3′);, forwardprimer (5′-TGAATAACAGTGGAGTGTGGAG-3′) and reverseprimer (5′-AACCTGCCACTCGTACCAAG-3′);, forwardprimer (5′-CCTCAGCCATGCCATTCAG-3′) and reverse primer (5′-CTTGTCCACGTCCAGGAACTC-3′);, forward primer (5′-AGGCTCCTCTCAACCCCAAG-3′) and reverse primer (5′-TTTCCTGGTCATAGTCCAGG-3′).
Accession numbers
The sequence data can be found in the MSU Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu) under the following accession numbers:(LOC_ Os04g52780),(LOC_Os08g42580),(LOC_ Os02g41680),(LOC_Os12g36880) and(LOC_Os03g18850).
ACKNOWLEDGEMENTS
This study was supported by the National Key Research and Development Program of China (Grant No. 2016YFD0100602) and the National Natural Science Foundation of China (Grant No. 31901868). We thank Li Huali for technical support, Professors Chu Chengcai and Lu Tiegang for sharing rice seeds.
SUPPLEMENTAL DATA
The following material is available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Genotyping ofandmutants generated using CRISPR/Cas9 system.
Akamatsu A, Wong H L, Fujiwara M, Okuda J, Nishide K, Uno K, Imai K, Umemura K, Kawasaki T, Kawano Y, Shimamoto K. 2013. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity., 13(4): 465‒476.
Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez- Ibanez S, Chinchilla D, Rathjen J P, de Vries S C, Zipfel C. 2012. Brassinosteroids inhibit pathogen-associated molecular pattern- triggered immune signaling independent of the receptor kinase BAK1., 109(1): 303‒308.
Anderson J C, Bartels S, González Besteiro M A, Shahollari B, Ulm R, Peck S C. 2011. Arabidopsis MAP kinase phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria., 67(2): 258‒268.
Ao Y, Li Z Q, Feng D R, Xiong F, Liu J, Li J F, Wang M L, Wang J F, Liu B, Wang H B. 2014. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity., 80(6): 1072‒1084.
Ausubel F M. 2005. Are innate immune signaling pathways in plants and animals conserved?, 6(10): 973‒979.
Bajaj S, Mohanty A. 2005. Recent advances in rice biotechnology- towards genetically superior transgenic rice., 3(3): 275‒307.
Boller T, Felix G. 2009. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors., 60(1): 379‒406.
Brutus A, Sicilia F, Macone A, Cervone F, de Lorenzo G. 2010. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides., 107(20): 9452‒9457.
Cao Y R, Liang Y, Tanaka K, Nguyen C T, Jedrzejczak R, Joachimiak A, Stacey G. 2014. The kinase LYK5 is a major chitin receptor inand forms a chitin-induced complex with related kinase CERK1., 3(2): e03766.
Chen X W, Zuo S M, Schwessinger B, Chern M, Canlas P E, Ruan D L, Zhou X G, Wang J, Daudi A, Petzold C J, Heazlewood J L, Ronald P C. 2014. An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors., 7(5): 874‒892.
Chuberre C, Plancot B, Driouich A, Moore J P, Bardor M, Gügi B, Vicré M. 2018. Plant immunity is compartmentalized and specialized in roots.,9: 1692.
Conrath U, Beckers G J M, Langenbach C J G, Jaskiewicz M R. 2015. Priming for enhanced defense., 53(1): 97‒119.
Ding B, del Rosario Bellizzi M, Ning Y, Meyers B C, Wang G L. 2012. HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice., 24(9): 3783‒3794.
Ferrari S, Savatin D V, Sicilia F, Gramegna G, Cervone F, De Lorenzo G. 2013. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development., 4: 49.
Gómez-Gómez L, Boller T. 2000. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in., 5(6): 1003‒1011.
Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in., 18(3): 277‒284.
Hann D R, Rathjen J P. 2007. Early events in the pathogenicity ofon., 49(4): 607‒618.
Howard R J. 1997. Breaching the outer barriers: Cuticle and cell wall penetration.: Carroll G C, Tudzynski P. The Mycota: Plant Relationships, Part A. Springer-Verlag Berlin Heidelberg: 43‒60.
Huang P Y, Yeh Y H, Liu A C, Cheng C P, Zimmerli L. 2014. The Arabidopsis LecRK-VI.2 associates with the pattern-recognition receptor FLS2 and primespattern- triggered immunity., 79(2): 243‒255.
Jiang L Y, Anderson J C, Besteiro M A G, Peck S C. 2017. Phosphorylation of Arabidopsis MAP kinase phosphatase 1 (MKP1) is required for PAMP responses and resistance against bacteria., 175(4): 1839‒1852.
Jones J D G, Dangl J L. 2006. The plant immune system., 444: 323‒329.
Laluk K, Luo H L, Chai M F, Dhawan R, Lai Z B, Mengiste T. 2011. Biochemical and genetic requirements for function of the immune response regulator BOTRYTIS-INDUCED KINASE1 in plant growth, ethylene signaling, and PAMP-triggered immunity in., 23(8): 2831‒2849.
Li Y, Zhang Q Q, Zhang J G, Wu L, Qi Y J, Zhou J M. 2010. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity., 152(4): 2222‒2231.
Li Y, Zhang Y, Wang Q X, Wang T T, Cao X L, Zhao Z X, Zhao S L, Xu Y J, Xiao Z Y, Li J L, Fan J, Yang H, Huang F, Xiao S Y, Wang W M. 2018. RESISTANCE TO POWDERY MILDEW 8.1 boosts pattern-triggered immunity against multiple pathogens in Arabidopsis and rice., 16(2): 428‒441.
Liu H J, Wang S F, Yu X B, Yu J, He X W, Zhang S L, Shou H X, Wu P. 2005. ARL1, a LOB-domain protein required for adventitious root formation in rice., 43(1): 47‒56.
Liu J L, Park C H, He F, Nagano M, Wang M, Bellizzi M, Zhang K, Zeng X S, Liu W D, Ning Y, Kawano Y, Wang G L. 2015. The RhoGAP SPIN6 associates with SPL11 and OsRac1 and negatively regulates programmed cell death and innate immunity in rice., 11(2): e1004629.
Liu T T, Liu Z X, Song C J, Hu Y F, Han Z F, She J, Fan F F, Wang J W, Jin C W, Chang J B, Zhou J M, Chai J J. 2012. Chitin- induced dimerization activates a plant immune receptor., 336: 1160‒1164.
Nekrasov V, Li J, Batoux M, Roux M, Chu Z H, Lacombe S, Rougon A, Bittel P, Kiss-Papp M, Chinchilla D, van Esse H P, Jorda L, Schwessinger B, Nicaise V, Thomma B P H J, Molina A, Jones J D G, Zipfel C. 2009. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity., 28(21): 3428‒3438.
Paulert R, Ebbinghaus D, Urlass C, Moerschbacher B M. 2010. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants., 59(4): 634‒642.
Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. 2011. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage- associated molecular patterns., 68(1): 100‒113.
Rebouillat J, Dievart A, Verdeil J L, Escoute J, Giese G, Breitler J C, Gantet P, Espeout S, Guiderdoni E, Périn C. 2009. Molecular genetics of rice root development., 2(1): 15‒34.
Robatzek S, Bittel P, Chinchilla D, Köchner P, Felix G, Shiu S H, Boller T. 2007. Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities., 64(5): 539‒547.
Roberts R, Liu A E, Wan L W, Geiger A M, Hind S R, Rosli H G, Martin G B. 2020. Molecular characterization of differences between the tomato immune receptors flagellin sensing 3 and flagellin sensing 2.,183: 1825‒1837.
Robin A H K, Saha P S. 2015. Morphology of lateral roots of twelve rice cultivars of Bangladesh: Dimension increase and diameter reduction in progressive root branching at the vegetative stage., 9: 34‒42.
Sanabria N, Goring D, Nürnberger T, Dubery I. 2008. Self/nonself perception and recognition mechanisms in plants: A comparison of self-incompatibility and innate immunity., 178(3): 503‒514.
Sasaki O, Yamazaki K, Kawata S. 1981. The relationship between the diameters and the structures of lateral roots in rice plants., 50(4): 476–480.
Shan Q W, Wang Y P, Li J, Gao C X. 2014. Genome editing in rice and wheat using the CRISPR/Cas system., 9(10): 2395–2410.
Shibuya N, Minami E. 2001. Oligosaccharide signalling for defence responses in plant., 59: 223‒233.
Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, Shibuya N. 2010. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice., 64(2): 204‒214.
Stegmann M, Anderson R G, Ichimura K, Pecenkova T, Reuter P, Žárský V, McDowell J M, Shirasu K, Trujillo M. 2012. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in., 24(11): 4703‒4716.
Takai R, Kaneda T, Isogai A, Takayama S, Che F S. 2007. A new method of defense response analysis using a transient expression system in rice protoplasts., 71(2): 590‒593.
Takai R, Isogai A, Takayama S, Che F S. 2008. Analysis of flagellin perception mediated by flg22 receptor OsFLS2 in rice., 21(12): 1635‒1642.
Wang S Z, Sun Z, Wang H Q, Liu L J, Lu F, Yang J, Zhang M, Zhang S Y, Guo Z J, Bent A F, Sun W X. 2015. Rice OsFLS2- mediated perception of bacterial flagellins is evaded bypvs.and., 8(7): 1024‒1037.
Xing H L, Dong L, Wang Z P, Zhang H Y, Han C Y, Liu B, Wang X C, Chen Q J. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants., 14(1): 327.
Xu J, Hong J H. 2013. Root development.: Zhang Q F, Wing R A. Genetics and Genomics of Rice.New York, USA:Springer-Verlag:297‒316.
Yang C, Yu Y Q, Huang J K, Meng F W, Pang J H, Zhao Q Q, Islam M A, Xu N, Tian Y, Liu J. 2019. Binding of thechitinase MoChia1 by a rice tetratricopeptide repeat protein allows free chitin to trigger immune responses., 31(1): 172‒188.
Zhang J, Shao F, Li Y, Cui H T, Chen L J, Li H T, Zou Y, Long C Z, Lan L F, Chai J J, Chen S, Tang X Y, Zhou J M. 2007. Aeffector inactivates MAPKs to suppress PAMP-induced immunity in plants., 1(3): 175‒185.
17 December 2020;
1 March2021
Qiu Jinlong (qiujl@im.ac.cn); Han Liebao (hanliebao@163.com)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.12.006
(Managing Editor: Wu Yawen)
杂志排行
Rice Science的其它文章
- Rice Drying, Storage and Processing: Effects of Post-Harvest Operations on Grain Quality
- UvWhi2 Is Required for Stress Response and Pathogenicity in Ustilaginoidea virens
- Fine Mapping of QTLs for Stigma Exsertion Rate from Oryza glaberrima by Chromosome Segment Substitution
- Ionomic Profiling of Rice Genotypes and Identification of Varieties with Elemental Covariation Effects
- Cold Plasma: A Potential Alternative for Rice Grain Postharvest Treatment Management in Malaysia
- Diversity of Sodium Transporter HKT1;5 in Genus Oryza
