Influence of water leaching on alkali-induced slagging properties of biomass straw
2022-01-05LIUYanjingYANTingguiANYanZHANGWeiDONGYang
LIU Yan-jing , YAN Ting-gui,* , AN Yan , ZHANG Wei , DONG Yang
(1.School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, China;2.Research Center of Karst Ecological Civilization, Guizhou Normal University, Guiyang 550025, China)
Abstract: Deposition or slagging problem caused by the alkali metal species is one of the major obstacles to utilize biomass fuel in combustion and gasification plant.The paper mainly studies the effect of water leaching on alkali-induced slagging properties of corn straw and rice straw.The original mineral form of alkali elements in biomass straw was studied with comparison of low temperature ash of biomass before and after water leaching.The release and transformation of alkali compounds in biomass straw at different temperatures during heating were analyzed with combination of the XRD result of the heated biomass and the chemical composition of the digested samples.The result shows that the potassium in corn and rice straw mainly exists in the form of KNO3, KClO4, K2SO4 and KAlSi3O8, in which KNO3, K2SO4 and KClO4 is mostly removed in water leaching.The fusion temperatures increased after water leaching, especially for rice straw ash, which is a typical sample that the basic compounds are mainly potassium-containing compounds.The decrease of potassium content with temperature in the original corn and rice was because the potassium-containing minerals decomposed and escaped at 25-1000 °C.The release of potassium in the range of 400-800 °C significantly reduced for corn and rice straw after water leaching, but the potassium content will still decrease above 800 °C.The decrease of magnesium content in corn with increasing temperature may be due to the volatilization of magnesium oxide under the action of carbon reduction.For fuels with high alkali metal content after water leaching, the residual alkali metal will still escape and cause deposition or slagging in the furnace, especially in the temperature range above 800 °C.
Key words: biomass;alkali metal;deposition and slagging;leaching;low temperature ash
With the growing concern about the impact of acid rain, stratospheric zone depletion, greenhouse warming on health of human being, public attention has been focused on ways to reduce these changes brought by fossil fuel, which supply 80% of the world's energy consumption every year[1,2].Biomass is known as a renewable energy source with CO2-neutral.Cofiring and co-gasification of biomass with coal are available methods to replace fossil fuel partially without rebuilding new plants[3,4].However, there are many problems need to be resolved during the coutilization of biomass with coal.The volatile elements,such as potassium and sodium, are usually more prevalent in biomass than in most coal.These alkali metal species may release and transport in the gas flow and then condense and react with convective heat exchange surface, causing deposition or corrosion,which is one of the major obstacles to ensure the longterm operation of combustion and gasification plant[5-8].
The main components of biomass ash are SiO2,K2O, CaO, Na2O, MgO, Fe2O3,Al2O3and so on[9,10],among which K and Na are considered to be the main cause of slagging.Niu et al[11]have classified the slagging into alkali-induced slagging and silicate meltinduced slagging.The former type is related with the release and re-condense of volatile alkali compounds and the latter involves the eutectic melt of nonvolatile alkali compound with silicate.Zhang et al[12,13]found that the alkali metal K release in the form of KNO3at 400 °C during the pyrolysis of wheat straw and in the form of KCl at 600 °C with the release amount increasing from 35% to 45.85%.During the combustion process, volatile alkali metals transfers in the form of chlorides, and also can reacts with SO2and CO2to form sulfates or carbonates[14,15], releasing in the form of KCl and K2SO4aerosol.Besides the volatile parts of alkali, the nonvolatile alkali compounds also involve the formation of eutectic melt with silicate at low temperatures.The migration and precipitation of K in the process of biomass heat utilization was simulatedby FactSage software in the study of Wei et al[16].The potassium compounds were released as gaseous form(KCl (g), K2SO4(g), KOH (g) and K (g) etc.), and the residual potassium left in the bottom ash form potassium silicate, potassium aluminosilicate,potassium sulfate etc.Zhao et al[17]found that the residual ratio of K in the combusted ash was 63% and 71% respectively after 0.5 and 2.5 h, indicating that the ratio of gaseous K reached 29% and 37% at the corresponding time.Chen et al[18]studied the migration and transformation of alkali metals during biomass combustion and found that alkali metals mainly exist in solid substances, as KCl, K2SO4, Na2SO4, Na2CO3,Na2SiO3etc at temperature below 600 °C, but alkali metals begin to release into the gas phase mainly as form of chloride as temperature exceed 600 °C.At 1000 °C, the alkali metal silicate transformed into aluminosilicate with high melting point as the temperature increases.Effective and low-cost countermeasures to reduce the alkali metals in biomass without changing the fuel properties should be considered as pretreatment to improve the slagging characteristics.
Research has shown that leaching, adding additives, or co-combustion with coal, sludge and other fuels can change the ash composition and improve slagging characteristics of biomass.Leaching especially can effectively remove the inherent inorganic substances, particularly alkalis, thereby reducing the problems related to ash.According to the solubility of alkaline metals in different solvents, alkali compounds can be classified into four categories:water-soluble substances such as alkali sulfate and chloride, exchangeable cations, soluble hydrochloric acid compounds, insoluble residues, such as sodium silicate[19].Saddawi et al[20]concluded that water washing can remove easily soluble metal salts, such as alkali metal chlorides, sulfates and carbonates.Ammonium acetate washing can remove organically bound salts, while acid washing can dissolve acidsoluble salts such as alkaline earth carbonates and sulfates.Mlonka-Mędrala et al[21]used H2O, NH4COOH and HCl to treat corn straws step by step, and found that the water-soluble K in the water leaching solution accounts for 75% of the total potassium content.Soluble K increase by 8% and 2% respectively in ammonium acetate and hydrochloric acid.Similar results, water leaching rate of K up to 75%, is also reported in the study of Xin[22], in which the rice straws was treated with the similar agents step by step.Deng et al[23]compared corn straws at different temperatures(30, 60, 90 °C) and found that with the increase of water temperature, the ash content of biomass decreased from 6.4% to 2.72%.In summary, the water leaching significantly reduce the ash content of biomass and the content of soluble alkali metals in the biomass, especially the potassium, and it has little effect on the content of elements such as Ca and Mg.Water leaching can increase the melting temperature of biomass[24]and similar process, such as rain wash,widely exists in the harvest, open-air stacking and transportation process.It is therefore water leaching should be preferred to improve the slagging characteristics with low cost.However, there are still alkali metal elements in the biomass after water leaching, and the sample with the highest potassium removal rate still has about 30% residues.If the biomass itself has high ash and potassium content, this part of potassium will still escape into the gas phase at high temperatures.The harm should not be underestimated.
Water leaching treatment reduces the ash content of biomass and the content of water-soluble K, Na, Cl and other elements[25-27].Leaching pretreatment can significantly reduce the content of soluble alkali metals in biomass, thereby reducing the content of potential hazards metals[28,29].However, for fuels with high content of alkali metal, such as corn straws, the considerable non-soluble alkali metal after leaching still exist, and there are few research reports on the influence of these alkali metals on the melting and slagging properties of biomass after water leaching.A large amount of straw biomass produced and consumed in China every year.Corn straw and rice straw are two types that account for a relatively high proportion, with 36.7% and 27.5% of the total respectively[30].In this study, the high content of alkali metals is their similarity, but the content of alkaline earth metal in corn straw is higher than that in rice.As the alkaline earth metals are also basic component that reduce the melting point of ash, the comparison between corn and rice will be helpful for the understanding the influence of alkaline earth metals on the ash slagging properties.This research is aim to study the release and transformation characteristics of residual alkali metals in biomass straw after water leaching at high temperatures.A mild ashing method for the removal of the organic components, low-temperature ashing, was used to make a detailed comparison of the occurrence forms of minerals in these two straws before and after water leaching.XRD analysis was employed to reflectthe original minerals in the biomass straws before and after leaching.The biomass straw prepared at different temperatures were digested and then the chemical compositions were analyzed to determine the alkali release during heating process.XRD analysis was also used to determine the crystal components in biomass ash at different temperatures.
1 Experimental
1.1 Characteristics of raw materials
The rice straw and corn straw used in this study were from Guanshan Lake District, Guiyang City,Guizhou Province.The biomass straw was pulverized into the particle size of 60 meshes, and stored at room temperature for further analysis.Proximate analysis and elemental analysis of corn straw and rice straw were carried out, and the corresponding results are listed in Table 1.The difference in moisture and fixed carbon content between corn straw and rice straw is small, but the ash content of corn straw is only 4.67%and the volatile content of corn straw is higher than that of rice straw.

Table 1 Proximate analysis and ultimate analysis of biomass straw
The biomass ash was prepared according to Chinese national standard GB/T 28731—2012 "Analysis method for solid biomass fuel industry".The chemical compositions of straw ash was analyzed by PANalytical Axios X-ray fluorescence (XRF), and the corresponding results are listed in Table 2.Both corn straw ash and rice straw ash contain a high level of K2O.The SiO2content of rice straw is higher than that of corn straw, and the content of other components is small.Additionally, there are more alkaline earth metals (CaO and MgO) in corn straw compared with rice straw.

Table 2 Ash composition of straw ash
1.2 Leaching pretreatment process
~50 g biomass sample was put in a three-necked flask with 1000 mL deionized water.The mixture was mechanically stirred at 60 °C for 6 h.After settling for 2 h, ordinary filter paper and 0.45 μm filter membrane were used for primary filtration and secondary filtering.The filtrate was sealed and stored, and the residue was dried at 105 °C for 12 h and stored for further analysis.
1.3 Preparation of low temperature ash
The organic matters in biomass were removed by oxygen plasma oxidation in a K1050X plasma furnace(Quorum Technologies Ltd.) so that the minerals in biomass can be analyzed by XRD analysis as oxygen plasma oxidation is a reliable method with minimal effect on the mineral species[31,32].~1 g biomass straw was put into the furnace chamber, and the sample was ashed repeatedly for 2 h until mass fluctuation was below 0.001 g to ensure the complete removal of organic matters.
1.4 Ash fusion temperature test
The ash fusion temperatures (AFTs) of synthetic ash samples were measured under oxidizing atmosphere.The ash cone was heated at 15 °C/min up to 900 °C, and then heated at 5 °C/min up to fusion temperature (FT) or 1550 °C.The heating program follows the Chinese national standard GB/T 219—2008, and 1550 °C is the upper limit for the equipment.During this process, deformation temperature (DT),softening temperature (ST), hemispherical temperature(HT) and FT were recorded according to the specific shapes of the ash cone.
1.5 Test of alkali metal and alkaline earth metal content in solid samples
Nitric acid, hydrogen peroxide and hydrofluoric acid (the mass ration of 6∶1∶1) were mixed as digestion solution.~0.1 g filter residue and 8 mLdigestion solution were blended in a polytetrafluoroethylene digestion tank, then the digestion tank was sealed in the autoclave and digested at 210 °C for 4 h.After cooling to room temperature,the digestion solution was transferred to a volumetric flask with washing the digestion tank 2-3 times and was diluted to 50 mL.The inductively coupled plasma emission spectrometer (ICAP 7400 model, Thermo Fisher Scientific Inc) was used to determine the contents of K, Ca, Na, and Mg in the digestion solution.
1.6 X-ray diffraction (XRD) analysis
XRD analysis was conducted using a X'Pert PRO MPD X-ray diffractometer (PANalytical).The scanning angle was 5°-80° and the scanning speed was 5 (°)/min.The radiation was CuKα (1.5406) under the conditions of 40 kV and 40 mA.
1.7 Inductively coupled plasma optical emission spectroscopy (ICP-OES)
ICP-OES (ICAP 7400) was used to test the content of alkali metal and alkaline earth metal elements in samples, exploiting the fact that excited electrons emit energy at a given wavelength as returned to the ground state.The default parameters of the instrument software: radio frequency power of 1300 W,observation height of 15 mm, atomizing gas flow of 0.8 mL/min, sample injection volume of 1.5 mL/min,and radial observation.
1.8 Thermogravimetric analysis
NETZSCH (STA-449 F3) differential thermal analyzer was used to test the pyrolysis characteristics of tested samples.~10 mg sample was put into alumina crucible with N2as carrier gas during the test.The gas flow rate of N2is 100 mL/min and the heating rate is 10 °C/min.
2 Results and discussion
2.1 Influence of water leaching on AFTs of biomass
The AFTs of biomass straw before and after water leaching are illustrated in Figure 1.The AFTs of corn straw and rice straw ash increase after water leaching,indicating that the water-soluble components are closely related with the low fusion temperatures of biomass.The DT of original rice straw is close to that of corn straw.However, the DT of rice straw after water leaching reaches 1388 °C and that of corn straw only increases to 1093 °C.Table 2 shows that the total contents of alkaline components (CaO+Fe2O3+K2O+Na2O) in rice straw ash is 28.54%, among which the K2O content reaches 23.06%, and the remaining acidic components (63.08%) are mainly SiO2(62.60%).In other words, the predominant alkaline component in the rice straw is K2O, which is usually closely related with the low ash fusion temperature.However, corn straw also contain alkaline earth metals such as CaO and MgO, which are not easily soluble in water.Alkaline components easily form a low-temperature eutectic with acidic components such as SiO2[33,34], so the melting temperature of corn straw is still relatively low.

Figure 1 AFTs of biomass straw ash
2.2 Influence of water leaching on the AAEMs contents of biomass
The alkali metal concentrations of the biomass before and after water leaching are shown in Figure 2.The K contents of corn straw and rice straw decrease significantly after water leaching, and the Ca and Mg contents show relatively slight decrease trends.The XRF results show that the Na content in corn straw and rice straw is only 0.138 and 0.176 mg/g, respectively,so the changes in Na content are relatively small.Deng et al[27]pretreated six types of biomass by water leaching at different temperatures and found that the increase in DT of biomass after water leaching was mainly resulted from the removal of most of K and a small amount of Na.As for the straws in this study, the partly removement of potassium in corn straw and rice straw is the main reason for the decrease of melting point after water leaching.
2.3 XRD analysis of low temperature ashing samples
Figure 3 shows the XRD spectrum of the lowtemperature ash samples.Figure 3(a) indicates that KNO3, KClO4and K2SO4exist in the original corn straw.The Ca and Mg contents of corn straw are also high, but no Ca- and Mg-containing minerals are detected as they may exist in the form of amorphoussubstances at low temperatures.The diffraction peak intensities of KNO3, KClO4and K2SO4of corn straw after water leaching are lower, indicating that these potassium-containing compounds were mostly removed.Figure 3(b) shows that K in rice straw mainly exists in the form of KNO3, KClO4, and K2SO4.For the leached rice straw, the diffraction peak intensities of KNO3, K2SO4and KClO4are also significantly reduced, while that of SiO2is enhanced.The XRD and ICP results demonstrates that the contents of KNO3,K2SO4and KClO4are reduced after water leaching,which may be the main reason for the increase of AFTs.

Figure 2 Changes of metal concentration in straw ash before and after water leaching

Figure 3 XRD patterns of low temperature biomass ash(a): corn straw; (b): rice straw
The water leaching treatment leads to the most removal of the soluble alkali components such as KNO3, K2SO4and KClO4, while insoluble alkali components like KAlSi3O8still remain in the biomass ash.The increase in AFTs of the biomass ash after leaching indicates that the soluble alkali metals is the key factor for the lower melting point of the biomass ash.The increase in AFTs of rice straw is more obvious, meaning that the insoluble alkali metal in the form of KAlSi3O8has less influence on the melting point.The mineral evolution process of soluble and insoluble alkali metals at high temperature could further explain the influence mechanism of different forms of alkali metals on AFTs of biomass.
2.4 Influence of water leaching on biomass pyrolysis process
TG/DTG curves of original straws and leached straws are illustrated in Figure 4, and it can be obviously found that the pyrolysis process of all samples can be roughly divided into three stages.The stage I is the volatilization stage of the moisture from ambient temperature to ~210 °C.The stage II corresponds to the temperature range of 210-420 °C,which is the main pyrolysis stage of biomass.The TG curves tend to be flat above 420 °C, where the residue carbonization and the slow ash decomposition occur.The TG/DTG curve difference between the sample before and after leaching in stage I is small, but the weight loss ratio in the stage II is higher (changing from 94% to 74%) for corn straw after leaching.The DTG peak in the temperature range of 210-420 °C also shows that the pyrolysis weight loss rate of the sample after leaching is faster.The weight loss ratios of corn straw and rice straw in the temperature range of 210-800 °C (76% and 64%, respectively) are close to those of volatile matter according to the proximate analysis results of biomass straw samples.Therefore,the increase in weight loss ratio after leaching may be resulted from the removal of some soluble minerals by water leaching treatment, meaning the ash content of tested sample decreases and the relative proportion of volatile matter increase accordingly.Therefore, the final weight loss ratio of leached biomass is higher.
The peak of the maximum weight loss rate shifts to the high temperature direction, which may be due to the fact that the water leaching reduces the content of alkali metal salts, and alkali metals originally have acatalytic effect on the pyrolysis process of biomass, so the maximum pyrolysis rate peak of the sample after pretreatment shifts to the higher temperature direction.
From Figures 4(c)-(d), it can be seen that the curve trends of TG and DTG of rice straw are similar to those of corn straw, and the weight loss ratio of pretreated rice is 9.12% higher than the original rice straw.This difference value is higher than that of corn straw, because the amount of soluble salt leached in rice is higher than that of corn, and its volatile content also increases more due to the higher ash content of rice straw.Therefore, the weight loss rate of pretreated samples and original rice is much different.

Figure 4 TG and DTG diagrams of pretreated and untreated straw
2.5 Migration of AAEMs in biomass at high temperature
Water leaching can extract most of the soluble potassium compounds and part of calcium and magnesium compounds.In order to explore the transportation of soluble and insoluble alkaline components at high temperatures, the content of alkaline components in samples after pyrolysis at different temperatures were determined.From the TG results of corn straw and rice straw, it can be seen that there is no significant difference between the original and leached samples below 400 °C.Therefore, the change in the alkaline content of the sample was investigated from 400 to 1000 °C.
Figure 5 shows the ICP-OES results of samples prepared at different temperatures.The potassium concentration in the original corn straw gradually decreases with the increase of temperature.The potassium concentration in corn straw prepared at 1000 °C is 0.68 mg/g, that is, 5.93% potassium is removed at high temperature.The volatilization of these potassium compounds into gas phase indicates that the potassium-containing compounds in the original corn straw have poor thermal stability in the temperature range of 400-1000 °C, and are easily released and then condensed on the walls of dust removal and heat exchange equipment to generate corrosion or slagging problems.The potassium concentration of leached corn straw is 2.41 mg/g and that of leached corn straw prepared at 800 °C decreased to 2.22 mg/g, indicating that the residual insoluble potassium in leached corn straw is stable and not easy to migrate into the gas phase below 800 °C.However,the potassium concentration in the corn straw prepared at 1000 °C decreased to 2.19 mg/g, indicating thatinsoluble potassium would volatilize into the gas phase above 1000 °C, causing alkali metal-derived slagging problems.
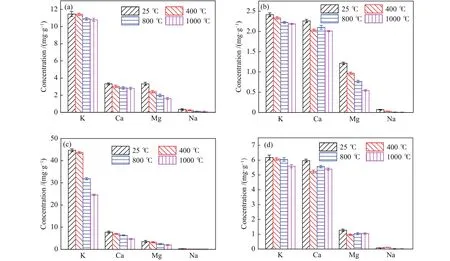
Figure 5 Changes in the AAEMs concentrations during biomass pyrolysis at different temperatures
The potassium concentration of the original rice straw is 44.70 mg/g, which is higher than that of corn straw.However, the concentration of this part of potassium decreases to 24.56 mg/g at 1000 °C,meaning 45.1% potassium in the rice straw is easily released as gas phase at 1000 °C.If the rice straw is used as feedstock in combustion or gasification plants without treatment, the unstable part of alkali metal in it will cause serious slagging problems.The potassium concentration of rice straw after water leaching decreased to 6.17 mg/g, and further decreased to 5.59 mg/g at 1000 °C, indicating that part of potassium-containing compounds are still unstable at low temperatures.These results are consistent with the XRD results.When temperature exceeds 400 °C, the Mg and Ca concentrations are basically stable,indicating that these components in leached rice straw hardly escape at high temperature.In summary, water leaching treatment can lead to the significant reduction of the alkali metals in corn straw and rice straw, but the differences in the forms of alkali metals in corn straw and rice straw results in different migration characteristics of alkali metals in original biomass and leached biomass.Water leaching is more suitable for the removal of potassium-containing compounds in rice straw that are unstable at low temperatures.
The relative change ratios of potassium versus temperature are shown in Figure 6.For the raw corn straw, the potassium concentration decreases sharply in the temperature range of 400-800 °C and shows a relatively slight decrease trend in the temperature ranges of 25-400 °C and 800-1000 °C.However, the potassium concentration of leached corn straw declines almost uniformly, indicating that the decomposition ratio of the leached corn straw decreases in the temperature range of 400-800 °C.However, the potassium concentration after water leaching is lower,meaning it is still easy to escape into gas phase.The potassium concentrations of the original and leached rice straw show decrease trend with temperature, which is similar with the variation trend of corn straw.The decrease curve of potassium concentration in the temperature range of 400-800 °C is changed from a steep hill into a gentle slope, but the decomposition ratio in the temperature range of 800-1000 °C is still near 8%, demonstrating that water leaching mainly influence the minerals that decompose and escape below 800 °C.
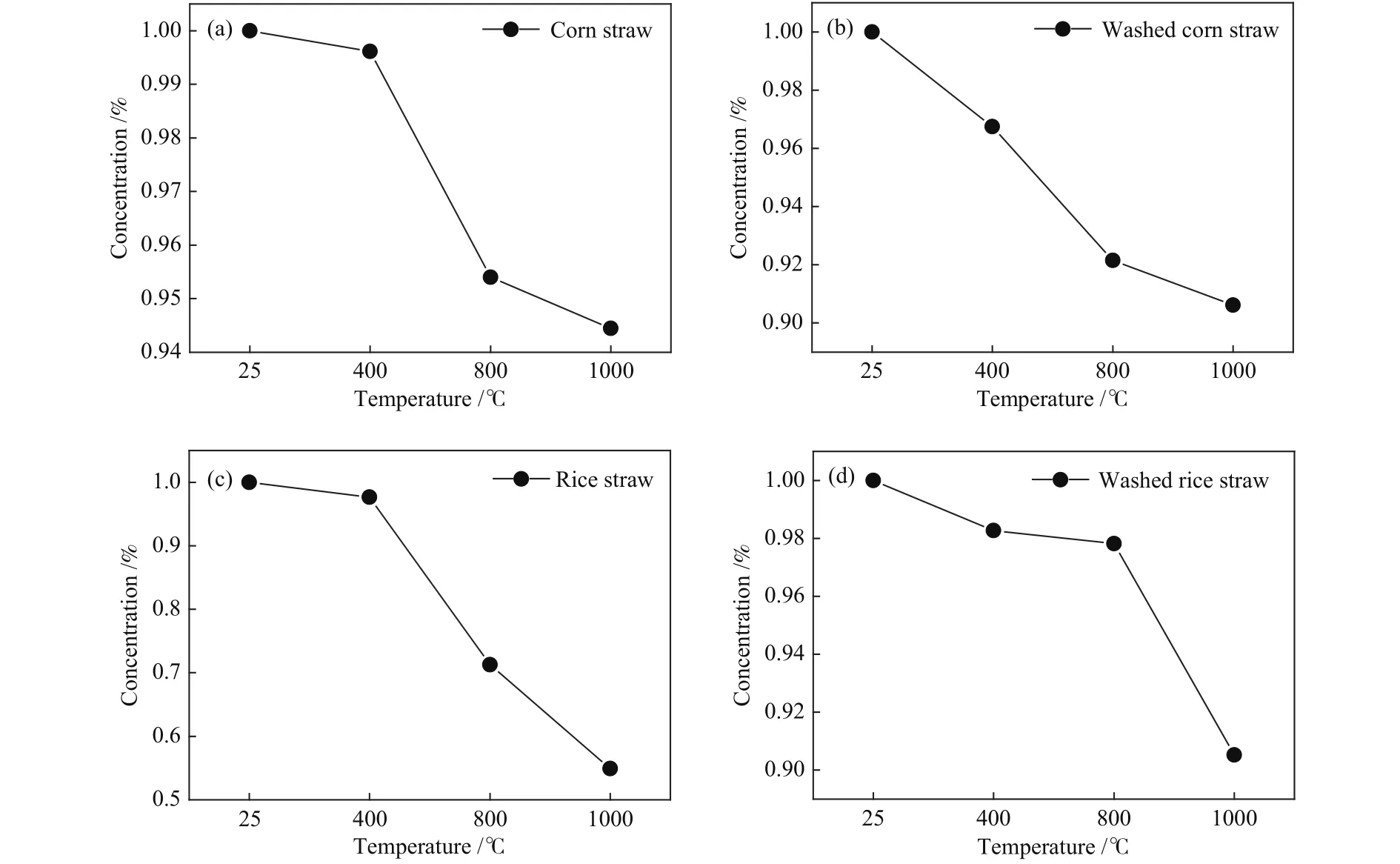
Figure 6 K concentration changes at different temperatures
2.6 Evolution of minerals in biomass ash at high temperature
In order to explore the mineral evolution at high temperature for the samples before and after water leaching treatment, XRD analysis was performed on biomass ash obtained at different temperatures.Figure 7 shows the XRD spectrum of straw ash at different temperatures.It can be seen in Figure 7(a) that the main K-containing minerals in the original corn straw at low temperature are KNO3, KClO4and K2SO4.When the combustion temperature rises up to 800 °C, the diffraction peaks of KNO3and KClO4disappear, and those of KCl, KAlSi3O8, and alkaline earth metal oxides (CaO and MgO) appear.Knudsen et al[35]reported that K, Cl and S in biomass release easily during the heating process at the temperature range of 500-1500 °C and then escape into the gas phase in large amount from 700 °C.As decomposition reactions of KNO3, KClO4and K2SO4are easy to happen at 700 °C, the disappearing of these minerals is reasonable[35-38].


At 1000 °C, the diffraction peaks of MgO and CaO become weak and that of K2SO4disappears.The ICP-OES results show that the magnesium content decreases versus temperature while that of CaO changes slightly.It have been verified that the reduction reaction of magnesium oxide with carbon will produce volatile magnesium.The diffraction peak of Ca2SiO4appears with increasing temperature[39,40].The main reactions are as follows:

Figure 7(b) shows that for the leached corn straw,the diffraction peak intensities of KNO3and K2SO4are greater, that of KClO4disappear.and that of silicate mineral like KAlSi3O8appear.As the temperature increases, the only K-containing crystal is KAlSi3O8,and its diffraction peak intensity firstly increases and then decreases.The original quartz is also transformed into tridymite.These changes were mainly owing to thereactions of KNO3and K2SO4mentioned above.Figures 5(a) and (b) also show that 5% K escapes at 1000 °C for original corn straw, but the escaping ratio of K increases to 10% for the leached corn straw,indicating the K-containing mineral in leached corn straw are also unstable.
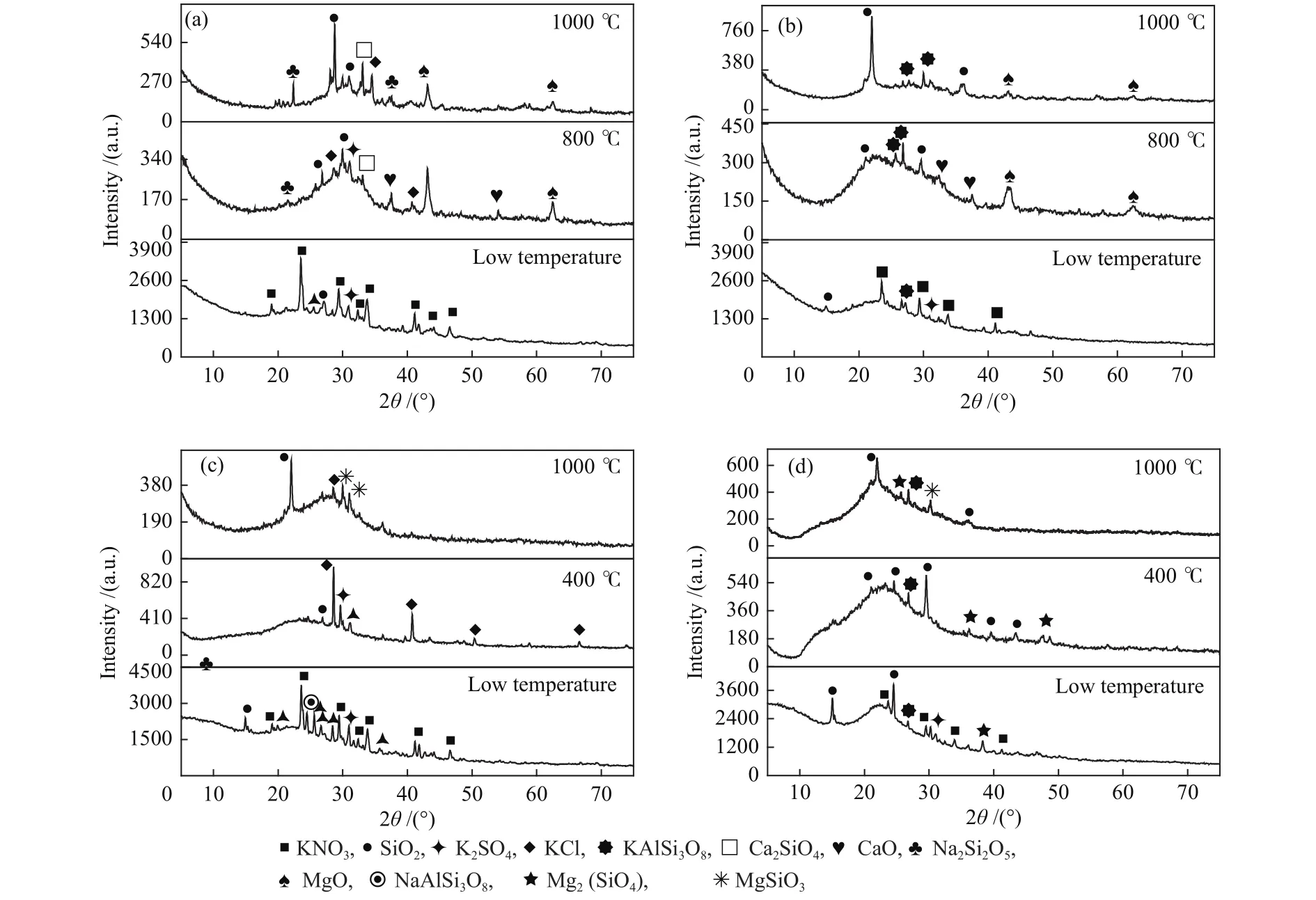
Figure 7 XRD of biomass at different combustion temperatures
After all, the total release amount of K is reduced considering the reduction of the absolute K concentration of the corn straw after leaching (decrease from 11.47 to 2.41 mg/g) as shown in Figures 6(a) and(b).Water leaching leads to the removal of the most of the soluble KNO3, KClO4and K2SO4, but residual potassium-containing minerals still escape at high temperatures.Therefore, if potassium content of leached biomass is still high, it is necessary to put attention on the slagging problems caused by these residual alkali metals.
Compared with corn straw, rice straw has a higher original alkali metal content, and its potassiumcontaining mineral types are similar with corn straws as shown in Figure 7(c), mainly KNO3, KClO4and K2SO4.At 400 °C, the diffraction peaks of KClO4and K2SO4still exist and that of KCl appears.Combined with the elemental analysis results, the K in rice straw is only reduced by 5.25% at 400 °C.while diffraction peak of KClO4and K2SO4disappear at 1000 °C and that of KCl is significantly reduced.The detected crystals are mainly silicate minerals like MgSiO3.Mg- and Cacontaining minerals are not detected in the lowtemperature ash samples, indicating this part of the minerals exist in the form of amorphous minerals in the early stage, and silicate crystals are formed at high temperatures.According to Figures 5(c) and (d), as the temperature increases, Mg and Ca will undergo different degrees of decomposition and oxidation,leading to their contents decrease.
Figure 7(d) shows for leached rice straw, the diffraction peak intensities of KNO3and K2SO4are significantly lower than those of original rice straw,and that of KClO4disappears.Combining with the changes in element content in Figure 5, it can be seen that the potassium content in rice dropped from 44.70 to 6.17 mg/g, so more than 86% of K was leached out of water.meaning that the ash composition has undergone major changes.Above 400 °C, the diffraction peaks of KNO3and K2SO4almost disappear,and most of the mineral crystals are silicate and calcium carbonate.The escape ratio of K in rice straw is ~2% in the range of ambient temperature to 800 °C as shown in Figure 6(d), indicating that the release may attribute to the decomposition of residual KNO3and K2SO4.The potassium content decreases significantly above 800 °C, but only some potassium-containing silicates are detected based on the XRD results.The escape rule in this high temperature range needs further study.
In summary, the alkaline components in corn straw and rice straw have similar migration rules at high temperatures.However, the potassium content in the original rice straw is higher, resulting in a higher residual amount after water leaching.If it is subjected to high temperatures such as combustion or gasification, the more severe slagging problem will exist.The potassium contents in corn straw and rice straw decrease significantly after water leaching.The decomposition temperature of water-soluble potassium salts such as KNO3, KClO4and K2SO4are in the range of 400-800 °C and the water leaching actually reduces the release amount of alkali metals in the range of 400-800 °C.Therefore, the slagging properties of fuel is improved.From 800 to 1000 °C, the K contents in corn straw and rice straw after water leaching treatment still decrease, indicating that the release of insoluble potassium at high temperatures can not been avoided.
3 Conclusions
In this research, the original mineral form of alkali elements in biomass straw was studied by comparing the XRD analysis results of low temperature ash of raw biomass and leached biomass.The release and transformation characteristics of alkali compounds in biomass straw at different temperatures during heating process were analyzed with combining the XRD results of the heated biomass and the chemical compositions of the digested samples.Thermogravimetric analysis was also carried out to explore the fuel performance for biomass straw before and after water leaching.The main conclusions were summarized as follows.
The potassium in corn straw and rice straw mainly exists in the form of KNO3, KClO4, K2SO4and KAlSi3O8, and a small amount of Na exists in the form of Na2Si2O5.Water leaching treatment can remove 79.0% and 87.5% of K (mainly the soluble KNO3,K2SO4and KClO4) in corn straw and rice straw,respectively, but has little effect on the types of minerals in the biomass.The maximum rate of pyrolysis of biomass after water leaching is shifted to the high temperature region because water leaching can reduce the K content in biomass straw.
Water leaching leads to the significant increase of the melting temperature of biomass ash, especially for rice straw ash, the basic compounds of which are mainly potassium-containing compounds.However,the melting temperature of corn straw is still low as there are some insoluble Ca- and Mg-containing compounds in corn straw after water leaching.
As the temperature increases, the contents of potassium and magnesium in corn straw and rice straw significantly decrease.The decrease of potassium content in the original corn straw and rice straw was mainly resulted from the decomposition and evaporation of the soluble potassium-containing minerals at 25-1000 °C.The release of potassium in the temperature range of 400-800 °C is significantly reduced for corn straw and rice straw after water leaching, but the potassium content will still decrease above 800 °C.The decrease of magnesium content in corn straw with increasing temperature may be resulted from the volatilization of magnesium oxide under the action of carbon reduction.Magnesium oxide in rice straw is not detected, thus the reduction of magnesium content of rice straw as temperature increases needs further study.In summary, water leaching leads to the reduction of a considerable amount of soluble potassium-containing minerals, that is, the release amount of alkali metal is reduced before 800 °C.However, for fuels with high alkali metal content after water leaching, the residual alkali metal will still escape and thus cause deposition or slagging in the furnace, especially when the temperature exceeds 800 °C.
Acknowledgements
The authors thank Dr.Jin Bai in State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences for supplying the low temperature ashing device.
