Synthesis,crystal structure and characterization of a Cu(II)⁃dien complex based on Keggin polyoxotungstates
2021-12-02GONGDelongLIUYunLIJie
GONG Delong, LIU Yun, LI Jie∗
(1.Laboratory and Equipment Management Service, Henan University, Kaifeng 475004, Henan, China;2.College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
Abstract: A new organic⁃inorganic hybrid with a one⁃dimensional zigzag chain, [Cu(dien)(H2O)]2[Cu(dien)(H2O)2]2{[Cu(dien)][α⁃GeW11.5Cu0.5O40]}2]·3H2O (dien = diethylenetriamine)(1), has been obtained under mild hydrothermal conditions by introducing polyoxotungstate into the Cu⁃dien system.Its structures have been determined by elemental analysis, infrared spectrometry and single⁃crystal X⁃ray diffraction.Results indicate that as⁃synthesized 1 is crystallized in the triclinic space group P⁃1.The basic molecular unit of 1 is composed of two Keggin⁃type anions {[Cu(dien)][α⁃GeW11.5Cu0.5O40]}4-, two discrete copper coordination cations [Cu(dien)(H2O)]2+and [Cu(dien)(H2O)2]2+, and three crystal water molecules.The adjacent {[Cu(dien)][α⁃GeW11.5Cu0.5O40]}4-polyoxoanions are interconnected together through terminal oxygens (Od) into 1D zigzag chains.
Keywords: Keggin cluster; transition metal complex; heteropolytungstate; synthesis; crystal structure
Polyoxometalates (POMs), asa classof structurally well defined inorganic building blocks,have exhibited a rich structural versatility and excellent physical and chemical properties in reported POM⁃based hybrid compounds[1-3].The construction and characterization of organic⁃inorganic hybrid POMs with versatilities have been the focus of intense research interest in recent years, owing to the following reasons:(a) in comparison with discrete POM or metal⁃organic framework, organic⁃inorganic hybrid POMs possess more complicated frameworks and more stable structures; (b) the incorporation of inorganic and organic units together reveal value⁃adding properties.In a general way,hybrid materials mainly consist of three building blocks, including POMs, metals and organic components.Until now, a variety of organic⁃inorganic hybrid Keggin POMs have been reported,owing to the large number of potential coordination sites and the relatively weak coordination ability[4-5].Many types of organic ligands with different donor groups have been used in the production of coordination polymers and organic⁃inorganic hybrids[6].The rigid ligands with amine donorare widely applied asdidentate or tridentate linkers to construct organic⁃inorganic hybrids, owing to their smaller steric hindrance[7-10].The dien molecule as a rigid bridging ligand can exhibit two coordination modes(Scheme 1), which is beneficial to the formation of complicated structures[9,11].Thus, metal ions play an important role in the formation of skeletons and provide persistent ties between inorganic POMs and various organic ligands to construct organic⁃inorganic hybrid POMs.Among the metal ions,CuIIion is a good candidate and more easily coordinates with organic ligands due to its larger radius, a wide variety of coordination modes,and special chemical properties[12-14].Keeping the above points in mind, Keggin anion [α⁃GeW12O40]4-was chosen as inorganic building unit and coupled with Cu ionsand dien molecules, to constructnovel organic⁃inorganic hybrid POMs. The POM⁃based hybrid [Cu(dien)(H2O)]2[Cu(dien)(H2O)2]2{[Cu(dien)][α⁃GeW11.5Cu0.5O40]}2]·3H2O (1)( dien = diethylenetriamine) with one⁃dimensional zigzag chain structure was obtained undermild hydrothermal conditions.Hence, we report synthesis and structuralcharacterization of1 by elemental analysis, single⁃crystalX⁃ray diffraction and IR spectrum.
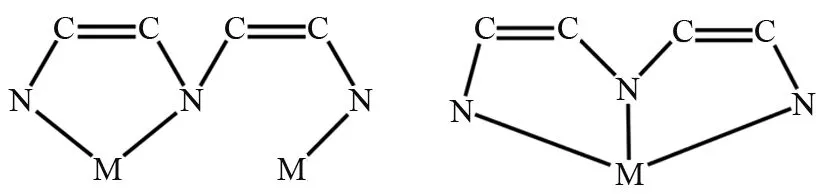
Scheme 1 Coordination modes of the dien ligands
1 Experiment
1.1 Materials and general methods
All starting materials are commercially obtained and used as received without further purification.K4[α⁃GeW12O40]·H2O was synthesized according to the literature[15]and characterized by IR spectrum.1 was identified by a Bruker APEX⁃II CCD X⁃ray single crystal diffractometer with graphite⁃monochromated Mo Kαradiation(λ = 0.071 073 nm) at 293 K.Elemental analysis of C,H and N was taken on a Perkine Elmer 2400⁃II CHNS analyzer.IR spectrum ( using KBr pellets)was recorded on a Bruker VERTEX 70 IR spectrometer.
1.2 Preparation of 1
1 was prepared by one⁃pot method.The K4[α⁃GeW12O40]·H2O (0.53 g), Cu(Ac)2·H2O (0.13 g) and diethylenetriamine (dien) (0.1 mL) were added in 20 mL of distilled water with a molar ratio of Ge ∶W ∶Cu ∶dien ∶H2O ≈ 2.79 ∶33.48 ∶1 ∶1.39∶1 660, and stirred for 1 h at room temperature.The pH of the mixture was adjusted to 5.73 with 2 mol/L HCl,and then transferred and sealed in a 30 mL Teflon⁃lined stainless steelautoclave with~80% filling.The mixture was heated at 160℃ for 4 d,and then the reactor was slowly cooled to room temperature over a period of 13 h.Rod⁃like black crystals of 1 were filtered, washed with water, and dried atroom temperature.Yield: 6.12% based on W.Elemental analysis data (%).Theoretical value: C, 4.20; H,1.13; N, 3.68.Experimental value: C, 4.41; H,1.09; N, 3.72.
1.3 X⁃ray crystallographic determination
A single crystal of 1 with a suitable size was carefully selected by observing under an optical microscope and glued on a thin glass fiber with epoxy resin.The structure was solved by direct methods and refined by full⁃matrix least squares on F2.All calculations were carried out by using the SHELXL⁃2014 /7 program package[16-17].All hydrogen atoms were refined isotropically as a riding mode using the defaultSHELXTL parameters.Their weighting details:
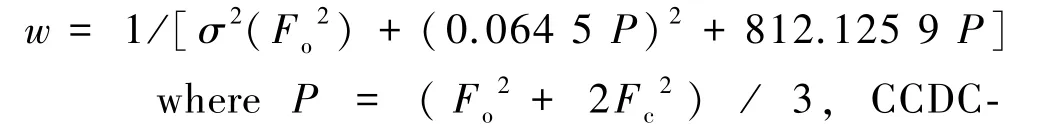
2091236.A summary of the crystallographic data and structural determination for 1 is provided in Table 1.The sectional bond lengths and angles are listed in Table 2.
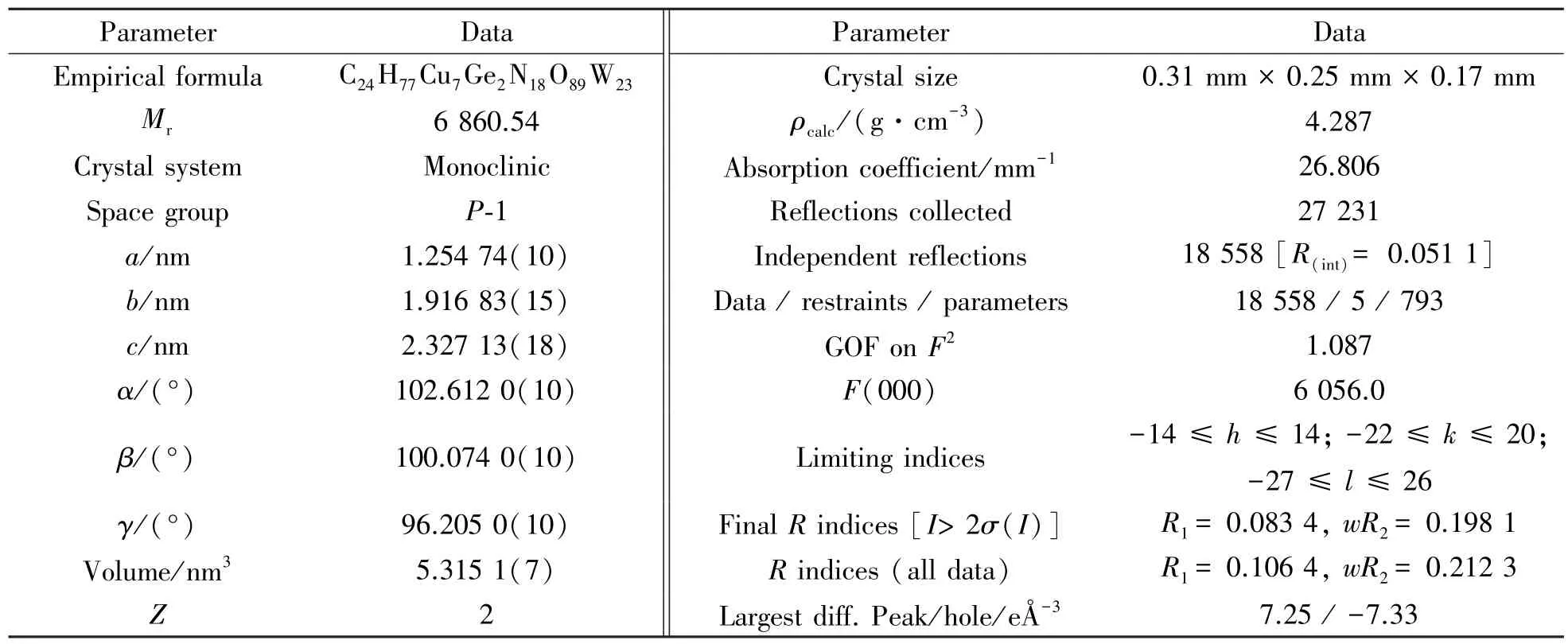
Table 1 Crystal data and structural refinements for 1

Table 2 Selected bond lengths in 1
2 Results and discussion
2.1 Structural description
1 was synthesized by the reaction of the germanotungstate K4[α-GeW12O40]·H2O and Cu(Ac)2·H2O in the presence of N-donor ligands underhydrothermalconditions.1 belongs to the triclinic space groupP⁃1. Single crystal X⁃ray diffraction analyses reveal that the basic structure unit of 1 consists of two negatively charged cluster{[Cu(dien)] [α⁃GeW11.5Cu0.5O40]}4-, two discrete four⁃coordinated copper coordination cations [Cu(dien)( H2O )]2+, two discrete five⁃coordinated copper coordination cations [Cu(dien)(H2O)2]2+, and three crystal water molecules (Fig.1).In the above⁃mentioned cluster, there exists a polyoxoanion [α⁃GeW11.5Cu0.5O40]6-and a copper coordination cation[Cu (dien)]2+.In other words, polyoxoanion [α⁃GeW11.5Cu0.5O40]6-serves as a monodentate ligand and coordinates to the copper centre of coordination cation[Cu ( dien)]2+( W2⁃O17⁃Cu3), forming a POM supported cluster {[ Cu ( dien )] [α⁃GeW11.5Cu0.5O40]}4-.The polyoxoanion [ α⁃GeW11.5Cu0.5O40]6-exhibits a plenary α⁃Keggin structure, in which a central GeO4tetrahedron (Ge-O distances: 0.172(2)-0.176(2) nm) is surrounded by twelve WO6octahedra.Every three of WO6octahedra constitute a{ W3O13} triad in an edge⁃sharing mode.In the polyoxoanion [α⁃GeW11.5Cu0.5O40]6-, 0.5 WVI(W23 or W24) and 0.5 CuIIatoms simultaneously occupy one position,leading to six negative chargeson the polyoxoanion.This disordered phenomenon is constantly encountered in POM chemistry[18-20].BVS calculation[21]shows that all W atoms exhibit the oxidation state+6,in consistent with the overall charge balance of 1.In the cluster, Cu atom is located in a distorted tetrahedral geometry by three N atoms from one dien ligand and one terminal oxygen atom of polyoxoanion [α⁃GeW11.5Cu0.5O40]6-.The bond lengths of Cu3-O17 (0.198(2) nm) and Cu3-N (0.197(2)-0.203(3) nm) are in the reasonable ranges.
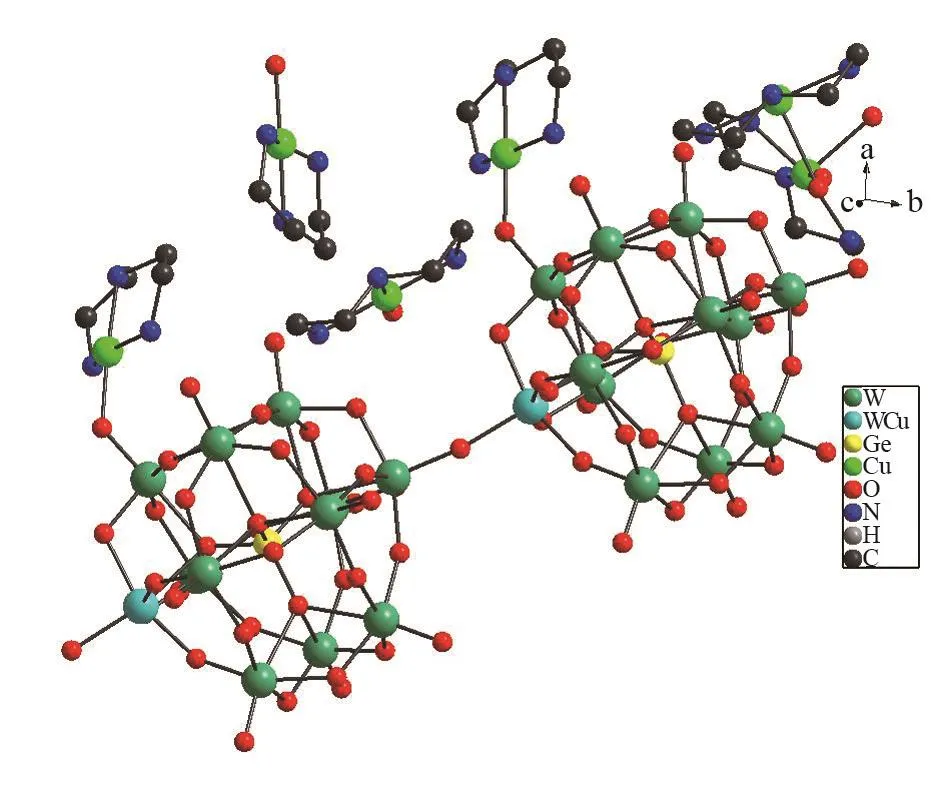
Fig.1 Ball⁃stick representation of 1.All the hydrogen atoms and crystal water molecules are omitted for clarity
In the polyoxoanion [ α⁃GeW11.5Cu0.5O40]6-, the oxygen atoms except for those linked to W2,W23 and W24 can be classified into three types, Od, Ob(c)and Oa, according to their coordination numbers.The WOd,W-Ob(c)and W-Oadistances are in the ranges of 0.168(2)-0.175(2), 0.182(2)-0.201 3(19) and 0.221 3(19) - 0.231 (2) nm, respectively.The distances of W2-O17, W23-O36 and W24-O65 are 0.176 (2), 0.186 (2) and 0.206 (2) nm,respectively.And the W-O-W bond angles change from 70.2(7)°to 178.6(16)°.The above bond lengths and angles are in accord with the results of the previous researches[22-23].
An unusual feature of 1 is that two types of copper complexes([Cu(dien)(H2O)]2+and [Cu(dien)(H2O)2]2+) are successfully introduced into the polyoxoanions.All of the dien ligands are in a μ3coordination mode and connect with one Cu atom.Each copper centre of [Cu(dien)(H2O)]2+exhibits a deformed tetrahedral geometry,constructing from three N atoms of one dien ligand and one O atom of a coordination water molecule with Cu-N distances of 0.194(4)-0.201(3) nm and Cu-O distances of 0.194(3)-0.199(3) nm.However, each copper center of[Cu(dien)(H2O)2]2+not only receives contributions of three N atoms from one dien ligand as plane of pyramid (Cu-N bond lengths: 0.196(3)-0.203(4)nm)but also receives the contribution of two O atoms from two coordinated water molecule as apexes of pyramid (Cu-O bond length: 0.195(5)-0.242(2)nm), exhibiting a trigonal bipyramid geometry.
The adjacent polyoxoanions {[Cu(dien)][α⁃GeW11.5Cu0.5O40] }4-are interconnected together through terminal oxygen atoms,constructing a 1D zigzag chain architecture (Fig.2).
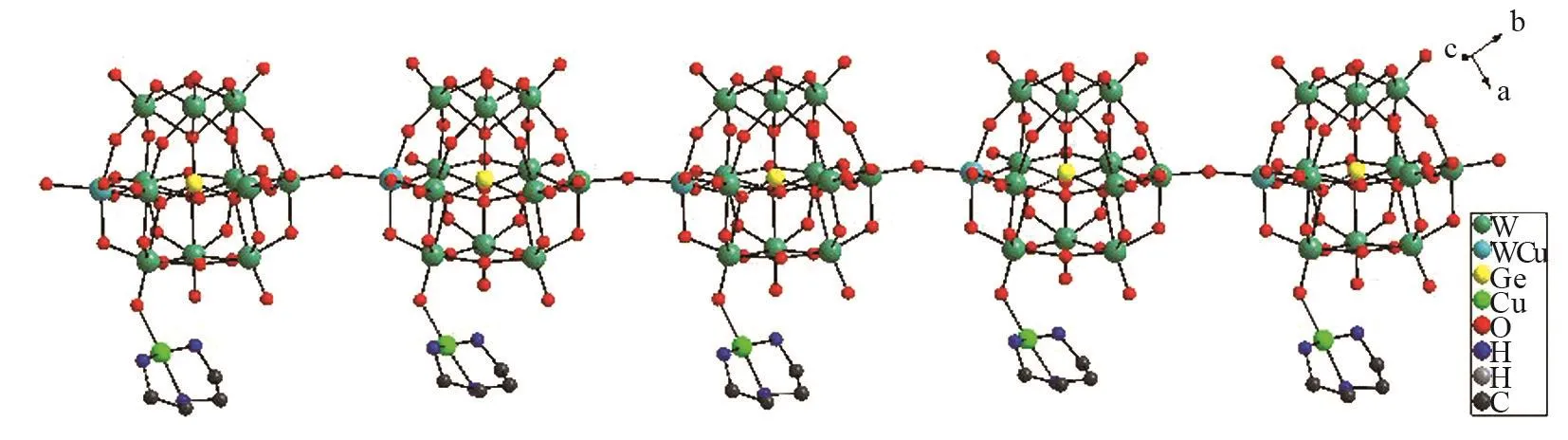
Fig.2 View of the 1D zigzag chain constructed by polyoxoanions {[Cu(dien)][GeW11.5Cu0.5O40]}4-in 1 along c⁃axis.The hydrogen atoms are omitted for clarity
2.2 IR spectrum of 1
As shown in Fig.3, there are four characteristic peaks of α⁃Keggin structure in 1: the peak at 938 cm-1is due to ν(W-Od), the peaks at 873 and 783 cm-1assigned to ν (W-Ob(c)-W) and the peak at 1 032 cm-1attributed to ν(Ge-Oa).This means that the polyoxoanion in 1 still retains the saturated Keggin structure[23].In addition, the characteristic peaks at 2 964 and 3 323 cm-1are attributed to ν(-CH2) and ν( - NH2) of the rigid ligands dien[9,11], and the peaks at 3 402 and 1 579 cm-1are assigned to ν(-OH)of the water molecules, respectively.
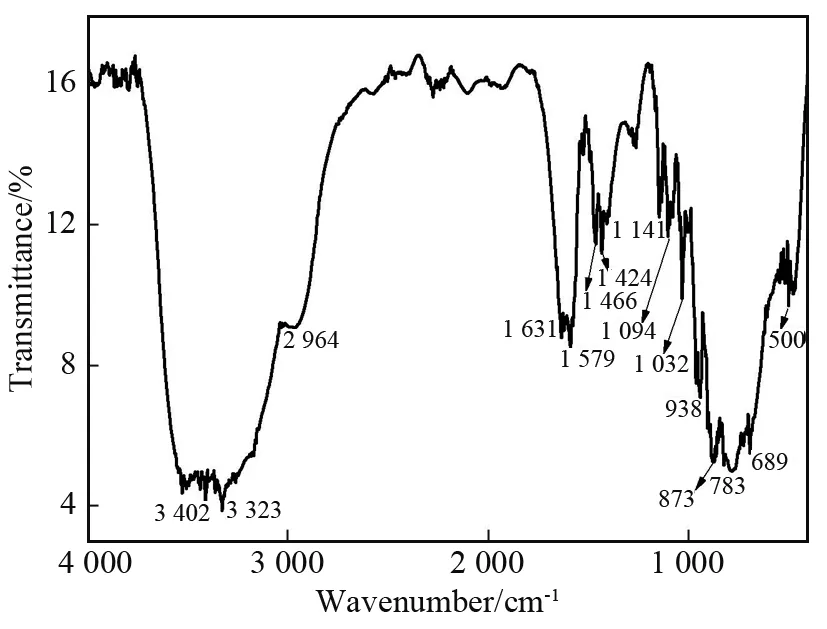
Fig.3 IR spectrum of 1
3 Conclusion
In summary,we have successfully isolated a new organic⁃inorganic hybrid with distinct structural feature based on the assembly of rigid diethylenetriamine ligand.The basic molecular unit of the isolated hybrid is composed of two Keggin⁃type anions {[Cu(dien)][ α⁃GeW11.5Cu0.5O40]}4-, two discrete copper coordination cations [Cu(dien)(H2O)]2+and[Cu(dien)(H2O)2]2+, and three crystal water molecules.The adjacent {[Cu(dien)] [α⁃GeW11.5Cu0.5O40]}4-
polyoxoanions are interconnected together through terminaloxygens (Od) into 1D zigzag chains.Furhtermore,synthesis of multidimensional hybrids by the incorporation of inorganic and organic units together into POMs also remain a challenge in POMs chemistry.
