Synergistic effects of anti-angiogenesis and immune checkpoint blockade -a new era of systemic chemotherapy for hepatocellular carcinoma
2021-11-08YujiEsoHiroshiSeno
Yuji Eso , Hiroshi Seno
Department of Gastroenterology and Hepatology, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Tumor growth requires oxygen and nutrient supplementation through angiogenesis from preexisting vascular beds. Hepatocellu-lar carcinoma (HCC) is a hyper-vascularized tumor with a predomi-nant arterial blood flow. Therefore, targeting angiogenesis is essen-tial for treating HCC. Currently approved molecular-targeted agents (MTAs) for HCC affect angiogenic pathways. Sorafenib, which was approved as the first MTA in 2008, targets vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor re-ceptor (PDGFR), c-Kit, and Raf kinases [1] . Sorafenib has a high in-cidence of adverse events (AEs) that impair quality of life, such as hand-foot skin reaction and diarrhea. Therefore, novel tolera-ble and effective first-line MTAs and second-line MTAs after so-rafenib failure were desired. Lenvatinib was approved as a novel first-line treatment in 2018 [2],and regorafenib and ramucirumab were approved as second-line treatments in 2017 and 2019, respectively [ 3,4 ]. Lenvatinib is an oral multi-tyrosine kinase in-hibitor targeting VEGFR, fibroblast growth factor receptor (FGFR), PDGFR-α, c-Kit, and rearranged during transfection (RET) [ 2,5 ]. Lenvatinib strongly inhibits angiogenesis by blocking VEGF and FGF pathways and has become a commonly used first-line MTA due to its high response rate and a relatively manageable AE profile [6] . Regorafenib targets VEGFR, RET, Kit, PDGFR, and Raf [3] . Ramu-cirumab is an anti-VEGFR-2 monoclonal antibody (mAb) that binds the extracellular domain of VEGFR-2 with high affinity and blocks VEGF-A, C, and D ligand binding [4] . These antiangiogenic MTAs have revolutionized the treatment for advanced HCC. However, the therapeutic outcomes are not satisfactory, and more effective ther-apies are expected to emerge.
Meanwhile, immunotherapy with immune checkpoint inhibitors (ICIs), including anti-programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) mAbs, has shown its benefit for patients with various types of cancers [7] . Recently, the synergistic effects of anti-angiogenesis and immune checkpoint in-hibition have been elucidated. Blocking the binding of PD-L1 to PD-1 with an ICI restores T cells’ ability to attack tumor cells. How-ever, angiogenesis in the tumor microenvironment (TME) can pre-vent immune cells from infiltrating the tumor tissue [8] . Vessels in the TME have poor vascular endothelial cell connections, result-ing in high vascular permeability to the tumor stroma. The gra-dient between intravascular pressure and tissue pressure becomes small, which reduces the oxygen diffusion capacity and leads to hypoxia. Hypoxia increases VEGF expression, promotes angiogen-esis, and increases PD-L1 expression [9] . Hypoxia also suppresses cytotoxic T cells and increases the recruitment of regulatory T cells (Tregs) [10] . In summary, the TME is altered in hypoxia, enhancing tumor survival ( Fig. 1 A).
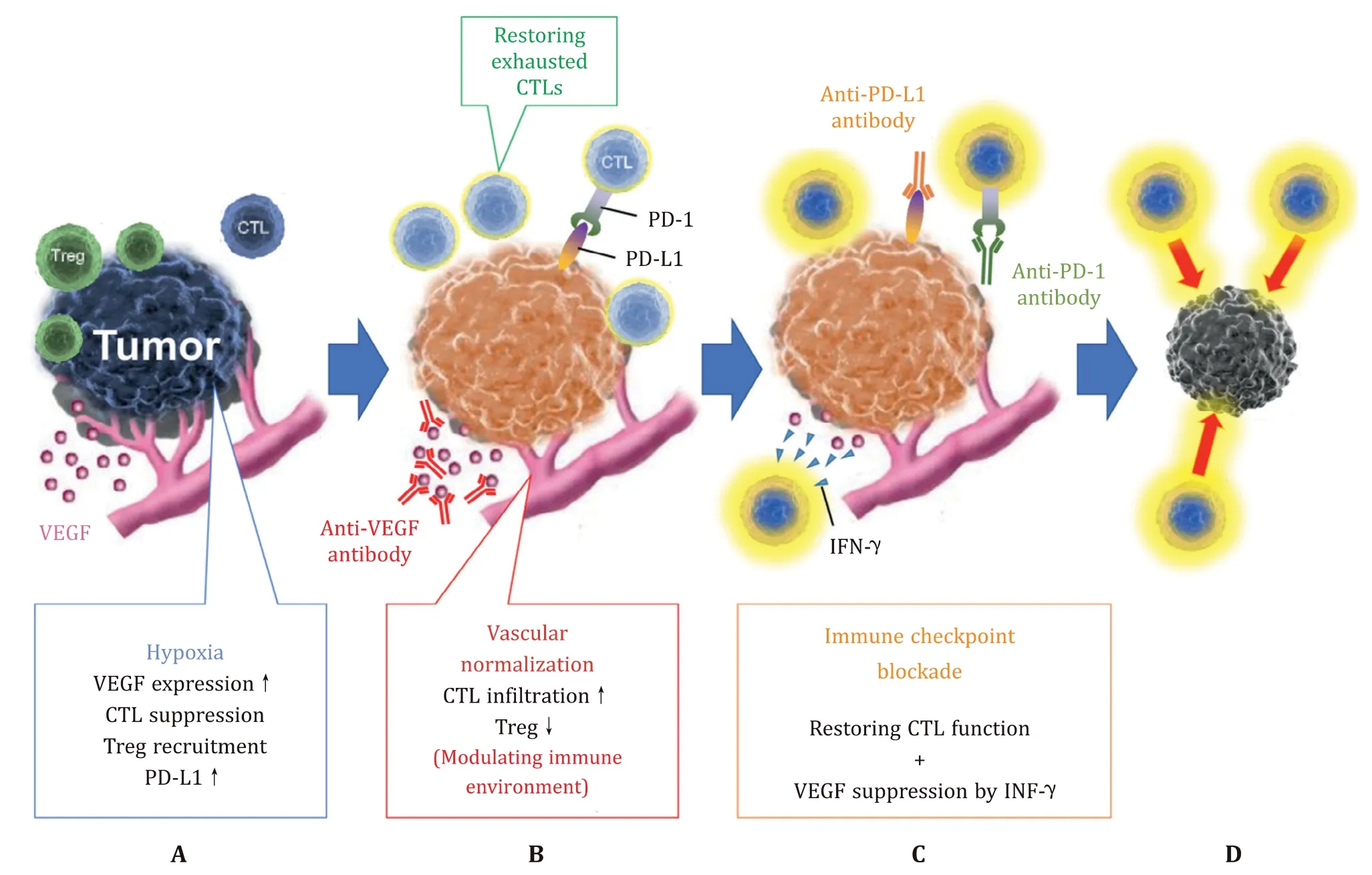
Fig. 1. The synergistic effects of anti-vascular endothelial growth factor (VEGF) therapy and immune checkpoint inhibitors (ICIs). A: Structural abnormalities of vessels in the tumor microenvironment cause tumors to become hypoxic. Hypoxia increases VEGF expression, suppresses the function of cytotoxic T cells (CTLs), and increases the recruitment of regulatory T cells (Tregs) and PD-L1 expression. B: Vascular normalization with anti-VEGF therapy increases intratumoral infiltration and survival of CTLs by modulating the immune microenvironment. C: Immune checkpoint blockade with anti-programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) antibodies restores the ability of CTLs to attack tumor cells. Interferon-gamma (IFN-γ), secreted by Th1 cells and activated by immune checkpoint inhibition, suppresses VEGF expression and promotes vascular normalization. D: Based on these mechanisms, anti-VEGF therapy and ICIs exert a synergistic anti-tumor effect.
Increased vascular permeability in tumor tissue appears to fa-cilitate immune cell migration and invasion, but they are sup-pressed in the TME. Migration of immune cells from the vascu-lar lumen to the tissue stroma requires adhesion factors such as intercellular adhesion molecule-1 expressed on vascular endothe-lial cells, which induces cell-cell adhesion between immune cells and vascular endothelial cells. The immune cells then extravasate and migrate into the tissue stroma according to the concentra-tion gradient of chemokines, which are formed around the vessels. However, overexpression of angiogenic factors in the TME sup-presses adhesion factors and prevents cell-cell adhesion [11] . In addition, the perivascular deposition of chemokines and the for-mation of their concentration gradients are suppressed in tumor vessels. This hampers immune cell infiltration of the tumor tis-sue, and even if successful, these cells are gradually exhausted by hypoxia. Moreover, vascular endothelial cells are constantly stimu-lated by inflammatory cytokines such as VEGF, prostaglandin E 2,and interleukin-10 [12] . VEGF-A induces the tumor endothelium to express Fas ligand. Fas ligand induces apoptosis as cytotoxic T cells (CTLs) pass through tumor vessels, whereas Tregs with high c-Flip expression, which suppress caspase expression, can es-cape apoptosis and infiltrate the tumor tissue [12] . Thus, the in-filtration of CTLs is suppressed in tumor blood vessels due to structural abnormalities and changes in ligand expression. There-fore, normalizing tumor blood vessels and improving the im-mune environment could enhance the therapeutic effect of cancer immunotherapy.
VEGF-A, mainly produced by tumor cells, tumor-associated macrophages, and tumor-associated fibroblasts, is overexpressed in HCC. As mentioned earlier, VEGF-A not only promotes angiogene-sis but also decreases T cell infiltration and increases the recruit-ment of Tregs. Therefore, anti-VEGF therapy increases intratumoral infiltration and survival of CTLs by normalizing vascularization and modulating the immune microenvironment ( Fig. 1 B), thus acting synergistically with ICIs. Furthermore, recent studies have revealed that thymocyte selection-associated high mobility group box pro-tein (TOX) plays a pivotal role in developing and maintaining ex-hausted T cells [ 13,14 ]. TOX reduces PD-1 degradation and pro-motes PD-1 translocation to the cell surface, thus maintaining high PD-1 expression at the surface of T cells [15] . A recent report re-vealed that VEGF-A drives TOX-dependent T cell exhaustion; there-fore, the combined blockade of PD-1/PD-L1 and VEGF pathways could effectively restore the antitumor function of T cells [16] . Fur-thermore, interferon-gamma (IFN-γ), secreted by Th1 cells and ac-tivated by ICIs, suppresses VEGF expression and promotes vascu-lar normalization ( Fig. 1 C) [17] . Altogether, the synergistic effect of anti-VEGF therapy and an ICI is not limited to the normalization of vascular permeability and the immune environment by anti-VEGF treatment; it represents a complementary synergy with ICI ( Fig. 1 D).
Several clinical trials of combined anti-VEGF and ICI ther-apy have been conducted, and the results of a phase III IM-brave 150 study evaluating the anti-PD-L1 mAb atezolizumab com-bined with the anti-VEGF mAb bevacizumab for unresectable HCC were recently reported [18] . Atezolizumab combined with beva-cizumab significantly improved overall survival compared to that of sorafenib [hazard ratio (HR) = 0.58; 95% confidence interval (CI): 0.42 to 0.79;P= 0.0 0 06]. Median progression-free survival (PFS) was 6.8 months and 4.3 months in the respective groups (HR = 0.59; 95% CI: 0.47 to 0.76;P<0.0 0 01). Interestingly, there was little difference between the treatment effect determined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and that determined by modified RECIST. Modified RECIST mainly evaluates decreased tumor vascularity, whereas RECIST focuses on changes in tumor size. The lack of differences in the determination via each guideline suggests that the success of the combination therapy may be caused more by a synergistic effect of normaliz-ing the immune microenvironment than by a reduction in tumor blood flow by bevacizumab.
Combined blockade of VEGF and PD-1/PD-L1 pathways has be-come a front-line treatment for non-small cell lung cancer, renal cell carcinoma, endometrial cancer, and HCC [19] . Currently, sev-eral other phase III studies evaluating the combination of antian-giogenic MTAs and ICIs for HCC are ongoing ( Table 1 ) [20] . In or-der to improve the treatment quality, biomarkers that can predict therapeutic efficacy are essential. However, it has been suggested that tumor mutation burden, an established predictive biomarker in ICI monotherapy, is of limited utility in anti-VEGF and PD-1/PD-L1 combination therapy [ 7,19,21 ]. Molecular profiling of the TME may be a useful approach to identify patients who may benefit from anti-VEGF and PD-1/PD-L1 combination therapy [22] . For ex-ample, exploratory data from a randomized phase II study of ate-zolizumab combined with bevacizumab in patients with renal cell carcinoma identified that myeloid inflammatory gene expression signature was strongly associated with longer PFS [21] . To improve treatment outcomes in advanced HCC, it is essential not only to identify predictive biomarkers but also to maintain liver reserve and take a multidisciplinary treatment approach, including surgery and locoregional therapy.
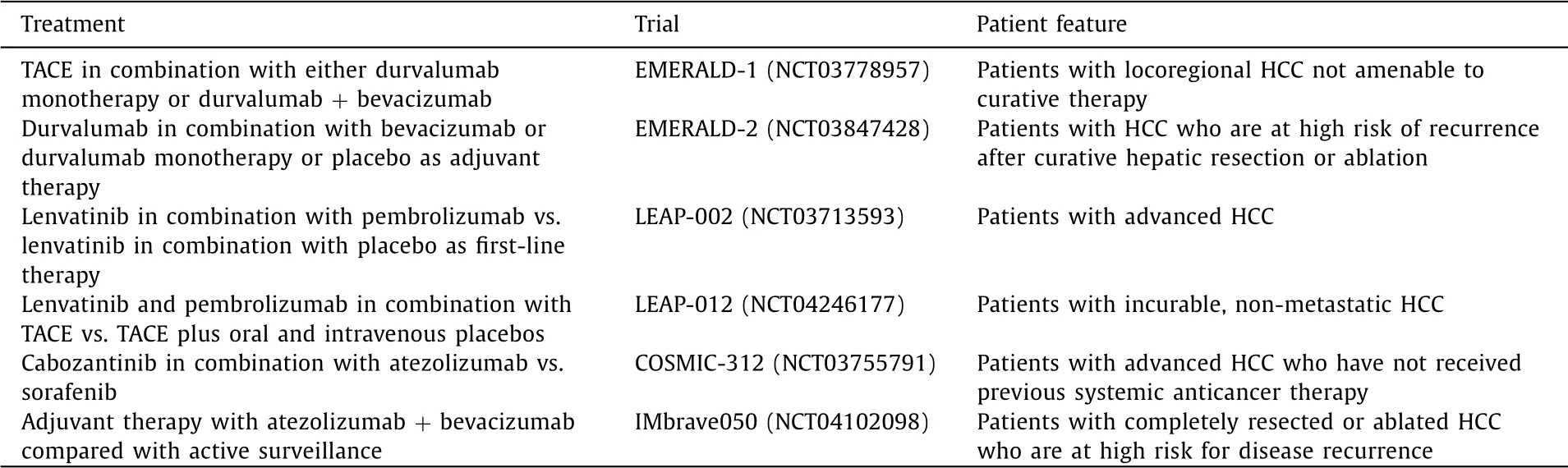
Table 1 Ongoing phase III studies evaluating the combination of antiangiogenic molecular-targeted agents and immune checkpoint inhibitors for hepatocellular carcinoma.
Acknowledgments
None
CRediT authorship contribution statement
Yuji Eso:Funding acquisition, Writing -original draft.Hiroshi Seno:Writing -review & editing.
Funding
This study was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (Grant number 19K17458 ).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub-ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Comparison and development of advanced machine learning tools to predict nonalcoholic fatty liver disease: An extended study
- Hepatobiliary&Pancreatic Diseases International
- Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment
- Key factors and potential drug combinations of nonalcoholic steatohepatitis: Bioinformatic analysis and experimental validation-based study
- LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver
- Cryptococcosis in patients with liver cirrhosis: Death risk factors and predictive value of prognostic models
