LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver
2021-11-08ZhongHuWngKnnthZhngXioDongWngJinQioYngYngLiLiZhngMingHuZhngJinWu
Zhong-Hu Wng ,Knnth I Zhng ,Xio-Dong Wng ,,,Jin Qio ,Yng-Yng Li ,Li Zhng ,Ming-Hu Zhng ,,,Jin Wu ,g,h,∗
a Department of Medical Microbiology and Parasitology, MOE/NHC/CAMS Key Laboratory of Medical Molecular Virology, School of Basic Medical Sciences, Fudan University Shanghai Medical College, Shanghai 20 0 032, China
b NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 3250 0 0, China
c Institute of Hepatology, Wenzhou Medical University, Wenzhou 3250 0 0, China
d Th e Key Laboratory of Diagnosis and Treatment for the Development of Chronic Liver Disease in Zhejiang Province, Wenzhou 3250 0 0, China
e Department of General Practice, Huaihai Middle Road Community Health Service Center of Huangpu District, Shanghai 20 0 025, China
f Department of Pathology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 3250 0 0, China
g Department of Gastroenterology and Hepatology, Zhongshan Hospital of Fudan University, Shanghai 20 0 032, China
h Laboratory of Fatty Liver and Metabolic Diseases, Shanghai Institute of Liver Diseases, Fudan University Shanghai Medi cal College, Shanghai 20 0 032, China
Keywords: Lipidomics Nonalcoholic steatohepatitis Nonalcoholic fatty liver disease Biomarker Nonalcoholic activity score Hepatic fibrosis
ABSTRACT Background: Nonalcoholic fatty liver disease (NAFLD) is one of the main liver diseases, and its pathologic profile includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). However, there is no reliable non-invasive parameter in distinguishing NASH from NAFL in clinical practice. The present study was to find a non-invasive way to differentiate these two categories of NAFLD via lipidomic analysis. Methods: Lipidomic analysis was used to determine the changes of lipid moieties in blood from 20 NAFL and 10 NASH patients with liver biopsy. Liver histology was evaluated after hematoxylin and eosin stain-ing and Masson’s trichrome staining. The profile of lipid metabolites in correlation with steatosis, inflam-mation, hepatocellular necroptosis, fibrosis, and NAFLD activity score (NAS) was analyzed. Results: Compared with NAFL patients, NASH patients had higher degree of steatosis, ballooning de-generation, lobular inflammation. A total of 434 different lipid molecules were identified, which were mainly composed of various phospholipids and triacylglycerols. Many lipids, such as phosphatidyl-choline (PC) (P-22:0/18:1), sphingomyelin (SM) (d14:0/18:0), SM (d14:0/24:0), SM (d14:0/22:0), phos-phatidylethanolamine (PE) (18:0/22:5), PC (O-22:2/12:0), and PC (26:1/11:0) were elevated in the NASH group compared to those in the NAFL group. Specific analysis revealed an overall lipidomic profile shift from NAFL to NASH, and identified valuable lipid moieties, such as PCs [PC (14:0/18:2), PE (18:0/22:5) and PC (26:1/11:0)] or plasmalogens [PC (O-22:0/0:0), PC (O-18:0/0:0), PC (O-16:0/0:0)], which were signifi-cantly altered in NASH patients. In addition, PC (14:0/18:2), phosphatidic acid (18:2/24:4) were positively correlated with NAS; whereas PC (18:0/0:0) was correlated positively with fibrosis score. Conclusions: The present study revealed overall lipidomic profile shift from NAFL to NASH, identified valuable lipid moieties which may be non-invasive biomarkers in the categorization of NAFLD. The cor-relations between lipid moieties and NAS and fibrosis scores indicate that these lipid biomarkers may be used to predict the severity of the disease.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the main liver diseases and its pathologic profile ranged from nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH) with varying de-grees of fibrosis and, in some cases, to cirrhosis [1] . NAFLD has be-come the most common metabolic disease underlying obesity, dys-lipidemia, metabolic syndrome and type 2 diabetes with a preva-lence of 15% in China [2],and 30% or higher in the North America and Europe [ 3–5 ]. The main difference between NASH and NAFL is the presence of hepatic inflammation, hepatocellular injury and cell death. Approximately 25% of patients with NAFL may develop NASH, 15%-20% of patients with NASH may further progress to fi-brosis and eventually cirrhosis, and at least 1%-2% of them may develop to HCC each year [ 3,5 ]. The progression from NAFL to NASH with fibrosis is a manifestation of disease activity, and the severity of liver fibrosis is a long-term determinant of complica-tions and death in NASH patients [ 6–8 ]. Thus, it is crucial to iden-tify NASH patients with fibrosis and take preventive measures to stop or reverse NASH progression. However, it is difficult to dis-tinguish NASH from NAFL with current laboratory tests and imag-ing modalities, and often requires invasive liver biopsy. Therefore, novel biomarkers are needed for noninvasive differentiating diag-nosis of NASH from NAFL and assessment of treatment responses in clinical practice.
Metabolomics has drawn great scientific attention due to its powerful measurement of numerous metabolites at accurate levels. Metabolomic methodology allows to understand overall metabolic alterations during the transition from healthy liver, NAFL to NASH [9] . Lipidomics is an important branch of metabolomics. It accurately analyzes the changes of lipid metabolites, determines the characteristics of lipid metabolism in different stages of dis-ease progression, and identifies valuable diagnostic indicators. As a metabolic disorder, NASH possesses tremendous metabolic ab-normalities in carbohydrates, lipids and other metabolites. General metabolomics may provide an overall profile of metabolic changes; whereas lipidomic analysis with ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS/MS) is able to re-veal lipids that are not seen in established analytical techniques, thus, may identify unusual lipid metabolic pathways or possible biomarkers for differentiating NASH from NAFL [10] . Moreover, routine lipid panels in clinical laboratory only examine limited range of lipids in the liver or bloodstream, and substantial changes in numerous lipids outside of these moieties are not commonly measured in a metabolomic panel. Given the important biological activity among lipids, especially for lipid species that are not rec-ognized, such as in informatory signaling pathways, lipidomic anal-ysis in pathologically confirmed patients may provide deep insights into the pathophysiology in the transition from NAFL to NASH as well as further fibrotic progression [11] . To explore the differenti-ating potential of lipidomic analysis in NAFLD patients, the UHPLC-MS/MS lipidomic platform was used to measure serum lipids of NASH patients and NAFL controls with liver biopsy, and to explore the relationship between abnormalities in blood lipid metabolism and extent of steatohepatitis and fibrosis in NASH patients. The ul-timate goal is to identify new biomarkers for noninvasive diagnosis and effective prevention of NASH.
Materials and methods
Patients and sample collection
Human serum samples of this study were obtained from the First Affiliated Hospital of Wenzhou Medical University. The study was approved by the Institutional Review Board of the First Affili-ated Hospital of Wenzhou Medical University and the Ethics Com-mittee of Fudan University School of Basic Medical Sciences. In our study, all subjects were enrolled in accordance with ethical guide-lines, and written informed consent was obtained prior to the par-ticipation. All clinical procedures involved in this study were per-formed in accordance with the relevant guidelines and regulations. All subjects were ruled out of alcoholic liver or other causes of liver diseases (hepatitis B, or C infection, autoimmune hepatitis, drug toxicity, etc.) by methods previously described [12] .
According to liver histology, serum from 30 subjects was di-vided into two groups: NAFL control group (n= 20) and NASH group (n= 10). Blood samples were collected from subjects who were fasted overnight (8 h) and placed in cold tubes containing ethylene diamine tetraacetic acid (EDTA) (6 mmol/L). Serum was separated by centrifugation (2500 rpm, 15 min) and aliquots were immediately frozen at -80 °C until analysis (avoiding repeated freezing and thawing) [13] . Table 1 summarizes the demographic information and results of clinical laboratory parameters of the enrolled subjects, including NAFLD activity score (NAS), index of homeostasis model assessment insulin resistance (HOMA-IR), con-trolled attenuation parameter (CAP) from ultrasound stiffness ex-amination, and semiquantitative score of fibrosis. The calculation of HOMA-IR was made according to the formula as described pre-viously [14] .

Table 1 Clinical and biological characteristics of NASH and NAFL patients.
Ultrasonographical examination for hepatic steatosis
All patients have undergone sonographic examination for hep-atic fatty content measurement of CAP by a well-trained special-ist. The CAP measures ultrasonic attenuation in the liver at 3.5 MHz using signals acquired by the FibroScan®M probe based on vibration-controlled transient elastography (VCTETM). The princi-ples were described previously [15] .
Tissue staining of liver biopsy
An ultrasound-guided liver biopsy was performed under se-dation using an 18-gauge Hepafix needle (Gallini, Modena, Italy). Biopsy specimens were fixed in 10% formalin, embedded in paraf-fin, cut and stained with hematoxylin and eosin and Masson’s trichrome. Histopathology of all biopsies was examined by an ex-perienced and board-certified liver pathologist (Dr. Yang-Yang Li), who was blinded to clinical and laboratory data of participants. The histologic features of NAFLD were scored according to the NASH Clinical Research Network classification [16] . Each slide was evaluated for two aspects, NAS (0–8) and fibrosis stage (1–4). NAS was calculated based on the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3) and hepatocellular bal-looning (0–2) [16] . Fibrosis was staged in both HE and trichrome staining sections according to Ludwig’s and Scheuer’s classifica-tions, from 0 to 4: 0 = no fibrosis; 1 = perisinusoidal or portal fi-brosis; 2 = perisinusoidal and portal/periportal fibrosis; 3 = bridg-ing fibrosis; 4 = highly suspicious or definite cirrhosis [ 17–19 ]. If NAS of a liver specimen is ≥5, the section is defined as “NASH”. If NAS is ≤4, it represents as “NAFL”[16] . All stained sections were photographed under light microscopy with ×200 amplification.
Non-targeted lipidomics
The ultra-high performance liquid tandem chromatography quadrupole time of flight mass spectrometry (UHPLC-QTOF/MS) analysis was performed utilizing an UHPLC system (1290 series, Agilent Technologies, Santa Clara, CA, USA) coupled to a quadru-ple time-of-flight (QTOF) mass spectrometer (Agilent 6550 iFunnel QTOF, Agilent Technologies). A Waters ACQUITY UPLC BEH Amide column [particle size, 1.7μm; 100 mm (length) ×2.1 mm (i.d.)] was used for the lipid extract (LE) separation, and the column temperature was maintained at 25 °C. The flow rate was set as 0.6 mL/min, and the sample injection volume was 2μL. The mo-bile phase A was 25 mM ammonium hydroxide (NH4OH) + 25 mM ammonium acetate (NH 4 OAc) in water, and B was acetonitrile (ACN) in positive mode (ESI + ). The gradient was set as follows: 0–0.5 min, 95% B; 0.5–7 min, 95% B to 65% B; 7–8 min, 65% B to 40% B; 8–9 min, 40% B; 9–9.1 min, 40% B to 95% B; 9.1–12 min, 95% B. The acquisition rate was set as 4 spectra/second, and the time-of-fight (TOF) mass range was set as m/z 50–1200 Da in a positive mode. The parameters of mass spectrometry (MS) data ac-quisition were set as follows: sheath gas temperature, 400 °C; dry gas temperature, 250 °C; sheath gas flow, 12 L/min; dry gas flow, 16 L/min; capillary voltage, 30 0 0 V; nozzle voltage, 0 V; and neb-ulizer pressure, 20 psi. Tandem mass spectrometry (MS/MS) data acquisition was performed using another QTOF S2 mass spectrom-eter (Triple TOF 5600 +,AB SCIEX, USA). Quality control samples were used for MS/MS data acquisition. To expand the coverage of MS/MS spectra, the mass range was divided into 4 segments: 50–30 0 Da, 290–60 0 Da, 590–90 0 Da, and 890–1200 Da. The acquired MS/MS spectra were matched against in-house tandem MS/MS li-brary for metabolite identification. The source parameters were set as follows: GAS1, 60; GAS2, 60; CUR, 30; TEM, 600 °C; and ISVF, 5500 V [20] . The analysis was performed in the Lipidomics Service Platform [21],Shanghai Life Science Institutes, Chinese Academy of Sciences, Shanghai, China.
Raw data preprocessing
The ionization source of the LC-QTOFMS platform is electro-spray ionization. There are two ionization modes: positive ion mode (POS) and negative ion mode (NEG). The raw data of the pos-itive ion mode contained 4 quality control samples, 20 NAFL and 10 NASH samples. A total of 327 peaks were extracted from them. In order to better analyze the data, a series of preparation and data management on the original data were undertaken. They mainly included the following steps: (1) filtering a single peak to remove noise. The deviation was filtered based on the relative standard de-viation (RSD), or coefficient of variation (CV). (2) Filtering on a sin-gle peak. Only peak area data with no more than 50% null values in a single group or no more than 50% null values in all groups were retained. Missing value recoding was performed on the orig-inal data. The numerical simulation method was to fill in half of the minimum value. Data normalization was performed using the total ion current (TIC) of each sample. After preprocessing, 327 sta-tistically significant peaks were retained.
Statistical analysis
All statistical analyses were conducted using the SPSS Version 2.0 (SPSS, Chicago, IL, USA). Data were presented as means ±stan-dard deviation (SD) or frequencies. Baseline characteristics of the study population were compared using the one-way ANOVA analy-sis for continuous variables. The final dataset containing the infor-mation of peak number, sample name and normalized peak area were imported to SIMCA15.0.2 software package (Sartorius Stedim Data Analytics AB, Umeå, Sweden) for multivariate analysis. Data were scaled and logarithmic transformed to minimize the impact of both noise and high variance of the variables. After these trans-formations, principle component analysis (PCA) was carried out to visualize the distribution and the grouping of the samples. We used 95% confidence interval in the PCA score plot as the thresh-old to identify potential outliers in the dataset. In order to visualize group separation and find significantly altered metabolites, super-vised orthogonal projection to latent structures-discriminate anal-ysis (orthogonal partial least-square, OPLS-DA) was applied. Then, a 7-fold cross validation was performed to calculate the value of R2and Q2. R2indicates how well the variation of a variable is ex-plained and Q2means how well a variable could be predicted. To check the robustness and predictive ability of the OPLS-DA model, 200 times of permutations were further conducted. Afterward, the R2and Q2intercept values were obtained. Here, the intercept value of Q2represents the robustness of the model, the risk of over-fitting and the reliability of the model, and the smaller the bet-ter. Furthermore, the value of variable importance in the projec-tion (VIP) of the first principal component in OPLS-DA analysis was obtained. It summarizes the contribution of each variable to the model. The metabolites with VIP>1 andP<0.05 (Student’sttest) were considered as significantly altered. Spearman rank cor-relation was used to analyze the correlation between lipids and liver pathological scores. At the same time, the lipidomic data were also verified by the PLS-DA model to confirm suitability and lia-bility of OPLS-DA modeling (Fig. S1) [22] . As shown in the figure, the PLS-DA model was used to re-analyze the data from two NASH and NAFL groups. Through the analysis, it is clear that there is no significant difference in the first and second principal component analyses between the groups. The parameters (PLS-DA model pa-rameter table) used in PLS-DA analysis are the same as in the pre-vious OPLS-DA model. The only difference is that PLS-DA lacks pos-itive exchange calculation analysis; whereas OPLS-DA model may filter out the signals that are not related to the model classifica-tion, hence its interpretation ability is stronger than PLS-DA.

Fig. 1. Lipid profiles of serum samples from the NAFL and NASH groups and preliminary data processing. A: Proportion of major lipid components. B: Principal component analysis (PCA) score plot of metabolic profile of the NASH and NAFL groups after mean-centering and not (Ctr) scaling. C: OPLS-DA score plot of lipid profile of the NASH and NAFL groups after unit variance (uv) scaling. D: Score plot of OPLS-DA model obtained from C . The resulting R 2 and Q 2 values were plotted. The red dots represent the R 2 Y values obtained from the displacement test; the blue square dots represent the Q 2 values obtained from the displacement test; and the two dashed lines represent the regression lines of R 2 Y and Q 2,respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)

Fig. 2. Heatmap of hierarchical cluster analysis of differential metabolites in the NASH and NAFL groups. The columns at the left side of the solid line represent the samples from the NASH group; while those at the right side of the solid line represent the samples from the NAFL group. Levels of the discriminating metabolites in all samples are shown in color. Up-regulated metabolites are presented with the red color, while the down-regulated metabolites are shown with the blue color. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Results
Demographics
Thirty NAFLD patients (20 cases of NAFL and 10 cases of NASH) were included in the present study. The detailed baseline charac-teristics including demographics, body mass index (BMI), biochem-ical tests, HOMA-IR, CAP value and histopathologic description of liver biopsy from all patients are detailed in Table 1 . It is clear that NASH patients had a higher BMI (P= 0.02), higher serum levels of AST/ALT ratio (P= 0.05) and slightly higher GGT (P>0.05) than NAFL patients. Fasting serum insulin levels tended to be higher in NASH patients than NAFL patients, however, statistical analysis did not show any significance (P>0.05). Routine liver biochemical and metabolic tests [such as serum glucose, bilirubin, high density lipoprotein (HDL), low density lipoprotein (LDL), triglyceride (TG), total cholesterol did not significantly differ between the NASH and NAFL groups ( Table 1 ). Due to very close serum glucose and fast-ing insulin levels, HOMA-IR did not show any difference between these two groups although both of them were above the normal range (5.80 and 8.17), and there was a tendency that NASH patients had a more severe insulin resistance than NAFL patients. Compared to the NAFL group, patients in NASH group had much higher NAS levels with a severe degree of steatosis (CAP and histology), bal-looning degeneration, lobular inflammation, and there was a ten-dency that NASH group had higher semiquantitative score of hep-atic fibrosis in Masson’s staining. Thus, AST/ALT ratio, CAP read-ing, NAS and extent of fibrosis were separable in patients between NAFL and NASH, and were consistent with clinical and patholog-ical diagnosis. As the most reliable pathologic variables, NAS and semi-quantitative fibrosis score were used as two major references to determine differential values of lipidomic measurements as well as the base for correlation analysis, although there was no sig-nificantly statistical difference in fibrosis score between these two groups.
Composition of serum lipid extracts
A total of 434 different lipid molecules, which were predom-inantly composed of various phospholipids (PLs), were identi-fied from analyzed serum samples. There were 166 phosphatidyl-cholines (PC), 108 phosphatidylethanolamines (PE), 59 sphin-gomyelins (SM), 37 lyso-phosphatidylcholines (LPC), 21 phos-phatidylglycerols (PG), 17 phosphatidylinositols (PI), 13 phospha-tidic acids (PA), 12 lyso-phosphatidylethanolamines (LPE), and 1 phosphatidylserines (PS) in the identified lipid pool, which were further analyzed. The percentage of each lipid in the total pool is shown in Fig. 1 A and Table S1.
The results of multivariate statistical analysis
To evaluate whether NASH affects lipid synthesis and compo-sition, 327 peaks remained after the removal of noise based on the interquartile range and normalization using TIC of each sam-ple. After obtaining the collated data, a series of multivariate pat-tern recognition analysis was undertaken. First, PCA was initially applied to the spectra to visualize inherent clustering between the NAFL control and NASH groups. As shown in the PCA score map, the isolated samples were basically within the 95% confi-dence interval (Hotelling’s T-squared ellipse); however, there was some overlap between the NAFL and NASH groups ( Fig. 1 B). In order to obtain a high level of group separation and for a better understanding of the variables responsible for classification, super-vised OPLS-DA was applied. From the OPLS-DA score ( Fig. 1 C), it is evident that isolated samples have a clear separation between these two groups. Then it was further verified whether the model established in this way reflects the actual situation of the lipid data by OPLS-DA permutation test. As shown in Fig. 1 D, the original R2Y model was very close to 1, indicating that the OPLS-DA model was consistent with the actual situation of the lipid data. In general, the original model may explain the differences in lipid distribution between these two groups in convincing robustness and there was no over-fitting phenomenon (R2Y = 0.65).
Dif ferences in lipid metabolites between the NAFL and NASH groups
In order to find out the lipid moieties whose concentrations were altered in the NASH group compared to those in the NAFL group, all the ion peaks were defined with their VIP in the OPLS-DA model, which was superior to 1. A Student’sttest was performed between these two groups. Peak areas withPvalue higher than 0.05 were excluded as indicated in Fig. S2. Then the heatmap was created to perform hierarchical clustering analysis on such features ( Fig. 2 ), which aids in classifying lipid moieties with the same features into one class and exhibits the signifi-cant difference between the NASH and NAFL groups. From the heatmap, it is clear that PC (P-22:0/18:1) (1.3:1,P= 0.037), SM (d14:0/18:0) (1.3:1,P= 0.003), SM (d14:0/24:0) (1.4:1,P= 0.012), SM (d14:0/22:0) (1.6:1,P= 0.033), PE (18:0/22:5) (1.4:1,P= 0.025), PC (O-22:2/12:0) (1.4:1,P= 0.045), and PC (26:1/11:0) (P= 0.011) in the NASH group were significantly higher than the NAFL group. Whereas PC (22:6/0:0) (0.6:1,P= 0.0 0 01), PC (16:1/0:0) (0.7:1,P= 0.001) and SM (d14:0/12:0) (0.77:1,P= 0.023) in the NASH group were lower than the NAFL group. Therefore, the value of these lipid moieties appears to be differentially distributed be-tween the NASH and NAFL groups. Relevant data are shown in Ta-ble S1.
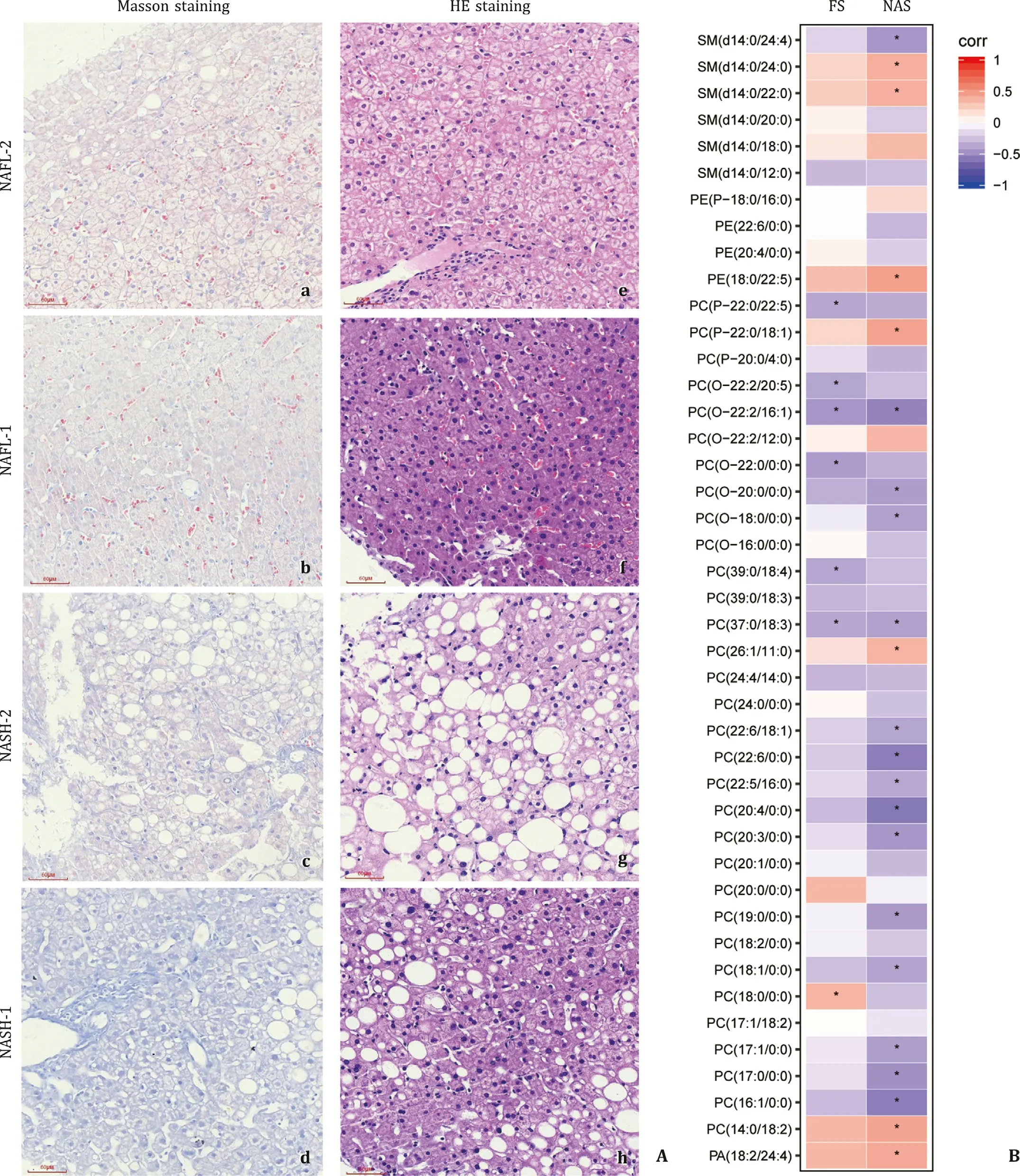
Fig. 3. Pathological staining and correlation analysis. A: Masson trichrome and hematoxylin-eosin (HE) staining of liver biopsy tissue from the NASH and NAFL groups. a, b, e, f represent the samples from the NAFL group, while c, d, g, h represent the samples from the NASH group. B : Correlation between lipid changes and liver pathological scores. FS: fibrosis score. Negative and positive regression value is presented in color. NAS: NAFLD activity score. Images were taken at original magnification ( ×200). Scale bars = 60 μm ( e,f,g,h )
Hepatocellular steatosis and fibrosis in NAFL and NASH
To determine the extent of hepatic fibrosis, Masson’s trichrome staining of liver biopsy tissue was performed. It is evident that collagenous fibrils were deposited in the pericellular space in the NASH group ( Fig. 3 Ac,d), which was more severe than the NAFL group ( Fig. 3 Aa,b). Hepatocytes in the NASH group appeared to be steatotic in severe degrees as shown by HE staining ( Fig. 3 Ag, h), whereas steatosis in the NAFL group ( Fig. 3 Ae, f) was mild and less visible in comparison to the NASH group. Thus, the main patho-logic difference of NASH from NAFL was the severity of steatosis, occurrence of inflammatory reaction, and fibrotic progression as observed in HE and Masson’s staining, and they were well reflected by NAS and fibrosis scores in Table 1 . The statistical difference in NAS between the NASH and NAFL groups was striking (P<0.0 0 01 in NAS), although the fibrosis score did not reach statistical sig-nificance (P= 0.08). The NAS and fibrosis score are well-accepted semiquantitative parameters to reflect the severity of NASH with fibrotic progression.
Correlation between lipid changes and semi-quantitative scores of pathological assessments
NAS and fibrosis scores were used to further define whether al-tered profile of lipid metabolites in the NASH group reflects un-derlying pathologic changes of steatosis, inflammation, hepatocel-lular necroptosis, and fibrosis. As shown in Fig. 3 B, PE (18:0/22:5) and PC (14:0/18:2) are positively correlated with NAS (regression values: 0.46 and 0.43,P= 0.01, respectively). In contrast, PCs (sat-urated or monounsaturated), such as PC (22:6/0:0), PC (20:4/0:0) and PC (16:1/0:0) were negatively correlated with NAS (regression value: -0.56, -0.58 and -0.56,P= 0.0 01, 0.0 0 06 and 0.0 01, respec-tively). Of note, PC (18:0/0:0) was positively correlated with liver fibrosis score (regression value: 0.36,P= 0.04). Moreover, a large proportion of plasmalogens, such as PC (O-22:2/16:1) and PC (O-22:0/0:0) were negatively correlated with liver fibrosis scores (re-gression values: -0.45 and -0.41,P= 0.01 and 0.02, respectively). In summary, lipid moieties are differentially distributed in the NASH and NAFL groups with positive or negative correlation to the sever-ity of NASH and fibrosis progression. These correlations may im-ply pathophysiologic importance and potential clinical references of significantly altered lipid moieties as biomarkers for NASH pro-gression. Presumably, a combination of these lipid moieties may help to differentiate NASH from NAFL.
Discussion
It is well known that the changes in lipid homeostasis, such as cholesterol esters, TG, diacylglycerol, and sphingomyelin in blood and liver tissues are the feature of NAFLD [ 23–26 ]. Existing studies have shown that there are significant differences in lipid profiles between healthy subjects and NAFLD patients [10] . However, it re-mains a real challenge in using lipid data to distinguish NASH from NAFL patients. The biological effects of changes in lipid composi-tion are very complex and multifaceted, and depend on their rel-ative cellular and subcellular locations [ 27,28 ]. They may function as key signaling molecules, transcription regulators or check-point substances in metabolism, immunity, or cellular responses [ 29–31 ]. They may further affect membrane fluidity. Lipid peroxidation may occur under oxidant stress, and cause lipotoxicity [32] .
Several studies employing metabolomics or lipidomics are avail-able to determine metabolic or lipidomic profile changes among controls, NAFL and NASH [ 33–35 ]. Puri and colleagues demon-strated that [33] serum 5-hydroxyeicosatetraenoic acid (5-HETE), 8-HETE, and 15-HETE are increased in a stepwise manner in the progression from normal to NAFL, further to NASH. Our present study identified the differences in lipid characteristics between NAFL and NASH patients. Through rigorous statistical analysis, we found that phospholipids: PC (P-22:0/18:1), PC (26:1/11:0), PE (18:0/22:5) and PE (18:0/22:5) were increased in patients with NASH; whereas PC (22:6/0:0), PC (16:1/0:0) and SM (d14:0/12:0) were decreased in these patients compared to NAFL. Therefore, NASH lipidomic profile was much shifted from NAFL, and the changes in individual PC, PE components demonstrated signifi-cant alteration in lipid metabolism in NASH patients, suggesting that abnormal phospholipid metabolism is involved in the tran-sition from NAFL to steatohepatitis. Furthermore, individual moi-eties of lipids which were either increased or decreased in NASH patients from this study were different from those previously re-ported [ 33,34 ]. Thus, our findings are additive to the previous stud-ies in terms of changes in specific individual lipid moieties in the transition from NAFL to NASH.
With the availability of pathological evidence, we found that the changes in individual lipid moieties were correlated with the severity of NASH and fibrotic progression. Through cluster analysis combined with small lipid molecules in the correlation with NAS and fibrosis score, we found that PC (18:0/0:0) was positively cor-related with fibrosis score, and PC (14:0/18:2), PA (18:2/24:4) were positively correlated with NAS. Therefore, the lipid moieties may be non-invasive serum markers indicating NASH progression. This is a significant advance in this field, since, to our knowledge, this is the first study to reveal these correlations in NAFLD patients. Moreover, a combinatory modeling with 10 increased lipid species predicts the presence of liver fibrosis in NASH patients [11] . With healthy controls and a better cross-section study, polyunsaturated fatty acid metabolites, such as 13,14-dihydro-15-keto prostaglandin D2 (dhk PGD2) and 20-carboxy arachidonic acid (20-COOH AA) were found to be of diagnostic values for NAFL and NASH [36] .
In conclusion, the findings of this study demonstrated overall lipidomic profile shift from NAFL to NASH, and identified valuable lipid moieties. The correlations of altered lipids to NAS and fibro-sis scores indicate differential value of these lipid biomarkers in NAFLD.
Acknowledgments
We are grateful to Prof. Hui-Yong Yin and his team from the Shanghai Life Science Institutes, Chinese Academy of Sciences, Shanghai, China for careful analysis of lipidomic and valuable assis-tance in data analysis. We are in debt to Prof. Xiu-Ping Liu from the Department of Pathology, Fudan University School of Basic Medical Sciences in helping with micrographs of histopathologic images.
CRediT authorship contribution statement
Zhong-Hua Wang :Data curation, Formal analysis, Investi-gation, Writing original draft.Kenneth I Zheng :Investigation.Xiao-Dong Wang :Investigation. Jin Qiao :Data curation, Formal analysis.Yang-Yang Li :Investigation.Li Zhang:Data curation, In-vestigation.Ming-Hua Zheng :Data curation, Investigation, Super-vision.Jian Wu :Conceptualization, Data curation, Funding acquisi-tion, Supervision, Writing review & editing.
Funding
This study was supported by grants from the Ministry of Sci-ence & Technology of China ( 2016YFE0107400 ), the National Nat-ural Science Foundation of China ( 81272436,81572356,81871997,81500 6 65 and 82070588 ) and High Level Creative Talents from De-partment of Public Health in Zhejiang Province (S203210260 0 032) and Project of New Century 551 Talent Nurturing in Wenzhou.
Ethical approval
The study was approved by the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University and the Ethics Committee of Fudan University School of Basic Medical Sci-ences. In our study all subjects were collected in accordance with ethical guidelines, and written informed consent was received. All patients were approached based on approved ethical guidelines, and patients who agreed to participate in this study were required to sign consent forms before being included in the study.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub-ject of this article.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.hbpd.2021.05.008 .
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Comparison and development of advanced machine learning tools to predict nonalcoholic fatty liver disease: An extended study
- Hepatobiliary&Pancreatic Diseases International
- Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment
- Key factors and potential drug combinations of nonalcoholic steatohepatitis: Bioinformatic analysis and experimental validation-based study
- Cryptococcosis in patients with liver cirrhosis: Death risk factors and predictive value of prognostic models
- Gene knockout or inhibition of macrophage migration inhibitory factor alleviates lipopolysaccharide-induced liver injury via inhibiting inflammatory response
