Cryptococcosis in patients with liver cirrhosis: Death risk factors and predictive value of prognostic models
2021-11-08QiHuiZhouCaiQinHuYuShiFengTianWuQinYangJunGuanAiChunLiZhiChen
Qi-Hui Zhou ,Cai-Qin Hu ,Yu Shi, Feng-Tian Wu, Qin Yang, Jun Guan, Ai-Chun Li, Zhi Chen
Department of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 0 0, China
Keywords: Cryptococcosis Cirrhosis Risk factors Prognosis
ABSTRACT Background: Liver cirrhosis is associated with immune deficiency, which causes these patients to be sus-ceptible to various infections, including cryptococcus infection. Mortality in cirrhotic patients with cryp-tococcosis has increased. The present study was to explore the risk factors of mortality and the predictive ability of different prognostic models. Methods: Forty-seven cirrhotic patients with cryptococcosis at a tertiary care hospital were included in this retrospective study. Data on demographics, clinical parameters, laboratory exams, diagnostic meth-ods, medication during hospitalization, severity scores and prognosis were collected and analyzed. Stu-dent’s t test and Mann-Whitney test were used to compare characteristics of survivors and non-survivors at a 90-day follow-up and cerebrospinal fluid (CSF) manifestations of cryptococcal meningitis. Multivari-ate Cox regression analysis was used to identify the independent risk factors for mortality. Kaplan-Meier curves were used to analyze patient survival. Receiver operating characteristic (ROC) curves were used to evaluate the different prognostic factors. Results: The 30-and 90-day survival rates were 93.6% and 80.9%, respectively, in cirrhotic patients with cryptococcosis. Cryptogenic liver diseases [hazard ratio (HR) = 7.567, 95% confidence interval (CI): 1.616-35.428, P = 0.010], activated partial thromboplastin time (APTT) (HR = 1.117, 95% CI: 1.016-1.229, P = 0.022) and Child-Pugh score (HR = 2.146, 95% CI: 1.314-3.504, P = 0.002) were risk factors for 90-day mortality in cirrhotic patients with cryptococcosis. Platelet count (HR = 0.965, 95% CI: 0.940-0.991, P = 0.008) was a protective factor. APTT (HR = 1.120, 95% CI: 1.044-1.202, P = 0.002) and Child-Pugh score (HR = 1.637, 95% CI: 1.086-2.469, P = 0.019) were risk factors for 90-day mortality in cirrhotic pa-tients with cryptococcal meningitis. There was significant difference in the percentage of lymphocytes in CSF between survivors and non-survivors [60.0 (35.0-75.0) vs. 95.0 (83.8-97.2), P < 0.001]. The model of end-stage liver disease-sodium (MELD-Na) score was more accurate for predicting 30-day mortality both in patients with cryptococcosis [area under curve (AUC): 0.826, 95% CI: 0.618-1.0 0 0] and those with cryptococcal meningitis (AUC: 0.742, 95% CI: 0.560-0.924); Child-Pugh score was more useful for predict-ing 90-day mortality in patients with cryptococcosis (AUC: 0.823, 95% CI: 0.646-1.0 0 0) and those with cryptococcal meningitis (AUC: 0.815, 95% CI: 0.670-0.960). Conclusions: These results showed that cryptogenic liver diseases, APTT and Child-Pugh score were as-sociated with mortality in cirrhotic patients with cryptococcosis and cryptococcal meningitis. MELD-Na score was important for predicting 30-day mortality, and Child-Pugh score was critical for predicting 90-day mortality.
Introduction
Liver cirrhosis is an end-stage liver disease that results from different kinds of chronic liver diseases. The 1-year mortality of liver cirrhosis ranges from 1% to 57%. Cirrhosis is a dynamic pro-cess: stage 1 is compensated with no esophageal varices; stage 2 is compensated with varices; stage 3 is decompensated with ascites; stage 4 is decompensated with gastrointestinal bleeding; and stage 5 is characterized by infections and renal failure, with a 1-year mortality of 67% [1] . Liver cirrhosis is also associated with immune dysfunction, including decreased cellular and humoral immunity, reduced phagocytic activity of innate immune cells, and comple-ment deficiency [ 2,3 ]. A previous study found that infections in cirrhotic patients quadrupled mortality [4] .
Cryptococcosis is an invasive fungal infection that primarily manifests as cryptococcal meningitis, pulmonary cryptococcosis, and disseminated cryptococcosis. Cryptococcosis generally occurs in individuals with compromised cellular immunity, including peo-ple with chronic renal diseases, rheumatic immune diseases, hu-man immunodeficiency virus (HIV) infection, diabetes, organ trans-plantation, and immunosuppressive drug administration [5] . How-ever, patients with cryptococcosis may also be immunocompe-tent [6] . Different manifestations are associated with varying co-morbidities. For example, HIV-positive patients primarily develop cryptococcal meningitis, but they also develop pulmonary crypto-coccosis.
Few studies focused on risk factors for death due to cryptococ-cosis in liver cirrhotic patients. Recognition of these risk factors is of great importance to improve the survival rate. The current study aimed to: (1) investigate the epidemiology of liver cirrhotic patients with cryptococcosis and cryptococcal meningitis, (2) iden-tify independent risk factors for mortality within 30 and 90 days, and (3) compare the value of prognostic models for liver cirrhotic patients with cryptococcosis.
Methods
Study design and patients
A total of 10 800 cirrhotic patients from the First Affiliated Hos-pital of Zhejiang University School of Medicine (Hangzhou, China) were screened between January 2010 and July 2020, and 47 cir-rhotic patients with cryptococcosis were included in this study (patients with cryptococcosis referred to those with cryptococcal meningitis, cryptococcal pneumonia, disseminated cryptococcosis and cryptococcosis in other organs). Of those, 31 (66.0%) had hep-atitis B virus infection, 3 (6.4%) had alcoholic liver diseases, 3 (6.4%) had autoimmune liver diseases, 2 (4.3%) had cholestatic liver disease, and the remaining 8 (17.0%) had cryptogenic liver diseases. Cirrhosis was diagnosed by (1) liver biopsy, (2) radiological evi-dence of lesions, and (3) clinical manifestations of decompensation, including ascites, hepatic encephalopathy, splenomegaly, and portal hypertension. Cryptococcosis was diagnosed by (1) positive culture of cryptococcus from biological samples, (2) a positive result of In-dia ink staining of cerebrospinal fluid (CSF), (3) histological stain-ing of tissues, and (4) positive results from the cryptococcal cap-sular polysaccharide antigen test. The following exclusion criteria were used: (1) under 18 or over 80 years old; and (2) incomplete clinical data. This study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Data collection
The following information was collected: age, sex, length of stay, etiologies and complications of liver cirrhosis, comorbid-ity, medication prior to admission, laboratory parameters, diag-nostic methods, medication during hospitalization, severity mod-els and prognosis. Complications including ascites, hepatic en-cephalopathy, hypersplenia, and variceal bleeding, were defined ac-cording to criteria from the European Association for the Study of the Liver and International Ascites Club [7] . The prognostic models used to predict 30-and 90-day mortality included the model of end-stage liver disease (MELD), MELD-Na, and Child-Pugh scores. The formula for MELD score is 3.78 × ln(total bilirubin, mg/dL) + 11.2 × ln(INR) + 9.57 × ln(creatinine, mg/dL) + 6.43 × etiology (0 for cholestatic and alcoholic cirrhosis; 1 for others). The formula for MELD-Na score is MELD + 1.59 ×(135-Na). Child-Pugh score is determined based on hepatic encephalopathy, ascites, total bilirubin, albumin and pro-thrombin time.
Statistical analysis
Continuous variables were presented as the means ±standard deviation (SD) or the median (interquartile rang, IQR). Student’sttest and Mann-Whitney test were used to compare continuous variables between survivors and non-survivors. Nominal variables were presented as frequency and percentage and were compared using Fisher’s Exact test. A difference was considered significant when theP<0.05. Cox proportional hazard regression was used to examine risk factors for time-dependent death. The factors re-lated to mortality were included in the univariate Cox regression analysis. After univariate Cox regression analysis, the factors with significant differences were included in the multivariate Cox re-gression analysis. Kaplan-Meier curves were used to analyze pa-tient survival. Receiver operating characteristic (ROC) curves were used to evaluate the different prognostic models. Statistical analy-ses were performed using SPSS software version 20 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 10 800 patients were diagnosed with liver cirrho-sis. Forty-seven liver cirrhotic patients with cryptococcosis were enrolled in this study, of them, 38 patients (80.9%) survived, and 9 (19.1%) died by the end of the 90-day follow-up. The median length of stay among survivors was 17 days, while the median length of stay among non-survivors was 25 days. Baseline char-acteristics, severity models, pathogen tests and treatments of the 90-day follow-up of the study were depicted in Table 1 . Of these patients, 9 (19.1%) had rheumatic immune diseases, 6 (12.8%) had chronic kidney diseases, 6 (12.8%) had diabetes mellitus, 2 (4.3%) had hematological malignancies, 2 (4.3%) had HIV infection, and 1 (2.1%) had cardiovascular diseases. There were no significant differ-ences in age, sex, co-morbidity, or medication prior to admission between survivors and non-survivors. Survivors differed from non-survivors in cryptogenic liver diseases (10.5% vs. 44.4%,P= 0.015). Non-survivors showed a higher frequency of certain complications, including hepatic encephalopathy (33.3% vs. 7.9%,P= 0.040), hep-atorenal syndrome (11.1% vs. 0,P= 0.038), and hepatopulmonary syndrome (11.1% vs. 0,P= 0.038). Regarding laboratory parame-ters, there were also significant differences in platelet count [109.5 (70.5-146.0) vs. 47.0 (33.5-89.0) ×109/L,P= 0.009], fibrinogen [2.5 (1.8-3.5) vs. 1.2 (1.0-2.0) g/L,P= 0.013], activated partial thromboplastin time (APTT) [30.6 (26.1-38.3) vs. 43.7 (37.4-62.9) s,P= 0.003], prothrombin time (PT) [13.3 (11.8-15.7) vs. 17.6 (15.2-19.7) s,P= 0.007], total bilirubin (TBIL) [14.5 (9.0-26.3) vs. 34.2 (26.0-82.0)μmol/L,P= 0.002], direct bilirubin (DBIL) [6.0 (4.0-10.3) vs. 16.0 (10.6-50.5)μmol/L,P= 0.001] and indirect biliru-bin (IBIL) [8.1 (5.0-15.0) vs. 23.0 (11.0-31.5)μmol/L,P= 0.003] be-tween survivors and non-survivors.
There were no significant differences in the positive rates of serum cryptococcal antigen and blood culture of cryptooccus be-tween survivors and non-survivors (39.5% vs. 22.2%,P= 0.333;5.3% vs. 22.2%,P= 0.101). Forty-two patients underwent lumbar puncture. There was a significant difference in cryptococcal smear between survivors and non-survivors (48.6% vs. 100%,P= 0.012), and the rates of CSF cryptococcal antigen, CSF culture, and CSF ink staining showed no significant differences ( Table 1 ).
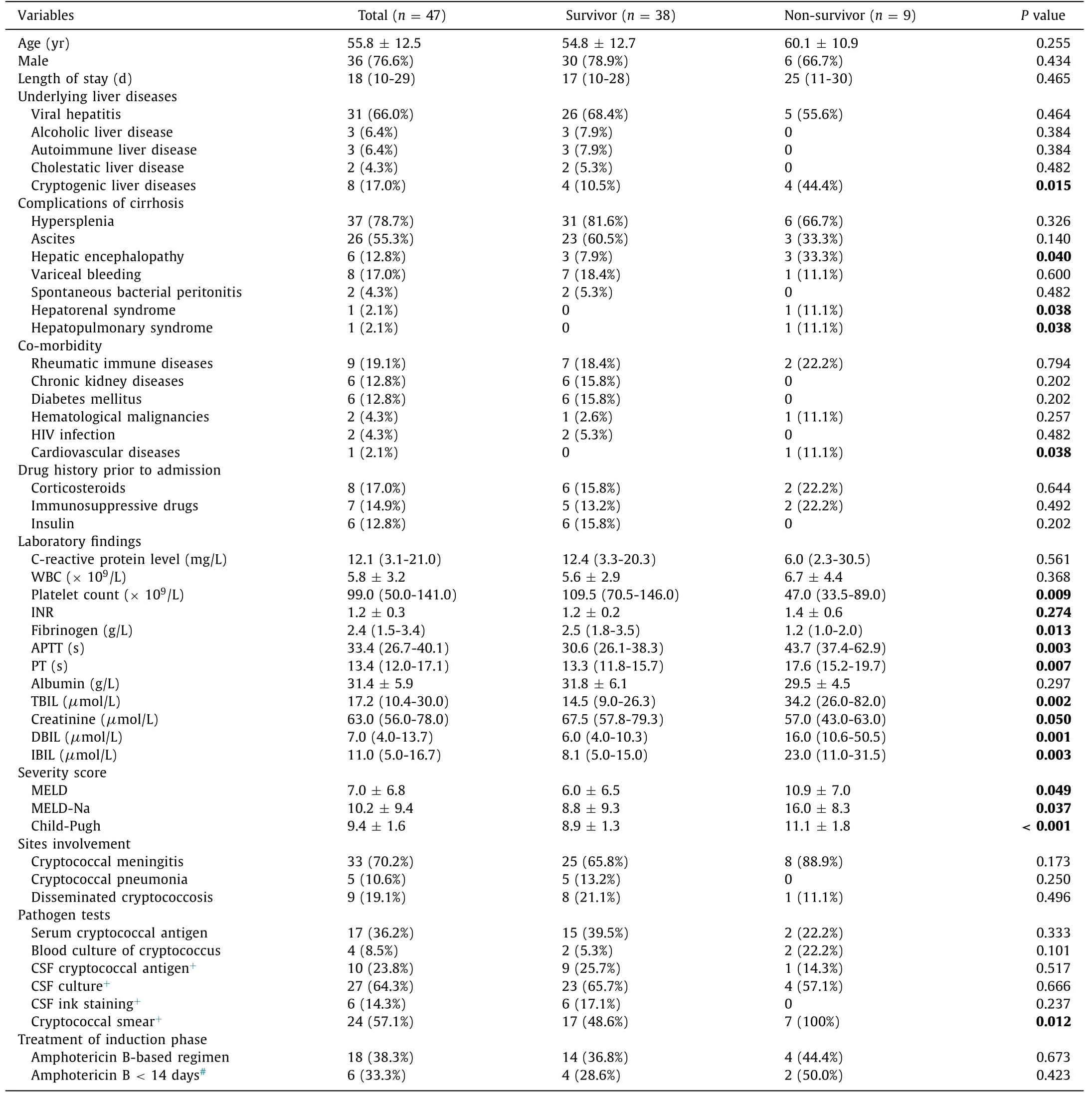
Table 1 Comparison of clinical characteristics between survivors and non-survivors for 90-day follow-up.
Thirty-three cirrhotic patients with cryptococcosis had crypto-coccal meningitis (70.2%); of these, twenty-five patients survived (75.8%), and eight died (24.2%). Baseline characteristics, severity models, pathogen tests and treatments of 90-day follow-up of the study were shown in Table 2 . Platelet counts [107.0 (58.5-148.0) ×109/L vs. 41.0 (33.3-97.8) ×109/L,P= 0.006], INR [1.2 (1.0-1.4) vs. 1.5 (1.2-1.7),P= 0.003], fibrinogen [2.5 (1.7-3.4) vs. 1.2 (1.0-2.3) g/L,P= 0.013], APTT [28.8 (26.1-37.5) vs. 39.9 (37.4-48.3) s,P= 0.001], PT [13.0 (11.7-15.5) vs. 17.5 (14.6-18.6) s,P= 0.003],TBIL [17.0 (8.7-27.5) vs. 33.1 (26.0-58.0)μmol/L,P= 0.001], creati-nine [62.0 (56.5-71.5) vs. 55.5 (43.0-63.0)μmol/L,P= 0.045], DBIL [6.0 (4.0-10.0) vs. 15.5 (10.3-28.8)μmol/L,P= 0.001], IBIL [10.0 (5.0-15.9) vs. 19.5 (11.0-29.3)μmol/L,P= 0.004] and Child-Pugh score (8.9 ±1.2 vs. 11.0 ±1.9,P= 0.001) were significantly differ-ent between survivors and non-survivors.
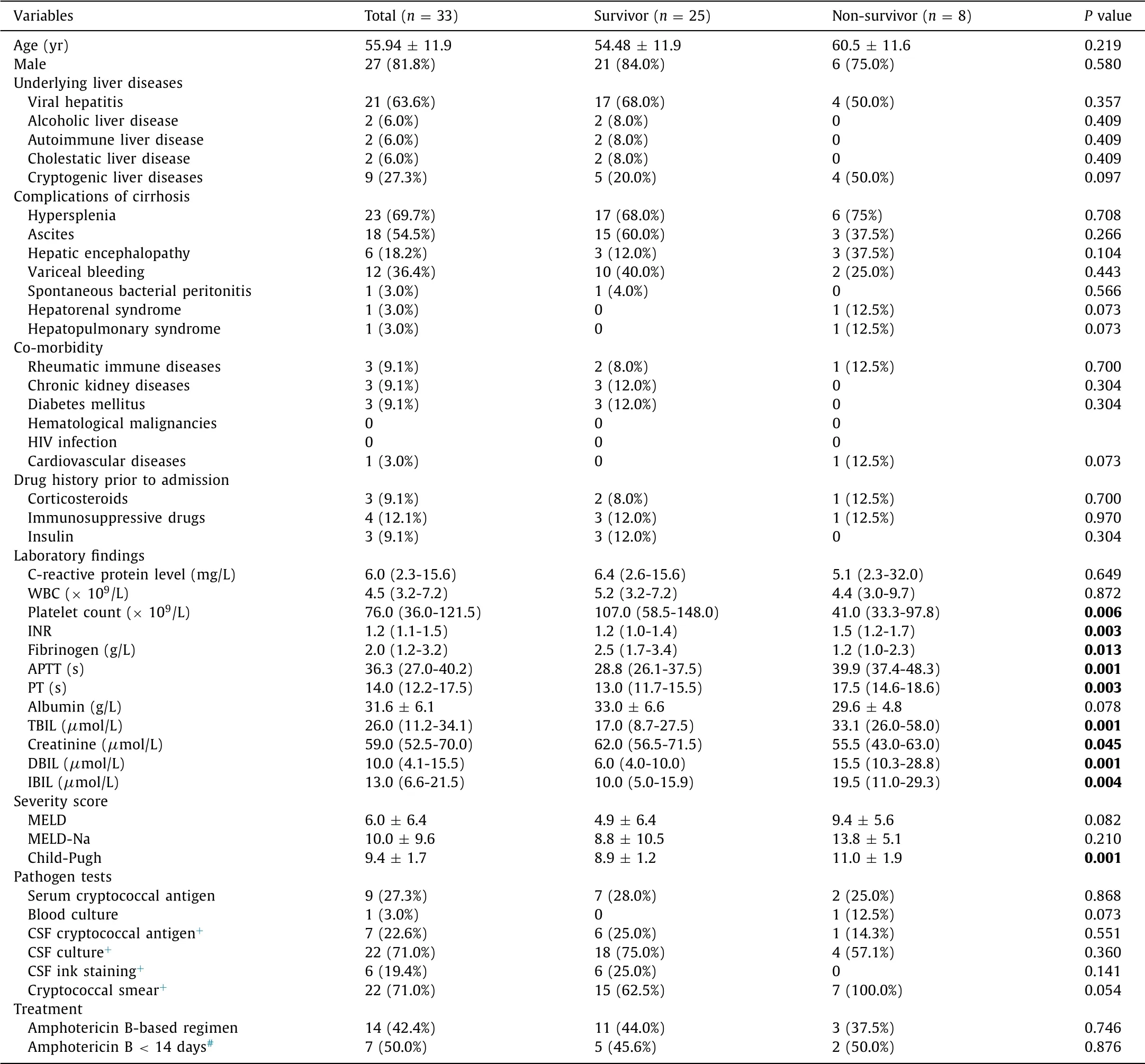
Table 2 Comparison of clinical characteristics between survivors and non-survivors of patients with cryptococcal meningitis for 90-day follow-up.
Clinical manifestations of cirrhotic patients with cryptococcal meningitis
Patients with isolated cryptococcal meningitis accounted for most cryptococcosis cases (n= 33), and we analyzed the clinicalmanifestations of these patients ( Table 3 ). Cirrhotic patients with cryptococcal meningitis exhibited various clinical signs, including fever, headache, vomiting, disturbance of consciousness, and coma.Fever and headache were the main complications and developed in 78.8% and 81.8% of the patients, respectively. In contrast, vomiting, unconsciousness, and coma were observed in fewer patients.

Table 3 Clinical manifestations of cryptococcal meningitis ( n = 33)

Table 4 Manifestations of CSF and the treatment of cryptococcal meningitis for 90-day follow-up.

Table 5 Risk factors correlated with 90-day mortality in cirrhotic patients with cryptococcosis.
CSF manifestations in cirrhotic patients with cryptoc occal meningitis
We also analyzed the manifestations of CSF at admission be-tween survivors and non-survivors in cirrhotic patients with cryp-tococcal meningitis at the 90-day follow-up ( Table 4 ). The per-centage of lymphocyte was higher in non-survivors than in sur-vivors [95.0% (83.8-97.2)% vs. 60.0% (35.0-75.0)%,P<0.001), and the protein level was higher in survivors than in non-survivors [1.2 (0.8-1.7) vs. 0.6 (0.5-1.1) g/L,P= 0.011). However, the intracranial pressure, nucleated cell count, glucose and chloride assessments showed no significant differences.
Treatments
Induction therapy is the most essential step of treatment for cryptococcal patients. As for non-HIV-infected patients with cryptococcosis, the combination of amphotericin B (AmB) and 5-flucytosine for at least 4 weeks is the preferred treat-ment. Apart from the abovementioned regimen, AmB monother-apy, AmB + fluconazole, fluconazole ±5-flucytosine, itraconazole ±5-flucytosine and voriconazole ±5-flucytosine are also considered when the preferred treatment is unavailable or impracticable. Ini-tial induction therapy is generally followed by consolidation and maintenance therapy with longer courses for suppression. In the present study, approximately 18 patients (18/47, 38.3%) received an AmB-based regimen ( Table 1 ). Fourteen cirrhotic patients with cryptococcal meningitis received an AmB-based regimen (14/33, 42.4%) ( Table 2 ). Patients also received flucytosine + fluconazole, flucytosine + voriconazole, fluconazole monotherapy and voricona-zole monotherapy as induction therapy. There were no significant differences in the percentages of patients who received AmB-based regimens or duration of AmB less than 14 days between survivors and non-survivors among all the patients and patients with cryp-tococcal meningitis (36.8% vs. 44.4%,P= 0.673; 28.6% vs. 50%,P= 0.423; 44% vs. 37.5%,P= 0.746; 45.6% vs. 50%,P= 0.876; Tables 1 and 2 ).
Factors associated with 30- and 90-day mortality
Liver cirrhotic patients with cryptococcosis in the present co-hort had 30-and 90-day survival rates of 93.6% and 80.9%, re-spectively ( Fig. 1 ). Age was an important factor in the progno-sis of cryptococcosis, and was included in the multivariate anal-ysis. As shown in Table 5,cryptogenic liver diseases were critical in predicting 90-day mortality (HR = 7.567, 95% CI: 1.616-35.428,P= 0.010) in multivariate Cox regression analysis. The laboratory exam results in model 2 showed that platelet count (HR = 0.965, 95% CI: 0.940-0.991,P= 0.008) and APTT (HR = 1.117, 95% CI: 1.016-1.229,P= 0.022) were associated with 90-day mortality. The severity models for liver cirrhotic patients with cryptococco-sis were shown in model 3. Child-Pugh score (HR = 2.146, 95% CI: 1.314-3.504,P= 0.002) was important in the prognosis of cirrhotic patients with cryptococcosis. As described above, platelet count was a protective factor, and cryptogenic liver diseases, APTT and Child-Pugh score were risk factors for predicting 90-day mortality. However, none of the factors affected 30-day mortality.
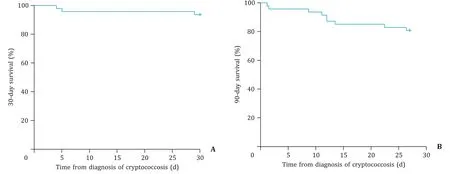
Fig. 1. Kaplan-Meier curve depicting 30-day ( A ) and 90-day ( B ) survival rates.
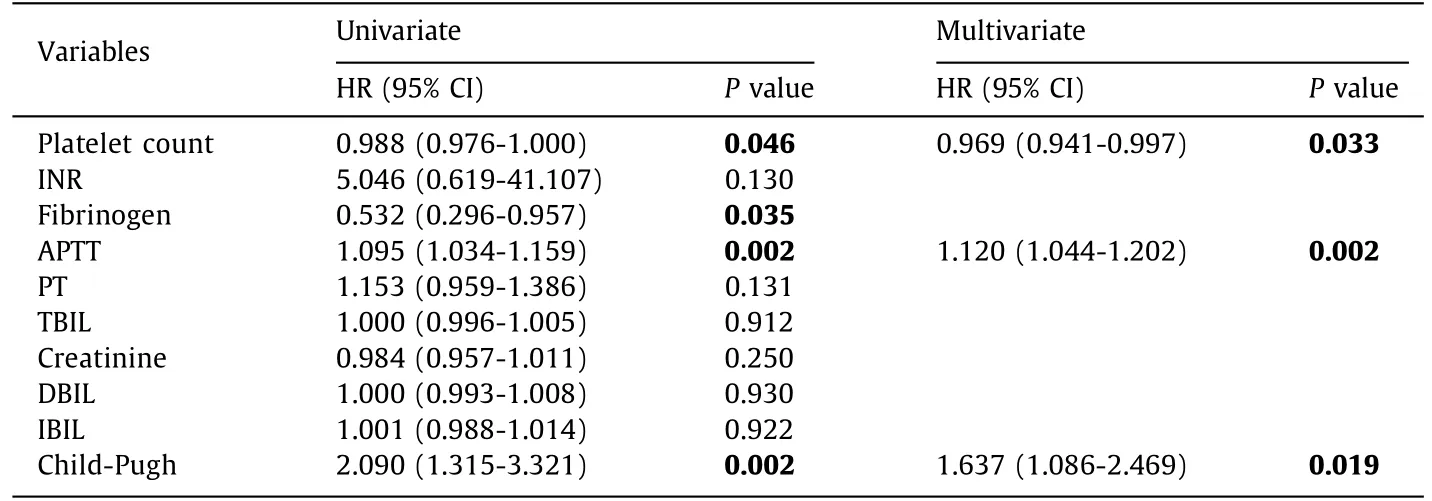
Table 6 Risk factors correlated with 90-day mortality in cirrhotic patients with cryptococcal meningitis.
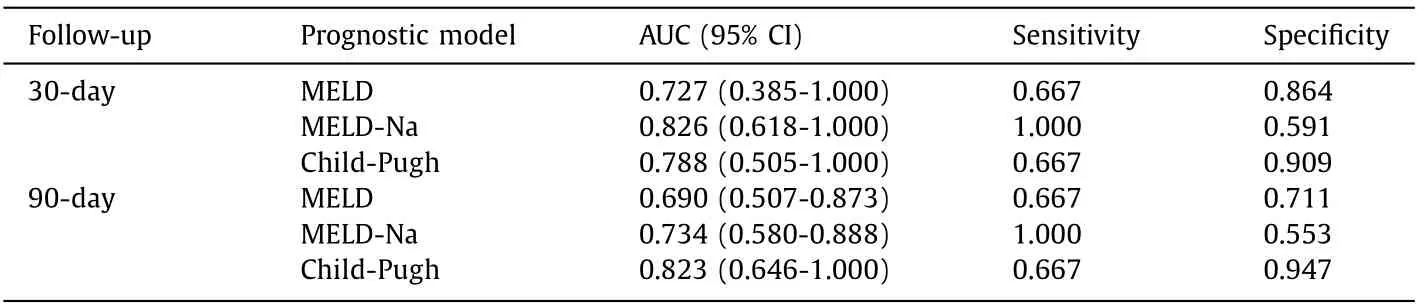
Table 7 Performance of three prognostic scores for predicting mortality of liver cirrhotic patients with cryptococcosis.
For the cirrhotic patients with cryptococcal meningitis, vari-ables with significantPvalues were included in the 90-day multivariate Cox regression ( Table 6 ). The results showed that platelet counts (HR = 0.969, 95% CI: 0.941-0.997,P= 0.033), APTT (HR = 1.120, 95% CI: 1.044-1.202,P= 0.002) and Child-Pugh score (HR = 1.637, 95% CI: 1.086-2.469,P= 0.019) were related with 90-day mortality. As described above, platelet count was also a protec-tive factor, APTT and Child-Pugh score were risk factors. However, these factors did not affect 30-day mortality.
Value of three prognostic scores in liver cirrhotic patients with cryptococcosis and cryptococcal meningitis
We evaluated the abilities of the three prognostic scores MELD, MELD-Na and Child-Pugh to predict 30-and 90-day mortality in liver cirrhotic patients with cryptococcosis ( Fig. 2 and Table 7 ). The area under curve (AUC) of MELD-Na score for predicting 30-day (AUC: 0.826, 95% CI: 0.618-1.0 0 0) mortality and the AUROC of Child-Pugh score for predicting 90-day (AUC: 0.823, 95% CI: 0.646-1.0 0 0) mortality at the onset of cryptococcosis were higher than those of the other two prediction scores.
For cirrhotic patients with cryptococcal meningitis, the AUC of MELD-Na score for predicting 30-day mortality (AUC: 0.742, 95% CI: 0.560-0.924) and the AUROC of Child-Pugh score for predicting 90-day mortality (AUC: 0.815, 95% CI: 0.670-0.960) at the onset of cryptococcal meningitis were higher than those of the other two scores. The Youden index revealed the best cutoff point for MELD-Na score was 10.725 for 30-day mortality (sensitivity: 1.0 0 0 and specificity: 0.613). The best cutoff point for the Child-Pugh score was 10.5 for 90-day mortality (sensitivity: 0.625 and specificity: 0.960) ( Fig. 3 and Table 8 ).

Table 8 Performance of three prognostic models for predicting mortality of liver cirrhotic patients with cryptococcal meningitis.
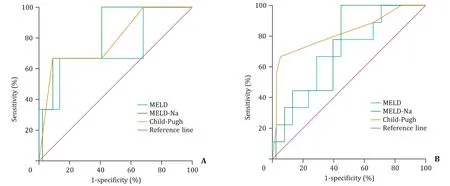
Fig. 2. Receiver operating characteristic (ROC) curves of prognostic models in predicting 30-day ( A ) and 90-day ( B ) mortality in cirrhotic patients with cryptococcosis. MELD: the model of end-stage liver disease; MELD-Na: the model of end-stage liver disease-sodium.

Fig. 3. Receiver operating characteristic (ROC) curves of prognostic models in predicting 30-day ( A ) and 90-day ( B ) mortality in cirrhotic patients with cryptococcal menin-gitis. MELD: the model of end-stage liver disease; MELD-Na: the model of end-stage liver disease-sodium.
Discussion
Cryptococcosis is observed in immunocompetent patients, but it primarily occurs in immunosuppressed patients, including HIV-positive patients and patients with organ transplantation, rheumatic immune diseases, diabetes mellitus, and malignancies. According to a study performed before 1983, the underlying dis-eases and the concurrent infections were more critical in deter-mining the survival rates of patients with cryptococcosis. The most common underlying disease in the present study was rheumatic immune diseases (9 patients, 19.1%), but another study reported HIV infection, HBV carrier status and malignancies as the main co-morbidities [8] . Steroid therapy, chronic lung and kidney diseases, malignancies and organ transplantation were associated with cryp-tococcal meningitis in a US cohort of cryptococcal infection [9] . Vi-ral hepatitis (31 patients, 66.0%) was the most common liver dis-ease that led to liver cirrhosis in the present study, which was con-sistent with the results of another study [3] .
Thompson et al. [10] found that serotypes A and D were more common in immunosuppressed patients, and serotypes B and C were prone to comprise immunocompetent patients. Liver cirrhotic patients had a higher risk than patients with other primary dis-eases. The immune dysfunction, including decreased cellular and humoral immunity, reduced phagocytosis by innate immune cells and promoted complement deficiency and portal-systemic shunt-ing of blood flow, which promotes the entry of intestinal micro-biota into the peripheral circulation, all led to cryptococcosis in cirrhotic patients [ 2,3,11 ]. Low complement levels were associated with increased mortality in laboratory animals with cryptococcosis and patient with cirrhosis.
Cryptococcosis is acquired via the respiratory system and begins in the lung. Fungal particles may also be ingested through contaminated food. Then cryptococci disseminate in the peripheral blood and localize in other organs, including the central nervous system (CNS), abdominal organs, and lymph nodes [3] . Therefore, disseminated cryptococcosis is diagnosed from a positive culture from two sites, or a positive blood culture. Cryptococcal meningitis often manifests as fever, headache, vomiting, seizure, confusion and unconsciousness. The median duration is approximately 6-12 weeks in non-HIV-infected patients and 2 weeks in HIV-infected patients [9] . Fever and headache were the two main signs in the current study, followed by vomiting, disturbance of consciousness and coma. These manifestations were consistent with a study in immunocompetent children [12] . Visual and hearing impair-ments, hemiplegia and altered mental status were not observed in our study but were common in a study of 94 non-HIV-infected patients with cryptococcal meningitis [13] . Lumbar puncture is indispensable in establishing the diagnosis. The CSF results indicate elevated intracranial pressure, increased lymphocytes and protein levels, and low glucose level [9],and the increased intracranial pressure and cerebral herniation are predictors of mortality. Cryptococcal antigen in CSF is always positive when CNS infection is established [14] . Williamson et al. [9] demon-strated that elevated cytokines in CSF, such as interleukin-6 (IL-6), interferon -γ(IFN-γ), IL-17 and tumor necrosis factor (TNF-α), were associated with a high CD4 + T cell count, high CSF white cell counts, quick clearance rates of infection and high survival rates. They also showed that survivors had a higher proportion of TNF-αand IFN-γ-producing CD4 + T cells than non-survivors. However, Shih et al. [13] reported that immunocompromised pa-tients who had lower CSF cell and lymphocyte counts showed less severe symptoms of meningitis than immunocompetent patients. Meningeal involvement in the prognosis may be underestimated. Therefore, it is urgent to diagnose cryptococcal meningitis early and manage intracranial pressure aggressively. The CSF lympho-cyte percentage in our study was higher in non-survivors than in survivors at the 90-day follow-up. Okurut et al. [15] showed no significant difference in CSF protein level between HIV patients with and without cryptococcal meningitis. Another study showed that increased CSF protein level was related to an increased like-lihood of cryptococcal meningitis compared to controls [16] . The manifestations of cryptococcal meningitis in CSF include increased protein level, elevated white cell counts and low glucose. High protein level may be the indicator of a poor prognosis. However, the level of CSF protein in our cohort was lower in non-survivors than in survivors. This significant difference between survivors and non-survivors was found at admission, and it may be due to the small size of the cohort. Another possibility is that high protein level in CSF may increase the opsonic effect which help to kill cryptococci. Cryptococcal antigen testing is more sensitive than blood culture in disseminated cryptococcosis. The titers of serum cryptococcal antigens in patients with cryptococcosis range from 1:2 to 1:32 678 or from 1:32 to 1:1024. However, a Korean study showed that positive rate of cryptococcal antigen testing in cirrhotic patients with cryptococcosis was low, and that screening for cryptococcal antigens may not be helpful [17] . Moreover, cryptococcal antigen testing had only 57% sensitivity in pulmonary cryptococcosis [5] . Cryptococcal smear positivity in cirrhotic patients with cryptococcosis was lower in survivors than in non-survivors in this study (48.6% vs. 100%). However, there were no significant differences in the results of cultivation of cryptococcus in CSF and blood or the measurement of cryptococcal capsular polysaccharide antigen in CSF and blood. An accurate and prompt diagnosis is indispensable regardless of the method used to confirm cryptococcal infection because the failure to diagnose may cause a poor outcome, especially neurological damage.
Our results showed that the survival rates were as high as 93.6% and 80.9% at the 30-and 90-day follow-ups, respectively. These percentages were similar to previous reports (0-47%) [13] . Because of the high mortality in cirrhotic patients with cryptococcosis and cryptococcal meningitis, the risk factors for mortality were ana-lyzed and discussed in our study. Cryptogenic liver diseases, APTT and Child-Pugh score were associated with 90-day mortality for cirrhotic patients with cryptococcosis. APTT and Child-Pugh score were also related with 90-day mortality for cirrhotic patients with cryptococcal meningitis. Notably, age did not influence 30-or 90-day mortality. The Child-Pugh scoring system was pivotal in pre-dicting the 90-day outcome. The Child-Pugh scoring system con-siders the complications of liver cirrhosis, liver function and blood clotting index, and is more comprehensive than the MELD and MELD-Na scoring systems.
The standard treatment course consists of induction, consol-idation and maintenance. AmB is the fastest acting drug against severe cryptococcal infection, especially meningitis, but it has several side effects. Renal injury and anemia are more commonly seen in the second week of AmB-based regimens than in the first week [18] . The combination of AmB and flucytosine is the first-line therapy because of its most potent fungicidal ability. Yan et al. [19] found that a low dose of AmB (0.4 mg/kg) in this combination reduced side effects without affecting effectiveness. Therefore, the combination of low-dose AmB and flucytosine was recommended for use in patients. The combination of AmB and fluconazole is recommended when flucytosine is not available because of the safety and low cost of fluconazole [ 20,21 ]. A study of HIV-infected patients advised two alternative options because of the short course of induction therapy: administration of AmB plus flucytosine for 1 week and flucytosine plus fluconazole for 2 weeks [18] . Day et al. [21] demonstrated that the use of AmB and flucytosine resulted in a higher survival rate without disability than AmB monotherapy, but AmB and fluconazole did not offer any benefit. The treatment regimens in this research followed the “Practice Guidelines for the Management of Cryptococcal Disease”and its 2010 updated version by Infectious Diseases Society of America (IDSA) [ 22,23 ]. However, the optimal treatment varies between patients according to individual differences. The admin-istration of AmB-based regimens did not improve the survival rate for cirrhotic patients with cryptococcosis and cryptococcal meningitis in the present study. There was no significant differ-ence in treatment with AmB-based regimens for less than 14 days between survivors and non-survivors at the 90-day follow-up. This result is not consistent with Xu et al. [24] . One explanation is that Xu’s study included the cryptococcal meningitis infec-tions underlying variety of diseases, whereas our cohort only included the patients underlying liver cirrhosis. Liver cirrhosis played an essential role in cirrhotic patients with cryptococcosis and cryptococcal meningitis. Another study also showed cirrhotic patients with cryptococcosis were older and the manifestations may not be obvious [25] . Therefore, these patients did not receive antifungal treatments promptly, which was not beneficial to their outcomes.
Our study also had some limitations. First, the study was a single-center cohort, the data need to be validated in multicenter studies. Second, the unavailability of cryptococcal capsular polysac-charide antigen measurements in earlier time was a big obstacle to acquire complete data for further analyses.
In conclusion, our study investigated the risk factors for mor-tality and the predictive value of prognostic scores in cirrhotic patients with cryptococcosis and cryptococcal meningitis. Cryp-togenic liver diseases, APTT and Child-Pugh score were indepen-dently associated with mortality in these patients. The MELD-Na score was important for predicting 30-day mortality, and the Child-Pugh score was critical for predicting 90-day mortality.
Acknowledgements
None.
CRediT authorship contribution statement
Qi-Hui Zhou:Data curation, Formal analysis, Writing -original draft.Cai-Qin Hu:Writing -original draft.Yu Shi:Conceptualiza-tion.Feng-Tian Wu:Investigation, Formal analysis.Qin Yang:In-vestigation, Formal analysis.Jun Guan:Investigation, Formal anal-ysis.Ai-Chun Li:Methodology.Zhi Chen:Funding acquisition, Su-pervision, Writing -review & editing.
Funding
The study was supported by grants from the National Sci-ence and Technology Major Project of China ( 2018ZX10302206 and 2017ZX10202203 ).
Ethical approval
This study was approved by Ethics Committee of the First Affil-iated Hospital, Zhejiang University School of Medicine.
Competing interestNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub-ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Comparison and development of advanced machine learning tools to predict nonalcoholic fatty liver disease: An extended study
- Hepatobiliary&Pancreatic Diseases International
- Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment
- Key factors and potential drug combinations of nonalcoholic steatohepatitis: Bioinformatic analysis and experimental validation-based study
- LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver
- Gene knockout or inhibition of macrophage migration inhibitory factor alleviates lipopolysaccharide-induced liver injury via inhibiting inflammatory response
