Efficacy and safety of conversion of percutaneous cholecystostomy to endoscopic transpapillary gallbladder stenting in high-risk surgical patients
2021-11-08HyungKuChonChanParkDongEunParkTaeHyeonKim
Hyung Ku Chon , Chan Park , Dong Eun Park , Tae Hyeon Kim b, ∗
a Division of Biliopancreas, Department of Internal Medicine, Wonkwang University College of Medicine and Hospital, 895 MuwangRo, Iksan, South Korea
b Institute of Wonkwang Medical Science, Wonkwang University College of Medicine and Hospital, Iksan, South Korea
c Department of Surgery, Wonkwang University College of Medicine and Hospital, Iksan, South Korea
Keywords: Cholecystitis Percutaneous cholecystostomy Endoscopic transpapillary gallbladder stenting High surgical risk
ABSTRACT Background: Endoscopic transpapillary gallbladder stenting (ETGBS) has been used as an alternative to percutaneous cholecystostomy in patients with acute cholecystitis who are considered unfit for surgery. However, there are few data on the efficacy and safety of ETGBS replacement of percutaneous cholecys-tostomy in high-risk surgical patients. This study aimed to evaluate the feasibility, efficacy, and safety of ETGBS to replace percutaneous cholecystostomy in high-risk surgical patients. Methods: This single center retrospective study reviewed the data of patients who attempted ETGBS to replace percutaneous cholecystostomy between January 2017 and September 2019. The technical success, clinical success, adverse events, and stent patency were evaluated. Results: ETGBS was performed in 43 patients (24 male, mean age 80.7 ±7.4 years) to replace percu-taneous cholecystostomy due to high surgical risk. The technical success rate and clinical success rate were 97.7% (42/43) and 90.5% (38/42), respectively. Procedure-related adverse events and stent-related late adverse events occurred in 7.0% (3/43) and 11.6% (5/43), respectively. Of the patients who success-fully underwent ETGBS ( n = 42), only one had recurrent acute cholecystitis during follow-up. The median stent patency was 415 days (interquartile range 240–528 days). Conclusions: ETGBS, as a secondary intervention for the purpose of internalizing gallbladder drainage in patients following placement of a percutaneous cholecystostomy, is safe, effective, and technically feasi-ble. Thus, conversion of percutaneous cholecystostomy to ETGBS may be considered as a viable option in high-risk surgical patients.
Introduction
Cholecystectomy is the gold standard management for pa-tients with acute cholecystitis, and early laparoscopic cholecystec-tomy is considered a safe and cost effective approach in low-risk patients [ 1,2 ]. However, high-risk patients with elderly or severe comorbidities may have increased early cholecystectomy related morbidity (up to 41%) and mortality (up to 18%) [3] . Percutaneous cholecystostomy (PC) is a useful procedure that can be used as an alternative to surgery in such patients [ 4,5 ]. Interval chole-cystectomy may be considered after stabilization of underlying medical conditions [ 6,7 ]. However, when the patient is considered high surgical risk through the multidisciplinary approach, there is no consensus guideline for post-PC. Classically, a cholecystogram may be performed from 1 week to 8 weeks after the improve-ment of the patient’s clinical manifestation. If the cholecystogram shows a patent cystic duct, removal following clamping of the PC tube in the absence of recurring symptoms may be considered. However, recurrent acute cholecystitis may occur in about 22%–47% of the patients after PC removal without subsequent chole-cystectomy [8–10] . A long-term indwelling PC catheter may also lead to various complications such as inadvertent dislodgement of catheter, cholecysto-colonic fistula, or catheter related infec-tion [ 11,12 ]. Low life quality or cosmetic problems are also im-portant issues. Therefore, the internalization of the PC may be considered.
Endoscopic gallbladder drainage, including endoscopic ultra-sound (EUS) guided transmural approach or endoscopic transpap-illary gallbladder stenting (ETGBS) followed by endoscopic ret-rograde cholangiopancreatography (ERCP), may be an alternative option to replace PC [13] . These approaches may improve the life quality of the patients, as well as provide more physiologic drainage. Recently, several studies relating to the conversion of PC to EUS guided gallbladder drainage (EUS-GBD) have shown excel-lent clinical outcomes [14–16] . However, to date, there has been no study about the conversion from PC to ETGBS in patients deemed medically unfit for subsequent cholecystectomy; thus, the efficacy and safety of this procedure have not yet been established. There-fore, this study aimed to evaluate the feasibility, efficacy, and safety of ETGBS to replace PC in high-risk surgical patients.
Methods
Patients
Between January 2017 and September 2019, we reviewed the data of patients who attempted ETGBS to replace PC. All en-rolled patients were diagnosed with acute cholecystitis based on the Tokyo Guidelines [17] and had previously undergone PC due to advanced malignancy and/or high surgical risk [ ≥5 on Charl-son Comorbidity Index (CCI)]. Patients were excluded if they had previously undergone primary ETGBS, or if interval cholecystec-tomy was performed. Clinical variables from the electronic medi-cal records included demographic features, underlying disease, eti-ology of acute cholecystitis, procedural details, and adverse events. All adverse events were graded according to the severity grading system of the American Society for Gastrointestinal Endoscopy lex-icon.
Endoscopic technique
The ETGBS was performed by two expert endoscopists (Chon HK and Kim TH) after the patients provided informed consent. A cholecystogram was obtained by contrast injection through the indwelling PC tube (8-Fr FleximaTMAPDL catheter, Boston Scien-tific Corp, Natrick, MA, USA) under fluoroscopy before performing ETGBS. After conscious sedation using midazolam and pethidine, the major papilla was identified using a duodenoscope (TJF 260V; Olympus Medical systems, Tokyo, Japan) and selective biliary can-nulation with endoscopic sphincterotomy (EST) was performed. We performed biliary endoscopic sphincterotomy in all cases of naïve papilla due to concern about post-ERCP cholangitis and pancreati-tis. An angled or straight hydrophilic coated guidewire (0.025-inch VisiGlide; Olympus Medical Systems) was passed through the cys-tic duct into the gallbladder. Over the guidewire, a 7-Fr, 12–15 cm double-pigtail plastic stent (Zimmon®, Cook medical, Blooming-ton, Ind, USA) was placed with one pigtail coiled into the gall-bladder and the other in the duodenal lumen ( Fig. 1 ). The PC tube was clamped on the day after the procedure. The removal follow-ing the clamp of PC tube was performed without recurred symp-toms. When guidewire advancement into the cystic duct was diffi-cult due to a tortuous, redundant, or invisible cystic duct, a digital cholangioscopy (SpyGlass DS Direct Visualization System; Boston Scientific Corp) was used to assist with the cystic duct cannulation. A digital cholangioscopy was advanced over the guidewire to the hilum and gently pulled back down until the cystic duct orifice was seen. A guidewire was inserted into the cystic duct under direct digital cholangioscopy and coiled in the gallbladder ( Fig. 2 ). Subse-quently, double pigtail plastic stent placement over the guidewire was performed. When concomitant common bile duct stones were recognized, stone removal was also performed at the same session.
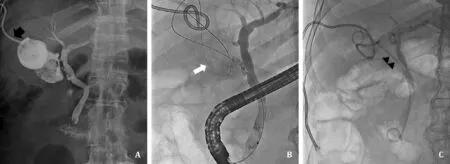
Fig. 1. The procedure of endoscopic transpapillary gallbladder stenting from percutaneous cholecystostomy (PC). A: Cholecystogram by PC tube (black arrow) showing cystic duct patency containing gallbladder stones. B: A guidewire (white arrow) was inserted into gallbladder through cystic duct and coiling in the gallbladder. C: A 7-Fr, 12 cm double pigtail stent (black arrowheads) was placed between the gallbladder and the duodenal lumen.
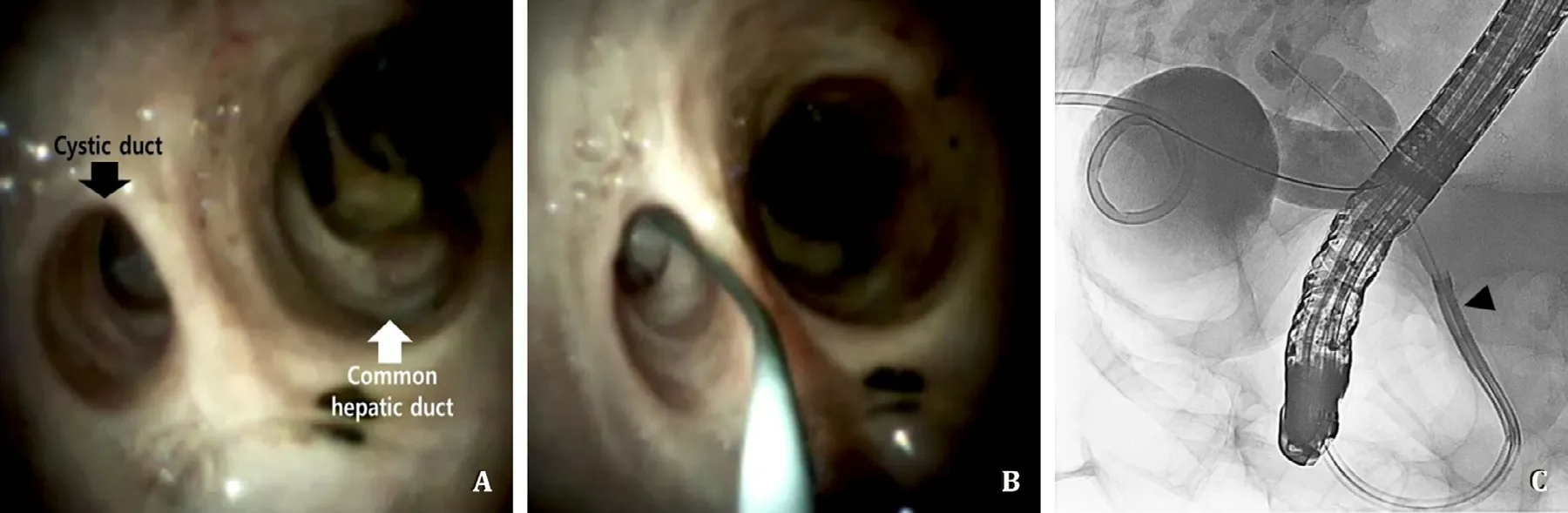
Fig. 2. Digital cholangioscopy assisted cystic duct cannulation procedure. A: Direct digital cholangioscopy using the Spyglass DS system showing the cystic duct orifice (black arrow) and common hepatic duct (white arrow). B: Guidewire advancement into the cystic duct under direct cholangioscopy. C: Cholangiography showing a successful guidewire insertion into the gallbladder by the Spyglass DS system (black arrowhead).
Follow-up
All patients were followed up every 3–6 months with labora-tory tests and abdominal X-ray until study termination (January 31, 2020) or death. Abdominal computed tomography, magnetic res-onance cholangiopancreatography, or abdominal ultrasound were performed as required. If the patient could not be contacted di-rectly, their family members were contacted to discuss the pa-tient’s clinical courses or symptoms by telephone. No regularly scheduled stent changes or removals were conducted in this study.
Outcome measurements
Technical success was defined as plastic stent placement be-tween the gallbladder and duodenal lumen using ERCP confirmed by fluoroscopy and endoscopy. Clinical success was defined as no recurred acute cholecystitis in the follow-up period after success-ful removal of the PC tube. Early adverse events were defined as any procedure-related adverse event within 2 weeks of the pro-cedure. Late adverse events were defined as any stent related ad-verse events that occurred 2 weeks after the procedure. Stent pa-tency was defined as the interval between stent placement by the endoscopic transpapillary approach and stent dysfunction or the patient’s death. Stent dysfunction was defined as the recur-rence of acute cholecystitis or occurrence of cholangitis. The recur-rence of acute cholecystitis was defined as the recurrence of typi-cal clinical symptoms and signs of acute cholecystitis with labora-tory results and radiologic findings in accordance with the Tokyo Guidelines [17] . The procedure time was defined as the time mea-sured from biliary cannulation to placement of a double pigtail stent for ETGBS.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). The categorical variables were reported as num-bers and percentages. The continuous variables were described as medians with interquartile range (IQR) or means with standard deviation. The stent patency was determined using Kaplan-Meier analysis.
Results
During the study period, 394 patients who were unfit for urgent cholecystectomy because of their serious underlying disease, severe grade acute cholecystitis, or refusal of surgery underwent PC. In-terval cholecystectomy was performed in 155, while the remaining 239 patients did not undergo surgical procedure. Of these, 43 pa-tients (24 male and 19 female; mean age 80.7 ±7.4 years) were converted from PC to ETGBS. The baseline characteristics and co-morbidity profiles of the enrolled patients are displayed in Table 1 . All patients had high surgical risk (higher than 5 on CCI) including advanced malignancies. The median time from PC to ETGBS con-version was 17.0 days (IQR 9.0–36.5 days). A total of 58.1% (25/43) had concomitant choledocholithiasis, and complete stone removal was performed during the same session. Details of the ETGBS pro-cedure are shown in Table 2 . A 7-Fr, 12 cm double-pigtail plastic stent was used in the vast majority of ETGBS, and most of the procedures used an angled-tip hydrophilic 0.025 inch guidewire (n= 40). A straight tip hydrophilic guidewire was only used for a digital cholangioscopy guided cystic duct cannulation (n= 3). Cystic duct dilatation with a 7-Fr screw-type dilation catheter was performed in two cases (4.7%) with severe cystic duct stricture. Digital cholangioscopy guided cystic duct cannulation was per-formed in three cases (7.0%). A total of 19 cases (44.2%) were of naïve papilla, which subsequently underwent endoscopic biliary sphincterotomy. The mean procedure time of cases, without digital cholangioscopy assisted ETGBS, was 15.1 min, while that of cases with cholangioscopy assisted ETGBS was 27.7 min.
The procedural outcomes are shown in Table 3 . The techni-cal success rate was 97.7% (42/43). One case of technical fail-ure was that a plastic stent was not inserted into the gallblad-der due to severe cystic duct stricture that did not respond to a 7-Fr screw type dilation catheter. During the study period, this pa-tient had PC tube removed; however, recurred acute cholecystitis occurred 35 days later and the patient was treated by repeated PC. The clinical success was 90.5% (38/42). Of the patients with ETGBS success, three died due to progression of underlying ma-lignancy without PC catheter removal at 30, 46, and 71 days af-ter the procedure. One patient had recurred acute cholecystitis 2 days after PC tube removal, and was treated by ETGBS following endoscopic naso-gallbladder drainage. Procedural adverse events, including mild post-ERCP pancreatitis (2/43, 4.7%) or hematoma around the cystic duct (1/43, 2.3%), related to the use of a dilation catheter occurred, but were improved with conservative treatment. Stent-related late adverse events included asymptomatic complete stent migration (2/43, 4.7%) and occurrence of cholangitis (3/43, 7.0%). Asymptomatic complete stent migration occurred in two cases, 106 and 210 days after the procedure respectively, but none of these patients had recurrence of acute cholecystitis during the study period. In three cases, acute cholangitis with common bile duct stones presented at 309, 333, and 413 days, respectively after the procedure and endoscopic treatments were required. The me-dian period from ETGBS to PC removal was 11.5 days (IQR 2.0–26.8 days). A total of 12 (27.9%) patients died of unrelated causes during the follow-up period. The median stent patency was 415 days (IQR 240–528 days) by Kaplan-Meier curves ( Fig. 3 ).
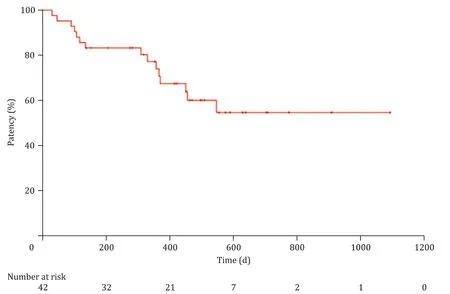
Fig. 3. Kaplan-Meier curve showing stent patency in patients who underwent successful endoscopic transpapillary gallbladder stent replacement of percutaneous drainage.

Table 1 Clinical characteristics of 43 patients who converted from PC to ETGBS.
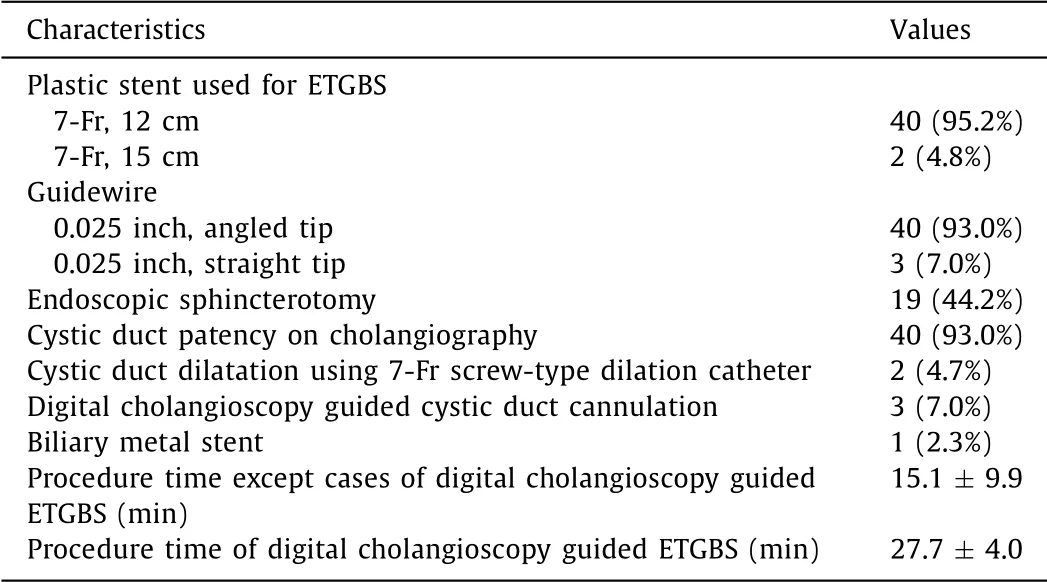
Table 2 Technical and procedural details of ETGBS.

Table 3 Procedural outcomes of patients who converted from PC to ETGBS.
Discussion
The present study reported the feasibility of ETGBS placement for internalization of a PC catheter in surgically high-risk patients who were unfit for interval cholecystectomy. PC has a pivotal role as a bridge to subsequent cholecystectomy. However, long-term ex-ternal gallbladder drainage may be required in high-risk patients who have advanced age or severe medical conditions. In such cases, leaving a PC tube may lead to various problems including catheter site infection, restriction on the patient’s movement, acci-dental catheter dislodgement, and need for re-intervention. More-over, PC tube removal without interval cholecystectomy can cause the recurrent acute cholecystitis. Thus, the internalization of the PC may be considered. To date, little is known about ETGBS re-placement of PC. ETGBS has been proposed as an alternative to PC in selected patients with severe coagulopathy, massive ascites, gallbladder malposition, or thrombocytopenia. This procedure has been performed as a bridging therapy before interval cholecystec-tomy [18] . The main advantage of primary ETGBS is that it omits the need for percutaneous intervention for gallbladder decompres-sion, as well as the physiologic drainage method with improved life quality of patients.
According to a previous systematic review, the technical and clinical success rates of primary ETGBS were 96% and 88%, respec-tively [19] . Compared to primary ETGBS, the advantage of ETGBS as a secondary intervention for internalization of PC is that the pro-cedure timing can be determined by the patients’ medical condi-tions, as well as cystic duct patency. Before performing ETGBS, if the cystic duct anatomy can be recognized by contrast injection through the PC tube, the success rate of a guidewire insertion into gallbladder may be increased. ETGBS was successfully performed in 39 of 40 patients with cystic duct patency. In addition, ETGBS conversion of PC may prevent the need for re-intervention or re-peated hospitalization associated with maintaining PC, and may re-duce the acute cholecystitis recurrence rate. In a recent study, the acute cholecystitis recurrence rate was compared in 64 consecutive high-risk surgical patients divided into the ETGBS group and ob-servation after PC group [20] . There was a significant difference in acute cholecystitis recurrence rate between the two groups (17.2% in the observation after PC group vs. 0% in the ETGBS group,P= 0.043). Our results also demonstrated the low recurrence of acute cholecystitis after ETGBS. Of 42 patients with successful ETGBS, re-current acute cholecystitis occurred in one case 2 days after PC catheter removal. In this patient, no cystic duct patency following the cholecystogram was observed, but the patient wished to re-move the PC tube. The cystic duct patency may be improved by ET-GBS placement. However, it is estimated that lots of sludge in the gallbladder caused early stent malfunction in this case. This patient was successfully treated by endoscopic re-interventions. According to our experience, a cystic duct patency with the clinical hallmarks of the patient with ETGBS should be checked before removal of the PC tube because the cystic duct patency was observed in all patents before removal of PC, except for one recurred case. Even if it was recurred, endoscopic transpapillary gallbladder drainage may be considered as a first line procedure.
Another option for conversion of PC is EUS-GBD, which re-portedly has an 84.6%–100% success rate [ 14,16,21,22 ]. The role of EUS-GBD as the primary drainage for acute cholecystitis has been shown in many studies [23–25],but EUS-GBD conversion from PC has been limited. Recently, several studies showed a high technical and clinical success rate of the EUS-GBD conversion of PC with low recurrence rate [ 21,22 ]. Law et al. [16] demonstrated that seven cases of EUS-GBD replacement with lumen apposing metal stent achieved a 100% technical success rate, and no occurrence of ad-verse events or recurrent acute cholecystitis was reported. Minaga et al. [14] described 21 cases of this technique using plastic or metal stents, yielding a 90.5% technical success rate and a 89.5% clinical success rate, but stent dysfunction or occlusion occurred in three cases (3/19, 15.8%). Those studies were conducted by experi-enced endoscopists in referral centers, and procedure related costs may be quite high when using metal stents [26] . Furthermore, EUS-guided internalization of PC may be technically more chal-lenging than the primary EUS-GBD. Thickening and fibrotic gall-bladder wall induced by indwelling PC catheter makes it difficult to perform endoscopic puncture or puncture tract dilation. In cases of inadequate inflation of gallbladder after contrast injection via the PC catheter, visualization of gallbladder during the procedure is limited; thus, guidewire coiling or metal stent deployment may be difficult within smaller gallbladder.
The early adverse events are post-ERCP pancreatitis. Only two patients (2/43, 4.7%) with difficult biliary cannulation developed mild post-ERCP pancreatitis, but there was no post-ERCP pancre-atitis in patients with a history of ERCP. A hematoma around a cystic duct occurred in one patient (1/43, 2.3%) with severe cys-tic duct stricture. A 7-Fr screw-type dilatation catheter was used to dilate a cystic duct stricture in two cases. A 7-F screw-type di-latation catheter, needle knife, or wire-guided snare tip has been described to bypass a very tight biliary or pancreatic stricture. The technique of needle knife or wire guided snare tip carries a poten-tial risk of either ductal perforation or creation of a false tract. A 7-Fr screw-type dilatation catheter was applied as a “screw drill”method over the guidewire across the stricture; thus, the ductal perforation risk may be low [27] . However, a buckling effect of a 7-Fr screw-type dilation catheter assembly over the tortuous in-serted guidewire into the cystic duct may hamper the direct trans-mission of axial force along the stricture, and could lead to cystic duct injury, such as hematoma, as in our study. Therefore, when a screw-type dilator catheter is applied to dilate a cystic duct stric-ture, the dilator catheter should be manipulated gently, and too much strength of dilators catheter insertion into the cystic duct should be avoided.
The late adverse events included stent migration (2/43, 4.7%) and cholangitis (3/43, 7.0%). Lee et al. [28] analyzed 20 patients with long-term ETGBS (median follow-up period, 586 days) and demonstrated that stent migration occurred in 10% of patients, while cholangitis developed in 5% of patients. If the gallbladder edema in acute cholecystitis improves after ETGBS, a plastic stent, which is placed along the gallbladder bottom, may be pushed out on the duodenal side. Additionally, the plastic stent may be af-fected by food material and bowel peristalsis. However, ETGBS can maintain gallbladder decompression by a bile wicking effect and prevent stone impaction of the cystic duct, even in the case of stent occlusion or a stent position change toward the gallbladder neck or cystic duct. Although re-intervention may be required in cases of complete stent migration with recurrent acute cholecysti-tis, no acute cholecystitis recurrence has been reported as of yet during the present study period. Recently, a newly developed plas-tic stent for ETGBS, which has a three-dimensional spiral-shaped structure with side hole, showed a significantly lower rate of stent migration than a pig-tail or straight type plastic stent (0% vs. 31.9%,P= 0.006) [29] . Although additional studies are required, the in-troduction of this new device may help reduce stent migration.
One of the concerning complications following the ETGBS is the development of cholangitis. A plastic stent may disturb the bile flow from the bile duct and could serve as the nidus for choledo-cholithiasis. Three patients (7.0%) developed cholangitis with com-mon bile duct stones at 309, 333, and 413 days after the ETGBS, and were treated endoscopically. The patients had a history of re-current cholangitis with common bile duct stones, periampullary diverticulum, and common bile ducts more than 15 mm in diame-ter. Many studies reported the various risk factors for recurrent bil-iary stones, including those using lithotripsy for stone fragmenta-tion, presence of biliary stricture, periampullary diverticulum, gall-bladder stone, and a dilated common bile duct [30–32] . Thus, it was supposed that not only plastic stent, but also patient factors were involved in the occurrence of cholangitis.
An important factor for ETGBS success is guidewire insertion into the gallbladder through the cystic duct. Thus, ETGBS is not possible if there is a cystic duct involved malignancy or indwelled biliary metallic stent covered with cystic duct orifice. However, even in benign disease, it is difficult to perform in cystic duct can-nulation because of the invisible cystic duct on cholangiography or the presence of a sharply angled cystic duct. A digital cholan-gioscopy makes it easy to find the cystic duct orifice with high resolution imaging, although these procedures are time consum-ing. Moreover, guidewire manipulation for cystic duct cannulation may become easier under direct visualization [33] . In the present study, digital cholangioscopy assisted ETGBS was successfully per-formed in three cases of invisible cystic duct on cholangiography; thus, a technical success rate of 97.7% was achieved.
The studies about long-term stent patency of ETGBS are still limited. One study demonstrated that the cumulative stent patency of ETGBS was 760 days (range 210–1403 days), and they suggested that observation without stent removal or exchange was possible for at least 2 years after the ETGBS [28] . The median stent pa-tency was 415 days (IQR 240–528 days) in our cases. To date, there was no consensus guideline for long-term follow-up or stent ex-change/removal of ETGBS. However, “watch-and-wait”approach to the patients with ETGBS would be more reasonable than a regular stent exchange until occurrence of acute cholecystitis or cholangi-tis, considering the patients undergone the ETGBS had severe co-morbidities.
There was no consensus guideline on timing of PC tube re-moval. However, a previous study showed that PC tube removal was safe and effective at minimum 3 weeks after the procedure because the tract maturation occurred within 20 days [34] . Accord-ing to these results, we removed PC tube at least 3 weeks after the PC. The median time from PC to ETGBS conversion was 17 days (9.0–36.5 days) and median period from ETGBS to PC removal was 11.5 days (IQR 2.0–26.8 day). The variant interval of PC removal was due to different time for ETGBS conversion from PC. Thus, fur-ther researches are needed to assess the timing of both ETGBS con-version from PC and PC removal after successful performing ETGBS.
The main limitations of the study are its retrospective design, a small sample size from a single center, and the lack of a com-parison group with other modalities such as EUS-GBD. In particu-lar, possibility of selection bias exists, thus technical success rate of the procedure may be overestimated. Furthermore, the proce-dure strategy and definitive criteria used for ETGBS conversion were not standardized, but as aforementioned approach, the proce-dure could be indicated in high-risk surgical patients with enable guidewire access to the cystic duct. Our results are limited by the lack of long-term outcomes. Therefore, a well-designed large-scale prospective randomized trial with long-term duration is desirable to confirm our results.
In conclusion, ETGBS as a secondary intervention for the pur-pose of internalizing gallbladder drainage in patients following placement of a PC is safe, effective, and technically feasible.There-fore, conversion of PC to ETGBS may be considered a viable option in patients requiring long-term indwelling PC with high surgical risk or advanced malignancies.
Acknowledgments
We thank Eun Ha Lee from the Wonkwang University Hospital for the first assistant for the procedures. In addition, we are grate-ful for the dedication of Hee Heun Kim and Ju hee Ahn from the Wonkwang University Hospital as physician assistants.
CRediT authorship contribution statement
Hyung Ku Chon:Formal analysis, Investigation, Writing -origi-nal draft.Chan Park:Resources, Writing –review & editing.Dong Eun Park:Data curation.Tae Hyeon Kim:Conceptualization, Su-pervision, Funding acquisition, Writing –review & editing.
Funding
This study was supported by Wonkwang University 2020.
Ethical approval
The study was approved by the Institutional Review Board of the Wonkwang University Hospital.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub-ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Comparison and development of advanced machine learning tools to predict nonalcoholic fatty liver disease: An extended study
- Hepatobiliary&Pancreatic Diseases International
- Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment
- Key factors and potential drug combinations of nonalcoholic steatohepatitis: Bioinformatic analysis and experimental validation-based study
- LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver
- Cryptococcosis in patients with liver cirrhosis: Death risk factors and predictive value of prognostic models
