Gene knockout or inhibition of macrophage migration inhibitory factor alleviates lipopolysaccharide-induced liver injury via inhibiting inflammatory response
2021-11-08YuLeiGuLiLiXioDeJinLiYnLiuChngJuZhuShuiJunZhng
Yu-Lei Gu ,Li-Li Xio ,De-Jin Li ,Yn-N Liu ,Chng-Ju Zhu ,Shui-Jun Zhng
a Emergency Department, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450 052, China
b Department of Cardiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450 052, China
c Department of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hosp ital of Zhengzhou University, Zhengzhou 450052, China
d Open and Key Laboratory of Hepatobiliary & Pancreatic Surgery and Digestive Organ Transplantation at Henan Province, Zhengzhou 450052, China
e Zhengzhou Engineering Laboratory of Organ Transplantation Technique and Application, Zhengzhou 450052, China
Keywords: Sepsis Liver injury Migration inhibitory factor Gene knockout Inflammation
ABSTRACT Background: Liver injury is one of the most common complications during sepsis. Macrophage migration inhibitory factor (MIF) is an important proinflammatory cytokine. This study explored the role of MIF in the lipopolysaccharide (LPS)-induced liver injury through genetically manipulated mouse strains. Methods: The model of LPS-induced liver injury was established in wild-type and Mif -knockout C57/BL6 mice. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil) were detected, and the expressions of MIF, tumor necrosis factor-α(TNF-α) and interleukin-1 β(IL-1 β) were measured. Liver histopathology was conducted to assess liver injury. Moreover, the inhibitions of MIF with (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1) and 4-iodo-6-phenylpyrimidine (4-IPP) were used to evaluate their therapeutic potential of liver injury. Results: Compared with wild-type mice, the liver function indices and inflammation factors presented no significant difference in the Mif −/ −mice. After 72 h of the LPS-induced liver injury, serum levels of ALT, AST, and TBil as well as TNF-αand IL-1 βwere significantly increased, but the knockout of Mif attenuated liver injury and inflammatory response. In liver tissue, mRNA levels of TNF-α, IL-1 βand NF-κB p65 were remarkably elevated in LPS-induced liver injury, while the knockout of Mif reduced these levels. More-over, in LPS-induced liver injury, the inhibitions of MIF with ISO-1 and 4-IPP alleviated liver injury and slightly attenuated inflammatory response. Importantly, compared to mice with LPS-induced liver injury, Mif knockout or MIF inhibitions significantly prolonged the survival of the mice. Conclusions: In LPS-induced liver injury, the knockout of Mif or MIF inhibitions alleviated liver injury and slightly attenuated inflammatory response, thereby prolonged the survival of the mice. Targeting MIF may be an important strategy to protect the liver from injury during sepsis.
Introduction
Sepsis is an organ dysfunction syndrome that occurs in pa-tients with infection [1] . It is estimated that sepsis affects 31.5 million people worldwide annually and is caused by recurrent or persistent organ dysfunction, with approximately 5.3 million deaths [2] . Sepsis usually originates in a community or health care facility, withStaphylococcusaureus(gam-positive),PseudomonasandEscherichiacoli(gam-negative) being the most commonly iden-tified microorganisms [3] . According to its severity, sepsis pro-gresses to three different states: severe sepsis, septic shock, and multiple organ dysfunction syndromes. Risk factors associated with the occurrence and development of sepsis and organ dysfunction include pathogen-related factors, complications, and host genetic factors [3] . Tissue hypoxia, mitochondrial dysfunction and apopto-sis may be important mediators in the development of organ dys-function [4] . Organ dysfunction is an important predictor of pa-tient prognosis, and multiple organ dysfunction often leads to a high risk of death [5] .
The liver is one of the earliest organs to be functionally dam-aged by sepsis, and the degree of damage is generally more pro-nounced than that of other organs. Liver injury caused by sep-sis mainly includes hypoxic hepatitis, sepsis-induced cholestasis, and secondary sclerosing cholangitis in severe patients [6] . Ab-normality of liver function in a variety of different organ fail-ures (e.g. circulatory, respiratory, and renal, etc.) is associated with prognosis of sepsis and can be a strong independent predictor of death [ 7,8 ]. Current diagnostic and treatment measures have failed to effectively reduce the morbidity and mortality of sepsis and sepsis-induced liver injury, prompting an urgent need to develop new therapies.
Macrophage migration inhibitory factor (MIF), an inflammatory cytokine produced by inflammatory and immune cells, can in-hibit macrophage migration and subsequently regulate host de-fense [ 9,10 ]. The neutralization of MIF played an important pro-tective role in septic shock [11] . MIF expression and secretion are increased in a variety of acute and chronic inflammatory diseases, including sepsis [12],arthritis [13] and asthma [14] . The serum MIF concentration of patients with sepsis is increased and is closely as-sociated with both disease severity and prognosis [ 11,14 ]. Blocking MIF with neutralizing antibodies or small-molecule MIF inhibitors has been shown to be effective in many animal models of systemic inflammation, including sepsis induced by gram-negative bacte-ria, gram-positive bacteria or mixed bacteria, arthritis, autoimmune diabetes, and chronic obstructive pulmonary disease [ 13,15–20 ]. These results confirm the therapeutic relevance of inhibiting the proinflammatory activity of MIF and suggest the potential role of MIF in the treatment of sepsis.
Although there are many evidences that MIF plays a role in a variety of inflammatory diseases, the trends in MIF expression and its specific mechanism of action in sepsis-induced liver injury still need to be defined. In the present study, we identified significant increases in serum MIF levels in mouse model of lipopolysaccha-ride (LPS)-induced liver injury. We found that the knockout of theMifgene reduced the severity of liver injury in septic mice and significantly improved their survival. We also used pharmacologi-cal MIF inhibitorsinvivoto evaluate the therapeutic potential of targeting MIF for liver injury during the sepsis.
Methods
Generation of Mif −/ −mice and groupings
All animal studies were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2019-KY-389).Mif+/−C57/BL6 mice were purchased from Nanjing Biomedical Re-search Institute of Nanjing University (Nanjing, China). The mice were kept in a cage in which the ambient temperature was main-tained at 22 °C, the light/dark cycle was 12 h, and standard labora-tory rodent food and water were given. Before mating and breed-ing, mice were acclimatized for 1 week.Mif−/−mice were gen-erated by crossing heterozygous littermates. LPS was used in the wild-type mice and theMifknockout mice to induce liver in-jury. MIF inhibitors with (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1) (Sigma, St. Louis, MO, USA) and 4-iodo-6-phenylpyrimidine (4-IPP) (Sigma) were used to evaluate their therapeutic potential of liver injury.
The experimental mice were divided into six groups with eight mice in each group: 1) control group, the wild-type C57/BL6 mice injected with normal saline; 2) theMifknockout (Mif−/−group),Mifknockout mice injected with normal saline; 3) LPS group, LPS was injected in the wild-type mice to induce liver injury; 4) LPS +Mif−/−group, LPS was injected inMifknockout mice to in-duce liver injury; 5) LPS + ISO-1 group, LPS was injected in the wild-type mice with ISO-1 pretreatment; 6) LPS + 4-IPP group, LPS was injected in the wild-type mice with 4-IPP pretreatment. Liver histopathology was observed, and serum parameters were mea-sured.
Induction of liver injury in mice by LPS
Male 8-to 10-week-old mice weighing 18–22 g were se-lected. After 1 week of adaptive feeding, each mouse were in-dividually weighed and intraperitoneally injected with 25 mg/kg LPS (5μg/μL in 25 μL aliquots, cryopreserved) and an MIF in-hibitor (ISO-1, 30 mg/kg; or 4-IPP, 30 mg/kg). The 72 h post-injection survival was observed. The mice were anesthetized with 1% pentobarbital (50 mg/kg) 72 h after the last injection, and 0.5–1 mL of venous blood was immediately collected from the angu-lar vein. After 1 h, the venous blood was centrifuged at 4 °C and 30 0 0 rpm for 15 min. The supernatant was used to analyze serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), the cytokines MIF, tumor necrosis factor-α(TNF-α), and interleukin-1β(IL-1β). The liver was removed from each mouse: one part was used for histology, and one part was immediately frozen at -80 °C for RT-qPCR.
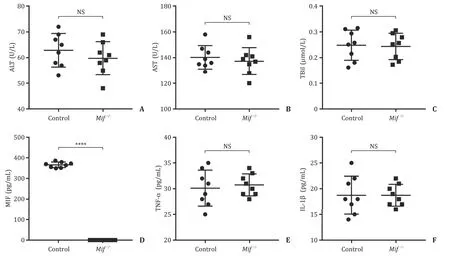
Fi g. 1. The liver function indices and inflammation factors levels of the wild-type C57/BL6 mice and Mif −/ −C57/BL6 mice. Serum levels of ALT ( A ), AST ( B ), and TBil ( C ) between the wild-type C57/BL6 mice and Mif −/ −C57/BL6 mice. ( D ) Serum level of MIF between the wild-type C57/BL6 mice and Mif −/ −C57/BL6 mice. Serum levels of inflammation factors including TNF-α( E ) and IL-1 β( F ) of the wild-type C57/BL6 mice and Mif −/ −C57/BL6 mice. Control group, the wild-type C57/BL6 mice; Mif −/ −, the Mif −/ −C57/BL6 mice. ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBil: total bilirubin; MIF: migration inhibitory factor; TNF-α: tumor necrosis factor-α; IL-1 β: interleukin-1 β; NS: not significant. ∗∗∗∗: P < 0.0 0 01.
Liver histopathology
Liver sections (4-μm thick) were stained with hematoxylin and eosin (HE) to show the degree of liver injury. All sections were photographed, and liver damage was evaluated in 10 random fields by two pathologists.
Extraction of liver tissue RNA and RT-qPCR
Total liver RNA was extracted using a TRIzol solution (Gibco, GrandIsland, NY, USA). Two micrograms of RNA were reverse-transcribed into cDNA using a reverse transcription kit (Thermo Scientific, Waltham, MA, USA). Real-time quantitative PCR was per-formed on a ProFlex real-time PCR system (Applied Biosystems, Waltham, MA, USA). GAPDH was served as an internal control. All primers are shown in Table 1 .
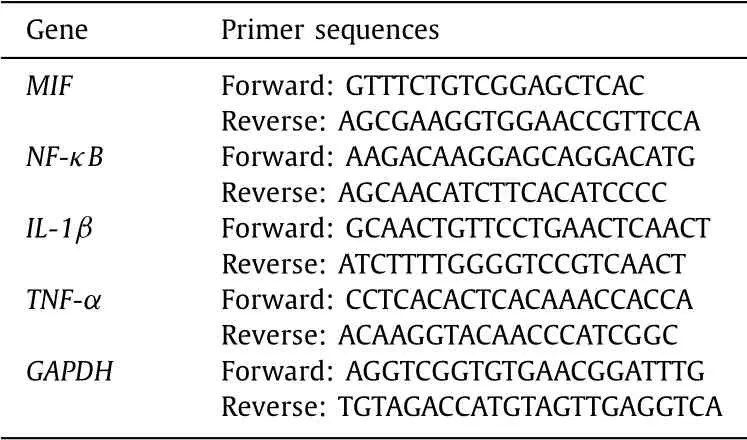
Table 1 The Primers for RT-qPCR in the study.
Analysis of human and mouse serum
Serum levels of ALT, AST, and TBil were evaluated enzymatically according to standard methods using 7180 Clinical Analyzer (Hi-tachi High-Technologies Corp., Tokyo, Japan) with reagents (Wako Pure Chemical Industries, Tokyo, Japan).
Mouse MIF, TNF-α, and IL-1βwere measured by enzyme linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, UK) accord-ing to the manufacturer’s instructions.
Statistical analysis
GraphPad Prism 8 (Graphpad Software Inc., LaJolla, CA, USA) and SPSS 20.0 (IBM, Armonk, NY, USA) were used for data analysis. Data were expressed as mean ±standard deviation (SD). Indepen-dent samplettest was used to compare the differences between two groups. One-way analysis of variance (ANOVA) was used for multiple comparisons among three groups. Log-rank (Mantel-Cox) test was used for comparing survival curves. APvalue of<0.05 was considered statistically significant.
Results
Establishment and identification of Mif −/ −C57/BL6 mice
The deletion ofMifin C57/BL6 mice was performed by Nanjing Biomedical Research Institute of Nanjing University. In brief, exon 1–3 deletion in theMifallele was generated using CRISPR/Cas9 technology.Mif−/−mice were generated by crossing heterozygous littermates. Genomic DNA from the mouse tail tip was used to screen for the specific mutation inMif.Mif−/−C57/BL6 mice devel-oped normally, behaved normally and were fertile. Heterozygous and homozygous litter sizes were normal, and the offsprings were produced in the expected genotype proportions. We used 8-to 10-week-oldMif−/−mice and their wild-type littermates for subse-quent sepsis experiments. Compared with wild-type C57/BL6 mice, the liver function indices including ALT, AST, and TBil presented no significant difference inMif−/−C57/BL6 mice ( Fig. 1 A-C). How-ever,Mifgene knockout in the C57/BL6 mice prevented the mice from producing MIF ( Fig. 1 D). Notably, inflammation factors TNF-αand IL-1βwere not significantly different between the wild-type C57/BL6 mice andMif−/−C57/BL6 mice ( Fig. 1 E, F).
Mif gene knockout reduced the inflammatory response and the severity of liver injury during sepsis
Serum levels of ALT, AST and TBil were the most sensitive and specific markers for liver injury during sepsis [6] . After inducing sepsis, we measured these markers. As shown in Fig. 2 A-C, 72 h after LPS injection, serum levels of ALT, AST, and TBil were sig-nificantly increased in the LPS group compared to those of the control group (allP<0.0 0 01). However, these markers were de-creased in the LPS +Mif−/−group compared with those in the LPS group (P<0.05). Compared with that in the control group, the serum level of MIF was significantly increased in the LPS group (P<0.0 0 01), while it was undetectable in the LPS +Mif−/−group ( Fig. 2 D). Moreover, serum levels of TNF-αand IL-1βwere signifi-cantly increased in mice with sepsis-induced liver injury and were considered to be involved in the pathophysiological process. Com-pared with LPS group, serum level of IL-1βwas significantly de-creased in LPS +Mif−/−group (P<0.05, Fig. 2 F), and serum level of TNF-αalso showed decreasing trend (P= 0.077, Fig. 2 E). Im-portantly, we further performed histological evaluation of liver tis-sues. Compared with those in the control group, LPS injection in-duced significant liver injury and widening of the sinusoid space in the liver, while liver injury was milder in the LPS +Mif−/−group ( Fig. 2 G).
Furthermore, the mRNA expression levels of MIF, TNF-α, IL-1βand NF-κB p65 in the liver were measured. Compared with that in the control group, MIF mRNA level relative to that of GAPDH was significantly increased in the LPS group (P<0.0 0 01), while MIF mRNA was undetectable in the LPS +Mif−/−group ( Fig. 3 A). Moreover, mRNA levels of TNF-αand IL-1βwere markedly ele-vated in the LPS group compared with those of the control group (P<0.0 0 01 andP<0.01, respectively), and TNF-αmRNA level was lower in the LPS +Mif−/−group compared with that of the LPS group (P<0.01) ( Fig. 3 B, C). Moreover, we measured the NF-κB p65 mRNA level in liver tissue and found a significant increase in NF-κB p65 mRNA level in the LPS group compared with that in the control group (P<0.01) ( Fig. 3 D).

Fig. 2. Mif gene knockout reduced the inflammatory response and the severity of liver injury in the context of sepsis. Serum levels of ALT ( A ), AST ( B ), and TBil ( C ) among the control group, LPS model group, and LPS model + Mif −/ −group. ( D ) Serum level of MIF among the control group, LPS group, and LPS + Mif −/ −group. Serum levels of inflammation factors including TNF-α( E ) and IL-1 β( F ) among the control group, LPS group, and LPS + Mif −/ −group. ( G ) The histological evaluation (original magnification ×200) of liver tissues among the control group, LPS group, and LPS + Mif −/ −group. Compared with the control group, LPS injection induced significant liver injury and widening of the sinusoid space in the liver, while liver injury was alleviated in the LPS + Mif −/ −group. The arrow indicated the widen sinusoid space and inflammatory cell infiltration in the liver. Control group, the wild-type C57/BL6 mice; LPS group, wild-type mice injected with LPS to induce liver injury; LPS + Mif −/ −group, Mif knockout mice injected with LPS to induce liver injury. LPS: lipopolysaccharide; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBil: total bilirubin; MIF: migration inhibitory factor; TNF-α: tumor necrosis factor-α; IL-1 β: interleukin-1 β; NS: not significant. *: P < 0.05; ∗∗: P < 0.01; ∗∗∗∗: P < 0.0 0 01.
The MIF inhibitors ISO-1 and 4-IPP reduced the inflammatory response and the severity of liver injury in the context of sepsis
Next, we validated whether targeting MIF as a therapeutic strat-egy could alleviate sepsis-induced liver injury in mice. ISO-1 and 4-IPP are small molecule tautomerase inhibitors of MIF that in-hibit the MIF-mediated secretion of inflammatory cytokines by monocytes and macrophages. Wild-type mice with sepsis-induced liver injury were pretreated with ISO-1 or 4-IPP before LPS induc-tion. As shown in Fig. 4 A-C, compared with those in the control group, serum levels of ALT, AST and TBil were significantly in-creased in the LPS group. However, pretreatment with ISO-1 and 4-IPP reduced the serum levels of AST and TBil in mice with sepsis-induced liver injury. Similarly, the serum level of MIF was also significantly reduced in the 4-IPP-pretreated mice with sepsis-induced liver injury (P<0.001, Fig. 4 D). Moreover, compared with those in the control group, serum levels of TNF-αand IL-1βwere markedly increased in the LPS group, while these expression lev-els showed a decreasing trend in the LPS + ISO-1 and LPS + 4-IPP groups ( Fig. 4 E, F). Histologically, pretreatment with ISO-1 or 4-IPP alleviated LPS-induced liver injury and reduced inflammatory cell infiltration and hepatocyte necrosis ( Fig. 4 G). mRNA expression lev-els of MIF, TNF-α, IL-1βand NF-κB p65 in liver tissues were signif-icantly upregulated in the LPS group compared with those of the control group (allP<0.05, Fig. 5 ). After ISO-1 or 4-IPP pretreat-ment, the MIF, TNF-α, IL-1βand NF-κB p65 mRNA expression lev-els in liver tissues showed decreasing trends compared with those in the LPS group.
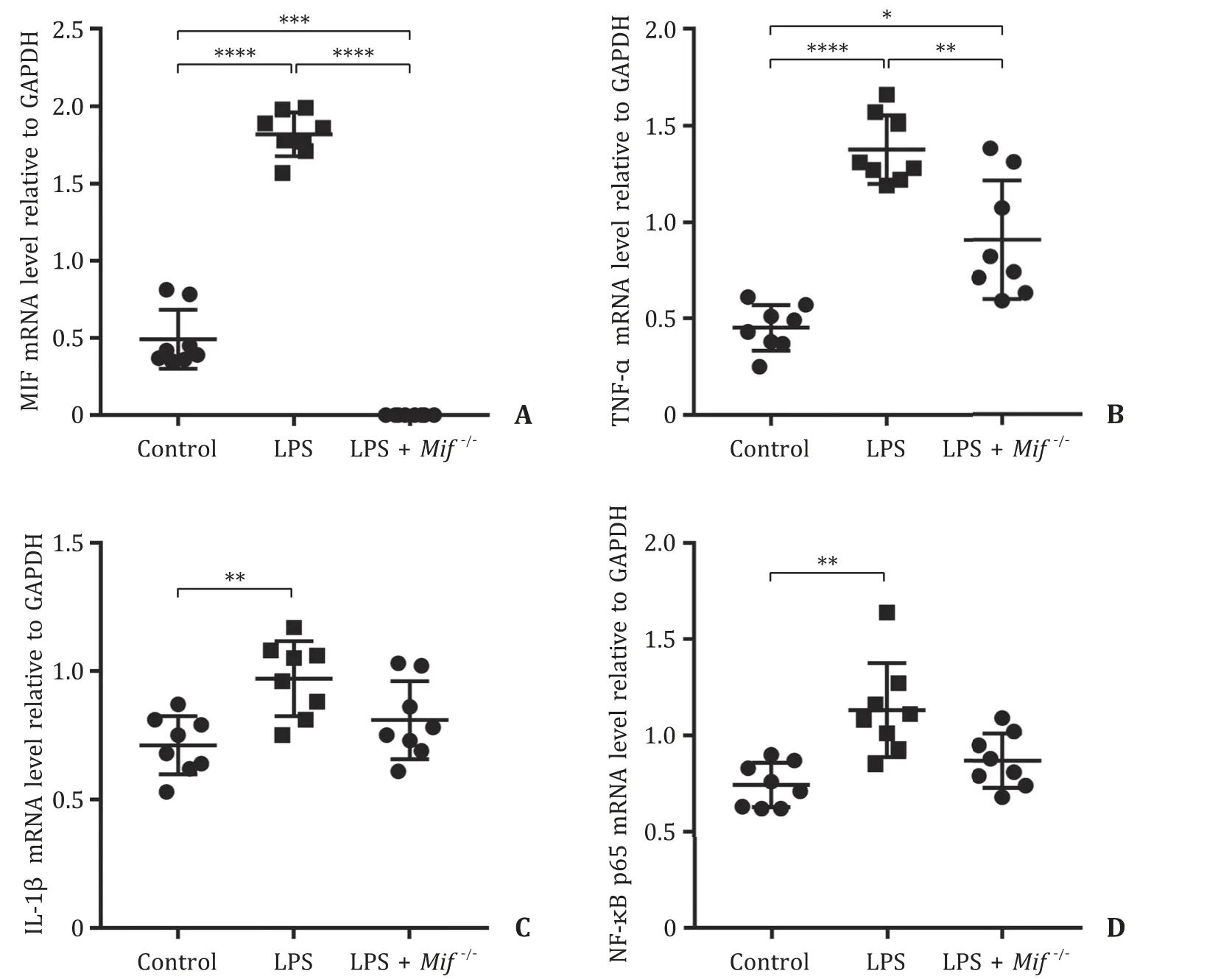
Fig. 3. The mRNA expression levels of MIF, TNF-α, IL-1 βand NF-κB p65 in the liver were measured by RT-qPCR among the control group, LPS group, and LPS + Mif −/ −group. ( A ) The mRNA level of MIF relative to GAPDH in the liver among the control group, LPS group, and LPS + Mif −/ −group. The mRNA levels of inflammatory factors including TNF-α( B ) and IL-1 β( C ) relative to GAPDH in the liver among the control group, LPS group, and LPS + Mif −/ −group. ( D ) The mRNA level of NF-κB p65 relative to GAPDH in the liver among the control group, LPS group, and LPS + Mif −/ −group. Control group, the wild-type C57/BL6 mice; LPS group, wild-type mice injected with LPS to induce liver injury; LPS + Mif −/ −group, Mif knockout mice injected with LPS to induce liver injury. LPS: lipopolysaccharide; MIF: migration inhibitory factor; TNF-α: tumor necrosis factor-α; IL-1 β: interleukin-1 β. *: P < 0.05; ∗∗: P < 0.01; ∗∗∗: P < 0.001; ∗∗∗∗: P < 0.0 0 01.
Prophylactic treatment with ISO-1 and 4-IPP before LPS in-duction of sepsis and liver injury had a similar effect asMifgene knockout, which reduced the inflammatory response and the severity of sepsis-induced liver injury.
Mif knockout improved the 72-hour survival rates of mice with LPS-induced liver injury
To evaluate the effect ofMifknockout on the survival time of mice with LPS induced liver injury, we compared the 72 h sur-vival rates of mice in the LPS,Mif−/−, LPS + ISO-1 and LPS + 4-IPP groups.Mifgene knockout and pretreatment with small molecule tautomerase MIF inhibitors significantly improved the 72 h survival rates of mice with LPS induced liver injury and provided signifi-cant protection against the lethal effects of LPS-induced liver injury ( Fig. 6 ).

Fig. 4. The MIF inhibitors ISO-1 and 4-IPP reduced the inflammatory response and the severity of liver injury in the context of sepsis. Serum levels of ALT ( A ), AST ( B ), and TBil ( C ) among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. ( D ) Serum level of MIF among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. Serum levels of inflammation factors including TNF-α( E ) and IL-1 β( F ) among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. ( G ) The histological evaluation (original magnification ×200) of liver tissues among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. The pretreatment with ISO-1 or 4-IPP alleviated sepsis-induced liver injury in the wild-type mice. Compared with the control group, LPS injection induced significant liver injury and widening of the sinusoid space in the liver, while liver injury was alleviated in the LPS + ISO-1 group and LPS + 4-IPP group. The arrow indicated the widen sinusoid space and inflammatory cell infiltration in the liver. Control group, the wild-type C57/BL6 mice; LPS group, wild-type mice injected with LPS to induce liver injury; LPS + ISO-1 group, wild-type mice injected with LPS to induce liver injury and ISO-1 pretreatment; LPS + 4-IPP group, wild-type mice injected with LPS to induce liver injury and 4-IPP pretreatment. LPS: lipopolysaccharide; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBil: total bilirubin; MIF: migration inhibitory factor; TNF-α: tumor necrosis factor-α; IL-1 β: interleukin-1 β; ISO-1: (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester; 4-IPP: 4-iodo-6-phenylpyrimidine; NS: not significant. *: P < 0.05; ∗∗∗: P < 0.001; ∗∗∗∗: P < 0.0001.
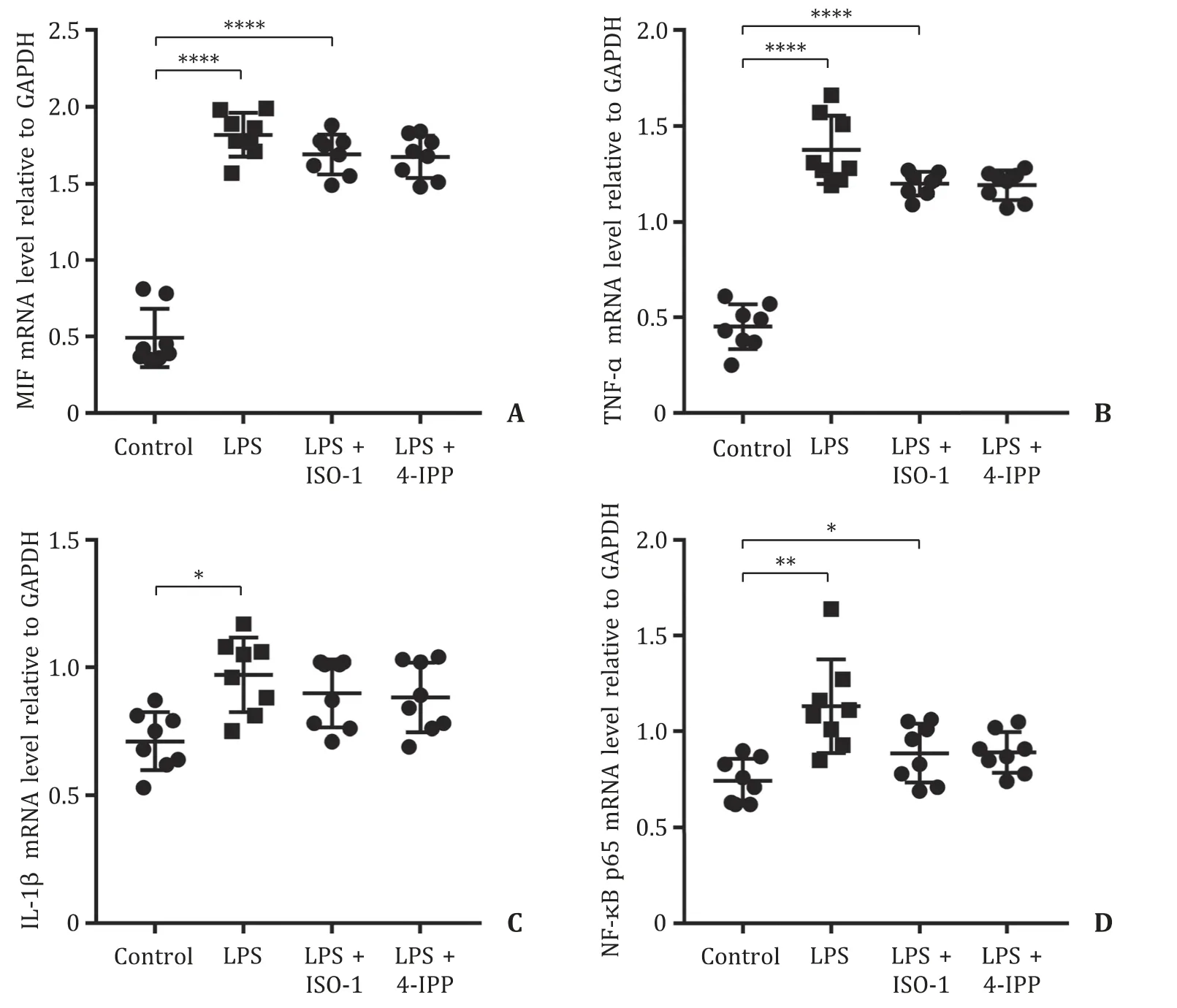
Fig. 5. The mRNA expression levels of MIF, TNF-α, IL-1 βand NF-κB p65 in the liver among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. ( A ) The mRNA level of MIF relative to GAPDH in the liver among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. The mRNA levels of inflammatory factors including TNF-α( B ) and IL-1 β( C ) relative to GAPDH in the liver among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. ( D ) The mRNA level of NF-κB p65 relative to GAPDH in the liver among the control group, LPS group, LPS + ISO-1 group, and LPS + 4-IPP group. Control group, the wild-type C57/BL6 mice; LPS group, wild-type mice injected with LPS to induce liver injury; LPS + ISO-1 group, wild-type mice injected with LPS to induce liver injury and ISO-1 pretreatment; LPS + 4-IPP group, wild-type mice injected with LPS to induce liver injury and 4-IPP pretreatment. LPS: lipopolysaccharide; MIF: migration inhibitory factor; TNF-α: tumor necrosis factor-α; IL-1 β: interleukin-1 β; ISO-1: (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester; 4-IPP: 4-iodo-6-phenylpyrimidine. *: P < 0.05; ∗∗: P < 0.01; ∗∗∗∗: P < 0.0 0 01.
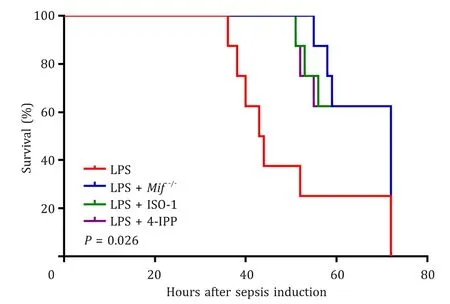
Fig. 6. Mif gene knockout improved the 72 h survival rates of mice with LPS in-duced liver injury. The log-rank (Mantel-Cox) test was used for comparing survival curves among the LPS group, LPS + Mif −/- group, LPS + ISO-1 group, and LPS + 4-IPP group. Mif gene knockout and pretreatments with small molecule tautomerase MIF inhibitors significantly improved the 72 h survival rates of mice with LPS in-duced liver injury ( P = 0.026). LPS group, wild-type mice injected with LPS to in-duce liver injury; LPS + Mif −/- group, Mif knockout mice injected with LPS to induce liver injury; LPS + ISO-1 group, wild-type mice injected with LPS to induce liver in-jury and ISO-1 pretreatment; LPS + 4-IPP group, wild-type mice injected with LPS to induce liver injury and 4-IPP pretreatment. MIF: migration inhibitory factor; LPS: lipopolysaccharide; ISO-1: (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester; 4-IPP: 4-iodo-6-phenylpyrimidine.
Discussion
Sepsis is a life-threatening organ dysfunction syndrome caused by a disordered host response to infection. Sepsis has a high mor-tality that increases with disease severity. The pathogenesis of sepsis is very complicated. The cascade-like release of inflamma-tory factors, imbalance in proinflammatory/anti-inflammatory me-diators, and immune dysfunction play important roles in the de-velopment of sepsis [ 11,21 ]. MIF, which is expressed and secreted by a variety of cell types, is a pleiotropic inflammatory cytokine. As an effective chemokine, MIF not only enhances the activity of macrophages [22],but also upregulates the expression of MHC-II molecules, costimulatory and adhesion molecules and cytokines in a variety of cell types [23] . In addition, MIF has the ability to prevent activation-induced apoptosis [24] . Therefore, MIF increases the expression of inflammatory mediators and plays a proinflam-matory role.
Recent studies [ 25,26 ] have shown that MIF plays an important role in both acute and chronic stress responses of the liver. For ex-ample, MIF is an important danger signal released from hepato-cytes in response to ethanol-induced damage [25] . However, MIF has a protective effect on hepatocytes in the face of high-fat diet-induced damage [26] . In sepsis, MIF promotes the synthesis and release of many inflammatory factors, including TNF-αand IL-1β. These inflammatory factors play important roles in the pathogen-esis of sepsis and sepsis-induced liver injury. MIF functions as a dopachrome tautomerase, and the small molecule compounds ISO-1 and 4-IPP specifically bind to their active site to inhibit MIF ac-tivity.
The mechanism that LPS causes liver damage in sepsis is com-plex. A recent study has shown that LPS activates Kupffer cells in the liver to release numerous inflammatory mediators, includ-ing TNF-αand IL-1β, which cause hepatocyte damage and necro-sis [27] . In our study, we found that MIF levels in mice with LPS-induced liver injury were significantly increased.Mifknockout mice were used to investigate the role of MIF in LPS-induced liver injury, which was further confirmed with the MIF inhibitors ISO-1 and 4-IPP in wild-type mice with sepsis. MIF is involved in the pathophysiological process of sepsis and mediates the lethal effect of LPS on mice, and anti-MIF antibodies reduce the mortality of mice with bacterial peritonitis [28] . While the injection of recom-binant MIF protein accelerates LPS-induced death in septic mice, anti-MIF antibody injection reduces the mortality of mice after the injection of a lethal dose of LPS, suggesting that MIF promotes LPS toxicity [29] . Our results clearly demonstrated that increased MIF activity had a worsening effect on the LPS-induced liver injury. Small molecule tautomerase inhibitors or neutralizing antibodies against MIF might be potential drugs against LPS-induced liver in-jury.
Cytokines are widely present in the body and play roles in sig-nal transduction and biological regulation; NF-κB is one of them. NF-κB plays a key role in the inflammatory response and is an upstream signal that promotes the secretion and release of vari-ous inflammatory factors. The NF-κB pathway is a classic inflam-matory activation pathway. Both TNF-αand IL-6 are regulated by this pathway, and their activation is also observed in systemic in-flammatory response syndrome. The inflammatory factors regu-lated by NF-κB include adhesion molecules (ICAM-1 and V-CAM), interferon, and some members of the interleukin family [ 30,31 ]. NF-κB can also be activated by inflammatory factors, potentiating the activation of NF-κB inflammatory signaling [32] . LPS effectively stimulates NF-κB, increases NF-κB synthesis and secretion, accel-erates the degradation of IκB, dissociates and activates the NF-κB-IκB complex, and regulates the activity of cytokines, including TNF-αand IL-1β[ 33,34 ]. In this study, we found that mRNA lev-els of NF-κB p65, MIF, TNF-αand IL-1βwere elevated in the liver tissue of LPS treated mice. Consistently, serum levels of TNF-αand IL-1βwere also significantly increased in mice with LPS-induced liver injury.
In summary, compared with wild-type mice, the liver function indices and inflammation factors presented no significant differ-ence in theMif−/−mice. After 72 h of the LPS-induced liver in-jury, serum levels of ALT, AST, and TBil as well as TNF-αand IL-1βwere significantly increased, and the knockout ofMifprotected mice from liver injury and inflammatory response. In liver tissue, mRNA levels of TNF-α, IL-1βand NF-κB p65 were remarkably el-evated in LPS-induced liver injury, while the knockout ofMifpre-cluded these increases. Moreover, in LPS-induced liver injury, the inhibitions of MIF with ISO-1 and 4-IPP alleviated liver injury and slightly attenuated inflammatory response. Importantly, compared with the LPS-induced liver injury,Mifknockout or MIF inhibitions significantly prolonged the survival of the mice. In conclusion, in LPS-induced liver injury, the knockout ofMifor MIF inhibitions al-leviated liver injury and slightly attenuated inflammatory response, thereby prolonging the survival of the mice. Targeting MIF may be an important strategy to protect the liver from injury during sepsis.
Acknowledgments
We thank Ji-Hua Shi and Bing Yan for their suggestions and guidance on the experiment, as well as the analysis and discussion of the experimental results.
CRediT authorship contribution statement
Yu-Lei Gu:Conceptualization, Data curation, Investigation, Writing -original draft, Writing -review & editing.Li-Li Xiao:Conceptualization, Data curation, Investigation, Validation.De-JianLi: Data curation, Investigation, Methodology, Validation, Visualiza-tion.Yan-Na Liu:Formal analysis, Software.Chang-Ju Zhu:Project administration, Supervision.Shui-Jun Zhang:Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing -review & editing.
Funding
This work was supported by grants from the National Natu-ral Science Foundation of China (81971881) and Medical Science and Technology Project of Henan Provincial Health Commission (SB201901045).
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2019-KY-389).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub-ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Comparison and development of advanced machine learning tools to predict nonalcoholic fatty liver disease: An extended study
- Hepatobiliary&Pancreatic Diseases International
- Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment
- Key factors and potential drug combinations of nonalcoholic steatohepatitis: Bioinformatic analysis and experimental validation-based study
- LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver
- Cryptococcosis in patients with liver cirrhosis: Death risk factors and predictive value of prognostic models
