全缘凤尾蕨化学成分的研究
2021-09-12邹娟毛效贤何康叶江海唐仁桃王喻雪张敬杰
邹娟 毛效贤 何康 叶江海 唐仁桃 王喻雪 张敬杰
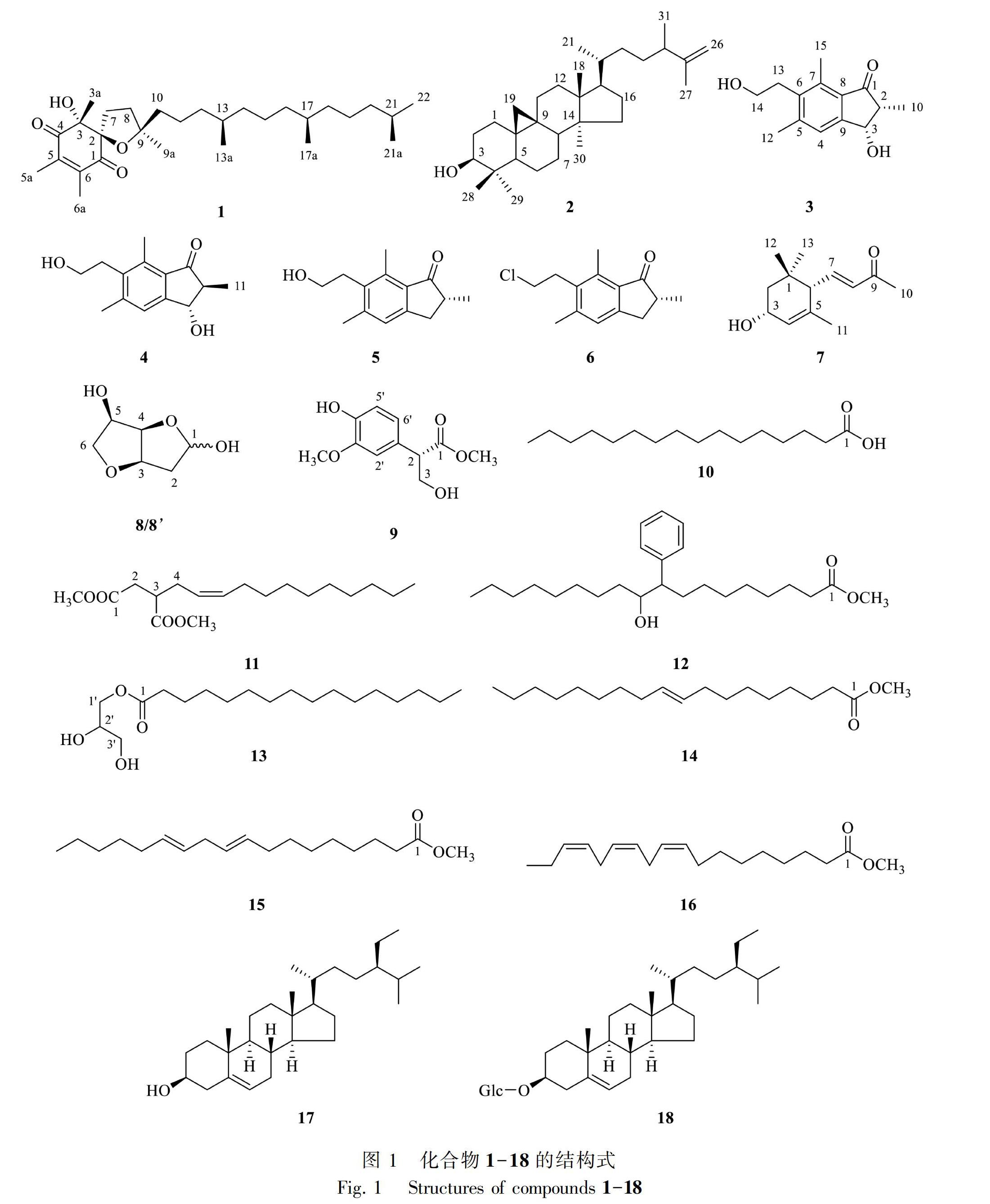
摘 要: 全缘凤尾蕨资源丰富且在贵州分布广泛,具有清热利湿、活血消肿的功效。为了探明该植物的物质基础、寻找相关的活性成分和先导化合物,该文用95%甲醇对全缘凤尾蕨地上部分浸渍提取,采用硅胶、MCI gel CHP 20P、YMC gel ODS-A-HG、Sephadex LH-20等色谱技术对全缘凤尾蕨的化学成分进行分离纯化,根据波谱数据鉴定化合物的结构。结果表明:从全缘凤尾蕨中分离得到18个化合物,分别鉴定为 (-)-α-tocospirone(1)、环鸦片甾烯醇(2)、(2S,3S)-pterosin C(3)、(2R,3S)-pterosin C(4)、pterosin B(5)、pterosin F(6)、α-紫罗兰酮A(7)、sauropunol C/D(8/8)、ficusol(9)、棕榈酸(10)、2-dodec-2-enyl-succinic acid dimethyl ester(11)、methyl-9-phenyl-10-hydroxyoctadecanoate(12)、十六烷酸甘油酯(13)、methyl elaidate(14)、(Z,Z)-9,12-十八烷二烯酸甲酯(15)、(Z,Z,Z)-9,12,15-十八烷三烯酸甲酯(16)、胡萝卜苷(17)、β-谷甾醇(18)。所有化合物均为首次从该植物中分离得到。该研究结果为合理利用其资源奠定了科学基础。
关键词: 凤尾蕨属, 全缘凤尾蕨, 化学成分, 分离纯化, 结构鉴定
中图分类号: Q946 文献标识码: A 文章编号: 1000-3142(2021)07-1046-08
Abstract: Pteris insignis, which has the medical function of clearing heat and draining dampness as well as activating blood to subside swelling, is widely distributed and rich in resources in Guizhou. As far as we know, there is no literature report on the study of its chemical constituents and biological activity. The purpose of this paper was to explore the material basis of this species, to reveal the related bioactive constituents and lead compounds. The aerial part of P. insignis was extracted with 95% methanol to give crude extract, which was then isolated and purified by silica gel, MCI gel CHP 20P, YMC gel ODS-A-HG and Sephadex LH-20 column chromatography. The structures of obtained compounds were deduced by the analysis of comprehensive spectral data. Eighteen compounds were isolated and identified as (-)-α-tocospirone (1), cyclolaudenol (2), (2S,3S)-pterosin C (3), (2R,3S)-pterosin C (4), pterosin B (5), pterosin F (6), α-ionone A(7), sauropunol C/D (8/8), ficusol (9), palmitic acid (10), 2-dodec-2-enyl-succinic acid dimethyl ester (11), methyl-9-phenyl-10-hydroxyoctadecanoate (12), hexadecanoic acid-2,3-dihydroxy-propyl ester (13), methyl elaidate (14), (Z,Z)-9,12-octadecadienoic acid methyl ester (15), (Z,Z,Z)-9,12,15-octadecatrienoic acid ester (16), carotenoid (17), β-sitosterol (18). All the compounds were isolated from P. insignis for the first time. The results of this study will lay a scientific foundation for the rational use of its resources.
Key words: Pteris L., Pteris insignis, chemical constituents, isolation and purification, structure identification
全緣凤尾蕨(Pteris insignis)为凤尾蕨科(Pteridaceae)凤尾蕨属(Pteris L.)植物,《中国蕨类植物图谱》《贵州蕨类植物志》《贵州石松类和蕨类植物志》等专著均有记载(秦仁昌,1930;王培善和王筱英,2001;王培善和潘炉台,2018)。该植物具有清热利湿、活血消肿的功效,内服治疗痢疾、黄疸、淋证、咽喉肿痛、瘰疬等,外用治疗风湿骨痛、跌打损伤等(潘炉台,2012)。贵州少数民族地区常用该植物全草治疗咽喉肿痛及颈、项、下颔等部位的各种肿块。近年来,本课题组在民族医药调研基础上,先后对其同属植物狭叶凤尾蕨(Li et al., 2015)、岩凤尾蕨(李宛霏等,2016)、银叶凤尾蕨(邹娟等,2019)等化学成分进行了研究,从中发现了系列具有抗HIV、抗肿瘤、抗菌作用的活性成分。为了继续寻找该属植物中活性成分和先导化合物,我们对全缘凤尾蕨的化学成分进行了研究,从该植物地上部分共分离得到18个化合物(图1),分别鉴定为(-)-α-tocospirone(1)、环鸦片甾烯醇(2)、(2S,3S)-pterosin C(3)、(2R,3S)-pterosin C(4)、pterosin B(5)、pterosin F(6)、α-紫罗兰酮A(7)、sauropunol C/D(8/8)、ficusol(9)、棕榈酸(10)、2-dodec-2-enyl-succinic acid dimethyl ester(11)、methyl-9-phenyl-10-hydroxyoctadecanoate(12)、十六烷酸甘油酯(13)、methyl elaidate(14)、(Z,Z)-9,12-十八烷二烯酸甲酯(15)、(Z,Z,Z)-9,12,15-十八烷三烯酸甲酯(16)、胡萝卜苷(17)、β-谷甾醇(18)。所有化合物均为首次从该植物中分离得到。
1 材料与方法
1.1 仪器、试剂和供试材料
仪器:质谱仪(JEOL 5973 MSD型,美国安捷伦公司);600 MHz核磁共振仪(Bruker Avance NEO型,布鲁克公司);500 MHz核磁共振仪(WNMR-I型,武汉中科牛津波谱技术有限公司);旋转蒸发仪(Buchi R210型,瑞士步琦有限公司);植物粉碎机(J209A-4型,河南黄骅齐家务科学仪器厂);电子天平(Metter-Toledo型,瑞士梅特勒-托利多公司);葡聚糖凝胶(Sephadex LH-20型,瑞士法玛西亚普强公司);暗箱三用紫外分析仪(ZF-7型,上海佳鹏科技有限公司);中压色谱分离凝胶(MCI GELCHP 20P 75-150 μm型,日本三菱化学公司);C18反相材料(YMC gel ODS-A-HG100-20/45型,日本维美希株式会社);制备薄层板及柱层析用硅胶(GF254型及200~300目,青岛海洋化工有限公司)。
试剂:NMR测试溶剂为氘代试剂,其余试剂均由工业纯经重蒸处理后使用。
供试药材于2016年10月采自贵州省荔波县,经贵州中医药大学赵俊华教授鉴定为凤尾蕨科凤尾蕨属植物全缘凤尾蕨(Pteris insignis)。植物标本存放于贵州中医药大学苗医药重点实验室(标本凭证20161003)。
1.2 实验方法
1.2.1 提取与萃取 首先,取干燥全缘凤尾蕨羽片粉末24.7 kg,用95%的甲醇进行浸渍提取(常温),得浸膏2.8 kg。浸膏用水分散溶解,分别用石油醚和乙酸乙酯萃取,得到乙酸乙酯部位335.61 g。然后,经MCI柱层析,用甲醇和水体系(1∶9→9∶1)梯度洗脱,得到A(25.42 g)、B(27 g)、C(80 g)、D(78.6 g)4个极性段。
1.2.2 分离与纯化 A部分经C18柱层析,用甲醇和水体系(1∶9→9∶1)梯度洗脱,得到4个部分(A1、A2、A3、A4):A1部分经过硅胶柱层析,用二氯甲烷和甲醇体系(19∶1、9∶1、4∶1)洗脱,得到化合物8/8(136 mg)、化合物3和化合物4(22 mg);A2部分经硅胶柱层析,用石油醚/乙酸乙酯体系(1∶1)洗脱,得到化合物9(7 mg);A3部分经硅胶柱层析,用石油醚/乙酸乙酯体系(19∶1、9∶1、5∶1)洗脱,得到化合物17(13 mg); A4部分母液经过硅胶柱层析分离, 用石油醚/乙酸乙酯体系(49∶1、19∶1、9∶1)洗脱,得到化合物11(23 mg)。B部分样品经C18柱层析,用甲醇和水体系(1∶9→9∶1)梯度洗脱,得到B1、B2、B3三个部分:B1部分经硅胶柱层析,用石油醚/乙酸乙酯体系(7∶3)洗脱,得到化合物7(13 mg);B2部分经硅胶柱层析,依次用石油醚/乙酸乙酯体系(50∶1、9∶1、1∶1)洗脱,得到化合物5(55 mg)、化合物6(300 mg)、化合物13(9 mg);B3部分经硅胶柱层析,用石油醚/乙酸乙酯体系(100∶1、50∶1、9∶1)洗脱,分离得到化合物16(44 mg)。C部分静置析出针状晶体,经甲醇反复重结晶,得到化合物18(3.2 g);C部分母液经硅胶柱层析,用石油醚/乙酸乙酯体系(7∶3、3∶2)洗脱,分离得到化合物14(583 mg)。D部分样品经硅胶柱层析分离,用石油醚/乙酸乙酯体系(20∶1、9∶1、7∶3、1∶1)洗脱,得到D1、D2两部分,其中9∶1流分析出晶体,经重结晶得到化合物10(1.1 g)。D1部分经硅胶柱层析,用石油醚/乙酸乙酯体系(4∶1)洗脱,得到化合物15(205 mg)和D1部分,D1经凝胶柱层析纯化得到化合物1(32 mg)和化合物3(12 mg);D2部分样品也同样经硅胶柱层析,用石油醚/乙酸乙酯体系(4∶1)洗脱,得到化合物12(20 mg)。
2 结果与分析
化合物1 无色油状,1H-NMR (600 MHz, CDCl3) δ: 0.83 (3H, d, J=2.3 Hz, H-18), 0.84 (3H, d, J=2.1 Hz, H-13a), 0.86 (3H, d, J=3.3 Hz, H-21a), 0.87 (3H, br s, H-22), 1.34 (3H, s, H-9a), 1.37 (3H, s, H-3a), 2.06 (3H, s, H-5a), 2.07 (3H, s, H-6a), 3.82 (1H, s, -OH); 13C-NMR (150 MHz, CDCl3) δ: 198.8 (C-1), 93.3 (C-2), 81.2 (C-3), 24.2 (C-3a), 201.7 (C-4), 142.0 (C-5), 13.1 (C-5a), 146.9 (C-6), 13.4 (C-6a), 32.0 (C-7), 36.4 (C-8), 87.1 (C-9), 25.7 (C-9a), 41.4 (C-10), 22.3 (C-11), 37.5 (C-12), 32.8 (C-13), 19.8 (C-13a), 37.5 (C-14), 24.8 (C-15), 37.4 (C-16), 32.7 (C-17), 19.7 (C-17a), 37.3 (C-18), 24.5 (C-19), 39.4 (C-20), 28.0 (C-21), 22.6 (C-21a), 22.7 (C-22)。以上数据与文献Lin et al.(2003)的报道基本一致,故鉴定化合物1为(-)-α-tocospirone。
化合物2 白色粉末,1H-NMR (600 MHz, CDCl3) δ: 0.33 (1H, d, J = 4.0 Hz, H-19), 0.55 (1H, d, J = 3.8 Hz), 0.81 (3H, s, H-29), 0.87 (3H, d, J=3.5 Hz, H-21), 0.88 (3H, s, H-30), 0.96 (3H, s, H-18), 0.97 (3H, s, H-28), 1.00 (3H, d, J=6.9 Hz, H-31), 1.64 (3H, s, H-27), 3.28 (1H, m, H-3), 4.67 (2H, br s, H-26); 13C-NMR (150 MHz, CDCl3) δ: 32.0 (C-1), 30.4 (C-2), 78.9 (C-3), 40.5 (C-4), 47.1 (C-5), 21.1 (C-6), 28.1 (C-7), 48.0 (C-8), 20.0 (C-9), 26.1 (C-10), 26.5 (C-11), 32.9 (C-12), 48.8 (C-13), 45.3 (C-14), 35.6 (C-15), 26.0 (C-16), 52.3 (C-17), 18.3 (C-18), 29.7 (C-19), 36.0 (C-20), 18.7 (C-21), 33.9 (C-22), 31.5 (C-23), 41.6 (C-24), 150.3 (C-25), 109.4 (C-26), 18.0 (C-27), 25.5 (C-28), 14.1 (C-29), 19.3 (C-30), 20.2 (C-31)。以上數据与文献Cantillo Ciau et al.(2001)的报道基本一致,故鉴定化合物2为环鸦片甾烯醇。
化合物3 白色油状液体,1H-NMR (500 MHz, CD3OD) δ: 1.29 (3H, d, J=7.0 Hz, H-11), 2.46 (1H, m, H-2), 2.48 (3H, s, H-12), 2.64 (3H, s, H-15), 3.01 (2H, m, H-13), 3.61 (2H, t, J=7.0 Hz, H-14), 4.68 (1H, d, J=5.9 Hz, H-3), 7.35 (1H, s, H-4); 13C-NMR (125 MHz, CD3OD) δ: 207.6 (C-1), 54.6 (C-2), 75.9 (C-3), 126.7 (C-4), 146.3 (C-5), 138.2 (C-6), 137.9 (C-7), 132.4 (C-8), 154.5 (C-9), 13.3 (C-10), 21.4 (C-12), 33.1 (C-13), 61.6 (C-14), 14.0 (C-15)。以上数据与文献Tian et al.(2011)和 Ng & Mcmorris(1984)的报道基本一致,故鉴定化合物3为(2S, 3S)-pterosin C。
化合物4 白色固体,1H-NMR (500 MHz, CD3OD) δ: 1.17 (3H, d, J=7.3 Hz, H-11), 2.46 (1H, m, H-2), 2.48 (3H, s, H-12), 2.64 (3H, s, H-15), 3.01 (2H, m, H-13), 3.61 (2H, t, J=7.0 Hz, H-14), 5.14 (1H, d, J=5.9 Hz, H-3), 7.35 (1H, s, H-4); 13C-NMR (125 MHz, CD3OD) δ: 210.3 (C-1), 49.7 (C-2), 70.2 (C-3), 126.7 (C-4), 138.2 (C-6), 138.1 (C-7), 132.2 (C-8), 155.1 (C-9), 10.7 (C-11), 21.4 (C-12), 33.1 (C-13), 61.6 (C-14), 14.1 (C-15)。以上数据与文献Ng & Mcmorris(1984)的报道基本一致,故鉴定化合物4为(2R, 3S)-pterosin C。
化合物5 无色透明块状晶体(乙酸乙酯),1H-NMR (600 MHz, CDCl3) δ: 1.27 (3H, dd, J=10.7, 4.3 Hz, H-10), 2.44 (3H, s, H-12), 2.56 - 2.83 (2H, m, H-3), 2.68 (3H, s, H-15), 3.02 (2H, t, J=7.5 Hz, H-13), 3.23 (1H, dd, J=16.7, 7.8 Hz, H-2), 3.75 (2H, t, J=7.4 Hz, H-14), 7.10 (1H, s, H-4); 13C-NMR (150 MHz, CDCl3) δ: 210.3 (C-1), 42.5 (C-2), 33.8 (C-3), 125.7 (C-4), 144.4 (C-5), 134.8 (C-6), 138.0 (C-7), 132.2 (C-8), 152.6 (C-9), 16.6 (C-10), 21.4 (C-12), 61.6 (C-13), 31.8 (C-14), 13.7 (C-15)。以上数据与文献Chen et al.(2008)的报道基本一致,故鉴定化合物5为pterosin B。
化合物6 白色粉末,1H-NMR (600 MHz, CDCl3) δ: 1.27 (3H, d, J=7.3 Hz, H-10), 2.43 (3H, s, H-12), 2.56-2.84 (2H, m, H-3), 2.67 (3H, s, H-15), 3.17 (2H, t, J=8.5 Hz, H-13), 3.23 (1H, dd, J = 16.7, 7.8 HZ, H-2), 3.53 (2H, t, J=8.8 Hz, H-14), 7.11 (1H, s, H-4); 13C-NMR (150 MHz, CDCl3) δ: 210.0 (C-1), 42.5 (C-2), 33.8 (C-3), 125.9 (C-4), 143.8 (C-5), 134.5 (C-6), 137.7 (C-7), 132.2 (C-8), 153.0 (C-9), 16.5 (C-10), 21.1 (C-12), 32.2 (C-13), 42.0 (C-14), 13.6 (C-15)。以上數据与文献Fukuoka et al.(1983)的报道基本一致,故鉴定化合物6为pterosin F。
化合物7 浅黄色油状液体,1H-NMR (600 MHz, CDCl3) δ: 0.89 (3H, s, H-12), 0.97 (3H, s, H-13), 1.39 (1H, m, H-2b), 1.63 (3H, s, H-11), 1.70 (1H, dd, J=12.9, 6.5 HZ, H-2a), 4.25 (1H, m, H-3), 2.26 (3H, s, H-10), 2.28 (1H, overlap, H-6), 5.59 (1H, br s, H-4), 6.07 (1H, d, J=15.8 HZ, H-8), 6.63 (1H, dd, J=15.8, 9.6 HZ, H-7); 13C-NMR (150 MHz, CDCl3) δ: 35.0 (C-1), 40.7 (C-2), 66.5 (C-3), 126.4 (C-4), 132.8 (C-5), 54.3 (C-6), 147.7 (C-7), 135.4 (C-8), 198.2 (C-9), 27.0 (C-10), 22.4 (C-11), 29.7 (C-12), 27.0 (C-13)。以上数据与文献Li & Jia(2003)的报道基本一致,故鉴定化合物7为α-紫罗兰酮A。
化合物8/8 无色油状,1H-NMR (500 MHz, CDCl3) δ: 2.05 - 2.25 (2H, m, H-2), 4.53 - 4.72 (1H, m, H-5), 5.72 - 5.54 (1H, m, H-1), 4.25 - 3.37 (3H, m, H-3, 4, 6); 13C-NMR (125 MHz, CDCl3) δ: 100.6 (C-1), 100.2 (C-1), 41.5 (C-2), 40.2 (C-2), 84.4 (C-4), 82.8 (C-4), 74.9 (C-3), 71.8 (C-3), 81.9 (C-5), 80.8 (C-5) 70.9 (C-6), 70.9 (C-6)。以上数据与文献Zhang et al.(2016)的报道基本一致,故鉴定化合物8/8为sauropunol C/D。
化合物9 白色油状液体,1H-NMR (600 MHz, CDCl3) δ: 3.71 (3H, s, H-OCH3), 3.82-3.76 (2H, m, H-3b, H-2), 3.89 (3H, s, H-OCH3), 4.11 (1H, dd, J=10.7, 8.5 Hz, H-3a), 6.76 (1H, dd, J=8.1, 1.9 Hz, H-6), 6.78 (1H, d, J=1.7 Hz, H-2), 6.88 (1H, d, J=8.0 Hz, H-5); 13C-NMR (150 MHz, CDCl3) δ: 173.8 (C-1), 53.5 (C-2), 64.7 (C-3), 127.3 (C-1), 114.7(C-2), 145.3 (C-3), 146.7 (C-4), 110.5 (C-5), 121.2 (C-6), 52.2 (C-OCH3), 56.0 (C-OCH3)。以上數据与文献Yi et al.(2011)的报道基本一致,故鉴定化合物9为ficusol。
化合物10 白色鳞片,通过与棕榈酸对照品共TLC,经3 种不同溶剂系统展开,其Rf值均一致,且混合熔点不下降。EI-MS m/z:256 [M]+, 239 [M-OH]+, 199, 185, 115, 73。1H-NMR (CDCl3, 500 MHz) δ: 0.89 (3H, t, J=6.9 Hz, H-16), 1.30 - 1.25 (24H, m, H-4 - H-15), 1.63 - 1.59 (2H, m, H-3), 2.31 (2H, t, J = 7.5 Hz, H-2); 13C-NMR (CDCl3, 125 MHz) δ: 14.1 (C-1), 34.0 (C-2), 31.9 (C-3), 29.7 (C-4 - C-8), 29.6 (C-9), 29.5 (C-10), 29.4 (C-11), 29.3 (C-12), 29.1 (C-13), 24.7 (C-14), 22.7 (C-15), 179.7 (C-16)。以上数据与文献鲁曼霞等(2009)的报道基本一致,故鉴定化合物10为棕榈酸。
化合物11 白色油状液体,1H-NMR (500 MHz, CDCl3) δ: 0.88 (3H, t, J = 6.7 Hz, H-15), 1.26-1.35 (14H, m, H-8-H-14), 2.03 (4H, m, H-4, 7), 2.30 (1H, t, J = 7.3 Hz, H-2b), 3.03 (1H, m, H-2a, 3), 3.66 (3H, s, H-OCH3), 3.68 (3H, s, H-OCH3), 4.13 (6H, dd, J = 5, 15 Hz), 5.54 (2H, m, H-5, 6); 13C-NMR (125 MHz, CDCl3) δ: 31.9 (C-1), 121.3 (C-2), 135.0 (C-3), 32.5 (C-4), 29.2 (C-5), 29.4 (C-6), 29.5 (C-7), 29.1 (C-8), 27.2 (C-9), 29.7 (C-10), 22.8 (C-11), 14.1 (C-12), 34.1 (C-13), 38.0 (C-14), 51.4 (C-15), 51.7 (C-16), 172.6 (C-17), 174.3 (C-18)。以上数据与文献Olejniczak(2010)的报道基本一致,故鉴定化合物11为2-dodec-2-enyl-succinic acid dimethyl ester。
化合物12 白色固体,1H-NMR (600 MHz, CDCl3) δ: 0.88 (3H, td, J = 6.9, 2.4 HZ, H3-18), 1.24-1.47 (26H, m, H-4-H-7, H-12-H-17), 1.63-1.56 (2H, m, H-3), 2.05-2.09 (1H, m, H-9), 2.30 (2H, t, J=7.5 Hz, H-2), 3.57-3.49 (1H, m, H-10), 3.67 (3H, s, H-OCH3), 7.27-7.10 (3H, m, P and O aromatic H), 7.28 (2H, m, M aromatic H); 13C-NMR (150 MHz, CDCl3) δ: 174.3 (C-1), 34.1 (C-2), 24.9 (C-3), 22.6 (C-4), 25.1 (C-5), 27.7 (C-6), 29.0 (C-7), 29.1 (C-8), 56.0 (C-9), 82.4 (C-10), 35.7 (C-11), 29.3 (C-12), 29.5 (C-13), 29.6 (C-14), 29.7 (C-15), 31.9 (C-16), 32.6 (C-17), 14.1 (C-18), 51.4 (C-OCH3), 127.7 (C-P aromatic), 129.5 (C-O aromatic), 130.9 (C-M aromatic), 139.1 (C-quaternary phenyl)。以上数据与文献Dailey et al.(2009)的报道基本一致,故鉴定化合物12为methyl-9-phenyl-10-hydroxyoctadecanoate。
化合物13 白色粉末,EI-MS m/z:330 [M]+,碎片离子峰239和一个十六碳脂肪酸 [M-OH] +的碎片质量相同,330与239的差值和甘油 [M-H] +的碎片质量相同。1H-NMR (600 MHz, CDCl3) δ: 0.88 (3H, t, J=7.5 Hz, H-16), 1.31-1.25 (24H, m, H-4-H-15), 1.65 (2H, m, H-3), 2.35 (2H, t, J=7.5 Hz, H-2), 3.60 (1H, dd, J=11.4, 5.8 Hz), 3.70 (1H, dd, J=11.4, 3.7 HZ, H-3a), 3.93 (1H, m, H-2), 4.21 (1H, dd, J=11.7, 4.5 HZ, H-1a), 4.15 (1H, dd, J=11.7, 6.2 HZ, H-1b); 13C-NMR (150 MHz, CDCl3) δ: 174.4 (C-1), 34.2 (C-2), 24.9 (C-3), 29.4 (C-4), 29.1 (C-5), 29.5 (C-6), 29.7 (C-7-C-12), 29.3 (C-13), 31.9 (C-14), 22.7 (C-15), 14.1 (C-16), 65.2 (C-1), 70.3 (C-2), 63.3 (C-3)。以上数据与文献柳全文等(2006)的报道基本一致,故鉴定化合物13为十六烷酸甘油酯。
化合物14 无色油状,1H-NMR (600 MHz, CDCl3) δ: 0.88 (3H, t, J=7.5 HZ, H3-19), 1.30-1.25 (22H, m, H-4-H-7, H-12-H-18), 1.63-1.59 (2H, m, H-3), 2.03-1.99 (4H, m, H-8, 11), 2.32 (2H, t, J =7.5 HZ, H-2), 3.67 (3H, s, H-OCH3), 5.35-5.34 (2H, m, H-9, 10); 13C-NMR (150 MHz, CDCl3) δ: 174.3 (C-1), 34.1 (C-2), 25.0 (C-3), 27.2 (C-4), 27.2 (C-5), 29.1 (C-6), 29.2 (C-7), 29.3 (C-8), 130.0 (C-9), 129.8 (C-10), 29.3 (C-11), 29.5 (C-12), 29.5 (C-13), 29.7 (C-14), 29.8 (C-15), 31.9 (C-16), 22.7 (C-17), 14.1 (C-18), 51.4 (C-OCH3)。以上数据与文献Thao et al.(2009)的报道基本一致,故鉴定化合物14为methyl elaidate。
化合物15 浅黄色油状液体,1H-NMR (600 MHz, CDCl3) δ: 0.90 (3H, t, J = 7.0 Hz, H-18), 1.38-1.27 (14H, m, H-4-H-7, H-15 - H-17), 1.67-1.62 (2H, m, H-3), 2.09-2.04 (4H, m, H-8, 14), 2.32 (2H, t, J = 7.6 Hz, H-2), 2.78 (2H, t, J = 6.5 Hz, H-11), 3.68 (3H, s, H-OCH3), 5.42-5.31 (4H, m, H-9, 10, 12, 13). 13C-NMR (150 MHz, CDCl3) δ: 174.3 (C-1), 34.2 (C-2), 25.0 (C-3), 29.0 (C-4), 29.4 (C-5), 29.4 (C-6), 29.5 (C-7), 27.1 (C-8), 129.9 (C-9), 128.0 (C-10), 25.7 (C-11), 128.4 (C-12), 130.4 (C-13), 27.3 (C-14), 29.4 (C-15), 31.6 (C-16), 22.7 (C-17), 14.2 (C-18), 51.5 (C-OCH3)。以上数据与文献Huh et al.(2010)的报道基本一致,故鉴定化合物15为(Z,Z)-9,12-十八烷二烯酸甲酯。
化合物16 浅黄色油状液体,1H-NMR (600 MHz, CDCl3) δ: 1.00 (3H, t, J=9.0 Hz, CH3, H-18), 1.41-1.26 (8H, m, H-4-H-7), 1.68-1.63 (2H, m, H-3), 2.11-2.07 (4H, m, H-8, 17), 2.33 (2H, J=7.6 Hz, H-2), 2.82 (4H, t, J=9.0 Hz, H-11, 14), 3.69 (3H, s, H-OCH3), 5.41-5.34 (6H, m, H-9, 10, 12, 13, 15, 16); 13C-NMR (150 MHz, CDCl3) δ: 174.2 (C-1), 34.1 (C-2),24.9 (C-3), 28.8 (C-4), 29.2 (C-5), 29.4 (C-6), 29.7 (C-7), 27.0 (C-8), 130.0 (C-9), 127.9 (C-10), 25.6 (C-11), 128.2 (C-12), 128.3 (C-13), 25.5 (C-14), 127.1 (C-15), 131.9 (C-16), 20.6 (C-17), 14.3 (C-18), 51.5 (C-OCH3)。以上數据与文献张龙等(2012)的报道基本一致,故鉴定化合物16为(Z,Z,Z)-9,12,15-十八烷三烯酸甲酯。
化合物17 白色粉末,1H-NMR (500 MHz, C5D5N) δ: 0.75 (3H, s, H-18), 0.94-1.08 (15H, m, 5 × H-CH3), 4.04-4.66 (5H, m, H-2-6), 5.14 (1H, d, J = 7.5 Hz, H-1), 5.38-5.43 (1H, m, H-6); 13C-NMR (125 MHz, C5D5N) δ: 37.3 (C-1), 30.1 (C-2), 71.5 (C-3), 42.3 (C-4), 140.9 (C-5), 121.7 (C-6), 31.9 (C-7), 32.0 (C-8), 50.2 (C-9), 36.7 (C-10), 21.1 (C-11), 39.2 (C-12), 39.8 (C-13), 56.6 (C-14), 24.3 (C-15), 28.4 (C-16), 56.1 (C-17), 12.0 (C-18), 19.2 (C-19), 36.2 (C-20), 19.0 (C-21), 34.0 (C-22), 26.2 (C-23), 45.8 (C-24), 29.5 (C-25), 20.0 (C-26), 18.9 (C-27), 23.2 (C-28), 11.8 (C-29), Glc: 102.4 (C-1), 78.4 (C-2), 78.3 (C-3), 75.1 (C-4), 77.9 (C-5), 62.6 (C-6)。以上数据与文献李玉林等(2004)的报道基本一致,故鉴定化合物17为胡萝卜苷。
化合物18 白色针晶(氯仿),1H-NMR (600 MHz, CDCl3) δ: 0.68 (3H, s, H-18), 0.81 (3H, br d, J=6.8 Hz, H-29), 0.83 (3H, t, J=2.1 Hz, H-26), 0.84 (3H, d, J = 2.2 Hz, H-27), 0.92 (3H, t, J=6.5 Hz, H-21), 1.01 (3H, s, H-19), 3.52 (1H, m, H-3), 5.35 (1H, d, J = 5.2 Hz, H-6); 13C-NMR (150 MHz, CDCl3) δ: 37.3 (C-1), 31.9 (C-2), 71.8 (C-3), 40.0 (C-4), 140.8 (C-5), 121.7 (C-6), 31.9 (C-7), 31.7 (C-8), 50.1 (C-9), 36.5 (C-10), 21.1 (C-11), 39.8 (C-12), 42.3 (C-13), 56.8 (C-14), 24.3 (C-15), 28.3 (C-16), 56.1 (C-17), 11.9 (C-18), 19.8 (C-19), 36.2 (C-20), 18.8 (C-21), 33.9 (C-22), 26.1 (C-23), 45.8 (C-24), 29.2 (C-25), 19.4 (C-26), 19.1 (C-27), 23.1 (C-28), 12.0 (C-29)。以上数据与文献刘志平等(2007)的报道基本一致,故鉴定化合物18为β-谷甾醇。
3 讨论与结论
奇特的地貌和湿润的气候,使得贵州成为我国盛产蕨类植物的地区之一,仅凤尾蕨科凤尾蕨属植物的分布就有34种(王培善和潘炉台,2018),入药18种(潘炉台,2012),药用蕨类资源丰富。因此,本课题组开展黔产凤尾蕨属植物的化学成分及活性研究,以寻找结构新颖又具活性的化合物,为该属植物资源的合理利用奠定了科学基础。
文献调研显示,迄今未见全缘凤尾蕨化学成分的文献报道,该文为其化学成分研究的首次报道,具有一定的新颖性和原创性。本次从该植物中共分离鉴定了18个化合物,结构类型包括2个三萜类、4个倍半萜类、1个紫罗兰酮类、7个脂肪族类、2个甾体类和2个其他类。所得化合物的类型丰富多样,较好地充实了凤尾蕨属植物和天然产物的研究内容,提供了相关化合物来源的数据和途径。
文献报道,凤尾蕨属植物主要含二萜类化合物及其苷、倍半萜类化合物及其苷和黄酮类化合物等。但是,本次研究未分离到二萜化合物,却分离到4个倍半萜,其骨架为1-氢-茚-1-酮(含有14个或15个碳原子),进一步印证了倍半萜类化合物是凤尾蕨属植物的特征性成分。其中,分离得到的pterosin B是一个具有抗肿瘤活性的倍半萜,对HL-60细胞显示出一定的细胞毒性作用(Chen et al., 2008)。课题组后期将进一步对所得倍半萜化合物进行活性筛选研究。另外,文献显示凤尾蕨属植物中所得其他化合物也有较好的抗肿瘤、抑制血小板聚集、抗炎、抗菌等作用(余有贵等,2001;龚先玲等,2007)。今后,我们将有目的、有侧重点地进行深入研究,期望能发现结构新颖、活性显著的化合物。
参考文献:
CANTILLO CIAU Z, BRITO LOEZA W, QUIJANO L, 2001. Triterpenoids from Tillandsia fasciculata [J]. J Nat Prod, 64(7): 953.
CHEN YH, CHANG FR, LU MC, et al., 2008. New benzyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm [J]. Molecules, 13(2): 255-266.
DAILEY JR OD, PREVOST NT, STRAHAN GD, et al., 2009. Conversion of methyl oleate to branched-chain hydroxy fatty acid derivatives [J]. J Am Oil Chem Soc, 86(7): 1101-1114.
FUKUOKA M, YOSHIHIRA K, NATORI S,et al., 1983. Carbon 13 nuclear magnetic resonance spectra of pterosin sesquiterpenes and related indan-1-one derivatives [J]. Chem Pharm Bull, 31(9): 3113-3128.
GONG XL, CHEN ZH, LIANG NC, 2007. Advances in study on chemical constituents and pharmacological activities of plants of genus Pteris [J]. Chin J Chin Mat Med, 32(14): 1382-1387. [龔先玲, 陈志红, 梁念慈, 2007. 凤尾蕨属植物化学成分及药理活性研究进展 [J]. 中国中药杂志, 32(14): 1382-1387.]
HUH S, KIM YS, JUNG E, et al., 2010. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis [J]. Biol Pharm Bull, 33(7): 1242-1245.
LI GQ, JIA ZJ, 2003. Two new ionone derivatives from Rhododendron przewalskii Maxim [J]. Chin Chem Lett, 14(1): 62-65.
LI WF, WANG J, ZHANG JJ, et al., 2015. Henrin A: A new anti-HIV ent-kaurane diterpene from Pteris henryi [J]. Int J Mol Sci, 16(11): 27978-27987.
LI WF, ZOU J, LI JX, et al., 2016. Chemical constituents from Pteris deltodon [J]. Chin Trad Herb Drugs, 47(8): 1278-1281. [李宛霏, 邹娟, 李继新, 等, 2016. 岩凤尾蕨化学成分的研究 [J]. 中草药, 47(8): 1278-1281.]
LI YL, WANG HH, SUO YR, 2004. Chemical constituents from Saussurea polycolea [J]. Acta Bot Boreal-Occident Sin, 24(7): 1292-1294. [李玉林, 王洪伦, 索有瑞, 2004. 尖苞雪莲的化学成分 [J]. 西北植物学报, 24(7): 1292-1294.]
LIN WY, KUO YH, CHANG YL, et al., 2003. Anti-platelet aggregation and chemical constituents from the rhizome of Gynura japonica [J]. Planta Med, 69(8):757-764.
LIU QW, FAN X, ZHANG T, et al., 2006. The seperation and identification of four compounds of Ceramium rubrum [J]. Mar Sci, 30(10): 4-6. [柳全文, 范晓, 张婷, 等, 2006. 三叉仙菜中4种化学成分的分离鉴定 [J]. 海洋科学, 30(10): 4-6.]
LIU ZP, CUI JG, LIU HX, et al., 2007. Chemica constituents from leaves of Livistona chinensis [J]. Guihaia, 27(1): 140-142. [刘志平, 崔建国, 刘红星, 等, 2007. 蒲葵叶化学成分研究 [J]. 广西植物, 27(1): 140-142.]
LU MX, HUANG KL, SHI SY, et al., 2009. Study on the chemical constituents of Selaginella involvens Spring and antibacterial activity [J]. Nat Prod Res Dev, 21(6): 973-975. [鲁曼霞, 黄可龙, 施树云, 等, 2009. 兖州卷柏化学成分及体外抗茵活性研究 [J]. 天然产物研究与开发, 21(6): 973-975.]
NG KME, MCMORRIS TC, 1984. An efficient synthesis of pterosin C and other pterosins [J]. Can J Chem, 62(10): 1945-1953.
OLEJNICZAK T, 2010. Microbial hydroxylation of non-activated carbon atoms in racemic 2-dodeceno-1-yl-succinic anhydride by Mortierella isabellina 212 [J]. World J Microbiol Biotechnol, 26(11): 2053-2060.
PAN LT, 2012. Medicinal ferns of Guizhou [M]. Guiyang: Guizhou Science Press: 114. [潘炉台, 2012. 贵州药用蕨类植物 [M]. 貴阳: 贵州科技出版社: 114.]
QIN RC, 1930. Icones Filicm Sinicarum [M]. Beijing: Beijing University of Technology Press: 11. [秦仁昌, 1930. 中国蕨类植物图谱 [M]. 北京: 北京大学出版社: 11.]
THAO TTP, ANH NV, ANH HN, et al., 2009. Study on chemical constituents of the vietnamese medicinal plant Fissistigma petelotii [J]. Z Naturforsch B, 64(3): 323-327.
TIAN SM, LI N, WANG LY, et al., 2011. Chemical constituents from Pteridium aquilinum var. latiusculum [J]. Chin Pharm J, 46(16): 1238-1241. [田圣梅, 李宁, 汪玲玉, 等, 2011. 蕨的化学成分研究 [J]. 中国药学杂志, 46(16): 1238-1241]
WANG PS, PAN LT, 2018. Flora of lycophytes and ferns of Guizhou [M]. Guiyang: Guizhou Science Press: 176. [王培善, 潘炉台, 2018. 贵州石松类和蕨类植物志 [M]. 贵阳: 贵州科技出版社: 176.]
WANG PS, WANG XY, 2001. Pteridophyte flora of Guizhou [M]. Guiyang: Guizhou Science Press: 13. [王培善, 王筱英, 2001. 贵州蕨类植物志 [M]. 贵阳: 贵州科技出版社: 13.]
YI B, HU LF, MEI WL, et al., 2011. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan [J]. Molecules, 16(12): 10157-10167.
YU YG, ZHAO LZ, DUAN LD, et al., 2001. Study Pteris multifida poir action and antimicroblal effect [J]. J Shaoyang Coll, 14(3): 199-203. [余有贵, 赵良忠, 段林东, 等, 2001. 凤尾草抗菌药物的提取与开发研究 [J]. 邵阳高等专科学校学报, 14(3): 199-203.]
ZHANG CX, WANG CC, WANG ZH, et al., 2016. Total synthesis, structural elucidation and anti-inflammatory activity evaluation of 2-deoxy-3, 6-anhydro hexofuranoside derivatives isolated from Sauropus rostratus [J]. Org Biomol Chem, 14(46): 10906-10913.
ZHANG L, ZHANG QJ, KANG WY, et al., 2012. Chemical constituents of Desmodium sequax Wall [J]. Chin Trad Pat Med, 34(10): 1943-1945. [張龙, 张前军, 康文艺, 等, 2012. 波叶山蚂蝗化学成分研究 [J]. 中成药, 34(10): 1943-1945.]
ZOU J, ZHAO CL, HE K, et al., 2019. Chemical constituents from Pteris cretica [J]. Chin Trad Pat Med, 41(10): 2388-2393. [邹娟, 赵臣亮, 何康, 等, 2019. 银叶凤尾蕨化学成分的研究 [J]. 中成药, 41(10): 2388-2393.]
(责任编辑 蒋巧媛)
