First-principles study of the growth and diffusion of B and N atoms on the sapphire surface with h-BN as the buffer layer
2021-08-26JianyunZhaoXuLiTingLiuYongLuandJicaiZhang
Jianyun Zhao, Xu Li, Ting Liu, Yong Lu,, and Jicai Zhang,
1College of Mathematics and Physics, Beijing University of Chemical Technology, Beijing 100029, China
2State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Abstract: Currently, the preparation of large-size and high-quality hexagonal boron nitride is still an urgent problem.In this study, we investigated the growth and diffusion of boron and nitrogen atoms on the sapphire/h-BN buffer layer by first-principles calculations based on density functional theory.The surface of the single buffer layer provides several metastable adsorption sites for free B and N atoms due to exothermic reaction.The adsorption sites at the ideal growth point for B atoms have the lowest adsorption energy, but the N atoms are easily trapped by the N atoms on the surface to form N–N bonds.With the increasing buffer layers, the adsorption process of free atoms on the surface changes from exothermic to endothermic.The diffusion rate of B atoms is much higher than that of the N atoms thus the B atoms play a major role in the formation of B–N bonds.The introduction of buffer layers can effectively shield the negative effect of sapphire on the formation of B–N bonds.This makes the crystal growth on the buffer layer tends to two-dimensional growth, beneficial to the uniform distribution of B and N atoms.These findings provide an effective reference for the h-BN growth.
Key words: hexagonal boron nitride; buffer layer; first-principles calculations; molecular dynamics
1.Introduction
With the advancement of science and technology, the earlier materials can no longer meet the performance requirements of current devices very well.Since the Geim groups successfully separated graphene as a single atomic layer material in 2004[1], a lot of graphene-like materials are drawing people's attention, which exhibit excellent physical and chemical performances.Hexagonal boron nitride (h-BN), as a wide bandgap semiconductor material sharing the similar crystal structure to graphite, is also destined to have great application potential and development prospects.The structure of h-BN is a six-membered cyclic honeycomb composed by B and N atoms alternately arranged, and its atomic layers overlap each other in a close-packed manner of AA'A...to form a crystal[2].The h-BN is called “white graphite” due to its white powder characteristics and the lattice difference between h-BN and graphite is only 1.7%[3,4].It has superior electrical insulation, thermal conductivity, corrosion resistance, good lubricity and chemical stability.
As a new type of wide-bandgap semiconductor material after gallium nitride (GaN) and aluminum nitride (AlN), h-BN shows excellent performance in many fields.Songet al.proved that the h-BN film exhibits significant deep ultraviolet absorption at a wavelength of 203 nm[5], and Dahalet al.conducted electron beam excitation experiments on h-BN and found that the deep ultraviolet (DUV) region with 225 nm produced laser action[6].These phenomena prove the potential application of single crystal h-BN film in deep ultraviolet materials and as a deep ultraviolet chip-level semiconductor material.In the application of neutron detectors, Jianget al.made metal–semiconductor–metal (MSM) neutrons detector by 0.3μm thick h-BN.An obvious steady-state current response is produced after continuously irradiating the detector with the thermal neutron beam, which corresponds to the effective conversion efficiency of absorbed thermal neutrons close to 80%[7,8].All of this research has shown the great application potential of h-BN in the semiconductor field.However, it is still difficult to grow large-size single crystal h-BN thick films currently.Thus, in-depth research on the adsorption and diffusion of B and N atoms on the surface of the sapphire substrate can help people understand the micro growth mechanism of h-BN.
In this study, we use thec-plane sapphire (Al2O3) as the substrate, which is often used in the growth of h-BN by the chemical vapor deposition method.The h-BN buffer layer is introduced to improve the crystal growth.In this way, the growth of h-BN tends to be the two-dimensional growth,which can thus reduce the generation of the amorphous to improve the quality of h-BN.We systematically calculated and compared the adsorption energies of the free B and N atoms on the Al2O3/h-BN-buffer-layer models.The diffusion behaviors of free B and N atoms on the buffer layer surface at different temperatures were also simulated to provide an effective guidance for h-BN growth in the experiment.
2.Calculation method
The first-principles calculations and molecular dynamics(MD) simulations of the growth and diffusion of B and N atoms on the surface of the Al2O3/h-BN-buffer-layer were carried out based on the density functional theory (DFT) with the projection-enhanced wave (PAW) method as implemented in the VASP package[9].The electron exchange and correlation potential was described with the generalized gradient approximation with Perdew–Burke–Ernzerhof (PBE) form[10].The plane-wave cut-off energy was set to 400 eV.For the self-consistent formation energy calculations with the primitive cell,the 12 × 12 × 1 k point mesh was used for the Brillouin zone integration[11].The convergence accuracies of the energy and force in the calculations are set to 1 × 10–5eV and 0.02 eV/Å,respectively.
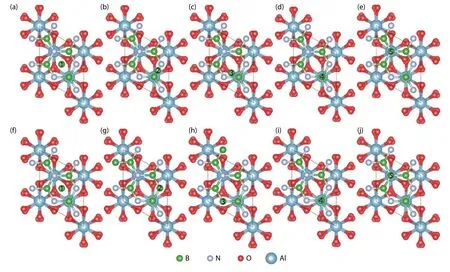
Fig.1.(Color online) The top view of free B atoms at different adsorption sites with one h-BN buffer layer.(a–e) show the initial adsorption sites and (f–j) show the final configurations after optimization.
The lattice parameters area= 4.81 Å andc= 13.11 Å for Al2O3anda= 2.51 Å andc= 7.19 Å for h-BN, consisting well with the experimental results ofa= 4.76 Å andc= 12.99 Å for Al2O3anda= 2.504 Å andc= 6.669 Å for h-BN[12,13].After optimizing the h-BN and Al2O3primitive cells, we constructed (001) h-BN and (001) Al2O3with different layers and combined them into a heterojunction structure, and h-BN was using the 2 × 2 × 1 supercell.In order to prevent the influence on the surface and the bottom caused by the periodicity of the model, a vacuum layer of 15 and 10 Å was introduced on the surface and the bottom respectively.Since h-BN and graphite have a very similar hexagonal structure, we selected four adsorption sites based on the high symmetry point of graphite when selecting the adsorption sites of free B and N atoms,and we also selected the fifth adsorption site considering other adsorption possibilities[14,15].The adsorption energy of the B and N atoms on the adsorption sites can be calculated by the following formula[16–18]:

whereEadsis the adsorption energy of atoms at the adsorption site,EallandEpartare the total energy of the overall model and the total energy of the model without free atoms.Eatomis the single-atom energy of free B and N atoms, and the smaller theEadsvalue, the easier to adsorb at this site.In the simulation process, we used different adsorption sites and different numbers of buffer layer to calculate the formation energy.The atomic positions and cell volume of Al2O3/h-BN-buffer-layer were fixed, and only the free B or N atoms on the surface were optimized.
For the first-principles molecular dynamics (MD) simulations of the adsorption and diffusion of B and N atoms on the surface, the simulated temperature were set to the room temperature (~300 K) and the high temperature of 1300 °C(1573 K), respectively.The canonical ensemble (NVT) was used for all the MD simulations and the temperature was controlled by Nosé thermostat[19].The simulation time is 10 ps with the time step of 1 fs.The process can be monitored by the atomic coordinates generated by the MD simulations.We can characterize the diffusion behavior of free B and N atoms by calculating the mean square displacement (MSD) and diffusion coefficientD[20,21].
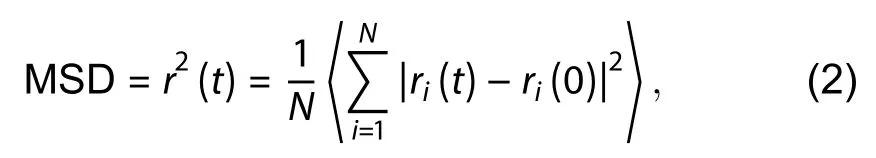
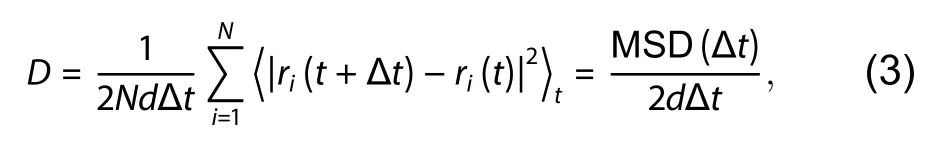
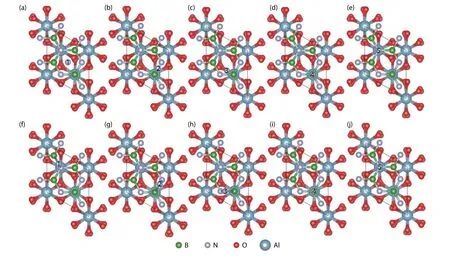
Fig.2.(Color online) The top view of free N atoms at different adsorption sites with one h-BN buffer layer.(a–e) show the initial adsorption sites and (f–j) show the final configurations after optimization.
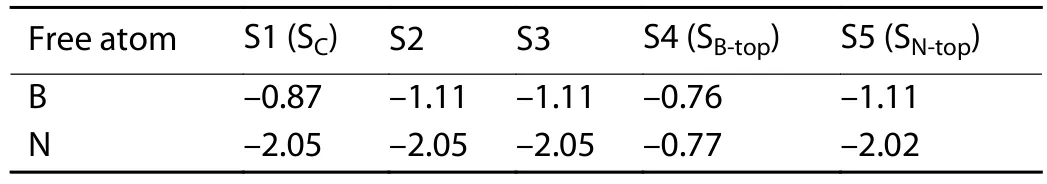
Table 1.The formation energies (eV) of free B or N atoms at the surface sites of the Al2O3/h-BN-buffer-layer model.
wheredis the dimensionality of the system (integer, 1 ≤d≤ 3).
3.Results and discussions
The initial absorption models of the free state B and N atoms at different absorption sites and the structures after the relaxation optimization of the surface atomic positions are shown in Fig.1 and Fig.2, respectively, where the five potential adsorption sites, i.e., S1–S5, are marked with numbers.All models adopt the heterojunction composed of Al2O3(0001) plane with the same thickness and the single layer h-BN along the normal direction.After optimizing the positions of the free B and N atoms on the surface, three final adsorption sites were obtained for both cases.As shown in Table 1, for the free B atoms, the top site of N (SN-top) with the adsorption energy of –1.11 eV is the most stable adsorption site, which is the ideal growth site for the close packed structure of h-BN.The central site of the h-BN six-membered ring(SC) and the top site of B (SB-top) are two meta-stable sites with the adsorption energies of –0.87 and –0.76 eV, respectively.We note that the SCcorresponds to the growth site of rhombohedral structure boron nitride (r-BN)[25].The formation energies are all negative, indicating the exothermic process of the adsorption of B atoms on these sites.For the free N atoms, the most stable adsorption site is the top site of N with a small deviation towards to one of the neighbor B atom (SN-top’), as shown in Figs.2(f)–2(h).The adsorption energy of SN-top’is –2.05 eV, which is slightly lower than that of–2.02 eV for the ideal top site of N (SN-top).In the SN-top’site,the free N atom bonds with the N atoms in the buffer layer to form N–N bond with a bond length of 1.502 Å, which restricts the diffusion of the surface N atom to other growth sites.The ideal growth site for h-BN is the top site of B (SB-top),which is a meta-stable site with the adsorption energy of–0.77 eV.By comparing the formation energies of free B and N atoms on the surface, the N atoms are more easily to be adsorbed on the SN-top’ site, which plays a negative role in the growth of h-BN.In contrast, although the adsorption capacity of B atoms is weaker than that of N atoms, they tend to combine into h-BN structure.
After calculating the relationship between the adsorption sites and the formation energy, we investigated the influence of the number of h-BN buffer layer on the formation energy.The Al2O3/h-BN-buffer-layer models with different numbers of buffer layer are shown in Fig.3 for B and Fig.4 for N atoms respectively.The SCsite is chosen as the initial absorption site for the free B and N atoms.By structural relaxation,the adsorption energies with different buffer layers were gathered in Table 2, and the final adsorption positions of B and N atoms on the surface were shown in Fig.3 and Fig.4, respectively.In general, the formation energy increases as the number of buffer layer increases.For B atom, the adsorption site after relaxation is not changed as the number of buffer layer increases from 1 to 3.But for the N atom, the final adsorption site for the N atom is changed to the SN-top’ site only with 1 buffer layer.As the buffer layer increases to 2 or 3, the initial SCsite for N atom is not changed.Both for the B and N atoms, the adsorption energy increases gradually as the number of buffer layer increases.When the number of h-BN buffer layer increases to 3, the adsorption process changes from an exothermic reaction to an endothermic reaction.As is known, during the growth process of h-BN, adjacent B and Natoms in the same atomic layer are combined to form a B–N bond by sp2 hybridization, and the inter layers are combined by van der Waals forces[2].According to the calculated results, it can be seen that the free B or N atom is affected by the coupling of Al2O3and h-BN buffer layer.This coupling effect will be sharply weakened when the distance between ad-sorption atom and the substrate increases.In previous experimental studies, it was found that N atoms can bond with Al and O atoms in the substrate when the nitriding process is applied to the surface of the sapphire substrate[26,27].This indicates that Al2O3has a strong adsorption effect on N atoms,which may cause the competition for forming an Al–N bond and B–N bond.

Fig.3.(Color online) (a–c) The top view and (d–f)front view of the optimized adsorption positions for B atom on the surface with different buffer layers.The SC site is chosen as the initial absorption site for the B atom.
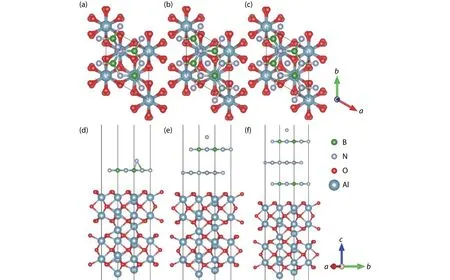
Fig.4.(Color online) (a–c) The top view and (d–f)front view of the optimized adsorption positions for the N atom on the surface with different buffer layers.The SC site is chosen as the initial absorption site for the B atom.
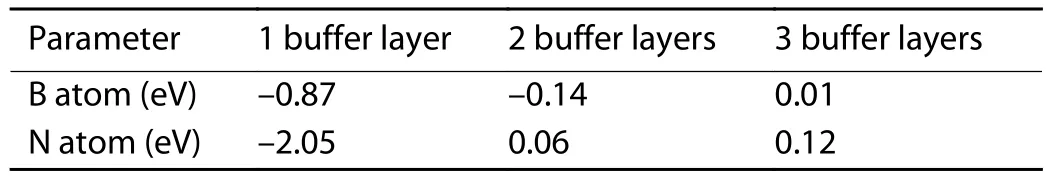
Table 2.The formation energies of free B and N atoms at the same position on the surface of the Al2O3/h-BN-buffer-layer model in a different number of buffer layers.
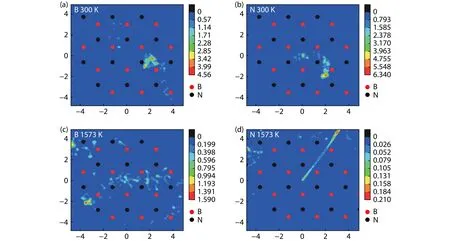
Fig.5.(Color online) The probability distribution functions of atomic displacements projected onto the xy plane for (a) the B atom at 300 K, (b)the N atom at 300 K, (c) the B atom at 1573 K and (d) the N atom at 1573 K, respectively.The color scale indicates the distribution probability.The positions of B and N atoms of the buffer layer are marked.
In addition to calculating the formation energy of one isolate B or N atom on the surface, we also set up a pair of free B and N atoms on the surface with one h-BN buffer layer.The calculated formation energy is –3.11 eV.Compared to the formation energies of isolate B and N atoms at their adsorption site, the formation energy of the coexisting B and N atoms are much lower, indicating that B and N atoms can interact with each other on the surface to form B–N bond.Namely,the free B and N atoms firstly bond with each other and then combine with the adsorption sites of the h-BN buffer layer.
The diffusion of free B and N atoms on the buffer layer surface was simulated by the first-principles molecular dynamics.The probability distribution functions of atomic displacements projected onto thexyplane is shown in Fig.5 to trace the diffusion of B and N atoms in the surface.At room temperature, the B and N atoms both show a relative localization distribution.In general, the B and N atoms tend to diffuse around the SN-topsite and SB-topsite respectively by overcoming the energy barrier between the adjacent adsorption sites.As shown in Figs.5(a) and 5(b), the trajectory of the N atom is more localized than that of B atom, which is more likely to jump between two adjacent adsorption sites.This can be compared quantitatively by calculating the MSD and diffusion coefficient of B and N atoms according to Eqs.(2) and (3).According to Fig.6 and Table 3, the B atom has a larger MSD with respect to the N atom.As a result, the diffusion coefficient of B atom is 1.07 × 10–9m2/s at 300 K, much higher than 0.35 ×10–9m2/s of N atom.As temperature increases to 1573 K, the activity of B and N atom are both increased and the distribution range is obviously expanded, as shown in Figs.5(c) and 5(d).The diffusion of B atom is delocalized, which can diffuse freely on the surface by overcoming the energy barrier between the most stable and meta-stable sites.Although the distribution of N atom also shows delocalization characteristics, the trajectory is limited between specific adsorption sites.The N atom tends to diffuse across the sites around the B–N bond, increasing the probability of N bonding with N and B atoms in the buffer layer.The MSD of B atom is still larger than that of the N atom.The diffusion coefficients of B and N atoms at 1573 K increase to 17.04 × 10–9and 7.18 × 10–9m2/s,respectively.The diffusion rate of B atom is much higher than that of the N atom.It can thus be concluded that B atoms play a leading role in the growth process of h-BN.At high temperature, B atoms distribute uniformly at the growth sites by diffusion, then promoting N atoms to the break away from the localization to form B–N bonds.
4.Conclusion
In summary, we studied the growth and diffusion of B and N atoms on the surface of the Al2O3/h-BN-buffer-layer through first-principles calculations based on the density functional theory.The results show that the surface of single-buffer-layer h-BN provides several metastable adsorption sites for free B and N atoms due to the exothermic reaction.The free B atoms have the lowest adsorption energy at the adsorption site of the ideal growth point for h-BN, but the free N atoms are most easily to be trapped by the N atoms on the h-BN buffer layer to form the N–N bonds.As the number of buffer layer increases, the binding capacity of the surface of the buffer layer with free B and N atoms decreases.When the number of the h-BN buffer layer increases to three, the adsorption of the free B and N atoms on the surface changes from an exothermic reaction to an endothermic reaction.The influ-ence of the Al2O3substrate on the surface atoms is largely weakened.As temperature increases from 300 to 1573 K, the activity and diffusion range of B and N atoms increase significantly.The B atoms can diffuse freely on the buffer layer surface by overcoming the energy barrier between the adsorption sites.But the diffusion trajectory of N atoms is restricted in the specific adsorption sites around the B–N bonds of buffer layer.The B atoms play a major role in the formation of B–N bonds on the surface, the diffusion rate of which is much higher than that of the N atom.The introduction of buffer layers make the crystal growth have the characteristics of two-dimensional growth, which can effectively shield the negative effect of the substrate on the formation of B–N bonds.Also,they are conducive to the uniform diffusion surface B and N atoms, which can thus reduce the generation of the amorphous to improve the growth quality of h-BN.
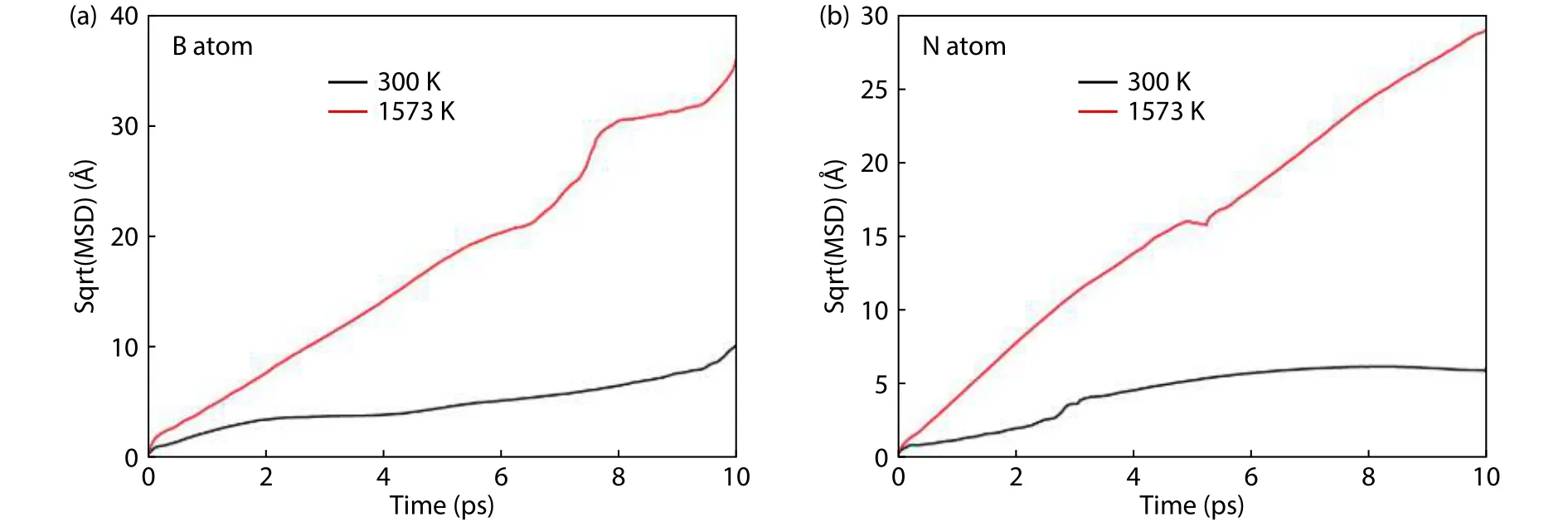
Fig.6.The MSD curves of (a) B atoms and (b) N atoms on the buffer layer surface at different temperatures.
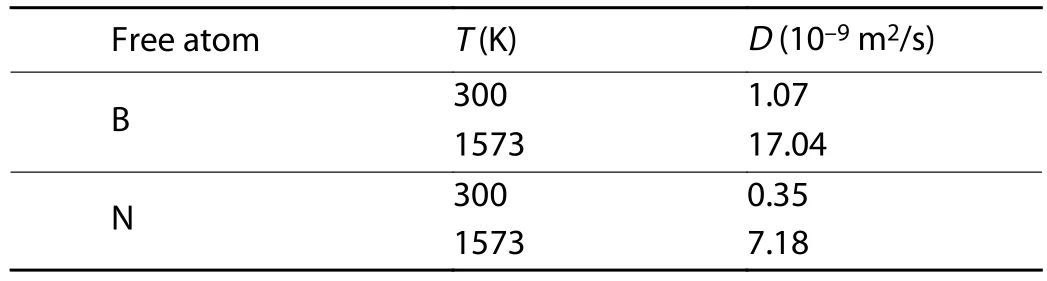
Table 3.Diffusion coefficients of free atoms on the surface of the model at different temperatures.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (61874007, 12074028), the Beijing Municipal Natural Science Foundation (4182046), Shandong Provincial Major Scientific and Technological Innovation Project (2019JZZY010209), Key-area research and the development program of Guangdong Province (2020B010172001),and the Fundamental Research Funds for the Central Universities (buctrc201802, buctrc201830, buctrc202127).
杂志排行
Journal of Semiconductors的其它文章
- Reliability evaluation on sense-switch p-channel flash
- I mpact of switching frequencies on the TID response of SiC power MOSFETs
- Targeted transfer of self-assembled CdSe nanoplatelet film onto WS2 flakes to construct hybrid heterostructures
- Low-cost dual-stage offset-cancelled sense amplifier with hybrid read reference generator for improved read performance of RRAM at advanced technology nodes
- Ultra-low Vpp and high-modulation-depth InP-based electro–optic microring modulator
- Extensive study of optical contrast between bulk and nanoscale transition metal dichalcogenide semiconductors
