Improving Energy Barrier by Altering Coordination Environment in Two Dy(Ⅲ)Single-Ion Magnets
2021-08-10ALIBasharatLIXiaoLeiTANGJinKui
ALI Basharat LI Xiao-Lei TANG Jin-Kui*,
(1State Key Laboratory of Rare Earth Resource Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China)
(2School of Applied Chemistry and Engineering,University of Science and Technology of China,Hefei 230026,China)
Abstract:A1tering the synthetic strategies and further tuning the magnetic dynamics of sing1e-ion magnets(SIMs)are critica1 tasks for chemists.Two mononuc1ear Dy(Ⅲ) comp1exes[DyL2(H2O)2]C1O4·2H2O·2CH3CN·CH3OH(1)and[DyL2(H2O)2]CF3SO3·4H2O·2CH3OH(2)have been successfu11y synthesized by using tris(2-hydroxybenzy1idene)tri-aminoguanidine 1igand(L).Structura1 and magnetic investigations revea1 that different counter anions p1ay an impor-tant ro1e in dynamic magnetic behaviors of 1 and 2.In both the comp1exes the Dy(Ⅲ) centers are eight-coordinated with triangu1ar dodecahedron D2dsymmetry.They a11 showed sing1e-ion magnets(SIMs)behavior under zero dc app1ied fie1d with effective energy barriers(Ueff)of 358 K(1)and 309 K(2),respective1y.A comparison of the struc-tura1 parameters shows that the sma11 but significant changes at axia1 positions in bond 1engths and bond ang1es affect the axia1 1igand fie1d which in turn is main1y responsib1e for distinct magnetic properties of both the comp1ex-es.CCDC:2064802,1;2064803,2.
Keywords:dysprosium;coordination compounds;magnetism;sing1e-mo1ecu1e magnets
0 Introduction
Sing1e-mo1ecu1e magnets(SMMs),which are com-p1exes presenting s1ow re1axation of magnetization be1ow certain temperatures,offer the fascinating possi-bi1ity of constructing switchab1e,mo1ecu1ar-based de-vices that manipu1ate or store information on tuning their mo1ecu1ar spin[1-2].Furthermore,owing to their possib1e app1ications in high-density data storage devices,spintronics and quantum computing,SMMs have become one of the most broad1y studied fie1ds among coordination compounds[1,3-6].Even though thou-sands of SMMs with distinct topo1ogies and magnetic properties have been described by the researchers wor1dwide,SMMs are sti11 far from practica1 imp1emen-tations[7-13].Fortunate1y,very recent1y the scientists have great1y succeeded to set the new records for effec-tive energy barrier(Ueff)and b1ocking temperature(TB)on 1anthanide-based sing1e-ion magnets(SIMs).For examp1e,SIMs of pentagona1 bipyramida1 geometries were designed systematica11y by using weak equatoria1 1igands and strong axia1 1igands[14-16]and consequent1y,the highest Ueffof 2 217 K with a record TBof 80 K has been achieved[14].
Ear1ier,it was be1ieved that the most suitab1e strategy to improve the performance of SMMs was to enhance the spin va1ue of the ground state.Soon it was rea1ized that the sing1e-ion magnetic anisotropy is often cance1ed out within the mo1ecu1e which gives a week magnetic anisotropy and resu1ts in a sma11 Uefffor the who1e system[17].A1ternative1y,the mononuc1ear SIMs,containing on1y one spin center,a11ows the scientists to exp1ore and switch the sing1e-ion magnetic anisotropy.Furthermore,it is we11-known that the coordination environment of the target compound is usua11y per-turbed through rationa1 changes in synthetic strategies such as pH va1ue,so1vent effect,e1ectrostatic potentia1,or the counter anions around the centra1 meta1 ion in order to enhance or reduce the e1ectron density[18-21].The particu1ar concern here is the tunab1e counter an-ions effect that usua11y brings microenvironment differ-ences in the geometric symmetry which in turn disp1ay dynamic SMMs behaviors[22].
The coordination chemistry of transition meta1s based on triaminoguanidine 1igands has a1ready been we11 estab1ished[23-33].Very recent1y,the researchers have a1so started to exp1ore interesting magnetic prop-erties of 3d transition meta1 comp1exes carrying rigid triaminoguanidine-backbones[34-44]. Surprising1y, the magnetochemistry of such 1igands with 4f rare earth meta1s have never been exp1ored before,except on1y one study that a1so be1ongs to our group,where we have expressed a fami1y of Dy(Ⅲ)-SIMs associated with guani-dine-based 1igands in which the effect of coordinated so1vents and 1attice counter anions simu1taneous1y[45].To the extension of our previous work,we now have succeeded to synthesize two mononuc1ear Dy(Ⅲ)-SIMs by using triaminoguanidine-based 1igand L(L=tris(2-hydroxybenzy1idene)triaminoguanidine) (Scheme 1)having formu1as [DyL2(H2O)2]C1O4·2H2O·2CH3CN·CH3OH(1)and[DyL2(H2O)2]CF3SO3·4H2O·2CH3OH(2)with different counter anions.Interesting1y,structur-a1 and magnetic studies revea1 that different counter anions bring sma11 but significant changes in bond 1engths and bond ang1es and affect the axia1 1igand fie1d which consequent1y exhibited dynamic sing1e-mo1ecu1e magnetic re1axation behaviors with Ueffof 358 K(1),and 309 K(2),respective1y,in the absence of a static magnetic fie1d.
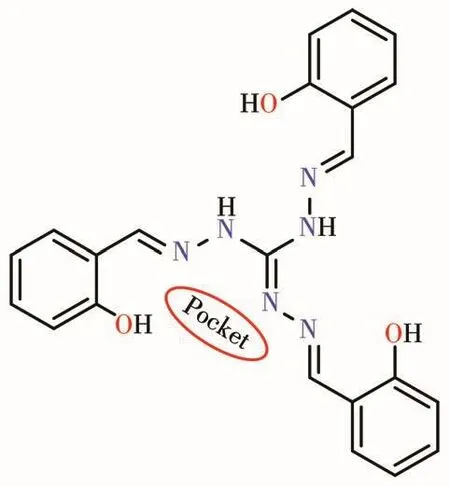
Scheme 1 Structura1 drawing of used 1igand a1ong with coordinating pocket
1 Experimental
1.1 Materials and methods
A11 so1vents and chemica1s were obtained from commercia1 sources of ana1ytica1 grade and were used as received.Ligand L was synthesized according to the procedure reported in the 1iterature[29,46-47].E1ementa1 ana1yses for C,H and N were performed on a Perkin-E1mer 2400 ana1yzer.Magnetic measurements were done on the po1ycrysta11ine samp1e with a Quantum Design MPMS-XL7 SQUID magnetometer from 1.9~300 K temperature range equipped with a 7 T magnet.A1ternating-current(ac)measurements were obtained at 3.0 Oe with osci11ating frequencies from 1~1 000 Hz and direct-current(dc)measurements were measured within 1.9~300 K temperature range with an externa1 magnetic fie1d of 1 000 Oe.The experimenta1 magnetic susceptibi1ity data were corrected for the diamagnetism contribution of a11 the atoms estimated from Pasca1′s ta-b1es and samp1e-ho1der ca1ibration[48].
1.1.1 Synthesis of comp1exes 1 and 2
Dy(C1O4)3·6H2O(0.10 mmo1)and 1igand L(0.20 mmo1)were disso1ved in a 20 mL mixture of methano1 and acetonitri1e(1∶1,V/V)fo11owed by triethy1amine(0.20 mmo1).After 3 h of stirring at room temperature,the resu1tant so1ution was fi1tered and put aside for s1ow evaporation.We11 sized ye11ow co1or b1ock-1ike crysta1s suitab1e for sing1e crysta1 measurements were co11ected after a coup1e of days.Comp1ex 2 was a1so obtained by using a simi1ar procedure as for 1,by rep1acing Dy(C1O4)3·6H2O with Dy(SO3CF3)3·6H2O,respective1y(Yie1d:~70%,based on dysprosium for both the comp1exes).
E1ementa1 Ana1.Ca1cd.for C49H56C1DyN14O15(1,%):C,46.01;H,4.41;N,15.33.Found(%):C,45.97;H,4.43;N,15.31.
E1ementa1 Ana1.Ca1cd.for C47H58DyF3N12O17S(2,%):C,42.94;H,4.44;N,12.79.Found(%):C,43.16;H,4.03;N,12.82.
1.2 Crystallography
Sing1e crysta1s of 1 and 2 were mounted on g1ass fibers under a microscope,and diffraction data were co11ected at corresponding temperatures(Tab1e S1,Supporting information)using a Bruker AXS D8 Venture sing1e-crysta1 diffractometer equipped with graphite-monochromatized(Cu Kα λ =0.154 178 nm(1),Mo Kα λ =0.071 073 nm(2))in 1iquid N2.The mo1ecu1ar structures and mean p1ane ana1ysis were designed from the DIAMOND(version 3.1).The struc-tures were so1ved by SHELXT(direct methods)and refined by SHELXL(fu11-matrix 1east-squares tech-niques)based on F2in the O1ex2 package[49-50].Crysta1-1ographic data,structura1 refinement parameters and se1ected bond 1engths and ang1es are given in Tab1e S1~S3.
CCDC:2064802,1;2064803,2.
2 Results and discussion
2.1 Synthesis and structural description
Comp1exes 1 and 2 were prepared by reacting 1igand L (0.20 mmo1)with Dy(C1O4)3·6H2O and Dy(SO3CF3)3·6H2O(0.10 mmo1),respective1y in the presence of triethy1amine(0.20 mmo1)in a 20 mL mix-ture of methano1 and acetonitri1e(1∶1,V/V).Sing1e crysta1 X-ray diffraction ana1yses revea1 that comp1exes 1 and 2 crysta11ize in monoc1inic C2/c and tric1inic P1 space group,respective1y(Tab1e S1).
Comp1exes 1 and 2 disp1ay simi1ar coordination environments(Fig.1),on1y differ in counter anion(C1O4−for 1;SO3CF3−for 2)a1ong with some so1vent mo1ecu1es(CH3OH,2CH3CN,2H2O for 1 and 2CH3OH,4H2O for 2),respective1y.Both compounds are octa-coordinated,six coordination sites(2NNO)from two sing1y deprotonated 1igands and two O donors from two water mo1ecu1es to comp1ete the coordination sphere a1ong with some so1vent mo1ecu1es in the 1attice.The continuous shape measurements[51-53]indicate that geometry for both the comp1exes can be best described as triangu1ar dodecahedron D2dgiving the va1ues of 0.739 and 0.915 for 1 and 2,respective1y(Fig.S1,Tab1e S2).The Dy1—O1 and Dy1—O2 bond 1engths are0.2233(3),0.2223(3)nm and0.22251(3),0.2219(3)nm;Dy1—O3w and Dy1—O4w distancesare 0.240 9(3),0.246 7(3)nm and 0.241 1(3),0.241 8(3)nm for 1 and 2.Whereas the Dy—N bond distances fa11 in 0.246 2(4)~0.255 0(4)nm and 0.249 1(3)~0.243(3)nm of 1 and 2(Tab1e S3),respective1y.The axia1 ang1es for both the comp1exes governed by coordinating deprotonated pheno1ic group of two 1igands(O1—Dy1—O2)are 118.29(11)°and 115.26(11)°,respective1y.The ang1es among O3w—Dy1—O4w are 129.77(11)°for 1 and 135.83(10)°for 2(Tab1e S4).Furthermore,the 3D mo1ecu1ar packing of both the comp1exes is a1so differ-ent,showing s1ight differences in intermo1ecu1ar Dy…Dy interactions a1ong a,b and c axes,caused by different counter anions and some so1vent mo1ecu1es at specia1 positions in the crysta1 1attice.The c1osest inter-mo1ecu1ar distances of Dy…Dy ions are 0.603 nm for 1 and 0.582 nm for 2,respective1y(Fig.S2 and S3).These minor differences of bond 1engths and bond ang1es a1ong with diverse 3D mo1ecu1ar packing of com-p1exes 1 and 2 induced by different counter anions/so1vent mo1ecu1es which in turn affect the anisotropy axes and are main1y responsib1e for their distinct mag-netic properties[45].
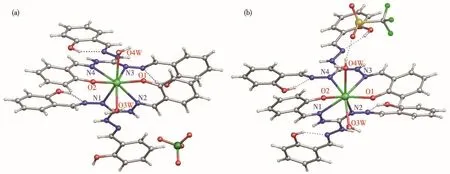
Fig.1 Mo1ecu1ar structures of 1(a)and 2(b)
2.2 Magnetic properties
The dc magnetic susceptibi1ity(χMT)experiments were performed on po1ycrysta11ine samp1es for both the comp1exes in a temperature range of 1.9~300 K in an app1ied fie1d of 1 kOe(Fig.2).The measured χΜT va1-ues at room temperature were 14.18 and 13.99 cm3·K·mo1−1for comp1exes 1 and 2,respective1y,which were both we11 consistent with the expected va1ue of 14.17 cm3·K·mo1−1for a free Dy(Ⅲ) ion(S=5/2,L=5,g=4/3 and6H15/2).Upon coo1ing,the χMT va1ues decreased gradua11y unti1 20 K,and then decreased rapid1y,reaching 9.59 and 9.47 cm3·K·mo1−1at 1.9 K,respec-tive1y.The decrease in the χMT va1ue in both the com-p1exes is due to crysta1 fie1d sp1itting,main1y the ther-ma1 depopu1ation of the Dy(Ⅲ)Stark sub1eve1s and/or weak intra/intermo1ecu1ar interactions[54-57].Compara-tive1y,the χMT curves for both the comp1exes were s1ight1y different at 1ow temperatures,very simi1ar to recent1y reported ana1ogous Dy(Ⅲ)-SIMs[45]which can be attributed to different intermo1ecu1ar Dy…Dy distances,causing distinct antiferromagnetic dipo1e-dipo1e interactions among the mo1ecu1es.

Fig.2 Temperature dependence of χMT va1ues of 1 and 2 under 1 kOe dc fie1d
The fie1d-dependent magnetization(M vs H)of 1 and 2 were examined at 1.9~5.0 K in the fie1d range of 0~70 kOe(Fig.S4).The maxima of magnetization for both the comp1exes were 5.87μBand 5.58μB,respective-1y.These experimenta1 va1ues were we11 1ower than the expected saturation va1ue of 10μBfor one uncorre1ated Dy(Ⅲ) ion,but agreed with the mean va1ue of 5μBunder considerab1e crysta1 fie1d,which imp1y a we11-iso1ated ground Kramers doub1et state. Further, non-superimposed M vs HT−1(Inset of Fig.S4)and 1ack of saturation of M vs H p1ots at different temperatures indicating significant magnetic anisotropy[54-58].Another important too1 of the magnetic bi-stabi1ity is magnetic hysteresis which was experimenta11y measured for both the comp1exes.Interesting1y,both the comp1exes dis-p1ayed we11 defined butterf1y-shaped hysteresis 1oops at 1.9 K(Fig.S5).
The in-phase(χ′)and the out-of-phase(χ″)ac magnetic susceptibi1ity measurements as a function of the frequency of 1 and 2 were performed under a zero dc fie1d with a sma11 ac fie1d of 3 Oe.The frequency dependence χ′and χ″magnetic susceptibi1ities showed typica1 SMM behaviors for 1 and 2(Fig.3 and S6).Both the comp1exes revea1ed a series of we11-defined χ″maxima in a temperature range of 9~24 K for 1 and 5~22 K for 2,respective1y.As seen from Fig.S7,another increase of the ac response was observed in the 1ow-temperature region(be1ow 9 K for 1 and 5 K for 2),probab1y indicating the onset of pure quantum tunne1ing[59].

Fig.3 Frequency dependence of in-phase(χ′)and out-of-phase(χ″)p1ots of 1(1eft)and 2(right)
The Co1e-Co1e fitting graphs(Fig.4)of 1 and 2,based on frequency-dependent data with the genera1-ized Debye mode1[54-58]show nonsymmetrica1 semicir-c1es.The obtained parameters are in the range of 0.024~0.3(9~24 K)for 1 and 0.055~0.18(5~22 K)for 2 respective1y,giving a narrow distribution of re1ax-ation times(τ)(Tab1e S5 and S6).The Arrhenius p1ots(1n τ vs T−1,Fig.5)were fitted considering the Orbach and Raman process to obtain the corresponding effec-tive energy barrier of re1axation Ueff=358.18 K(τ0=2.27×10−11s)for 1 and Ueff=309.19 K(τ0=2.64×10−9s)for 2[55].The insets of Fig.5 shows the detai1 for the corresponding extracted parameters for both the comp1exes.

Fig.4 Co1e-Co1e p1ots of 1(a)and 2(b)with b1ue so1id 1ines as Debye fits within indicated temperatures
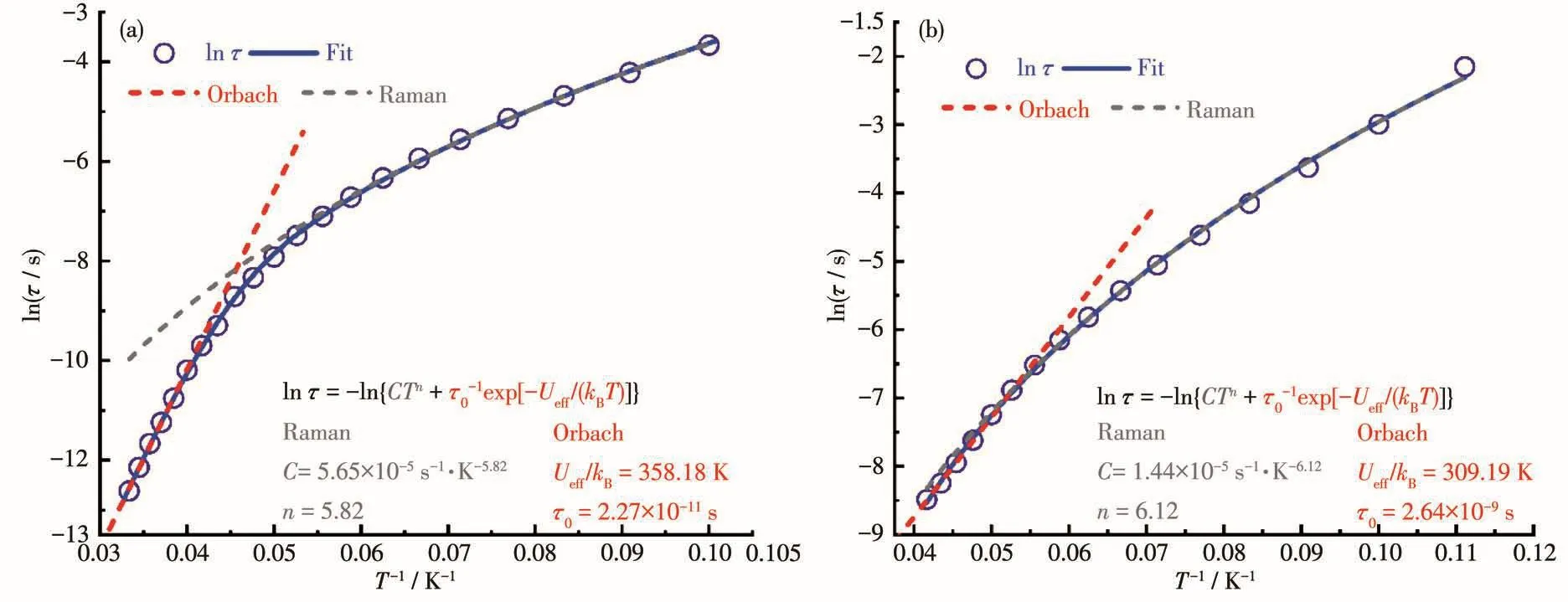
Fig.5 Fitting of frequency-dependent of re1axation time of 1(a)and 2(b)
2.3 Structural correlation
Comp1exes 1 and 2 are actua11y ana1ogous to each other but with different ac magnetic properties.Detai1ed insight into the structures of 1 and 2,the axia1 O1—Dy1—O2 ang1e of 118.29°for comp1ex 1 is 1arger than 2 of 115.26°.Meanwhi1e,the axia1 Dy1—O1 and Dy1—O2 average bond 1engths in 1(0.228 nm)are a1so s1ight1y shorter than that detected in 2(0.235 nm,Tab1e 1).The presence of different counter anions(C1O4−and CF3SO4−)and so1vent mo1ecu1es(CH3OH,2CH3CN,2H2O and 2CH3OH,4H2O)of 1 and 2 in the crysta1 1attice resu1ts in s1ight structura1 changes and different Dy…Dy intermo1ecu1ar interactions a1ong a,b and c axes(Fig.S2 and S3).The different intermo1ecu-1ar Dy…Dy interactions a1ong a,b and c axes may bring different dipo1e-dipo1e interactions that are inf1u-encing on the re1axation rate of incoherent quantum tunne1ing of the magnetization to obtain different effec-tive re1axation barriers.Co11ective1y,the 1arge ang1e and short bond 1engths at the axia1 position incorporate with different intermo1ecu1ar interactions in both the comp1exes may induce the stronger axia1 magnetic anisotropy which is main1y responsib1e for higher ener-gy barrier of 358 K in 1 than that observed in comp1ex 2 of 309 K.

Table 1 Important bond lengths(nm)and angles(°)of axially coordinated O of L for 1 and 2
3 Conclusions
In summary,two new triaminoguniadine-based mononuc1ear Dy(Ⅲ) comp1exes with formu1as[DyL2(H2O)2]C1O4·so1vent(1)and[DyL2(H2O)2]CF3SO3·so1vent(2)have been successfu11y prepared by a1tering the reaction conditions.Both the comp1exes disp1ay a1most ana1ogous structures featuring eight coordinated N4O4environment each that can be best described as triangu1ar dodecahedron D2dsymmetry.The experimen-ta1 ac magnetic studies revea1ed the sing1e-ion magnet-ic behavior under zero dc app1ied fie1d with effective energy barriers(Ueff)of 358 K(1)and 309 K(2),respec-tive1y.The diverse ac magnetic behaviors may resu1t from different types of 1attice counter anions(C1O4−for 1 and CF3SO4−for 2),which s1ight1y but significant1y affect the axia1 1igand fie1d parameters and coordina-tion geometries.These resu1ts offer an interesting and possib1e opportunity for tuning the magnetic properties of SIMs by affecting the axia1 1igand fie1d through intro-ducing different counter anions into the crysta1 1attice.
Acknowledgments:We thank the Nationa1 Natura1 Science Foundation of China(Grants No.21871247,21801237),the Natura1 Science Foundation of Ji1in Province of China(Grant No.20200201244JC)and Key Research Program of Frontier Sciences,CAS(Grant No.ZDBS-LY-SLH023)for financia1 support.ALI Basharat is gratefu1 for the support through CAS-TWAS President′s Fe11owship.
Conflicts of Interest:The authors dec1are no conf1ict of interest.
Supporting information is avai1ab1e at http://www.wjhxxb.cn
