Two-Dimensional Luminescent Coordination Polymer Based on Dinuclear{Zn2(COO)4}Second Bulidings Units:Crystal Structure and Detection of Fe3+
2021-08-10HUANGJiaXiangZHAOHeLIUShuQinZHANGJianJun
HUANG Jia-Xiang ZHAO He LIU Shu-Qin ZHANG Jian-Jun
(School of Chemical Engineering,Dalian University of Technology,Dalian,Liaoning 116024,China)
Abstract:A two-dimensiona1 1uminescent coordination po1ymer based on dinuc1ear{Zn2(COO)4}second bui1dings units,[Zn2(L)2(DMSO)2(DMF)](1,H2L=2-(1,3-dioxo-1H-benzo[de]isoquino1in-2(3H)-y1)terephtha1ic acid,DMSO=dmethy1 su1foxide,DMF=dimethy1formamide),was synthesized by hydrotherma1 reaction using H2L 1igand and Zn2+.Topo1ogica1 ana1ysis shows that the dinuc1ear{Zn2(COO)4}unit can be regarded as a 4-1inked node and forms a(4,4)-net topo1ogy with L2− 1inker.1 a1so exhibited a se1ective 1uminescence quenching type response to Fe3+with detec-tion 1imit as 1ow as 2.8 µmo1·L−1.1 has good anti-interference abi1ity for the detection of Fe3+,and can be regenerat-ed and recyc1ed for severa1 times by washing with DMF so1vent.CCDC:2079394.
Keywords:1uminescent coordination po1ymer;π-π stacking interaction;crysta1 structure;Fe3+;detection
0 Introduction
Iron is one of the most abundant e1ements in the earth′s crust,and it is not on1y found in 1arge quanti-ties in the natura1 environment,but a1so p1ays an important ro1e in the circu1atory system of 1iving organ-isms[1].In addition,Fe ion is a1so one of the major industria1 po11utants that can po11ute the eco1ogica1 environment.Some ana1ytica1 means,inc1uding atomic absorption spectrometer,inductive1y coup1ed p1asma spectrometry(ICP),etc.have been used to detect Fe3+.A1though these methods have high detection accuracy,they a1so have many disadvantages,such as that the in-strumentation is too 1arge and not easy to carry,and the detection cost is high.Therefore,it is important to de-ve1op an inexpensive and portab1e method for the de-tection of iron ion[2].
Luminescent coordination po1ymers(LCPs)are 1uminescent materia1s formed by meta1 ions/c1uster nodes(second bui1ding units,SBUs)and organic 1ink-ers[3-5].When LCPs come into contact with the guest substance,the resu1ted host-guest interactions may change the 1uminescence properties of LCPs,which can be used for detection of the guest substance[6-7].
Herein,we se1ected a 1igand H2L(H2L=2-(1,3-dioxo-1H-benzo[de]isoquino1in-2(3H)-y1)terephtha1ic acid)to react with zinc ion to prepare a two-dimension-a1(2D)LCP with(4,4)-net topo1ogy,name1y[Zn2(L)2(DMSO)2(DMF)](1,DMSO=dimethy1 su1foxide,DMF=dimethy1formamide).The abi1ity of 1 as a 1umines-cence probe for the detection of Fe3+was investigated.The resu1ts showed that 1 had a high sensitivity for Fe3+andthe 1imit of detection(LOD)was ca1cu1ated to be 2.8 µmo1·L−1.The probe can be recyc1ed after DMF washing,indicating a good potentia1 for Fe3+detection.
1 Experimental
1.1 Materials and methods
A11 the reagents and so1vents were commercia11y purchased,and used as received without further purifi-cation.H2L was synthesized according to reported method[8].The IR spectra(650~4 000 cm−1)were recorded from a Nico1et-20DXB spectrometer using KBr pe11ets.Thermogravimetric ana1yses(TGA)were carried out on a TA-Q50 thermogravimetric ana1yzer under N2atmosphere with the heating rate of 10℃·min−1.E1ementa1 ana1yses(C,H and N)were performed on a Vario EL Ⅲ e1ementa1 ana1yzer.Powder X-ray diffraction(PXRD)patterns were co11ected on D/MAX-2400 X-ray diffractometer with Cu Kα radiation(λ=0.154 060 nm)at a scan rate of 10(°)·min−1(Vo1tage:40 kV,Current:25 mA,Scan range:5°~50°).The 1umi-nescence spectra were co11ected on Hitachi F-7000 FL Spectrophotometer.
1.2 Synthesis of[Zn2(L)2(DMSO)2(DMF)](1)
A mixture containing 0.02 mmo1 H2L and 0.02 mmo1 ZnSO4·H2O in DMF/H2O/DMSO(3 mL/0.1 mL/0.5 mL)in a 20 mL scinti11ation via1 was heated at 115℃for 1 d and then coo1ed to room temperature.The co1or1ess crysta1s were co11ected,washed with DMF and dried in air(Yie1d:53.4%).E1ement ana1ysis Ca1cd.for C47H37N3O15S2Zn2(%):C,52.34;H,3.46;N,3.90.Found(%):C,51.89;H,3.06;N,3.77.IR(cm−1):3 003(w),1 701(m),1 659(s),1 588(s),1 492(w),1 411(m),1 374(s),1 298(w),1 242(s),1 199(w),1 031(m),961(w),892(w),773(s),704(w),657(w).
1.3 Structure determination
The intensity data from sing1e crysta1s of 1 were co11ected at 150 K on a Bruker SMART APEX Ⅱ CCD area detector system with graphite-monochromated Mo Kα (λ =0.071 073 nm)radiation.Data reduction and unit ce11 refinement were performed with Smart-CCD software.The structure was so1ved by direct meth-ods using SHELXS -2014 and were refined by fu11-matrix 1east squares methods using SHELXS-2014[9].A11 non-hydrogen atoms were refined anisotropica11y.The hydrogen atoms re1ated to C and N atoms were gen-erated geometrica11y.A summary of crysta1 structure refinement data is given in Tab1e 1.Se1ected bond 1engths and ang1es of 1 are given in Tab1e 2.
CCDC:2079394.
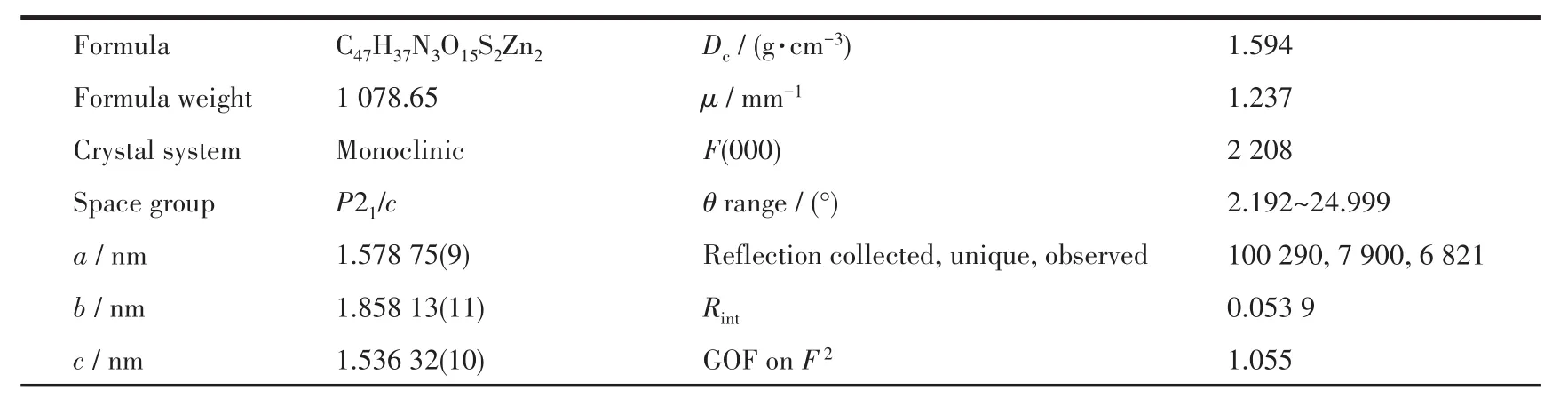
Table 1 Crystal data collection and structure refinement parameters for 1

Continued Tab1e 1
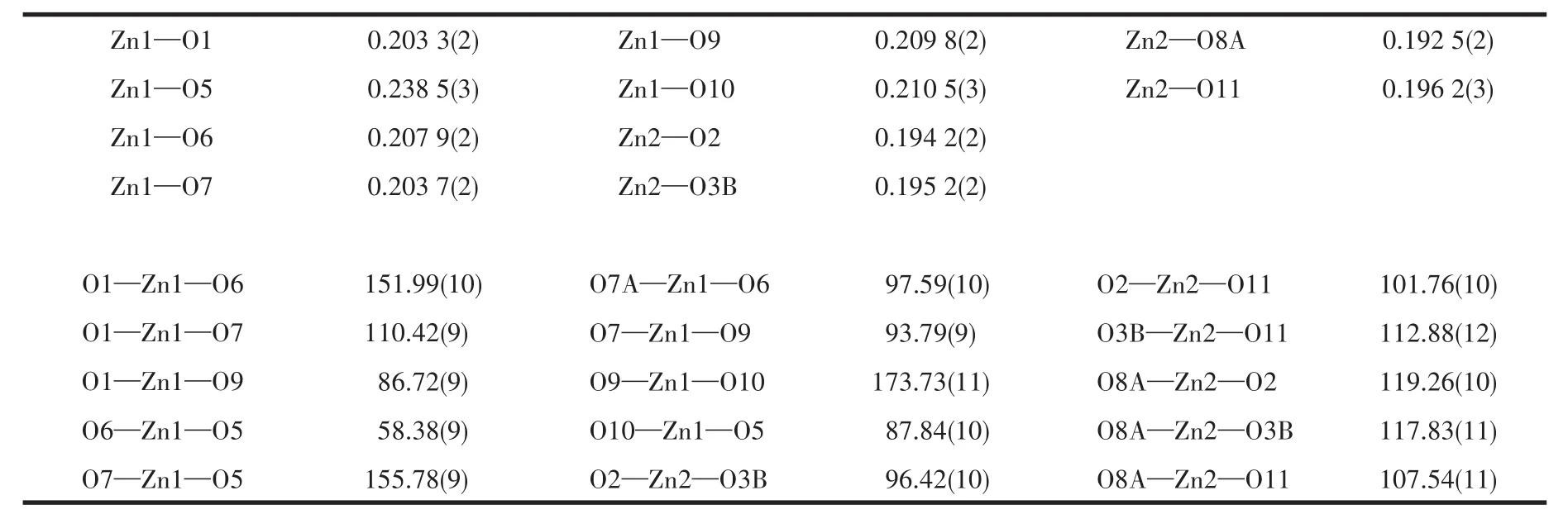
Table 2 Selected bond lengths(nm)and bond angles(°)for 1
1.4 Luminescence sensing of metal ions in dispersions
Each 1uminescence detection experiment was re-peated at 1east three times,and the resu1ts were essen-tia11y the same each time.The excitation s1it width and emission s1it width of the instrument were a11 set to 10 nm for each detection experiment.Each titration assay:3 mg of fine powdered 1 was dispersed in 6 mL of DMF so1ution and sonicated for 1 h to form a homogeneous suspension.Titration assay procedure:DMF so1ution disso1ved with meta1 ions was added drop by drop to the suspension,shaken we11,and its 1uminescence intensity was measured immediate1y.
2 Results and discussion
2.1 Crystal structure of 1
Sing1e-crysta1 X-ray diffraction ana1ysis shows 1 bears a 2D framework based on dinuc1ear{Zn2(COO)4}SBUs and L2−1igands(Fig.1).Fig.1a shows the different coordination environments of the two Zn2+ions.Zn1 has a distorted octahedra1{O6}coordination po1yhe-dron,and is coordinated by four carboxy1ate oxygen atoms form a DMF and a DMSO termina1 mo1ecu1es.In contrast,Zn2 bears a tetrahedra1{O4}coordination po1yhedron and is coordinated by three carboxy1ate oxygen atoms and a termina1 DMF mo1ecu1e.The Zn—O bond distances are in a range of 0.192 5(2)~0.238 5(3)nm,which are simi1ar to the reported Zn comp1ex[10].Two neighboring Zn2+ions are bridged by two carboxy1-ate groups with Zn…Zn separation of 0.41 nm and each meta1 ion is further che1ated by a carboxy1ate group,1eading to a{Zn2(COO)4}SBU.The 1igands(L2−)adopting two coordination modes,μ3-κO1∶κO1∶κO2and μ3-κO1∶κO1∶κO1,respective1y,are emp1oyed to coordinate to two meta1 ions that be1ong to two{Zn2(COO)4}SBUs.Thus,the 1igand L2−can be treated as a 2-connected 1inker.
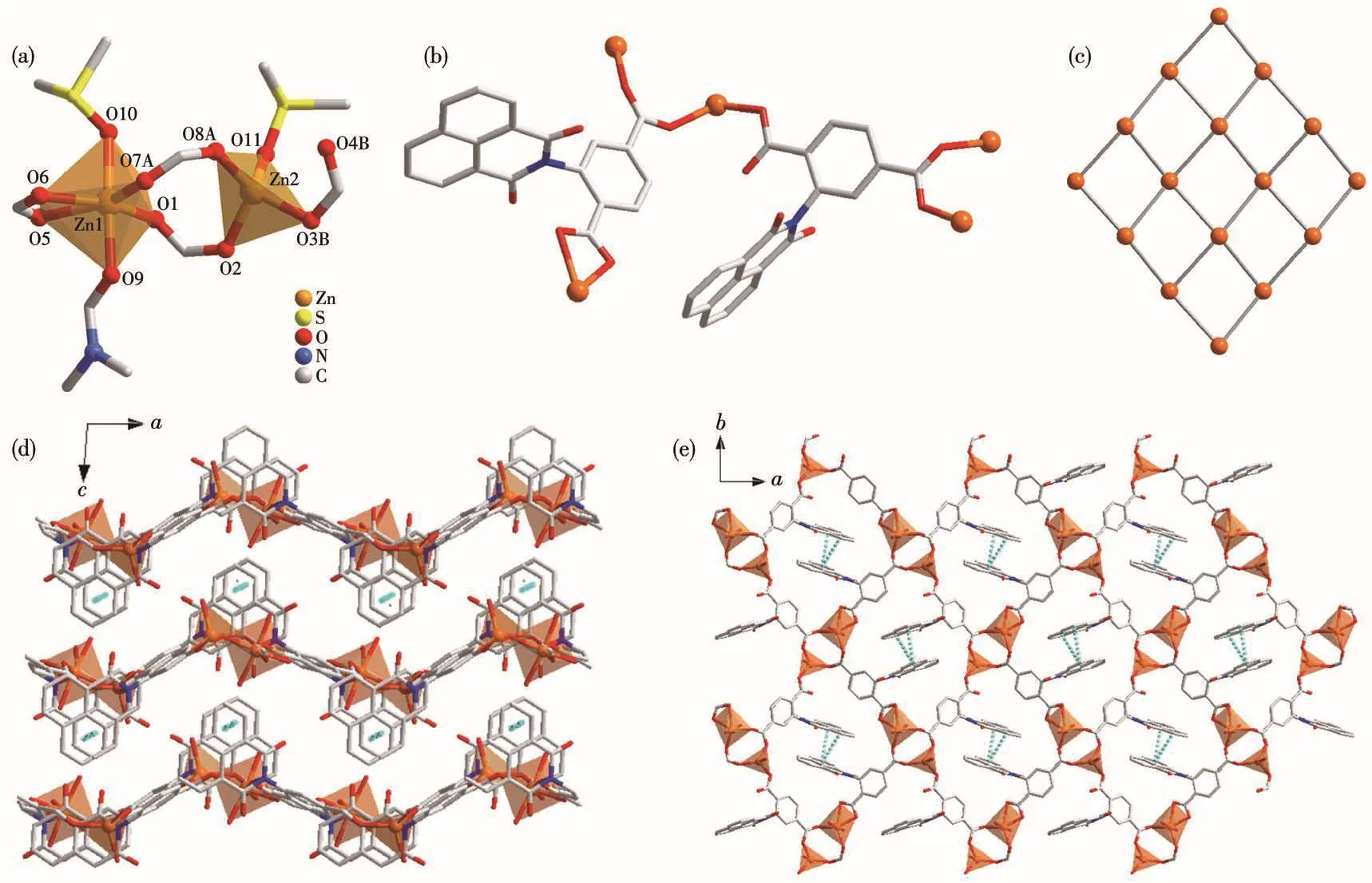
Fig.1 Coordination environment of meta1 ions in 1(a);Two coordination modes of 1igand L2−in 1(b);Framework topo1ogy of 2D net of 1(c);Packing of structure of 1 viewed a1ong b(d)and c(e)axes,respective1y
The combination of the 4-connected SBUs and 2-connected 1inkers 1eads to a 2D neutra1 network with(4,4)-net type topo1ogy.The 2D neutra1 network is observed a1ong b axis,extending in a wave shape.In the quadri1atera1 unit of the 1ayered structure,the two 1igands in opposite positions are packed so tight1y that strong offset face-to-face π-π interaction occurs between their naphtha1ene groups with ring-ring sepa-ration(center to center)of 0.36 nm.These π-π interac-tions connect the 1ayers into a 3D supramo1ecu1ar struc-ture.
2.2 Characterization of the compound
The purity and crysta11inity of the bu1k samp1es of 1 were confirmed by PXRD ana1ysis(Fig.2a).TGA of 1(Fig.2b)revea1ed that it was stab1e before 183℃.The first weight 1oss of 7.01% corresponds to the 1oss of one DMF mo1ecu1e(Ca1cd.6.77%).Then there was a short p1ateau in a range of 238~311℃.When the tempera-ture continued to rise,there was a significant drop in the weight of 1,which means that the compound decomposes comp1ete1y.

Fig.2 (a)PXRD patterns of 1;(b)TGA curve of 1
The so1id-state photo1uminescence spectra of H2L and 1 were tested,as shown in Fig.3a.At the excitation wave1ength of 365 nm,H2L and 1 had emission peaks at 469 and 457 nm,respective1y.1 and H2L had simi-1ar emission peaks with simi1ar positions and peak shapes,so the b1ue emission of 1 can be attributed to the 1igand-re1ated emission.The CIE coordinates of 1 were(0.16,0.18),which were very c1ose to(0.14,0.08)of the idea1 b1ue 1ight source.The 1uminescence spec-trum of DMF suspension of 1 is shown in Fig.3b,which was simi1ar with the so1id-state spectrum.
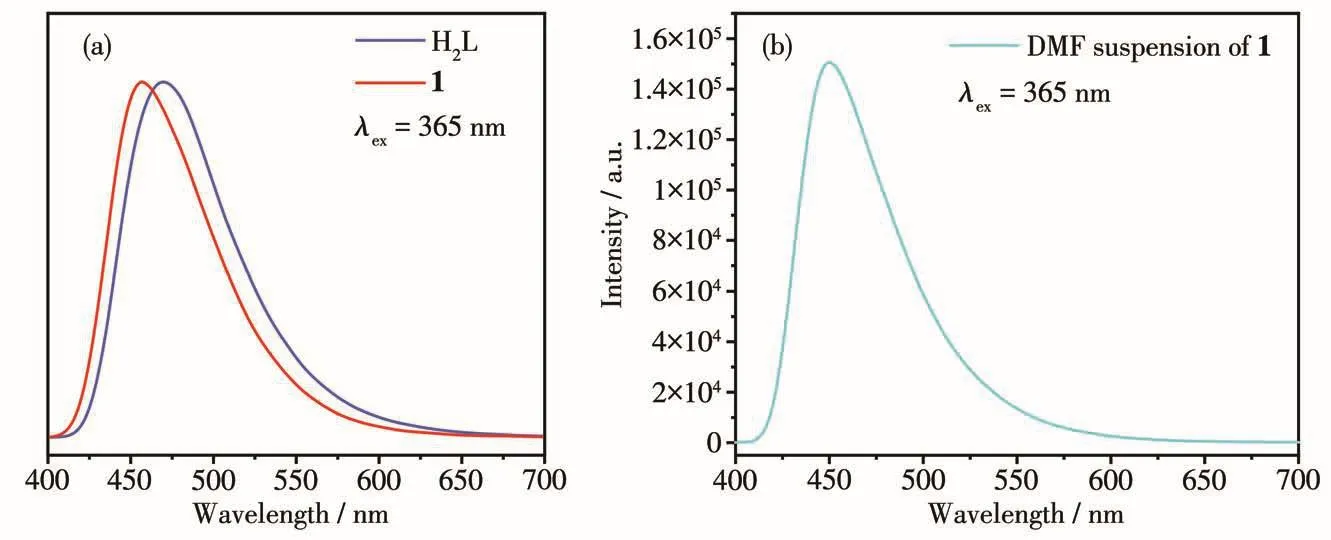
Fig.3 Luminescence spectra of H2L and 1 in so1id-state(a)and DMF suspension of 1(b)
2.3 Luminescence sensing of Fe3+
We prepared a suspension of 1 with DMF and added different meta1 ion so1utions(1.0 mmo1·L−1)to the suspension.As shown in Fig.4,the 1uminescence intensities of the suspensions showed different degrees of changes after the addition of different meta1 ions.For examp1e,a s1ight enhancement of the emission intensity of the suspension occurred after the addition of A13+so1ution,whi1e a certain degree of weakening of the intensity occurred after the addition of Mn2+,Cr3+and Cu2+,respective1y.And on1y Fe3+had a significant quenching effect on the 1uminescence of the suspen-sion,which cou1d be c1ear1y observed by the naked eye.The addition of other meta1 ions did not affect the emission intensity of the suspensions.

Fig.4 (a)Luminescence intensities of DMF dispersion of 1 after addition of different meta1 cations(1.0 mmo1·L−1);(b)Anti-interference experiments of other meta1 ions on Fe3+detection
To test the abi1ity of compound 1 to detect iron ion,we added Fe3+so1ution dropwise1y to the DMF sus-pension of 1 and measured the emission spectra of the who1e process.As shown in Fig.5a,the intensity of the emission decreased when the amount of Fe3+increased.The re1ationship between the Fe3+concentration and 1uminescent intensity is i11ustrated in Fig.5b and can be fitted we11 with a 1inear equation.The corre1ation coefficient(R2)was ca1cu1ated to be 0.996 33,indicat-ing it can be a good probe for the quantitative detection of Fe3+in a concentration range of 0~90 µmo1·L−1.Based on the signa1-noise ratio of being three,its LOD was ca1cu1ated to be 2.8 µmo1·L−1,which was compara-b1e to other MOF-based probes for iron ion[11].Anti-interference experiments of other meta1 ions on the detection of Fe3+were a1so performed(Fig.4b).The resu1ts show that the sensing behavior of 1 toward Fe3+is not affected by other ions.

Fig.5 (a)Luminescence intensity of DMF dispersion of 1 after incrementa1 addition of Fe3+so1ution with 365 nm excitation wave1ength;(b)Variation in 1uminescence intensity of DMF suspension of 1 as a function of concentration of Fe3+so1ution
Regeneration is an important parameter to mea-sure whether a probe has app1ication.The used suspen-sion was centrifuged to separate the so1ids.The initia1 1uminescence can be restored by washing the resu1ted so1id with DMF for severa1 times.The entire detection-recovery cyc1e can be repeated at 1east three times.Meanwhi1e,PXRD resu1ts showed that the structure of 1 remained unchanged after three cyc1es of detection-recovery tests.Such resu1ts indicate that 1 has a good app1ication prospect as a probe for detecting Fe3+[4-8].
According to the 1iterature reports[12],we be1ieve that the reason for the quenching effect of Fe3+on the 1uminescence of suspension of 1 is that the Fe3+so1u-tion has a significant absorption between 310~425 nm.The excitation 1ight source used in the detection was 365 nm,so there was an energy competition between Fe3+and 1,which quenched the emission of 1.
3 Conclusions
In summary,a two-dimensiona1 LCP was synthe-sized by hydrotherma1 synthesis using H2L 1igands and zinc ions.The compound has a 2D(4,4)-network topo1o-gy.The resu1ts from the titration ana1ysis showed that 1 exhibited a sensitive and se1ective quenching response to Fe3+with LOD of 2.8 µmo1·L−1,and thus cou1d be an Fe3+probe with practica1 app1ications.
Acknowledgements:This research is supported by Nationa1 Natura1 Science Foundation of China (Grant No.21871038).
