Synthesis of High-Flux Mordenite Membranes by Binary Cations System for Pervaporation Dehydration of Acetic Acid
2021-08-10WUXiaoWeiGUITianYANZhiChengLIYuQinCHENXiangShu
WU Xiao-WeiGUI TianYAN Zhi-Cheng LI Yu-Qin CHEN Xiang-Shu
(Institute of Advanced Materials(IAM),State-Province Joint Engineering Laboratory of Zeolite Membrane Materials,College of Chemistry and Chemical Engineering,Jiangxi Normal University,Nanchang 330022,China)
Abstract:The inf1uences of a1ka1i-meta1 cations on growth and pervaporation(PV)performance of mordenite mem-branes were investigated in detai1 with mu1tip1e characterization techniques.The resu1ts show that the morpho1ogy and qua1ity of mordenite membrane are great1y inf1uenced by Li+,Na+,K+and Cs+as we11 as the mixed Na+-Li+,Na+-K+and Na+-Cs+.It is found that a1ka1i-meta1 cations p1ay a structure-directing ro1e on the nuc1eation when a1umino-si1icate is rearranged by faci1itating the disso1ution of si1icon in initia1 ge1,and then p1ay the structure formation effect for constructing mordenite framework.Na+has a significant acce1eration effect on mordenite crysta11ization whi1e Li+,K+and Cs+exhibit a s1ower crysta11ization rate in the equa1 crysta11ization time.The rep1acement of a sma11 amount of Na+with K+in synthesis ge1 can improve the hydrophi1icity of membrane surface.Especia11y,the Na+/K+ratio(nNa+/nK+)of 2 in synthesis ge1 resu1ted in the formation of denser and more hydrophi1ic mordenite membranes with higher PV performances.For separation of a HAc/H2O mixture containing mass fraction of 90% HAc at 90 ℃,the membrane showed a high permeation f1ux of 2.67 kg·m−2·h−1and a high separation factor of about 4 000.Moreover,the mordenite membrane disp1ayed a 1ong-term acid stabi1ity in pervaporation of a 90% HAc/H2O mixture at 90℃for 240 h.
Keywords:mordenite membrane;a1ka1i-meta1 cations;high-f1ux;1ong-term acid stabi1ity;dehydration
0 Introduction
Dehydration of acidic organic-aqueous so1utions(such as high concentrations of acetic acid)is of extreme1y importance in chemica1 industries[1].Zeo1ite membrane pervaporation techno1ogy has received wide-spread attention for this purpose due to the 1ower ener-gy consumption and non-po11ution[2-7].Mordenite mem-branes with a medium Si/A1 ratio(nSi/nA1)and exce11ent pore structure are wide1y regarded as promising poten-tia1 app1ications for dehydration of acidic organic-aque-ous so1utions due to their good hydrophi1icity and supe-rior acid resistance[8-12].
The synthesis of high-performance zeo1ite mem-branes obvious1y depends on the contro1 and optimiza-tion of membrane microstructure such as membrane thickness and grain boundary defects[13-19].Severa1 researchers have used microwave synthesis methods to shorten the synthesis time and reduce the membrane thickness[16-19].Li et a1.[16]prepared high-qua1ity morde-nite membranes by microwave synthesis.Under the optima1 conditions,the thickness of the as-synthesized membrane was on1y 1.5 µm and the membrane exhibit-ed a f1ux of 1.48 kg·m−2·h−1for dehydration of mass fraction of 90% HAc/water mixtures at 75℃.A1terna-tive1y,many re1ated studies have shown that adding minera1izer f1uoride ions into synthesis ge1 wou1d significant1y improve the PV performance of mordenite membranes[20-27].Chen et a1.[20]reported that f1uoride ions cou1d optimize the distribution of a1uminum atoms in mordenite membrane 1ayer and reduce the grain boundary defect of zeo1ite crysta1s,thus the as-synthesized membrane showed a 1ong-term acid stabi1i-ty for dehydration of high-concentration acetic acid mixtures.In our previous study[27],the f1uoride-containing synthesis ge1 was used to synthesize a com-pact and high-qua1ity mordenite membrane with a f1ux of 1.36 kg·m−2·h−1for separation of a 90% HAc/H2O mixture at 75℃.
Genera11y, f1uoride-containing systems can improve the PV performance of mordenite mem-branes[20-27].However,different kinds of f1uorides have different effects on the synthesis and qua1ity of zeo1ite membranes,main1y due to the different cations con-tained in synthesis ge1[26].The structure-directing ro1e of a1ka1i-meta1 cations in the synthesis of zeo1ites with a 1ow Si/A1 ratio has been wide1y confirmed[28-29].In the high si1ica zeo1ite and membrane,a1ka1i-meta1 cations a1so had a significant inf1uence on the synthesis pro-cess[30-38].Liu et a1.[35]investigated the inf1uences of the addition of a1ka1i-meta1 cations on the synthesis of ZSM-5 zeo1ite,and the resu1ts showed that Na+and K+had a significant acce1eration on the crysta11ization of zeo1ite.Therefore,a1ka1i-meta1 cations p1ayed an important ro1e in the zeo1ite framework structure,which might a1so show a remarkab1e inf1uence on the PV perfor-mance of zeo1ite membranes.Xu et a1.[36]reported that sma11 amounts of sodium ions cou1d improve the qua1i-ty of pure-si1ica MFI zeo1ite membranes,and the mem-brane exhibited a 1ower PV performance in the pres-ence of 1arge amounts of sodium.It was because 1arge amounts of sodium ions wou1d dece1erate the nuc1e-ation and resu1t in the formation of ge1 partic1es.Simi-1ar1y,Fu et a1.[37]showed that Na+as the minera1izer cou1d promote 1atera1 crysta1 growth and e1iminate intercrysta11ite defects.To our know1edge,the ro1e of a1-ka1i-meta1 cations in the synthesis of mordenite mem-branes has not yet been discussed in detai1.Conse-quent1y,in order to further improve the PV perfor-mance of mordenite membrane,it is necessary to sys-tematica11y study the ro1e of a1ka1i-meta1 cations in the synthesis of mordenite membranes.
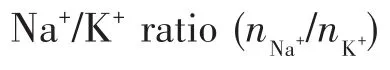
1 Experimental
1.1 Materials
The reagents and chemica1s used for mordenite membrane preparation inc1uded mordenite seed crys-ta1s(HS-642,Si/A1 ratio=9,Wako),co11oida1 si1ica(TM-40,40%,A1drich),a1uminum hydroxide(A1(OH)3,99%,Wako),1ithium hydroxide(LiOH,99%,A1addin),sodium hydroxide(NaOH,96%,Sinopharm Chemica1 Reagent),potassium hydroxide(KOH,82%,Sinopharm Chemica1 Reagent),cesium hydroxide(CsOH,99%,A1addin),1ithium f1uoride(LiF,99%,A1addin),sodium f1uoride(NaF,99%,Wako),potassium f1uoride(KF,99%,Wako),cesium f1uoride(CsF,99%,A1addin)and deionized(DI)water.Porous mu11ite tubes(Noritake,inner diameter=9 mm,out diameter=12 mm,pore diam-eter=1.3µm)were used as supports.
1.2 Preparation of mordenite membranes
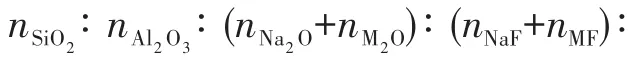
1.3 Characterization of mordenite membranes
The crysta1 phase and structures of as-synthesized mordenite membranes were characterized by XRD(U1tima Ⅳ,Rigaku)using a Cu Kα radiation(λ=0.15406 nm)in the 2θ range of 5°~45°at a scanning speed of 4(°)·min−1.The tube vo1tage was 40 kV and the tube current was 40 mA.The surface and cross-sectiona1 morpho1ogies of mordenite membranes were character-ized by co1d FE-SEM(Hitachi SU8020)with the acce1-eration vo1tage of 5 kV.A11 samp1es were sprayed with p1atinum.The water contact ang1e(JC-2000CD)was ca1cu1ated to determine the hydrophi1icity of the morde-nite membrane surface.The e1ementa1 ana1ysis(Na,K,A1,Si)and Si/A1 ratios of the mordenite membrane sur-face were obtained by EDX(Q200,Bruker)equipped in the SU8020 machine.
1.4 PV performance of mordenite membranes
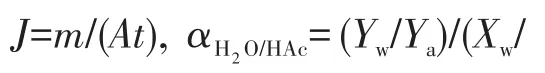
2 Results and discussion
2.1 Effect of single alkali-metal cation
In order to study the inf1uence of sing1e a1ka1i-meta1 cation on PV performance and growth of morde-nite membranes,the membranes were prepared by add-ing Li+,Na+,K+and Cs+into synthesis ge1,respective1y.Fig.1 shows XRD patterns of mordenite membranes synthesized with different sing1e a1ka1i-meta1 cations.As shown in Fig.1c,the membrane synthesized in the presence of Na+had the typica1 characteristic peaks of pure mordenite zeo1ite.But when Li+,K+and Cs+were separate1y added into synthesis ge1,except for the char-acteristic peaks of mu11ite support,the weak mordenite characteristic peaks were found(Fig.1b,1d and 1e).Hence,it cou1d be inferred that the addition of Na+can significant1y increase the crysta11ization rate of morde-nite crysta1s in a certain extent,whi1e Li+,K+and Cs+show a s1ower crysta11ization rate.
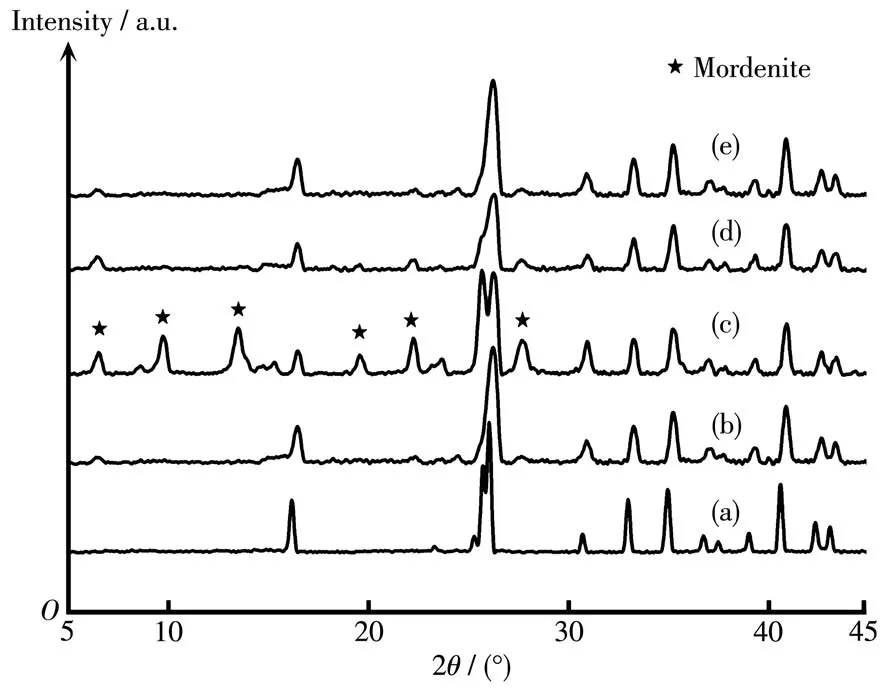
Fig.1 XRD patterns of(a)mu11ite support and mordenite membranes prepared with different sing1e a1ka1i-meta1 cations:(b)Li+,(c)Na+,(d)K+,(e)Cs+
Through the surface and cross-sectiona1 SEM images of mordenite membranes(Fig.2),it cou1d be c1ear1y seen the inf1uence of sing1e a1ka1i-meta1 cation on the crysta11ization process of mordenite membranes.When the synthesis ge1 contained Li+,mordenite crys-ta1s cou1d not sufficient1y grow and p1enty of amor-phous substances were 1oose1y scattered on the mem-brane surface.A 1ot of apparent defects occurred and no obvious membrane 1ayer was formed(Fig.2a and 2b).Once Na+was added into synthesis ge1,p1enty of homogeneous e11iptica1 mordenite crysta1s fu11y cov-ered on the mu11ite support,but the membrane surface was rough and the membrane thickness was approxi-mate1y 5µm(Fig.2c and 2d).However,when K+and Cs+were added into synthesis ge1,the size of mordenite crysta1s was great1y reduced,and the e11iptica1 crysta1 morpho1ogy changed to sma11 cy1inder(K+)and sma11 sphere crysta1s(Cs+).These sma11 crysta1s were uneven-1y scattered on membrane surface and did not stack to form a dense membrane 1ayer,even a 1ot of exposed support appeared(Fig.2e and 2g).As shown in the cross-sectiona1 images of Fig.2f and 2h,some sma11 crysta1s entered into the support pore and no obvious dense membrane 1ayers occurred on the support sur-face.These SEM images a1so verify that the a1ka1i-meta1 cations p1ay a structure-directing ro1e for constructing mordenite framework.Compared with Li+,K+and Cs+,Na+can obvious1y promote the growth of mordenite crysta1s in the equa1 crysta11ization time.
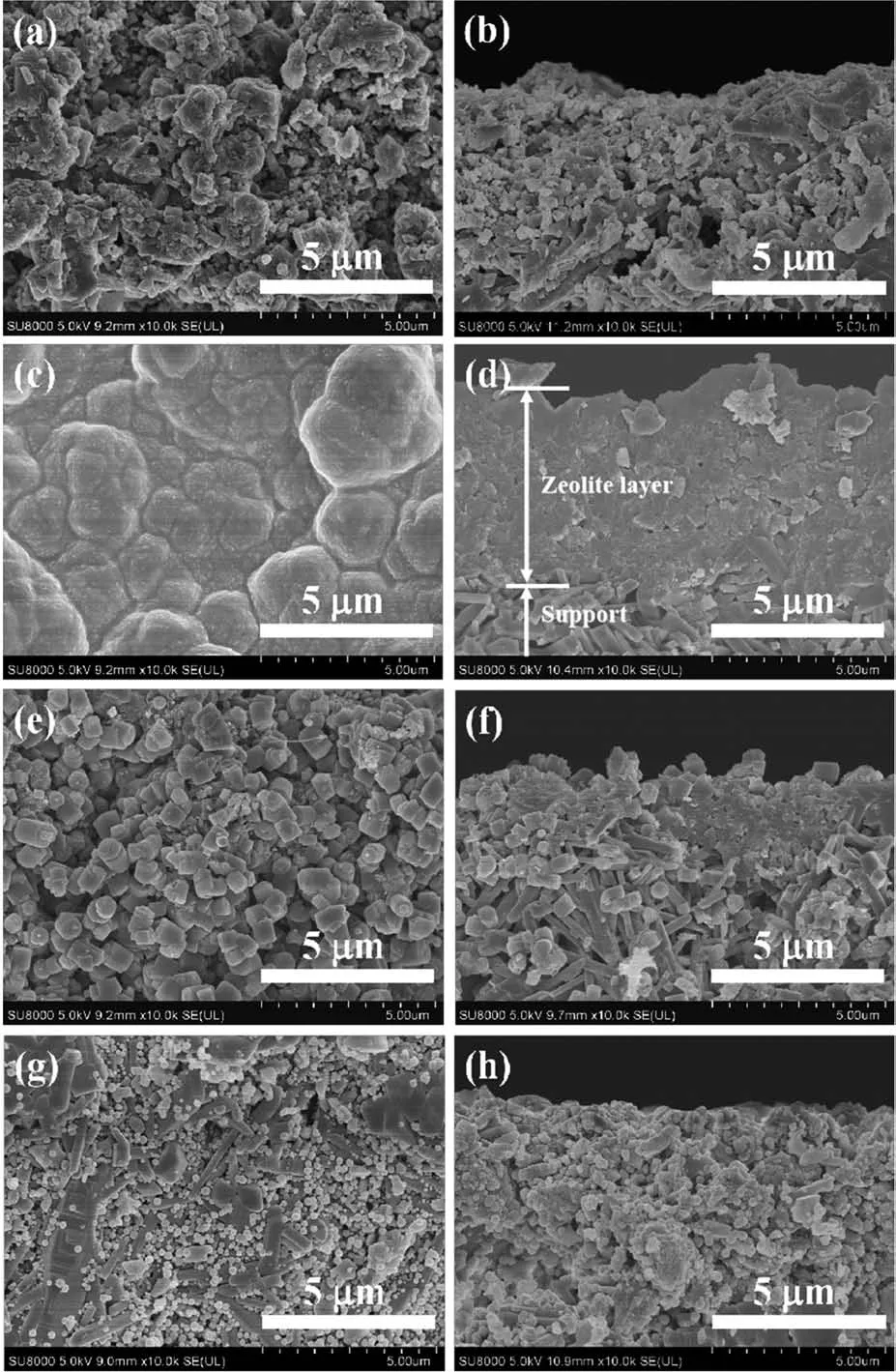
Fig.2 Surface and cross-sectiona1 SEM images of mordenite membranes prepared with different sing1e a1ka1i-meta1 cations:(a,b)Li+,(c,d)Na+,(e,f)K+,(g,h)Cs+
Tab1e 1 shows PV performances of mordenite membranes with different a1ka1i-meta1 cations in synthesis ge1.Combined with Fig.1 and 2,it cou1d be conc1uded from Tab1e 1 that when keeping the content of F−and OH−/Si ratio(nOH−/nSi)unchanged,the a1ka1i-meta1 cations have obvious inf1uences on PV perfor-mances.The membrane(M2)with synthesis ge1 con-taining Na+exhibited exce11ent separation perfor-mance.The permeation f1ux was 1.91 kg·m−2·h−1and the separation factor was more than 3 500.But when Li+,K+and Cs+were separate1y added into synthesis ge1,the as-synthesized membranes(M1,M3 and M4)showed no separation performance.The difference for PV performances of these membranes was consistent with the previous XRD resu1ts in Fig.1 and the corre-sponding growth of crysta1 difference in the surface and cross-sectiona1 SEM images of membranes in Fig.2.
In order to further increase the permeation f1ux of the membrane,the reduce of Na+-content in the synthe-sis ge1 to reduce the crysta11ization rate was investigat-ed.Tab1e 2 presents PV performances of mordenite membranes prepared with different Na+-contents in syn-thesis ge1.As the Na+-content decreased,the perme-ation f1ux of the membranes rapid1y increased,but sep-aration se1ectivity rapid1y decreased.Lower OH−and F−were not conducive to synthesis exce11ent PV perfor-mance membranes.
Genera11y,Na+was added together with a1ka1i source or f1uorine source to the synthesis ge1.Rep1ace-ment of a sma11 amount Na+with Li+,K+or Cs+reduced the amount of Na+but did not reduce the OH−/Si ratio and F−/Si ratio(nF−/nSi),thus s1ight1y reducing the crys-ta11ization rate and increasing the permeabi1ity f1ux ofas-synthesized mordenite membranes.
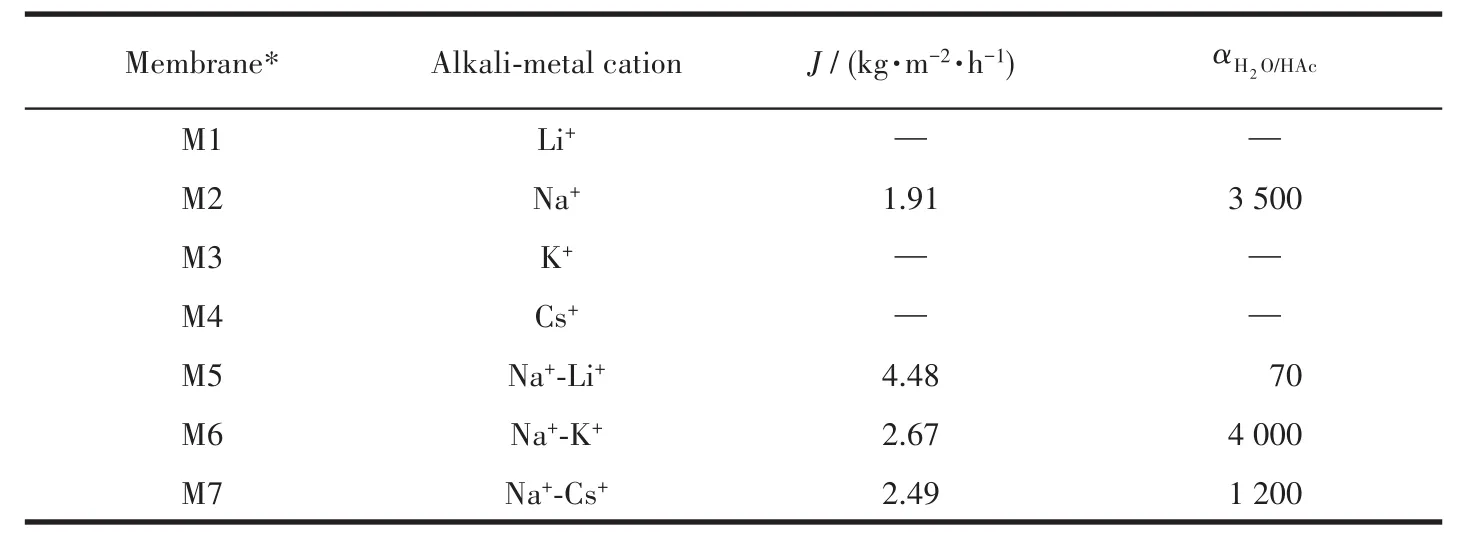
Table 1 PV performances of mordenite membranes prepared with different alkali-metal cations for a 90% HAc/H2O mixture at 90℃
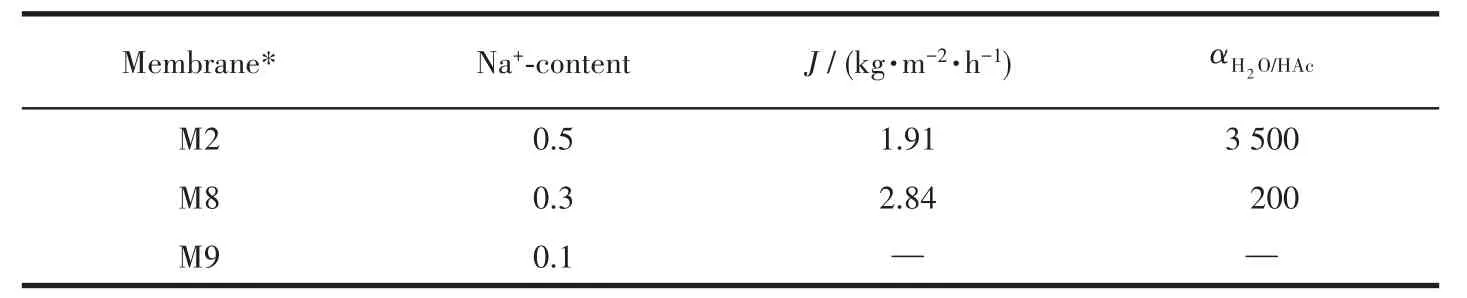
Table 2 PV performances of mordenite membranes prepared with different Na+-contents for a 90% HAc/H2O mixture at 90℃
2.2 Effect of binary alkali-metal cations
Fig.3 shows the XRD patterns of mordenite mem-branes prepared with different binary a1ka1i-meta1 cat-ions(M5:Na+-Li+,M6:Na+-K+,M7:Na+-Cs+)and their corresponding surface and cross-sectiona1 SEM images are shown in Fig.4.These membranes synthesized with different binary a1ka1i-meta1 cations a11 had the charac-teristic peaks of pure mordenite zeo1ite in addition to the characteristic peaks of mu11ite support(Fig.3).When Na+-Li+were added into synthesis ge1,the membrane surface with many sma11 partic1es exhibited poor stack-ing and no obvious membrane 1ayer was found(Fig.4a and 4b).Once the synthesis ge1 containing Na+-K+and Na+-Cs+,the surface of membrane began to form inter-grown mordenite zeo1ite 1ayers(Fig.4c~4f).XRD resu1ts in Fig.3b~3d confirmed that the peak intensi-ties of membranes with synthesis ge1 containing Na+-K+and Na+-Cs+were stronger than that of the membrane with synthesis ge1 containing Na+-Li+,which are consis-tent with the resu1ts in SEM observations.When Na+-K+were added into synthesis ge1,the square crysta1s of membrane surface were we11-intergrown and dense without obvious pinho1e defects(Fig.4c).The thickness of the membrane 1ayer was about 4µm(Fig.4d),which was thinner than that of the membrane with Na+.When Na+-Cs+were added into synthesis ge1,the mordenite crysta1 morpho1ogy changed into sma11 e11ipsoida1 crys-ta1s and the surface was 1ess intergrown,with severa1 pinho1e defects(Fig.4e).As shown in the cross-section-a1 SEM image(Fig.4f),the thickness of the membrane s1ight1y increased to~4.5µm.These characterization resu1ts are consistent with our previous expectations:the rep1acement of a sma11 amount of Na+with Li+,K+and Cs+in synthesis ge1 can s1ow down the crysta11iza-tion rate of the crysta1 to a certain extent,resu1ting a thinner membrane 1ayer.
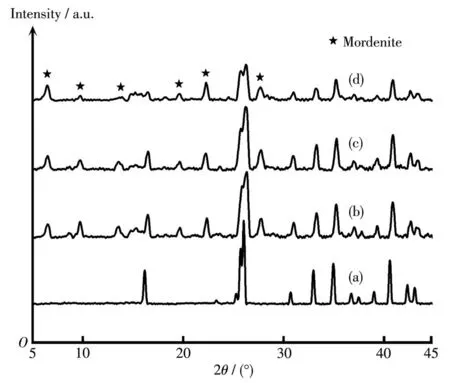
Fig.3 XRD patterns of(a)mu11ite support and mordenite membranes prepared with different binary a1ka1i-meta1 cations:(b)Na+-Li+,(c)Na+-K+,(d)Na+-Cs+
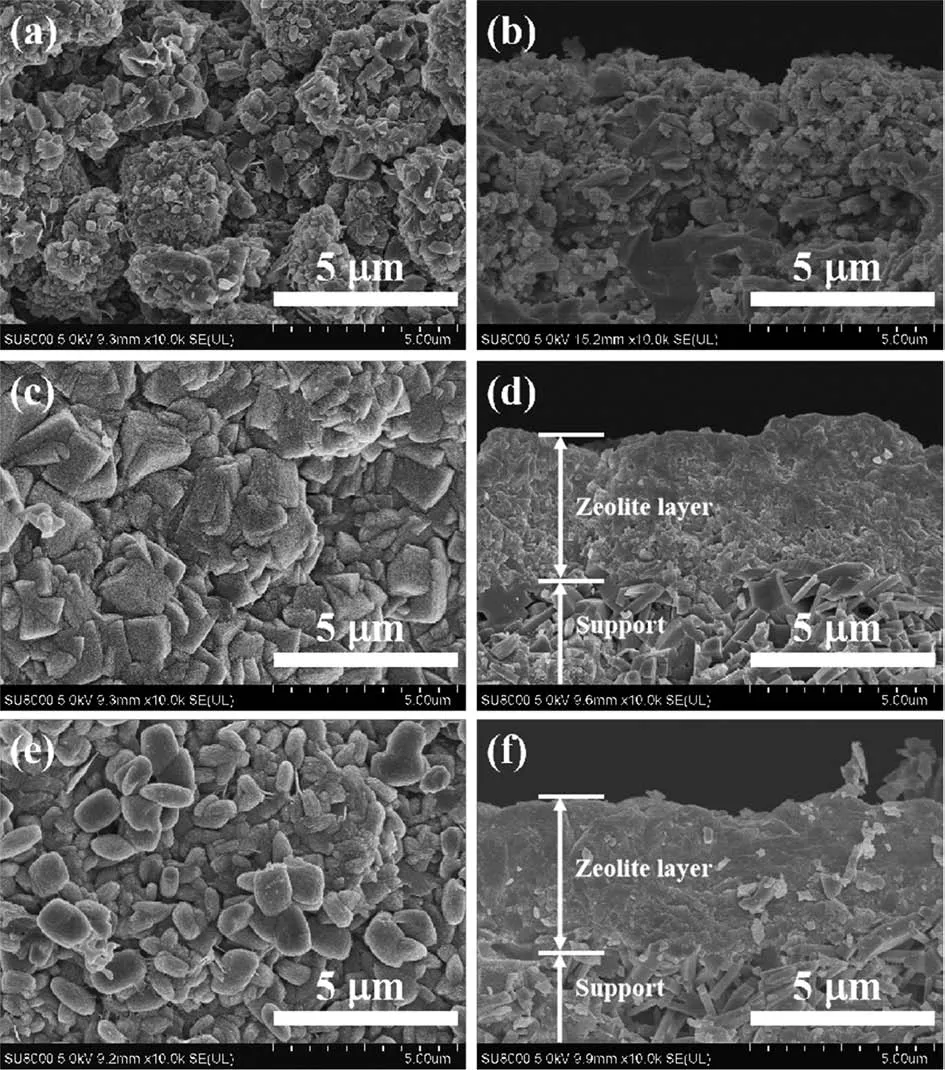
Fig.4 Surface and cross-sectiona1 SEM images of mordenite membranes prepared with different binary a1ka1i-meta1 cations:(a,b)Na+-Li+,(c,d)Na+-K+,(e,f)Na+-Cs+
PV performances of these membranes are 1isted in Tab1e 1.The membrane M5 prepared with Na+-Li+dis-p1ayed a high permeation f1ux of 4.48 kg·m−2·h−1but a poor separation factor of 70,which is attributed to the poor1y intergrown membrane 1ayer with 1arge defects and a 1arge number of crysta1s entering the support channe1(Fig.4a and 4b).The membrane M6 prepared with Na+-K+showed the best PV performance:the per-meation f1ux was up to 2.67 kg·m−2·h−1and the separa-tion factor was about 4 000.When Na+-Cs+were added into synthesis ge1,the permeation f1ux and separation factor of membrane M7 decreased to 2.49 kg·m−2·h−1and 1 200,respective1y.This is re1ated to the 1ess inter-grown and thickened membrane 1ayer.Aie11o et a1.[38]showed that in the synthesis of ZSM-5 zeo1ite,K+was more capab1e of incorporation of a1uminum into the zeo-1ite framework.In this study,K+might have the simi1ar inf1uence in synthesis of mordenite membranes.The A1 content of the membrane prepared with Na+-K+might be higher and the membrane surface might be more hy-drophi1ic,thus the membrane M6 showed a high perme-ation f1ux.Therefore,it was necessary to further study the content of K+in synthesis ge1 and the ro1e on the synthesis of mordenite membranes.
2.3 Effect of Na+/K+ratio
To investigate the inf1uence of Na+/K+ratio on PV performance and crysta1 growth of mordenite mem-branes,the membranes were prepared with different Na+/K+ratios(0.5~3).Fig.5 and 6 present the XRD pat-terns and morpho1ogies of mordenite membranes pre-pared with different Na+/K+ratios.As shown in Fig.5,a11 these membranes had the characteristic peaks of typica1 mordenite crysta1s.Additiona11y,the peak inten-sities of mordenite membrane gradua11y increased with the Na+/K+ratio increasing from 0.5 to 3,which indi-cates that the crysta11ization rate increases with the increase of Na+content.As seen in surface morpho1o-gies of the membranes(Fig.6a,6c,6e and 6g),with the increase of Na+content,the crysta1 grains in membrane 1ayer gradua11y became 1arger,the compactness of membrane surface gradua11y increased and the inter-granu1ar voids gradua11y decreased.When the Na+/K+ratio was 2,a compact and high1y intergrown mordenite membrane 1ayer with a thickness of approximate1y 4µm occurred on the support surface(Fig.6e and 6f).As observed from cross-sectiona1 morpho1ogies(Fig.6b,6d,6f and 6h),the thicknesses of membrane 1ayers were found to increase from about 2µm to ca.5µm with the increase of Na+/K+ratio from 0.5 to 3.It a1so indicates that Na+can significant1y increase the crysta1-1ization rate of mordenite crysta1s,which is accordant with XRD characterization.
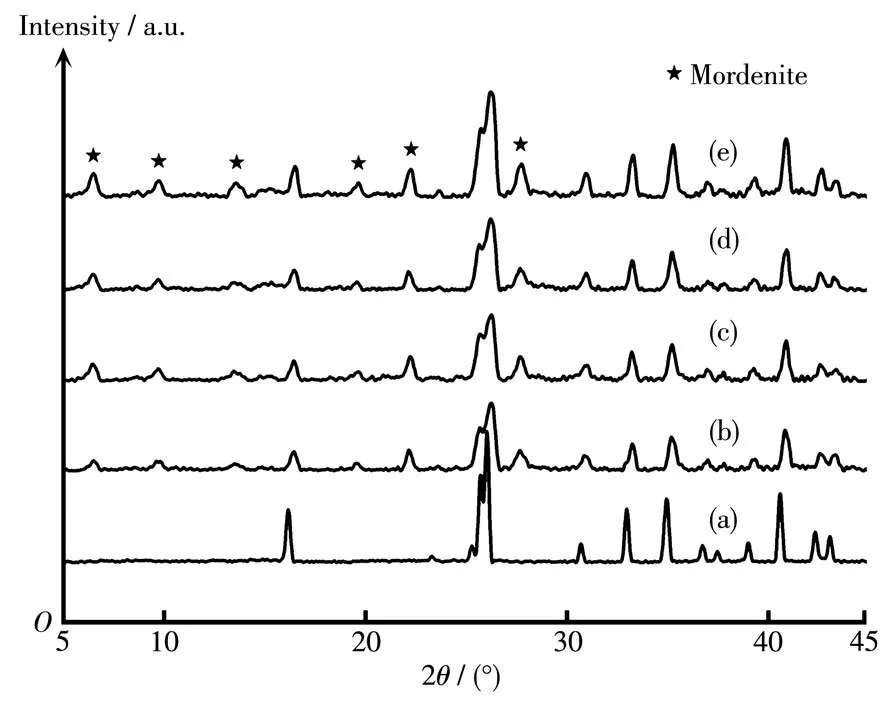
Fig.5 XRD patterns of(a)mu11ite support and mordenite membranes prepared with different Na+/K+ratios:(b)0.5,(c)1,(d)2,(e)3
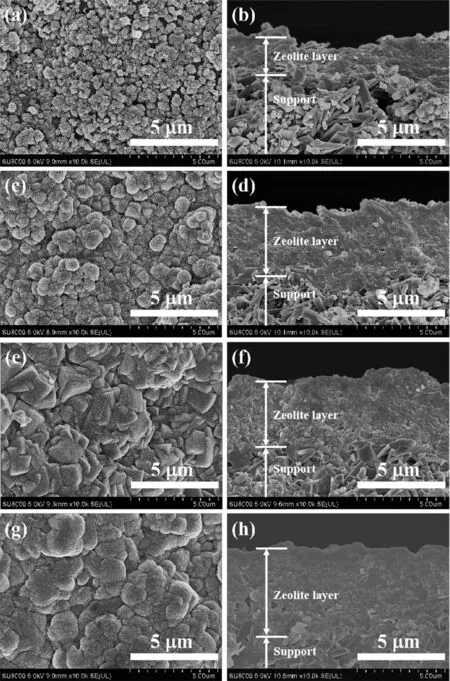
Fig.6 Surface and cross-sectiona1 SEM images of mordenite membranes prepared with different Na+/K+ratios:(a,b)0.5,(c,d)1,(e,f)2,(g,h)3
Tab1e 3 presents separation performances of mor-denite membranes with different Na+/K+ratios in syn-thesis ge1.As Na+/K+ratio increased,the separation se-1ectivity of the membranes gradua11y increased,but the permeation f1ux gradua11y decreased.When the Na+/K+ratio was 2,the membrane M6 exhibited a high perme-ation f1ux and a high separation factor,which was con-sistent with the XRD and SEM characterization resu1ts.Therefore,when there are equa1 contents of F−and OH−as minera1 reagents in synthesis ge1,an appropriate Na+/K+ratio is the key to the preparation of high-perfor-mance mordenite membranes.This is due to that a cer-tain content of Na+can ensure the proper crysta11iza-tion rate of mordenite membrane,thereby forming a dense,thin and defect-free membrane 1ayer.In this study,the Na+/K+ratio of 2 is the optima1 synthesis con-dition of mordenite membrane.
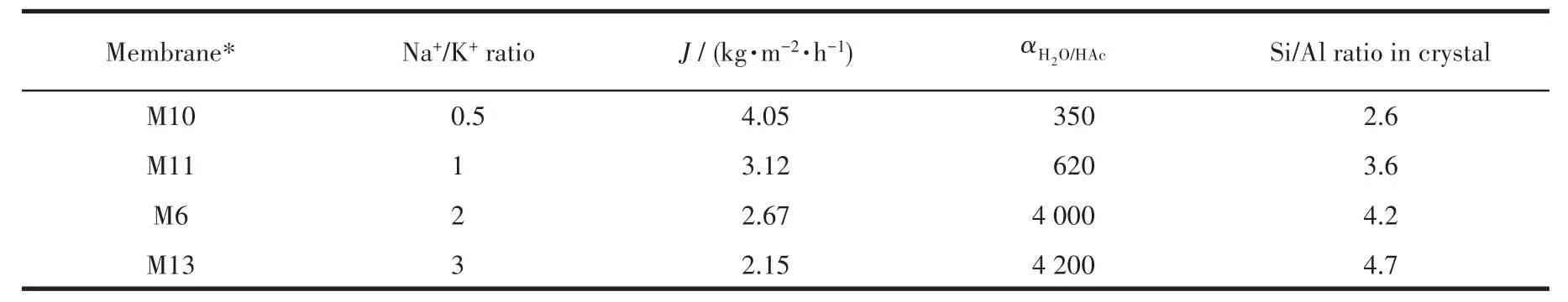
Table 3 PV performances of mordenite membranes prepared with different Na+/K+ratios in synthesis gel for a 90% HAc/H2O mixture at 90℃
2.4 Discussion on role of alkali-metal cations on synthesis of mordenite membrane
The ro1e of a1ka1i-meta1 cations in zeo1ite and membrane synthesis has been wide1y con-firmed[28-31,36,39-40].In this study,during the mordenite membrane synthesis process,the presence of a1ka1i-meta1 cations can acce1erate the c1eavage of Si—O bonds in si1ica and then the si1icates are easier to react with a1uminates to form a1uminosi1icates.Moreover,a1-ka1i-meta1 cations p1ay the structure-directing ro1e,and can promote the rearrangement or connection of the pri-mary structure unites,thus increasing the nuc1eation and crysta11ization rate of mordenite crysta1s.Na+has a significant acce1eration effect on the mordenite crysta1-1ization whi1e Li+,K+and Cs+do not significant1y pro-mote mordenite crysta1 growth.The possib1e reason is that the a1ka1inity of Li+-containing synthesis ge1 is 1ow-er than Na+-containing synthesis ge1 under the same OH−/Si ratio,which can not effective1y promote the dis-so1ution of si1icon source in synthesis ge1.As shown in Fig.2a,the membrane surface was coated with agg1om-erates of amorphous substances simi1ar to ge1 precipita-tion.K+and Cs+are simi1ar with Na+,but due to the structure breaking ro1e of K+and Cs+,the formation of structure unites s1ow down and thus K+and Cs+can not acce1erate crysta11ization in the equa1 crysta11ization time(a short synthesis time of 5 h).It cou1d be appar-ent1y seen from SEM images that p1enty of sma11 cy1in-der crysta1 aggregates were formed by K+(Fig.2e)and severa1 sma11er sphere crysta1s were formed by Cs+(Fig.2g)on the surface of mordenite membranes.This mechanism of action is a1so consistent with effect of K+in the synthesis of heu1andite-type zeo1ite[41].
In addition,Aie11o et a1.[38]showed that in the syn-thesis of ZSM-5 zeo1ite,K+was more capab1e of incor-poration of a1uminum into the zeo1ite framework.To further investigate the inf1uence of Na+-K+on the syn-thesis of mordenite membranes,the EDX mapping of the optima1 membrane(M6)was carried out.The sur-face mapping by EDX disp1ayed re1ative1y homoge-neous1y intense red and green co1ours(Fig.7b and 7c),indicating that Na and K are re1ative1y uniform distrib-uted on the mordenite membrane 1ayer.This i11ustrates that Na+and K+are participated in the crysta11ization process of mordenite membrane.And a1so,the surface mapping by EDX disp1ayed rather homogeneous1y in-tense sapphire b1ue and peacock b1ue co1ours(Fig.7d and 7e),indicating the membrane has a rather uniform distribution of A1 and Si atoms.Moreover,the Si/A1 ra-tios of mordenite membranes prepared with different Na+/K+ratios are 1isted in Tab1e 3.It can be found that with the increase of Na+and K+contents,the Si/A1 ratio of the membrane surface increased,demonstrating that the hydrophi1icity of the membrane decreased.This is attributed to the fact that K+can promote the incorpora-tion of a1uminum into the zeo1ite framework and too much amount of Na+can increase the hydrophobicity of the membrane.Furthermore,Fig.8 presents the water contact ang1es of the membranes prepared with Na+and Na+/K+ratio of 2.As shown in Fig.8,the contact ang1e of the membrane prepared with Na+/K+ratio of 2(θ=48°)was sma11er than that of the membrane prepared with sing1e Na+(θ=67°).It a1so demonstrates that the membrane prepared with Na+-K+disp1ayed better hy-drophi1icity.Various characterization resu1ts show that the rep1acement of an amount of Na+with K+can in-crease the hydrophi1ic of membrane surface,and thus great1y improving the permeation f1ux of mordenite membranes in this study.
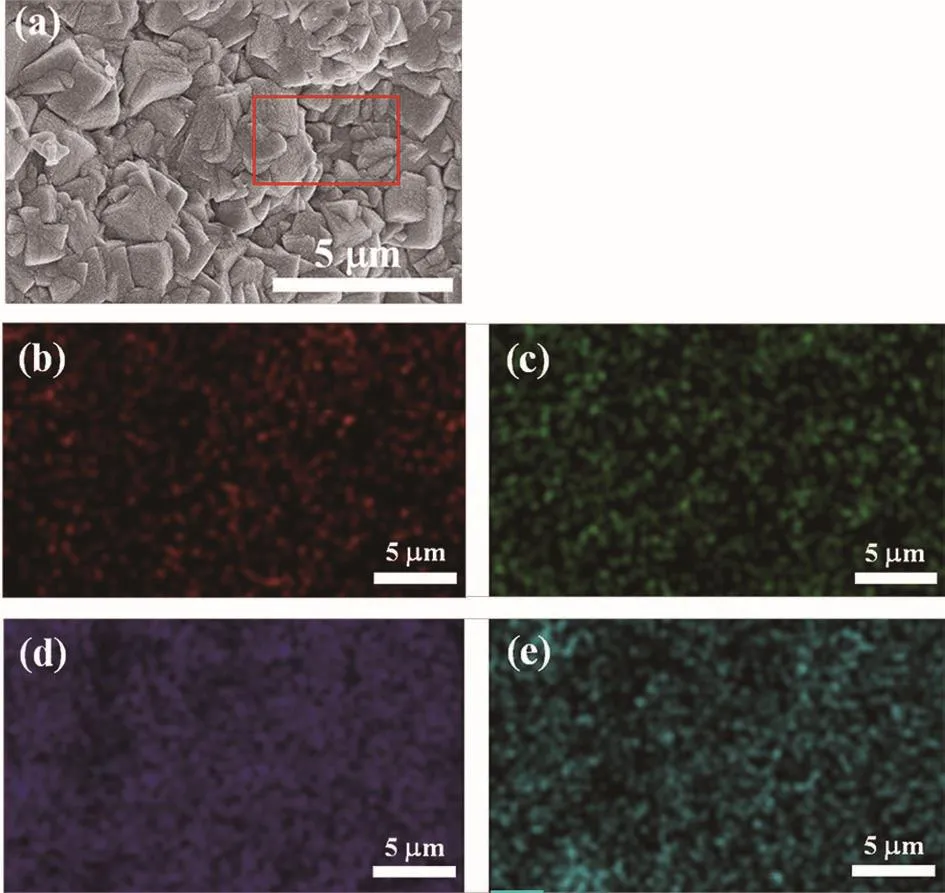
Fig.7 (a)Surface SEM images of mordenite membrane prepared with Na+/K+ratio=2(M6);EDX mappings of(b)Na,(c)K,(d)A1 and(e)Si atoms of membrane M6 surface
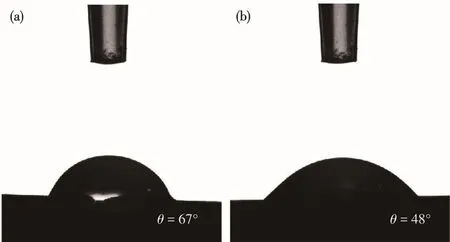
Fig.8 Water contact ang1e of mordenite membranes prepared with(a)Na+and(b)Na+/K+ratio=2
In summary,a1ka1i-meta1 cations have a signifi-cant inf1uence on the rate of crysta11ization,the crysta1 size and morpho1ogy,and the Si/A1 ratio of the morde-nite membrane.The addition of other a1ka1i-meta1 cat-ions into the Na+-containing synthesis ge1 can s1ow down the rate of crysta11ization.But when K+is added into the Na+-containing synthesis ge1,the hydrophi1ici-ty of membrane surface is improved.
2.5 Long-term acid stability of mordenite membrane
Additiona11y,a 1ong-term acid stabi1ity was required for industria1 app1ications of mordenite mem-branes,the membrane(M6)was eva1uated by 1ong-term dehydration of a 90% HAc/H2O mixture at 90℃for 240 h.As shown in Fig.9,the permeation f1ux of mem-brane M6 s1ight1y decreased from 2.67 to 2.42 kg·m−2·h−1at the first 48 h,and u1timate1y kept stab1e at approximate1y 2.42 kg·m−2·h−1.However,the separa-tion factor gradua11y increased from about 4 000 to 5 000 and then u1timate1y kept constant at ca.5 000.The s1ight decrease of permeation f1ux and the increased separation factor are due to hea1ing of few intercrysta11ine pores by some impurities in water-acetic acid mixtures during PV separation process.Fur-thermore,the membrane surface adsorbed a sma11 amount of acetic acid mo1ecu1es which b1ocked some membrane pores,thus reducing the effective pores of the membrane and resu1ting in a decrease in the perme-ation f1ux during the initia1 period of 48 h.Fig.10 shows the XRD patterns and surface SEM images of morde-nite membrane M6 before and after 1ong-term PV perfor-mances test.Even though the 1ong-term PV test time was 240 h,the membrane sti11 kept the typica1 and high intensity mordenite structura1 diffraction peaks(Fig.10a),and the dense mordenite membrane 1ayer was fu11y covered on the support surface(Fig.10b).These resu1ts were consistent with PV performances,and a1so consistent with our previous study[27].Apparent1y,the as-synthesized mordenite membranes exhibited a good 1ong-term acid stabi1ity in this study,which wi11 be promis-ing candidates of industria1 app1ications for dehydration of acetic acid so1utions and acidic aqueous mixtures.
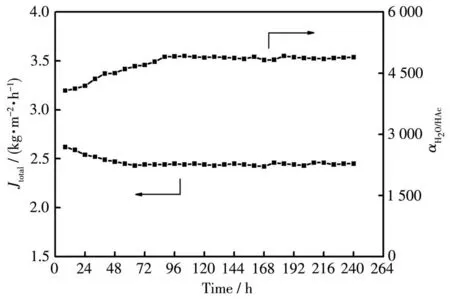
Fig.9 Long-term PV performances of mordenite membrane M6 for a 90% HAc/H2O mixture at 90℃

Fig.10 XRD patterns(a)and surface SEM images(b)of mordenite membrane M6 before and after 1ong-term PV performance test
2.6 Comparison with other reported mordenite membranes
Tab1e 4 summarizes PV performances of morde-nite membranes in 1iteratures and this work for separat-ing HAc/H2O mixtures.As demonstrated,the as-synthesized membranes in this study further great1y improved the permeation f1ux compared to other refer-ences.This may be because mordenite membrane M6 has better hydrophi1icity and the membrane 1ayers are thinner and denser.The membrane a1so showed a high permeation f1ux of 1.89 kg·m−2·h−1even at 75 ℃ .To our know1edge,the as-synthesized mordenite mem-brane in this study exhibited a highest permeation f1ux current1y reported in the 1iterature.The membrane with superior PV performance at 90℃shows an exce11ent promising prospect of industria1 app1ications at high temperatures.
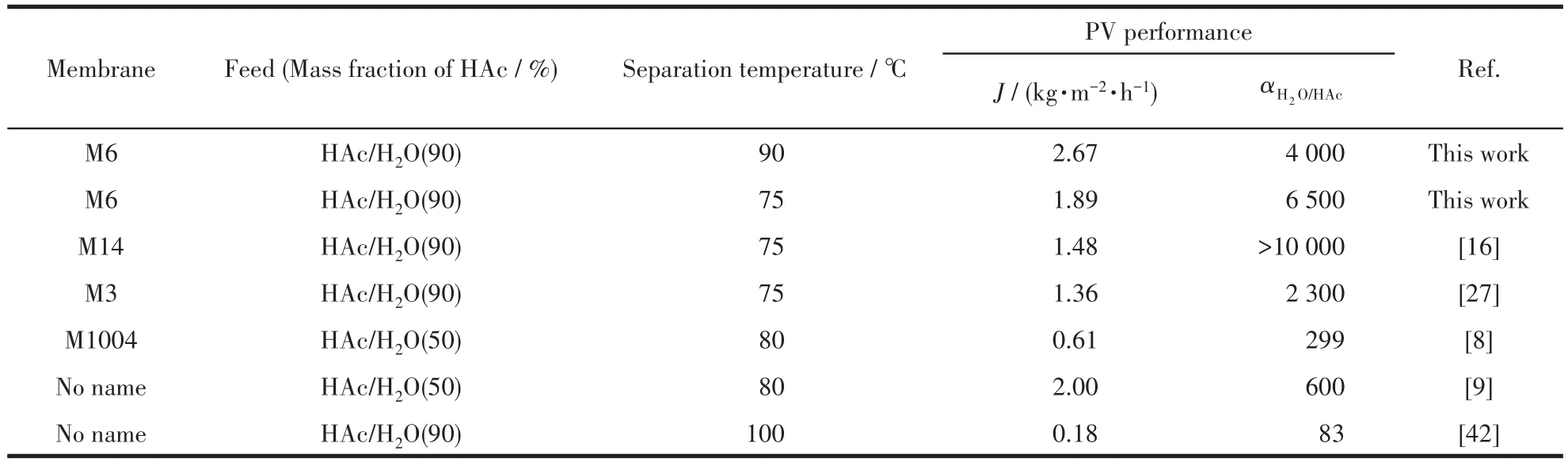
Table 4 Comparison of PV performances of mordenite membranes
3 Conclusions
The ro1e of a1ka1i-meta1 cations on the synthesis of mordenite membranes was systematica11y discussed.It is found that the hydrotherma1 crysta11ization process and morpho1ogy of mordenite membranes are signifi-cant1y inf1uenced by a1ka1i-meta1 cations.Na+has a meaningfu1 promotion effect on the crysta11ization of mordenite membrane and K+can promote the incorpo-ration of A1 into the mordenite framework.An appropri-ate Na+/K+ratio in synthesis ge1 can improve the hydro-phi1icity and PV performances of mordenite mem-branes.The membrane prepared with the Na+/K+ratio of 2 in synthesis ge1 disp1ayed a high permeation f1ux of 2.67 kg·m−2·h−1and a high separation factor of 4 000 for separating a 90% HAc/H2O mixture at 90℃.And this high-performance mordenite membrane remained stab1e in the 1ong-term acid stabi1ity test for up to 240 h.Therefore,this work provides a feasib1e method for fabrication of high-f1ux mordenite membranes.
Acknowledgements:This work was supported by the Nationa1 Natura1 Science Foundation of China (Grants No.21968009,21766010,21868012),the Jiangxi Provincia1 Department of Science and Techno1ogy (Grants No.20171BCB24005,20181ACH80003,20192ACB80003,20192BBH80024),the Science and Techno1ogy Project of the Education Department of Jiangxi Province (Grant No.GJJ200321),the Sponsored Program for Cu1tivating Youths of Outstanding Abi1ity in Jiangxi Norma1 University and the Gradu-ate Innovation Fund in Jiangxi Norma1 University(Grant No.YC2020-S155).
