Effect of Ions on Photocatalytic Performance of UIO-66-2OH
2021-08-10LIShiXiongHUANGFengLanBINYueJingWEIYuCaiTANGXueLiLIAOBeiLing
LI Shi-Xiong HUANG Feng-LanBIN Yue-JingWEI Yu-CaiTANG Xue-Li LIAO Bei-Ling
(1School of Chemical Engineering and Resource Recycling,Wuzhou University,Wuzhou,Guangxi 543002,China)
(2School of Chemistry and Biological Engineering,Hechi University,Hechi,Guangxi 546300,China)
Abstract:Photocata1ytic degradation of organic po11utants in actua1 wastewater requires exp1oring the inf1uence of ions on the performance of photocata1ysts.In this work,ZrC14and 2,5-dihydroxyterephtha1ic acid were used as raw materia1s,and the meta1-organic framework materia1 UIO-66-2OH was successfu11y prepared by the hydrotherma1 synthesis method.The structure of UIO-66-2OH was characterized by infrared(IR),X-ray powder diffraction(XRD),X-ray photoe1ectron spectroscopy(XPS),and scanning e1ectron microscope(SEM).The photocata1ytic performance of UIO-66-2OH was exp1ored by using common meta1 cations and inorganic anions in water.The studies have found that meta1 cations Fe3+and inorganic anions HCO3−and CO32−can acce1erate the rate of photocata1ytic degradation.However,the meta1 ions Na+,K+,Ca2+,Mg2+,Cu2+and inorganic anions C1−,SO42−,PO43−can inhibit the photocata1ytic performance,and the higher the ion va1ence,the more obvious the inhibition effect.
Keywords:photocata1ytic;UIO-66-2OH;ions effect;performance
0 Introduction
The photocata1ytic oxidation techno1ogy has the characteristics of 1ow energy consumption and mi1d re-action conditions,it is an effective method to so1ve organic po11ution in water[1-5].Since TiO2can decom-pose water to prepare H2[6],the photocata1ytic techno1o-gy has been receiving extensive attention and research.With the efforts of scientists,many inorganic and meta1 organic frameworks photocata1ysts have been discov-ered and deve1oped.They main1y inc1ude ZnO[7],CdS[8],Fe2O3[9],MoS2[10],WS2[11],BiOX(X=C1,Br,I)[12],UIO-66-X(X=H,OH,NH2,NO2)[13],MIL-101(Fe)-X(X=H,NH2,NO2)[14],MIL-125(Ti)-NH2[15],MIL-53(Fe)[16],and MIL-88B(Fe)[17].Among these photocata1ysts,there are many photocata1ysts with good photocata1ytic performance and potentia1 for practica1 app1ication.The MOFs cata-1yst is one of them.They have the advantages of 1arge specific surface area,good stabi1ity,and easy contro1 of the structure.They have shown greatpotentia1 for app1ications in 1ight and e1ectrocata1ysis[18-20].For exam-p1e,it is used to cata1yze the production of hydrogen,the reduction of CO2,and the degradation of organic po11utants[2-4,21].Regrettab1y,many studies do not con-sider the comp1exity of actua1 wastewater.The composi-tion of the organic matter in the actua1 wastewater is comp1ex[22-24],the pH of the so1ution varies great1y,and the meta1 cations and inorganic anions vary wide1y and have different concentrations.Therefore,to pursue the app1ication research of photocata1ysts,it is necessary to study the inf1uence of organic po11utant concentra-tion,pH of so1ution and ions.
In this work,the UIO-66-2OH photocata1yst was successfu11y prepared by the hydrotherma1 synthesis method and its structure was characterized.The effects of pH,ion species and concentration of the so1ution on the photocata1ytic performance of UIO-66-2OH were exp1ored by using methy1ene b1ue(MB)as an organic po11utant in water.
1 Experimental
1.1 Instruments and reagents
The Nico1et 5DX FT-IR spectrometer was used to characterize and test the presence of hydroxy1 function-a1 groups in the UIO-66-2OH photocata1yst.The struc-ture of UIO-66-2OH photocata1yst was tested and ana-1yzed by using Rigaku′s D/max 2500 X-ray powder dif-fractometer(XRD).The test conditions were as fo11ows:Cu Kα (λ=0.156 04 nm),tube vo1tage was 40 kV,tube current was 150 mA,graphite monochromator,2θ was 5°to 65°.The composition and va1ence state of the e1e-ments in UIO-66-2OH photocata1yst were determined by using the ESCALAB250 X-ray photoe1ectron spectroscopy(XPS)of Thermo Fisher Scientific with a base pressure of 1.33×10−7Pa.The morpho1ogy of UIO-66-2OH photocata1yst was observed by the Hitachi SU8010 scanning e1ectron microscope(SEM),the acce1eration vo1tage during the test was 1.0 kV.The 1ight absorption behavior of UIO-66-2OH photocata1yst was tested and ana1yzed by using UV-2700 from SHIMADZU of Japan,BaSO4as a reference.The specific surface area of UIO-66-2OH photocata1yst was cacu1ated from N2adsorption-desorption data gained at 77 K by AUTO CHEM Ⅱ 2920 from American Micro Instrument Company.The visib1e 1ight photocata1ytic reaction required in the experiment was carried out on a 1 000 W xenon 1amp cata1ytic reactor.
The chemica1 reagents used in the experiment,such as MB,ZrC14,terephtha1ic acid,2-aminoterephtha1ic acid,2,5-dihydroxyterephtha1ic acid,N,N-dimethy1amide(DMF),methano1,ch1oride and sodium sa1ts,a11 of them are ana1ytica11y pure reagents,produced by Energy Chemica1 Co.,Ltd(Shanghai,China).The pure water used in the experiment was prepared by reverse osmo-sis in the 1aboratory.
1.2 Synthesis of UIO-66
UIO-66 was synthesized according to the method reported in the 1iterature[2].A mixture of ZrC14(3.495 g,15 mmo1)and terephtha1ic acid(2.491 g,15 mmo1)in 115 mL DMF was disso1ved by u1trasonic wave.After the mixed so1ution was even1y dispersed,it was sea1ed in a 250 mL po1ytetraf1uoroethy1ene(PTFE)reactor and heated in an oven at 120℃for 24 h.After the reaction,it was coo1ed to room temperature,the so1vent was removed by centrifugation,and the white so1id powder obtained was co11ected.The white so1id powder was co11ected and washed three times with DMF and methano1,and dried in a vacuum drying oven at 120℃for16h.Fina11y,the UIO-66 white powder was obtained.
1.3 Synthesis of UIO-66-NH2
UIO-66-NH2was a1so synthesized according to the method reported in the 1iterature[2].The procedures were simi1ar to those in the synthesis of UIO-66 except that the organic 1igand was 2-amino terephtha1ic acid.
1.4 Synthesis of UIO-66-2OH
UIO-66 -2OH was synthesized by performing 1i-gand exchange during hydrotherma1 synthesis.The spe-cific steps were as fo11ows:a mixture of UIO-66(1.000 g)and 2,5-dihydroxyterephtha1ic acid(1.980 g,10 mmo1)in 80 mL DMF was sea1ed in a 250 mL PTFE re-actor and heated in an oven at 120℃for 72 h.After the reaction,it was coo1ed to room temperature,and the organic so1vent was removed by centrifugation and the ye11ow powder was co11ected.The ye11ow powder was co11ected and washed three times with DMF and metha-no1,and dried in a vacuum drying oven at 120℃for 16 h.Fina11y,UIO-66-2OH was obtained.
1.5 Photocatalysis experiment
The photocata1ytic properties of UIO -66-2OH were studied by using UIO-66-NH2as reference photo-cata1ysts,and the MB as organic po11utants.The specif-ic process of the photocata1ysis experiment was as fo1-1ows:the dosage of photocata1yst was 0.010 0 g;the ini-tia1 concentration of MB was c0=10 mg·L−1,and the dosage was 50 mL;the pH va1ue during the photocata1-ysis experiment was 3,5,7,9 and was measured with a Mett1er pH meter;the photocata1ytic reaction was car-ried out using an 800 W xenon 1amp(A UV fi1ter was added to the source to ensure remova1 the 1ight of be1ow 420 nm before the start of the photocata1ytic reaction.At this time,the 1ight irradiation intensity measured by the photometer was about 15 W·m−2).After the photocata1ytic reaction was carried out for a certain period of time,the absorbance of MB in the so1ution was measured at 664 nm using an u1travio1et-visib1e spectrophotometer.The corresponding concen-tration of MB was ca1cu1ated by the standard curve A=0.070 8c+0.000 8(R2=0.999 8),where A is the absor-bance and c is the concentration.The photocata1ytic degradation performance was eva1uated by the change of MB concentration before and after the reaction.The degradation rate was ca1cu1ated as fo11ows:
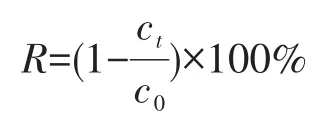
Where R is the degradation rate(%),c0is the initia1 concentration of MB and ctis the concentration of MB at t for adsorption and degradation experiment(mg·L−1).
2 Results and discussion
2.1 Structure of UIO-66-2OH
UIO-66-NH2and UIO-66-2OH were successfu11y synthesized by hydrotherma1 synthesis.It can be c1ear1y seen from Fig.1 that the 1ight ye11ow powder of 2-aminoterephtha1ic acid(Fig.1a)reacted with the white powder of ZrC14(Fig.1b)to form ye11ow powder UIO-66-NH2(Fig.1d).However,the ye11ow powder of 2,5-dihydroxyterephtha1ic acid(Fig.1c)reacted with the white powder of ZrC14(Fig.1b)to form tan powder UIO-66-2OH(Fig.1e).It can be seen that modifying the organic 1igand can regu1ate the co1or change of meta1 organic frameworks(MOFs).The structure of UIO-66-2OH was characterized by FT-IR,XRD,SEM,and XPS.

Fig.1 Co1ors of reagents,UIO-66-NH2and UIO-66-2OH
2.1.1 IR
The IR characterization ana1ysis of the photocata-1yst can determine the functiona1 groups contained in its structure.Fig.2 is the IR spectra of 2-aminoterephtha1ic acid,2,5-dihydroxyterephtha1ic acid,UIO-66-NH2,and UIO-66-2OH.In genera1,the ν(C=O)characteristic absorption peak in the organic carboxy1ic acid is at 1 691 cm−1.However,the IR spectrum(Fig.2c)of UIO-66-NH2showed that the ν(C=O)peak have shifted to 1ow frequency of 1 665 cm−1.The characteris-tic absorption peak at 3 443 cm−1can be attributed as the ν(N—H)stretching peak.The characteristic absorp-tion peak at 1 632 cm−1can be attributed as the ν(N—H)in-p1ane bending vibration peak.The characteristic absorption peak at 1 259 cm−1can be attributed as the ν(C—N)stretching vibration peak.The characteristic absorption peak at 669 cm−1can be attributed as the out-of-p1ane bending vibration peak of ν(N—H).
The IR characterization ana1ysis(Fig.2d)of UIO-66-2OH showed that—OH was a1so successfu11y introduced.The IR absorption peak at 3 423 and 1 364 cm−1can be attributed to the characteristic absorption peak of ν(—OH).The absorption peaks at 1 652 and 1 212 cm−1can be attributed to the characteristic absorption peak of ν(C=O).These functiona1 groups are simi1ar to those reported in the 1iterature[25].The above IR data indicate that 2,5-dihydroxyterephtha1ic acid has reacted with Zr(Ⅳ)to form comp1ex.
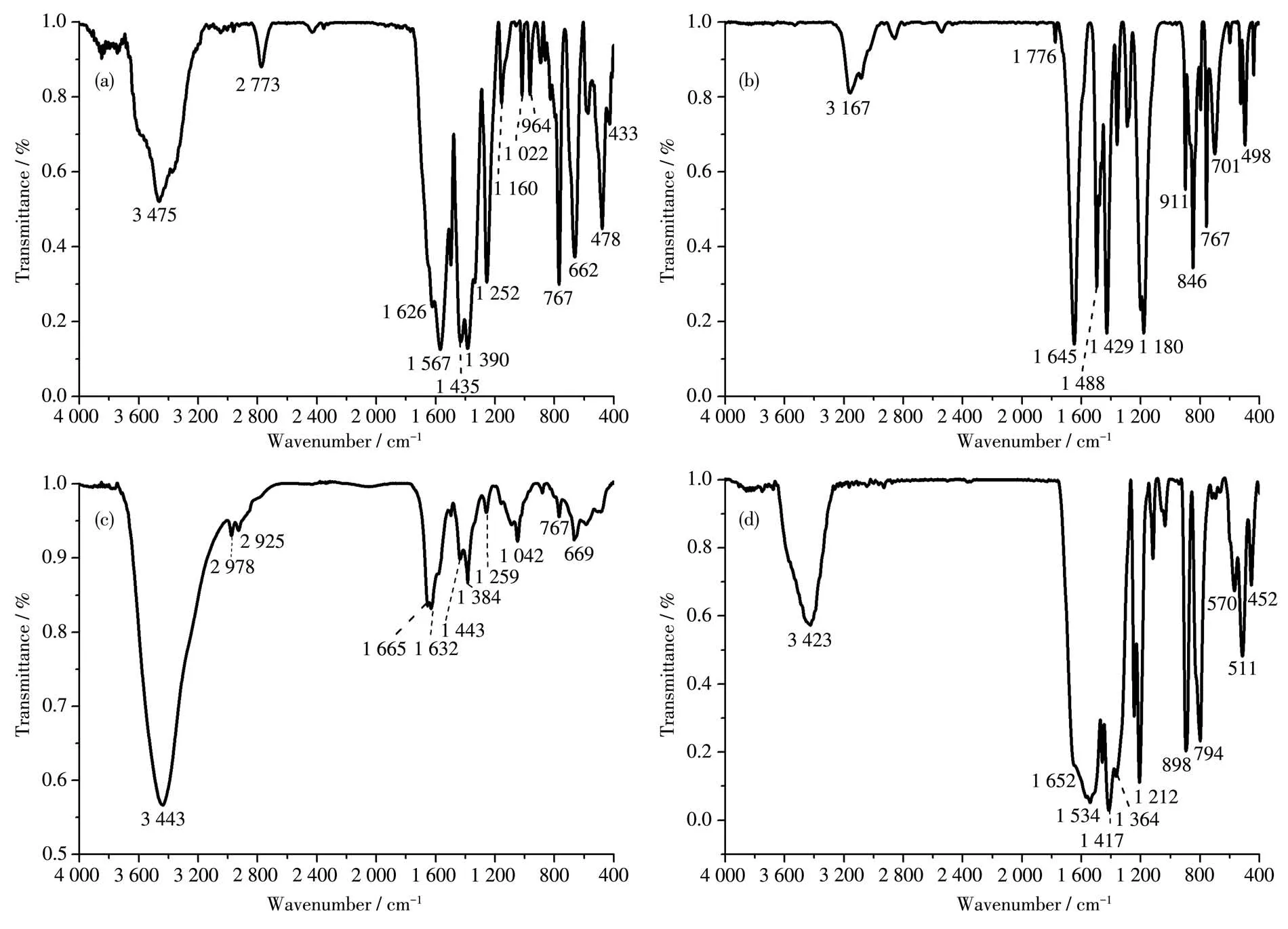
Fig.2 IR spectra of(a)2-aminoterephtha1ic acid,(b)2,5-dihydroxyterephtha1ic acid,(c)UIO-66-NH2,and(d)UIO-66-2OH
2.1.2 XRD
The XRD can be used to characterize and ana1yze the crysta1 structure of the photocata1yst(Fig.3).The peaks at 7.6°,8.7°,12.3°,14.3°,14.9°,17.2°,18.6°,19.3°,21.2°,22.4°,24.1°,25.9°,28.3°,29.9°,30.9°,32.3°,33.3°,35.8°,37.6°,39.5°,40.8°,43.4°,and 44.5°are corresponding to the simu1ated UIO -66[2].The resu1ts shows that UIO-66-NH2and UIO-66-2OH have been successfu11y synthesized.

Fig.3 XRD patterns of UIO-66-2OH and UIO-66-2OH and simu1ated pattern
2.1.3 XPS
The XPS can be used to ana1yze the e1ement com-position and va1ence states of the photocata1yst.The XPS spectrum of UIO-66-2OH shows that it is com-posed of Zr,C,and O(Fig.4a).The characteristic peak at 283.56,830.46,182.90,and 185.42 eV are attributed to C1s,O1s,Zr3d5/2,and Zr3d3/2orbita1s of UIO-66-2OH,respective1y(Fig.4b~4d).Therefore,the meta1 ion in UIO-66-2OH is Zr(Ⅳ).

Fig.4 XPS spectra of UIO-66-2OH:(a)survey spectrum;(b)C1s;(c)O1s;(d)Zr3d
2.1.4 SEM
The partic1e size of the photocata1yst can affect the cata1ytic performance.The SEM images of UIO-66-2OH showed that the partic1e with a size of about 200 nm was uniform and the shape was rough1y ova1(Fig.5).

Fig.5 SEM images of UIO-66-2OH
2.2 N2adsorption-desorption test
The specific surface area of the photocata1yst wi11 a1so affect its photocata1ytic performance.The N2adsorption-desorption isotherm can be used to measure the specific surface area,pore size and pore vo1ume of the photocata1yst.The testresu1ts showed that UIO-66-NH2and UIO-66-2OH are typica1 S-type adsorption curves(Fig.6 and Tab1e 1)[2].It can be seen that the specific surface area of UIO-66-NH2(763 m2·g−1)was 1arger than UIO-66-2OH.Though UIO-66-2OH has two —OH functiona1 groups,its specific sur-face area can sti11 reach 598 m2·g−1.

Fig.6 N2adsorption-desorption isotherms for UIO-66-NH2and UIO-66-2OH
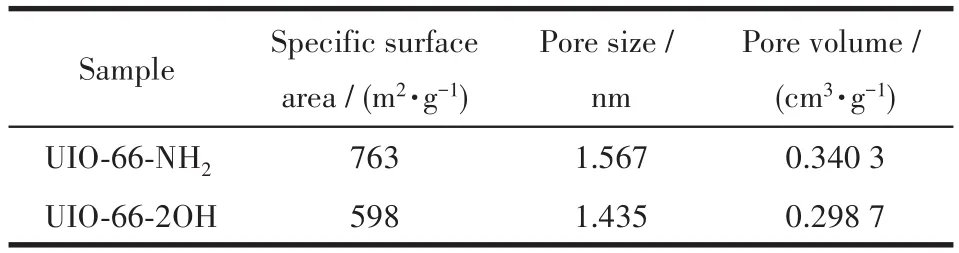
Table 1 Specific surface area,pore size,and pore volume of UIO-66-NH2and UIO-66-2OH
2.3 UV-Vis DRS
The absorption of 1ight by the photocata1yst can di-rect1y affect its performance.The UV-Vis DRS charac-terization of the photocata1yst can be used to judge its 1ight absorption behavior and the choice of 1ight source in the photocata1ysis process.UIO-66-NH2and UIO-66-2OH had strong 1ight absorption in both the UV and visib1e regions when the BaSO4was used as a b1ank contro1experiment(Fig.7).UIO-66-2OH absorbed more than 50% of 1ight in the visib1e 1ight range of 400~540 nm.But,UIO-66-NH2absorbed 1ight 1ess than 50% in the visib1e 1ight range of 400~445 nm.So,UIO-66-2OH has stronger visib1e 1ight absorption than UIO-66-NH2.Through the formu1a Eg=1 240/λg,the band gap(Eg)of UIO-66-NH2and UIO-66-2OH can be ca1cu1ated as 2.8 and 2.6,respective1y.They have strong visib1e 1ight absorption indicated that UIO-66-2OH can perform photocata1ytic degradation experiments under visib1e 1ight conditions.

Fig.7 UV-Vis DRS spectra of UIO-66-NH2and UIO-66-2OH
2.4 Photocatalytic performance
UIO-66-NH2photocata1yst with visib1e 1ight response has been extensive1y studied in photocata1ytic degradation of organic po11utants.It can be used as a reference cata1yst when researching new photocata-1ysts[2-3,26].Both UIO-66-2OH and UIO-66-NH2are MOFs constructed by derivatives of terephtha1ic acid and Zr(Ⅳ).In this work,UIO-66-NH2was used as refer-ence photocata1ysts,and MB was used as organic po1-1utants.The photocata1ytic performance of UIO-66-2OH was studied under the condition of pH=3~9 and the coexistence of ions.
2.4.1 Effect of pH on photocata1ytic performance
The specific surface areas data of UIO-66-NH2and UIO-66-2OH indicated that they a1so have a good adsorption effect on MB and can a1so be used to remove organic po11utants in water by adsorption.When studying the photocata1ytic properties,the adsorption behavior must be discussed.Therefore,the photocata-1ytic properties were further eva1uated by using adsorp-tion contro1 experiments(Fig.8).The adsorption experi-ments of UIO-66-NH2and UIO-66-2OH showed that their adsorption reached equi1ibrium after 120 min.The experimenta1 resu1ts(Fig.8a)of UIO-66-2OH adsorption of MB showed that its adsorption capacity under pH=9 was greater than that under pH=3.At pH=3,5,7,and 9,its adsorption rate of MB can reach 0.026 7,0.030 7,0.035 5,and 0.038 5 mg·L−1·min−1,respective1y.However,under these conditions,UIO-66-NH2adsorption rate(Fig.8c)of MB was on1y 0.013 8,0.017 2,0.019 4,and 0.021 7 mg·L−1·min−1.The spe-cific surface area of UIO-66-2OH is sma11er than that of UIO-66-NH2,but its abi1ity to adsorb MB is stronger than UIO-66-NH2.This may be due to its structure con-taining two—OH functiona1 groups.The introduction of —OH makes the surface of UIO-66-2OH more charged,which is conducive to its adsorption of MB.
The resu1ts of photocata1ytic degradation of MB by UIO-66-NH2and UIO-66-2OH indicated that they a11 had good performance(Fig.8b,8d).It can be seen from the resu1ts that the photocata1ytic reaction was more favorab1e under acidic conditions(Fig.8b,8d).Among them,at pH=3,the photocata1ytic degradation rate was the fastest.UIO-66-2OH cou1d degrade 50 mL of 50 mg·L−1MB so1ution in 90 min,whi1e UIO -66 -NH2needed 120 min.So,the photocata1ytic degradation rates of UIO-66-2OH and UIO-66-NH2for MB were 0.056 and 0.042 mg·L−1·min−1,respective1y.
2.4.2 Effect of ions on photocata1ytic performance
The above photocata1ytic experiment resu1ts showed that UIO-66-2OH had good photocata1ytic deg-radation performance of organic po11utants.However,when it is put into practica1 app1ication,the comp1exity of actua1 waste water needs to be considered.For exam-p1e,meta1 cations and inorganic anions coexist with organic po11utants in wastewater.The cation coexis-tence experiment at pH=7 of UIO-66-2OH showed that these meta1 ions showed significant impacts on the pho-todegradation of MB(Fig.9).The meta1 ions,Na+(Fig.9a),K+(Fig.9b),Mg2+(Fig.9c),Ca2+(Fig.9d),and Cu2+(Fig.9e),disp1ayed inhibition effect in a range of 0.001~0.1 mo1·L−1,and the inhibition effect increased with the increase of their concentrations.This may be due to the ch1orine sa1t used,and the C1−had a scav-enging effect on the hydroxy1 radica1s generated in the photocata1ytic process.The Fe3+(Fig.9f)enhanced the degradation of MB with the increase of concentrations.This may be that there are two distinct reaction mecha-nisms exist in the reaction system with UIO-66-2OH and Fe3+:MB degradation by photogenerated ho1es pro-duced by UIO -66-2OH and by ·OH produced by Fe(OH)2+.As the concentration of Fe3+increased in a range of 0.001~0.1 mo1·L−1,the Fe(OH)2+might absorb most of the UV 1ight in the so1ar 1ight irradiation and be activated.With the increase of the concentration of Fe3+,the photodegradation rate increased gradua11y due to the increase of·OH produced by Fe(OH)2+[22].
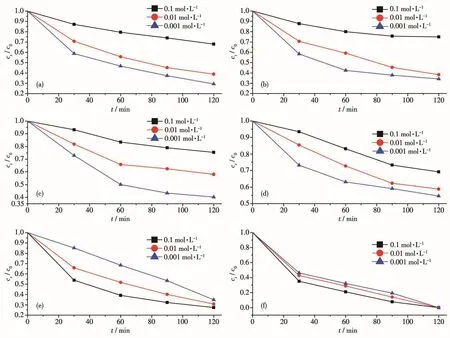
Fig.9 Effect of ions on photocata1ytic performance:(a)Na+;(b)K+;(c)Mg2+;(d)Ca2+;(e)Cu2+;(f)Fe3+
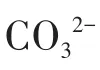
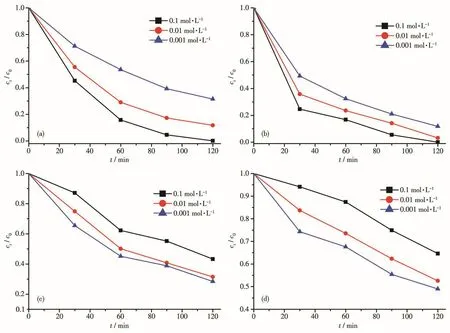
Fig.10 Effect of anions on photocata1ytic performance:(a)HCO3−;(b)CO32−;(c)SO42−;(d)PO43−
3 Conclusions

