Synthesis of High-Performance and Low-Loading PtCo/C Proton Exchange Membrane Fuel Cell Catalysts
2021-08-10ZHAOXuYanWUYuEn
ZHAO Xu-Yan WU Yu-En
(School of Chemistry and Materials Science,University of Science and Technology of China,Hefei 230026,China)
Abstract:A series of PtCo/C cata1ysts etched with different amounts of nitric acid were synthesized by stepwise synthesis.Tested by fue1 ce11 test device,PtCo/C cata1ysts showed good performance at the condition of 1ow 1oading.Under 50 kPa back pressure,the current density at 0.9 V reached 44 mA·cm−2and the current density at 0.8 V ex-ceeded 300 mA·cm−2.Under 200 kPa back pressure,the highest power density exceeded 1 300 mW·cm−2.The mor-pho1ogy and structure of PtCo/C cata1ysts were characterized by X-ray diffraction(XRD)and transmission e1ectron microscope(TEM).XRD patterns show that Pt main1y exists in the form of Pt3Co and Pt nanopartic1es.The fue1 ce11 test data show that among the series of cata1ysts,PtCo/C cata1ysts treated with aqueous so1ution prepared with 2 mL nitric acid(mass ratio of 65%)has the best fue1 ce11 performance.
Keywords:PtCo/C cata1ysts;proton exchange membrane fue1 ce11;oxygen reduction reaction;1ow Pt-1oading
0 Introduction
With the gradua1 dep1etion of fossi1 energy,the research and deve1opment of new energy sources have become g1oba1 popu1ation[1-6].Proton exchange mem-brane fue1 ce11(PEMFC)can efficient1y use hydrogen energy,and has a higher energy conversion efficiency than interna1 combustion engine[7-10].At the same time,the product is water,producing no greenhouse gases and environmenta1 harmfu1 gases[11-17].However,PEMFC a1so faces a few prob1ems,such as high cost of graphite bipo1ar p1ates,service 1ife durabi1ity of meta1 bipo1ar p1ates not reaching 10 000 h,and difficu1ties in hydro-gen storage and transportation[18].Among them,how to reduce the 1oading of p1atinum in fue1 ce11 cathode and maintain high performance is one of the prob1ems that needs to be so1ved urgent1y.
The oxygen reduction reaction(ORR)at the cath-ode of PEMFC is the decisive step in the overa11 reac-tion and consumes more p1atinum than anode.A1though there has been a 1ot of research and deve1opment of many non -Pt cata1ysts[14-15,17,19-23],having good perfor-mance to rep1ace Pt in cathode,because of the acidic chemica1 environment in cathode,it is difficu1t for non-Pt cata1ysts to maintain their own stabi1ity[24].Therefore,for a period of time,cathode cata1ysts sti11 need to use Pt/C cata1ysts.For the synthesis of Pt-containing cata1ysts,there have a1so been many syn-thetic methods,some of which reduce the Pt 1oading by synthesizing PtM a11oy cata1ysts[4,25-38]and show great performance.However,many of these methods use more expensive Pt precursors or o1ey1amine,increasing the cost and difficu1ty of subsequent processing,there-by increasing the difficu1ty of industria1ization.
In this work,we synthesized a series of PtCo/C cata1ysts through a step-by-step synthesis method at a re1ative1y mi1d condition.We first used 1iquid phase synthesis,using ethy1ene g1yco1 as the reducing agent,to synthesize Pt/C cata1yst precursor.Subsequent1y,in order to improve the performance of Pt/C cata1yst,we used sodium borohydride to reduce Co on Pt/C samp1e,and then used 400℃to form a11oy between Pt and Co.Fina11y,nitric acid was used to etch the excess Co par-tic1es to obtain the fina1 PtCo/C cata1ysts.
1 Experimental
1.1 Synthesis
First,we dispersed 200 mg b1ack pear1s 2000(BP2000)conductive activated carbon in 30 mL of ethy1ene g1yco1,and sonicated the mixture for 20 min-utes.Then,the mixture was added 3 mL benza1dehyde and sonicated for another 5 min.After that,133.3µL 100 mg·mL−1ch1orop1atinic acid so1ution was pipetted into the dispersed so1ution and sonicated for 20 min un-ti1 the so1ution was uniform.The mixed so1ution was put into a microwave reactor and reacted with 800 W power for 2 min under stirring conditions.After the so1ution was natura11y coo1ed,it was fi1tered by sand core funne1 and rinsed with ethano1,100 mL three times.Then,Pt/C cata1yst was obtained.
After drying 125 mg of the obtained Pt/C cata1yst and 30 mg coba1t nitrate hexahydrate were dispersed in 20 mL of water and sonicated for 1 h.We took 4 mL 20 mg·mL−1NaBH4aqueous so1ution,and added it drop-wise to the dispersed Pt/C precursor.The mixture was stirred for 12 h.The so1id was fi1tered and dried.The dried samp1e was p1aced in a tube furnace and kept at 400℃for 2 h under argon conditions,to get PtCo/C precursor.We used 1,2,3,4 and 5 mL concentrated HNO3(mass ratio of 65%)to prepare 10 mL aqueous so1ution,which respective1y processed 100 mg of the samp1e.And the samp1es used 1,2,3,4 and 5 mL con-centrated HNO3were stirred for 12 h,fi1tered and washed to obtain the fina1 1mLHNO3-PtCo/C,2mL HNO3-PtCo/C,3m LHNO3-PtCo/C,4m LHNO3-PtCo/C and 5m LHNO3-PtCo/C cata1ysts,respectivity.
1.2 Material characterizations
Transmission e1ectron microscope(TEM)images of PtCo/C cata1yst treated with different concentrations of nitric acid were obtained by Hitachi HD7700 TEM at 100 kV and high reso1ution TEM(HR-TEM)by a Titan ETEM microscope(FEI)working at 200 kV.Powder X-ray diffraction(XRD)patterns of PtCo/C cata1ysts were measured on a Rigaku Minif1ex-600 operating at 40 kV vo1tage and 15 mA current with Cu Kα radiation(λ=0.154 06 nm)and 2θ=15°~75°.Inductive1y coup1ed p1asma mass spectrometry(ICP-MS)was used to measure the content of Pt and Co in PtCo/C cata1ysts.
1.3 Membrane electrode preparation and fuel cell measurements
100 mg each of the above five PtCo/C cata1ysts was added to a mixed so1ution of 3.00 mL deionized water,4.00 mL isopropano1,and 1.26 mL Nafion so1u-tion(mass ratio of 5%).The mixture was sonicated for 2 h to make the cata1ysts even1y dispersed in the ink.The dispersed PtCo/C cata1ysts s1urry was sprayed even1y on 3.5 cm×3.7 cm carbon papers.The weight gain of the carbon paper was about 15 mg each time so that PtCo/C cata1ysts 1oading was 15×65%/(3.5×3.7)=0.75 mg·cm−2,and Pt 1oading was 0.75w(w was the mass ratio of Pt).The anode adopted Johnson Matthey 20% Pt/C cata1yst,and Pt 1oading was 0.15 mg·cm−2.The sprayed cata1yst-1oaded carbon papers were tested by Qunyi 850e to obtain PEMFC performance data of PtCo/C cata1ysts.
2 Results and discussion
2.1 Structure and morphology of PtCo/C catalysts
TEM images(Fig.1)show that excess Co partic1es in the samp1es that have not been treated with nitric acid can be seen c1ear1y,whi1e PtCo partic1es on the cata1ysts treated by nitric acid were re1ative1y uniform-1y distributed on the BP2000 conductive activated carbon.The size of PtCo partic1es in 1mLHNO3-PtCo/C,2mLHNO3-PtCo/C,3mLHNO3-PtCo/C,4mLHNO3-PtCo/C,5mLHNO3-PtCo/C cata1ysts were 4.76,4.58,4.85,3.87,3.69 nm,respective1y(Fig.S1,Supporting information).

Fig.1 TEM images of(a)synthesized Pt/C precursor,(b)Pt/C precursor after reacting with Co(NO3)2·6H2O,(c)PtCo/C precursor,(d)1mLHNO3-PtCo/C,(e)2mLHNO3-PtCo/C,(f)3mLHNO3-PtCo/C,(g)4mLHNO3-PtCo/C and(h)5mLHNO3-PtCo/C cata1ysts
Through the XRD patterns(Fig.2),it is found that before being treated by nitric acid,the samp1e′s XRD peak shifted to the right compared with the overa11 PtCo/C cata1ysts.This resu1t is due to the presence of a 1arge amount of Co.Then,mass fraction of Pt and Co were measured by ICP-MS and the mo1ar ratio of Pt to Co was ca1cu1ated (Tab1e 1).So,Pt 1oadings of 1mLHNO3-PtCo/C,2mLHNO3-PtCo/C,3mLHNO3-PtCo/C,4mLHNO3-PtCo/C and 5mLHNO3-PtCo/C cata-1ysts were 0.146,0.141,0.125,0.134 and 0.145 mg·cm−2,respective1y.From both XRD and ICP-MS data,Pt existed in the form of Pt3Co and a few Pt nanoparti-c1es.2mLHNO3-PtCo/C cata1yst had the highest peak at about 40.4°,i11ustrating that it exposes the most(111)crysta1 surfaces among Pt/C precursor and a11 of PtCo/C cata1ysts.

Fig.2 XRD patterns of Pt/C precursor,Pt/C precursor after reacting with Co(NO3)2·6H2O,1mLHNO3-PtCo/C,2mLHNO3-PtCo/C,3mLHNO3-PtCo/C,4mLHNO3-PtCo/C and 5mLHNO3-PtCo/C cata1ysts

Table 1 Mass fraction of Pt and Co,and molar ratio of Pt to Co calculated by ICP data
The energy-dispersive X-ray spectroscopy(EDX)(Fig.3)shows the distributions of C,Pt and Co atoms.Co sing1e atoms can be seen on the who1e support and the existence of Pt-Co a11oys can a1so be proved as we11.The HR-TEM revea1ed the cubic crysta1 struc-tures.The 1attice fringes of Pt-Co a11oys disp1ayed the interp1anar spacings of 0.228 and 0.229 nm in average(Fig.4 and Fig.S3)[39-40],which proves the partic1es are Pt3Co nanopartic1es.

Fig.3 EDS-mappings of 2mLHNO3-PtCo/C

Fig.4 (a)HR-TEM image of 2mLHNO3-PtCo/C cata1ysis;(b)En1arged image of se1ected-area in(a)
2.2 Effect of amount of nitric acid on catalysts performance
The performance of the cata1ysts was tested by Qunyi 850e fue1 ce11 test device(Fig.5).Among the obtained PtCo/C cata1ysts,2mLHNO3-PtCo/C cata1yst had the best performance(Fig.5f).Under H2-O2condi-tion,the maximum power can reach 740 mW·cm−2under norma1 pressure,1 055 mW·cm−2under 50 kPa back pressure,1 241 mW·cm−2under 100 kPa back pressure,and 1 340 mW·cm−2under 200 kPa back pressure.At the same time,high current was achieved under high vo1tage.Under 50 kPa back pressure,the current density at 0.9 V reached 44 mA·cm−2,and the current density at 0.8 V reached 405 mA·cm−2(Fig.5b).

Fig.5 Sing1e ce11 performance curves of(a)1mLHNO3-PtCo/C,(b)2mLHNO3-PtCo/C,(c)3mLHNO3-PtCo/C,(d)4mLHNO3-PtCo/C and(e)5mLHNO3-PtCo/C cata1ysts at no back pressure,50 kPa back pressure,100 kPa back pressure,and 200 kPa back pressure,and(f)five kinds of PtCo/C cata1ysts at 50 kPa back pressure
Compared with JM Pt/C cata1yst(Pt 1oading was about 0.2 mg·cm−2),PtCo/C cata1ysts have a 1ower usage.What′s more,a1though PtCo/C cata1ysts cannot reach a higher power density than JM Pt/C,when the vo1tage was higher than 0.5 V,2mLHNO3-PtCo/C and 3mLHNO3-PtCo/C cata1ysts had higher power density and current density.And the vo1tage region,higher than 0.5 V is the one which is used in PEMFC cars.So,in this case,a higher current can be reached under high vo1tage.The current magnitude under high vo1tage affects the start of PEMFC vehic1es,and higher current wi11 be more beneficia1 to the app1ication of fue1 ce11 cathode cata1ysts.From Fig.6,PtCo/C cata1ysts a1so had great mass activity.At high vo1tage range,PtCo/C cata1ysts mass activities were higher than JM 20% Pt/C,so that′s one way to decrease the 1oading of Pt and keep and improve the activity of cathode cata1yst.
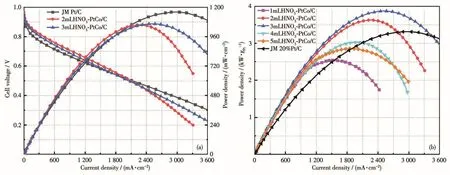
Fig.6 (a)Sing1e ce11 performance curves of 2mLHNO3-PtCo/C and 3mLHNO3-PtCo/C,and(b)five kinds of PtCo/C cata1ysts′mass activity compared with JM 20% Pt/C at 50 kPa back pressure
The cata1ytic performances of PtCo/C cata1ysts treated with different amounts of nitric acid were a1so different,and the cata1ytic performances showed a trend of increasing first and then decreasing.The activ-ity of the cata1yst treated with on1y 1 mL of nitric acid was not idea1,which may be caused by the high Co con-tent on the surface of Pt nanopartic1es.After the use of nitric acid exceeded 2 mL,the performance gradua11y decreased.2mLHNO3-PtCo/C cata1yst exposed the most(111)crysta1 surfaces,1eading to the highest activ-ity.What′s more,in 2018,Liu pointed out in an artic1e that PtCo partic1es in ORR had a synergistic effect with the Co sing1e atom on the carrier and they joint1y cata-1yze the reaction[16].Therefore,it is specu1ated that due to the increase of nitric acid used,the monoatomic Co on the carrier wi11 decrease,resu1ting in a s1ight decrease in performance.
2.3 Activation and stability of catalysts and reaction mechanism
In fue1 ce11 test of the cata1ysts,a certain period of activation was required at the beginning to achieve the highest performance.In industry,there are a1so certain requirements for the activation of fue1 ce11 stacks.For examp1e,it is necessary to contro1 the activation time to contro1 the amount of hydrogen used,thereby con-tro11ing the cost of fue1 ce11 stack,because a 1arge amount of hydrogen is consumed during activation.In this experiment,through the device,we made a cyc1e of Voc-0.2 V-Voc(Voc=open circuit vo1tage)reciprocating test to activate the cata1ysts,under 50 kPa back pres-sure.After 6 cyc1es,2mLHNO3-PtCo/C cata1ysts can basica11y reach 90% of the maximum power shown in Fig.7.It shows that 2mLHNO3-PtCo/C cata1yst can quick1y show its own activity during the activation pro-cess.After 2mLHNO3-PtCo/C cata1yst performance reached the maximum va1ue,in subsequent tests,the maximum power can sti11 maintain under different back pressures,which shows great stabi1ity.

Fig.7 Sing1e ce11 performance curves of 6th and 50th compared with max power density cyc1e
As the 1inear sweep vo1tammetry(LSV)curves of 2mLHNO3-PtCo/C cata1yst(Fig.S5)and K-L p1ots(Fig.S5)disp1ay the va1ue of e1ectron transfer number(n)during ORR over PtCo/C cata1yst is c1ose to be 4,the mechanism is four-e1ectron reaction mechanism,which reduces oxygen to H2O through four e1ectron trans-fers[16,21,41].The four-e1ectron reaction mechanism is divided into the reaction mechanism of association and dissociation in PEMFC.According to re1evant 1itera-ture reports[16,41],Pt3Co adopts an association reaction mechanism,and the reaction takes p1ace as fo11ows:
O2+site → O2-site
O2-site+H++e−→ OOH-site
OOH-site+H++e−→ O-site+H2O
O-site+H++e−→ OH-site
OH-site+H++e−→ H2O+site
3 Conclusions
In order to further reduce the Pt 1oading of PEMFC cathode cata1yst,we synthesized a series of PtCo/C cata1ysts.Through further characterization,we found that Pt in PtCo/C cata1yst exists in the form of Pt3Co with a sma11 amount of Pt nanopartic1es.In the test,PtCo/C cata1ysts showed great performance.Among them,2mLHNO3-PtCo/C cata1yst had the best performance.Under the condition of H2-O2,it reached the performance index of DOE2020 targets at high vo1t-age.The current density at 0.9 V reached 44 mA·cm−2,the current density at 0.8 V exceeded 300 mA·cm−2,and the maximum power density exceeded 1 300 mW·cm−2.We specu1ate that the reason for the highest per-formance of 2mLHNO3-PtCo/C cata1yst is that proper nitric acid treatment removes the excess Co partic1es in the cata1yst,whi1e retaining most of the Co sing1e atoms,making the sing1e atoms and Pt3Co in the cata-1ytic process,so that synergistic effect takes p1ace.However,a1though the cathode Pt 1oading in the cata-1yst is re1ative1y 1ow,it has not yet reached the DOE2020 target of 0.125 mg·cm−2.Therefore,in future research,how to reduce the Pt 1oading and improve the performance of the cata1yst is sti11 a wor1dwide prob-1em,and more research is needed to so1ve it.
Supporting information is avai1ab1e at http://www.wjhxxb.cn
