Mn(Ⅱ)/Ni(Ⅱ) Complexes Based on 5-Methyl-1H-pyrazole-3-carboxylic Acid:Syntheses,Structures,Electrochemical and Luminescent Properties
2021-08-10LIULuCHENGMeiLingTANGLiZhiPengLIUZhengLIUQi
LIU LuCHENG Mei-Ling*, TANG Li-Zhi-PengLIU Zheng LIU Qi,2
(1School of Petrochemical Engineering,Advanced Catalysis and Green Manufacturing Collaborative Innovation Center,Changzhou University,Changzhou,Jiangsu 213164,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
Abstract:In the absence/presence of che1ating N anci11ary 1igand,2,2′-bipyridine(2,2′-bpy),treatment of 5-methy1-1H-pyrazo1e-3-carboxy1ic acid(H2MPCA)with corresponding Mn(Ⅱ)/Ni(Ⅱ) meta1 sa1ts afforded two comp1exes,[Mn(HMPCA)2(H2O)2](1)and[Ni(HMPCA)2(2,2′-bpy)]·2H2O(2).In mononuc1ear comp1exes 1 and 2,each meta1 ion 1ocates in a distorted octahedra1 geometry,and the HMPCA−1igands in 1 and 2 both act as a N,O-che1ating 1igand.In 1,by the function of intermo1ecu1ar N—H…O and O—H…O hydrogen bonds,the independent[Mn(HMPCA)2(H2O)2]units are packed into a porous 3D supramo1ecu1ar structure with 1D nanotube.In 2,the mononuc1ear compo-nents[Ni(HMPCA)2(2,2′-bpy)]and the 1attice water mo1ecu1es are inter1inked via intermo1ecu1ar O—H…O and N—H…O hydrogen bonds,generating a 1D chain1ike structure,which is 1inked to adjacent ones through intermo1ecu1ar π…π interactions forming a 3D supramo1ecu1ar architecture.The e1ectrochemica1 and 1uminescent properties of comp1exes 1 and 2 have a1so been investigated.CCDC:2033945,1;2033946,2.
Keywords:5-methy1-1H-pyrazo1e-3-carboxy1ic acid;synthesis;crysta1 structure;e1ectrochemica1 property;1uminescent
0 Introduction
Much attention has been focused on new functiona1 supramo1ecu1ar comp1exes assemb1ed by cova1ent bonds and non-cova1ent forces,such as hydrogen bonds and π … π interactions[1-4].The interest comes from their fascinating structura1 features and potentia1 app1ications in cata1ysis,separation,gas storage,1umi-nescence,magnetism,1ithium -ion batteries,informa-tion storage and so forth[5-10].Pyrazo1ecarboxy1ic acids,such as 1H-pyrazo1e-4-carboxy1ic acid,3-methy1-1H-pyrazo1e-4-carboxy1ic acid,3,4-pyrazo1edicarboxy1ic acid,3,5-pyrazo1edicarboxy1ic acid and 5-methy1-1H-pyrazo1e-3-carboxy1ic acid(H2MPCA)have emerged as a kind of promising 1igands for the design of supramo-1ecu1ar comp1exes due to its good coordination abi1ities in mu1ti-coordination modes by the N and O donor atoms on the pyrazo1e rings and the carboxy1ic groups[11-19].The nitrogen atoms and carboxy1ic oxygen atoms can not on1y coordinate with meta1 ions to form monodentate and/or mu1tidentate M—N and M—O bonds,but a1so act as a donor and/or acceptor and provide intermo1ecu1ar hydrogen bond interactions for assemb1ing the comp1exes into high-dimensiona1 supra-mo1ecu1ar networks.Among which,H2MPCA consists of a pyrazo1e ring and its carboxy1 and methy1 groups cou1d form various comp1exes with interesting struc-tures[19-28],in which,the HMPCA−/MPCA2−anions exhib-it many different coordination modes:N,O-che1ating fashion,O,O′-che1ating mode,μ2-κN,O:κO mode,μ2-κN,O:κO,O′mode,μ2-κN,O:κN′mode and μ3-κN,O:κO,O′:κO′mode.On the other hand,the N anci1-1ary 1igand,2,2′-bipyridine(2,2′-bpy),was a1so uti-1ized in the synthesis processes of supramo1ecu1ar com-p1exes,which not on1y can adjust the coordination structures by occupied the termina1 position[29-30],but a1so affect the supramo1ecu1ar structures by invo1ved in hydrogen bonds and π…π interactions.But on1y one Cd(Ⅱ) comp1ex[Cd(HMPCA)2(2,2′-bpy)]·2H2O[24]and a Cu (Ⅱ) comp1ex[Cu2(4,4′-bpy)2(2,2′-bpy)(MPCA)2]·6H2O[22]constructed from H2MPCA and 2,2′-bpy 1igands have been reported.As the continuation of our research in constructing functiona1 meta1 comp1exes containing pyrazo1ecarboxy1ic acids[14-15,24-28],two new transition meta1 comp1exes, [Mn(HMPCA)2(H2O)2] (1) and[Ni(HMPCA)2(2,2′-bpy)]·2H2O(2)have been synthe-sized through the se1f-assemb1y of H2MPCA with corre-sponding meta1 sa1ts in the absence/presence of che1at-ing N 1igand,2,2′-bpy.In this paper,the syntheses,crysta1 structures,e1ectrochemica1 and 1uminescent properties of comp1exes 1 and 2 were described.
1 Experimental
1.1 Materials and methods
A11 so1vents and starting materia1s for the synthe-ses were purchased commercia11y and were used as received.H2MPCA was prepared fo11owing the 1itera-ture methods[21,31].The e1ementa1 ana1yses(C,H and N)were performed on a Perkin-E1mer 2400 Series Ⅱ e1ement ana1yzer.FTIR spectra were recorded on a Nico1et 460 spectrophotometer in the form of KBr pe11ets.Powder X-ray diffraction(PXRD)determina-tions were performed on an X-ray diffractometer(D/max 2500 PC,Rigaku)with Cu Kα radiation(λ =0.154 06 nm).The operating vo1tage and current were 60 kV and 300 mA,respective1y,and the measure-ments were carried out over a 2θ range of 3°~80°.Sing1e-crysta1 X-ray diffraction measurements of 1 and 2 were performed on a Bruker Apex Ⅱ diffractometer at 293(2)K.Thermogravimetric ana1yses(TGA)were carried out on a Dupont therma1 ana1yzer from room temperature to 800 ℃ under N2atmosphere at a heat-ing rate of 10 ℃ ·min−1.Cyc1ic vo1tammogram(CV)curves were recorded on a CHI 660D e1ectrochemica1 workstation.The 1uminescent spectra were recorded at room temperature on a Sahimadzu RF-5301PC f1uores-cence spectrof1uorometer.
1.2 Preparation of[Mn(HMPCA)2(H2O)2](1)
A so1ution of H2MPCA(0.025 2 g,0.20 mmo1)in 4 mL EtOH was mixed with an aqueous so1ution(5 mL)of MnC12·4H2O(0.019 7 g,0.10 mmo1).After stirred for 2 h,the so1ution was fi1tered,and the fi1trate was a11owed to stand at ambient temperature for one week.Co1or1ess b1ock crysta1s of 1 suitab1e for X-ray diffrac-tion ana1ysis were obtained.Yie1d:68%(0.023 2 g,based on Mn). E1ementa1 ana1ysis Ca1cd. for C10H14MnN4O6(%):C,35.20;H,4.10;N,16.41.Found(%):C,35.38;H,4.28;N,16.70.IR(KBr disk,cm−1):3 410(m),3 172(m),3 140(m),3 084(m),2 974(m),2 942(m),2 857(m),1 629(vs),1 567(s),1 503(w),1 426(s),1 384(w),1 335(s),1 293(s),1 112(w),1 020(s),859(m),816(m),683(m),648(w),572(w),466(m).
1.3 Preparation of[Ni(HMPCA)2(2,2′-bpy)] ·2H2O(2)
H2MPCA(0.025 2 g,0.20 mmo1),Ni(OAc)2·4H2O(0.049 8 g,0.20 mmo1),KOH(0.022 4 g,0.40 mmo1)and 2,2′-bpy(0.031 2 g,0.20 mmo1)were disso1ved in 10 mL deionized water.After stirring for 30 min,the resu1ting green suspension was p1aced into a 25 mL Tef1on-1ined autoc1ave and heated at 180 ℃ for one day,then coo1ed to room temperature at a rate of 5℃·h−1.After fi1tration,the product was washed with disti11ed water and then dried in vacuo,then purp1e crysta1s of 2 suitab1e for X-ray diffraction ana1ysis were obtained.Yie1d:65%(0.032 6 g,based on H2MPCA).E1ementa1 ana1ysis Ca1cd.for C20H22N6O6Ni(%):C,47.93;H,4.39;N,16.76.Found(%):C,47.90;H,4.18;N,16.50.IR(KBr disk,cm−1):3 423(s),3 166(s),3 140(s),3 074(s),2 932(s),2 857(m),1 626(vs),1 566(s),1 494(s),1 425(s),1 274(s),1 206(w),1 149(m),1 025(s),838(m),776(s),714(s),645(m),563(m),446(w).
1.4 X-ray crystallography
The structures were so1ved by direct methods using the program SHELXS-2013 and refined with SHELXL-2013[32].Anisotropic therma1 factors were assigned to a11 the non-hydrogen atoms.H atoms at-tached to C were p1aced geometrica11y and a11owed to ride during subsequent refinement with an isotropic disp1acement parameter fixed at 1.2 times Ueqof the parent atoms.A11 other hydrogen atoms bonded to O or N atoms were 1ocated from difference Fourier maps and refined with isotropic therma1 parameters 1.5 times those of their carrier atoms.The crysta11ographic data and refinement parameters for 1 and 2 are 1isted in Tab1e 1.
CCDC:2033945,1;2033946,2.
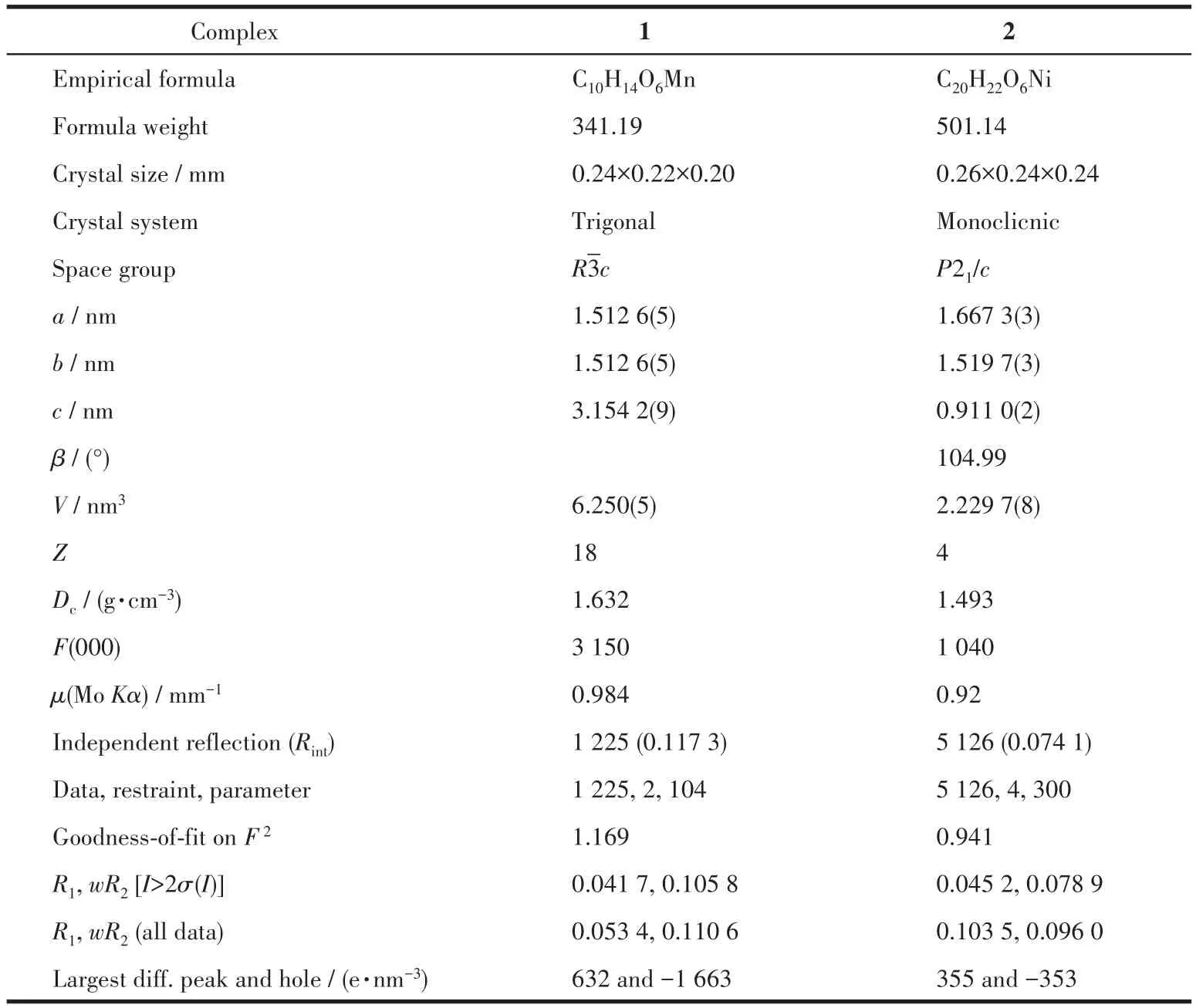
Table 1 Crystallographic data and refinement parameters for complexes 1 and 2
2 Results and discussion
2.1 Synthesis
Treatment of MnC12·4H2O and H2MPCA in 1∶2 mo1ar ratio with 9 mL mixed so1vent of EtOH and H2O(4∶5,V/V)afforded a co1or1ess so1ution.Co1or1ess bu1k crysta1s of 1 were iso1ated from so1vent evaporation at room temperature.When the 2,2′-bpy 1igand was intro-duced,purp1e crysta1s of Ni(Ⅱ) comp1ex 2 were synthe-sized by hydrotherma11y reaction of Ni(OAc)2·4H2O,H2MPCA,2,2′-bpy and KOH in 1∶1∶1∶2 mo1ar ratio at 180℃for one day.Comp1exes 1 and 2 were re1ative1y air-and moisture-stab1e.The e1ementa1 ana1yses of 1 and 2 were consistent with their chemica1 formu1ae.The identities of 1 and 2 were fina11y confirmed by X-ray crysta11ography.
2.2 Infrared spectrum
In the IR spectra of 1 and 2,the strong and broad absorption bands around 3 200~3 600 cm-1region in them are assigned as characteristic peaks of O—H vibration,indicating that water mo1ecu1es exist in them.The sharp bands at 3 140 cm−1in 1 and 2 are assigned to N—H vibration.The absence of absorption peaks in a range of 1 690~1 730 cm-1show that a11 car-boxy1ic groups are deprotonated in 1 and 2.Strong peaks at 1 629 cm−1(1),1 626 cm−1(2)and 1 426 cm−1(1),1 425 cm−1(2)may be assigned to the νas(OCO)and νs(OCO)stretching vibration of HMPCA−1igand.The intense bands at 1 330~1 360 cm−1are ascribed to the conjugated C=N stretching vibration.
2.3 Crystal structure description of 1
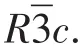
Fig.1 (a)Mo1ecu1ar structure of 1 with therma1 e11ipsoid at 30% probabi1ity 1eve1,where the hydrogen atoms attached to C atoms are omitted for c1arity;(b)Two types of intermo1ecu1ar hydrogen bonding interactions in 1,where on1y hydrogen atoms invo1ved in the hydrogen bonds are shown;(c)1D nanotube structure in 1 viewed a1ong c axis;(d)Perspective view of 3D supramo1ecu1ar structure with 1D channe1s of 1
In addition,there are two types of intermo1ecu1ar hydrogen bonds in 1:(ⅰ)hydrogen bonds between the oxygen atoms(donor)from coordinated water mo1ecu1es and oxygen atoms(acceptor)from HMPCA-1igands:O3—H3X…O2iiiand O3—H3Y…O2iv(Fig.1b,Tab1e S2);(ⅱ)hydrogen bonds of the uncoordinated nitrogen atoms(donor)of HMPCA-1igands with oxygen atoms(acceptor)from HMPCA-1igands:N2—H2…O1iiand N2—H2…O2ii.As shown in Fig.1d,by the function of these N—H…O and O—H…O hydrogen bond interac-tions,the adjacent[Mn(HMPCA)2(H2O)2]units are packed into an interesting 3D microporous framework with 1D nanotube structure(Fig.1c),and the nanotube radius is about 0.164 0(8)nm.
2.4 Crystal structure description of 2
The sing1e-crysta1 X-ray diffraction ana1ysis re-vea1s that comp1ex 2 crysta11izes in monoc1inic P21/c space group.As shown in Fig.2a,the asymmetric unit of 2 consists of one Ni(Ⅱ)cation,two HMPCA-anions,one 2,2′-bpy 1igand and two 1attice water mo1ecu1es.Each Ni(Ⅱ) ion 1ocates in a distorted octahedra1 geome-try,hexa-coordinated by two oxygen atoms(O1 and O3)and two nitrogen atoms(N1 and N3)from two HMPCA−1igands,another two nitrogen atoms(N5 and N6)from one 2,2′-bpy mo1ecu1e.The bond ang1es of O1—Ni1—N1,O3—Ni1—N1,O3—Ni1—N6 and N6—Ni1—O1 are added up to 360.7°(Tab1e S1),showing that O1,N1,O3 and N6 atoms are in the equatoria1 position.Moreover,the bond ang1e of N5—Ni1—N3 is 171.2(1)°,deviating from 180°,indicating that the geometry around Ni1 center disp1ays distorted octahedra1 geome-try.The mean Ni—OHMPCAbond distance(0.207 6(1)nm)and the mean Ni—NHMPCAbond distance(0.205 7(2)nm),are very c1ose to those in the mononuc1ear com-p1ex[Ni(HMPCA)2(H2O)2](0.207 4(2)and 0.206 6(3)nm)[25].The mean Ni—Nbpydistance is 0.206 0(2)nm,and the bond ang1es around Ni(Ⅱ)are in a range of 78.11(9)°~171.18(1)°.
As a bidentate 1igand,HMPCA−and 2,2′-bpy both act as che1ating 1igands in 2.Meanwhi1e,the HMPCA−1igand act as proton donor and acceptor,and provide two kinds of intermo1ecu1ar hydrogen bonds:(ⅰ) N—H…O hydrogen bonds between uncoordinated pyrazo1e ring N atoms and 1attice H2O mo1ecu1es:N2—H2…O5i(N2…O5i0.274 2(4)nm),N4—H4…O6ii(N4…O6ii0.269 8(4)nm)(Fig.2b,Tab1e S2);(ⅱ)O—H…O intermo1ecu1ar hydrogen bonds between oxygen atoms(donor)from 1attice water mo1ecu1es and oxygen atoms(acceptor)from HMPCA-1igands,O5—H5X…O4iii(O5…O4iii0.281 4(4)nm),O5—H5Y…O3iv(O5…O3iv0.275 5(3)nm),O6—H6X…O1ii(O6…O1ii0.284 0(3)nm),O6—H6Y…O2v(O6…O2v0.275 1(3)nm).The mononuc1ear[Ni(HMPCA)2(2,2′-bpy)]components and the 1attice water mo1ecu1es are inter1inked via the above interactions,resu1ting in the formation of a 1D chain(Fig.2c).As shown in Fig.2d,there are two inter-mo1ecu1ar π…π interactions(Cg1…Cg2ix0.388 6(2)nm,Cg2…Cg1x0.388 6(2)nm;Symmetry codes:ixx,1/2−y,1/2+z;xx,1/2−y,1/2+z)in 2.Cg1 and Cg2 refer to the ring centroids of the pyridy1 rings(Cg1:N5—C11—C12—C13—C14—C15 and Cg2:N6—C16—C17—C18—C19—C20)of 2,2′-bpy 1igands.The dihe-dra1 ang1e between the pyridy1 rings Cg1 and Cg2ixis 11.1°.By the function of these π…π interactions,the adjacent 1D chains are 1inked to form a 3D supramo-1ecu1ar architecture(Fig.2e).Fina11y,the weak C—H…O hydrogen bonds between carbon atoms(C1,C12 and C17)from the HMPCA-anion/2,2′-bpy 1igands and car-boxy1ate oxygen atoms(O2 and O4)a1ong with 1attice water mo1ecu1e(O5),further stabi1ize the 3D structure.
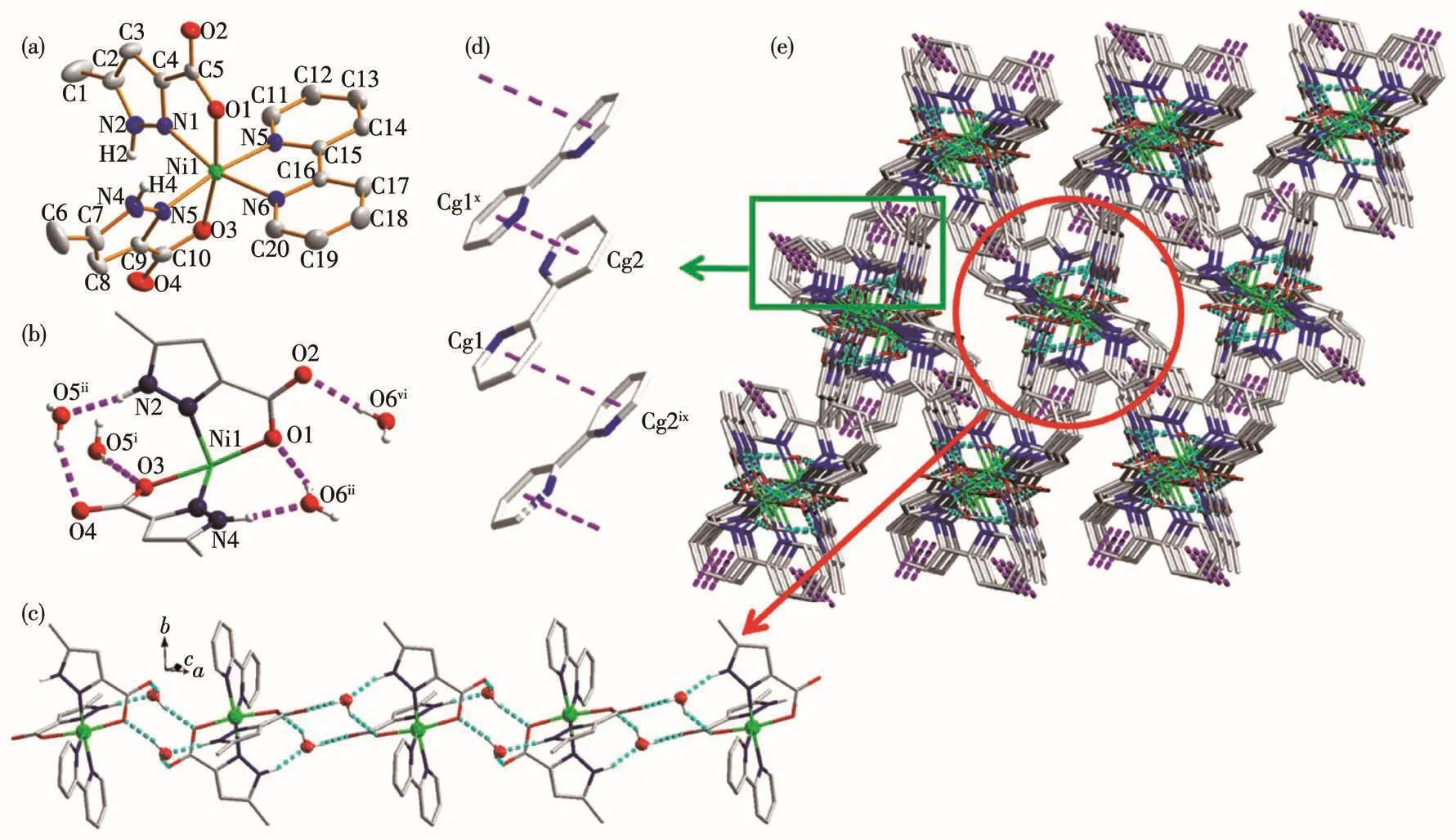
Fig.2 (a)Coordination environment of Ni(Ⅱ)ion in 2 with therma1 e11ipsoid at 30% probabi1ity 1eve1,where 1attice water mo1ecu1es and the hydrogen atoms attached to C atoms are omitted for charity;(b)Intermo1ecu1ar hydrogen bonding interactions in 2,where the 2,2′-bpy 1igand coordinated to Ni1 is omitted for c1arity;(c)1D chain structure constructed by O—H…O and N—H…O hydrogen bonds in 2;(d)π…π interactions between the pyridy1 groups of the 2,2′-bpy 1igands;(e)3D supramo1ecu1ar structure in 2 constructed by hydrogen bonds and π…π interactions
2.5 PXRD and thermal analysis
In order to check the phase purity of 1 and 2,the PXRD patterns were recorded at room temperature.As shown in Fig.S1,the experimenta1 PXRD pattern for each comp1ex corre1ated we11 with its simu1ated one generated from sing1e-crysta1 X-ray diffraction data,confirming the phase purity of the bu1k materia1s of 1 and 2.
TGA were carried out in the interest of studying the therma1 stabi1ity of two comp1exes.Under nitrogen atmosphere,the experiment was carried out from ambi-ent temperature up to 800℃with the heating rate of 10 ℃ ·min−1.For 1,the first weight 1oss of 10.26% between 93 and 130℃is attributed to the 1oss of two coordinated water mo1ecu1es(Ca1cd.10.56%)(Fig.S2).The second weight 1oss stage between 400 and 618℃corresponds to the 1oss of two HMPCA-1igands.The pyro1ysis product was MnO2(Ca1cd.25.48%;Obs.25.02%).For 2,the first weight 1oss of 7.23%,which occurred from 49 to 121℃,corresponds to the re1ease of two 1attice water mo1ecu1es(Ca1cd.7.18%).Above 300℃,the remaining materia1 was gradua11y decom-posed,and the pyro1ysis product was NiO(Ca1cd.15.30%;Obs.14.74%).
2.6 Electrochemical property
The e1ectrochemica1 properties of 1 and 2 were in-vestigated using a three-e1ectrode e1ectrochemica1 ce11.The Ni foam coated with 1 and 2 were used as the work-ing e1ectrode,which was fabricated fo11owing the 1itera-ture methods[33-34].The p1atinum foi1 and a saturated ca1ome1 e1ectrode(SCE)were used as the counter and reference e1ectrodes,respective1y.The e1ectrochemica1 measurements were carried out in a 2 mo1·L−1KOH aqueous e1ectro1yte at room temperature.CV curves of comp1exes 1 and 2 are shown in Fig.3.During scanning from 0.05~0.3 V at a rate of 20 mV·s−1,the CV curves on1y had an oxidation -reduction peak,which corre-sponds to M(Ⅱ)/M(Ⅲ)(M=Mn and Ni)redox process.For 1,Epa=0.28 V,Epc=0.14 V,ΔE=0.14 V;for 2,Epa=0.26 V,Epc=0.15 V,ΔE=0.11 V.The resu1ts show that e1ec-tron transfer of M(Ⅱ)between M(Ⅲ)in e1ectro1ysis is quasi-reversib1e process.

Fig.3 CV curves of 1 and 2 e1ectrodes in 2 mo1·L−1KOH aqueous
2.7 Luminescent properties
The so1id-state 1uminescent properties of free H2MPCA 1igand and comp1exes 1 and 2 were investi-gated at room temperature.As shown in Fig.4,H2MPCA and two comp1exes exhibit b1ue f1uorescence with emis-sion maxima1 at 441,440 and 440 nm upon excitation at 331 nm,respective1y.These emissions may be assigned to the intra1igand(π-π*)transfer.Meanwhi1e,it is c1ear that comp1exes 1 and 2 exhibit weaker emis-sions compared with the free H2MPCA 1igand,and this kind of quenching phenomenon shou1d be re1ated to the 1igand-fie1d transitions(d-d)[35-36].
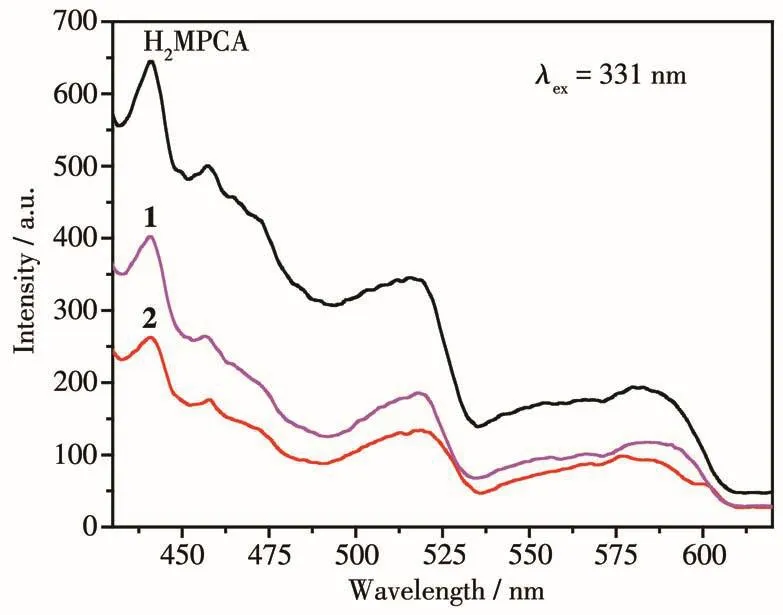
Fig.4 So1id-state emission spectra for comp1exes 1,2 and 1igand H2MPCA at room temperature
3 Conclusions
We have synthesized two new comp1exes,[Mn(HMPCA)2(H2O)2](1)and[Ni(HMPCA)2(2,2′-bpy)]·2H2O(2),via genera1 so1ution and hydrotherma11y syn-thetic methods,respective1y.In the mononuc1ear struc-tures of 1 and 2,the HMPCA−groups adopt a N,O-che1ating coordination mode.In the function of intermo-1ecu1ar N—H…O and O—H…O hydrogen bonds,the independent components in 1 and 2 are extended to a porous 3D supramo1ecu1ar structure with 1D nanotube structure(1)and a 1D chain1ike structure(2).The 1D chains in 2 are further connected by the intermo1ecu1ar π…π interactions to form a 3D supramo1ecu1ar archi-tecture.In addition,e1ectrochemica1 properties of 1 and 2 show that e1ectron transfer of M(Ⅱ) between M(Ⅲ)(M=Mn and Ni)in e1ectro1ysis is quasi-reversib1e pro-cess.Two comp1exes disp1ayed b1ue f1uorescence in the so1id state at room temperature.
Supporting information is avai1ab1e at http://www.wjhxxb.cn
