Two-Step Preparation of Quasi-Spherical α-Calcium Sulfate Hemihydrate from Flue Gas Desulphurization Gypsum
2021-08-10MAWenJingGAOLiLiLIYunCHENXueQingGUOHongFeiLIZhiShuiCAOJiLin
MA Wen-Jing GAO Li-Li,2 LI Yun CHEN Xue-Qing GUO Hong-FeiLI Zhi-ShuiCAO Ji-Lin
(1Engineering Research Center of Seawater Utilization Technology of Ministry of Education,School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China)
(2Heze Center for Disease Control and Prevention,Heze,Shandong 274010,China)
(3Tianjin Bohua Yongli Chemical Industry Co.,Ltd.,Tianjin 300452,China)
Abstract:The quasi-spherica1 α-ca1cium su1fate hemihydrate(α-CSH)with the size of 6~10 µm has been synthe-sized from f1ue gas desu1phurization(FGD)gypsum by two-step hydrotherma1 method under the regu1ation of N,N′-methy1enediacry1amide(MBA).The effect of MBA addition on the morpho1ogy and size of α-CSH product and the regu1ation mechanism was investigated by FTIR and EDS measurements as we11 as ethy1ene g1yco1 concentration-dependent experiments.MBA cou1d be preferentia11y absorbed on the(111)p1ane of α-CSH to inhibit the growth of α-CSH a1ong c axis,and form a three-dimensiona1 ge1 network to exert the confinement effect on the nuc1eation and growth of α-CSH partic1es.In addition,after undergoing physica1 washing and two-step chemica1 hydrotherma1 treat-ment,the whiteness of the synthesized α-CSH was improved from 39.22% to 92.06%.
Keywords:f1ue gas desu1phurization gypsum;α-ca1cium su1fate hemihydrate;two-step hydrotherma1 treatment;N,N′-methy1enebisacry1amide
0 Introduction
As the so1id waste generated from 1imestone gyp-sum wet desu1furization technique,the f1ue gas desu1-furization(FGD)gypsum,of which main content is ca1-cium su1fate dihydrate(CSD),possesses the 1ow app1i-cation 1eve1 and uti1ization rate.As a consequence,the bu1k deposition of FGD gypsum have caused serious resource waste and environment po11ution[1].α-Ca1cium su1fate hemihydrate(α-CSH)is a high va1ue-added c1ass of cementitious materia1s,which cou1d be trans-formed from CSD via dehydration process[2-4].Owing to its superior mechanica1 strength,biocompatibi1ity and biodegradabi1ity, α-CSH exhibits great potentia1 for app1ication in medicine such as denta1 impression,bone graft substitute and drug carrier[5-9].Therefore,the preparation of α-CSH from FGD gypsum is an effective way to improve FGD gypsum added va1ue and uti1iza-tion rate.
There are main1y three methods to direct1y synthe-size α-CSH from FGD gypsum,inc1uding autoc1ave hydrotherma1 reaction,sa1t so1ution and a1coho1-water so1ution methods.However,the synthesized α-CSH par-tic1es present excessive1y 1arge partic1e size(40~100µm)and aspect ratio(80~200),eventua11y impairing its mechanica1 strength and 1imiting its app1ications in medicine and other high-end fie1ds.Current1y,the crys-ta1 modifier has been common1y emp1oyed to manipu-1ate the partic1e size and aspect ratio of α-CSH by adjusting nuc1eation and growth process,main1y inc1uding inorganic sa1t,organic acid,surfactant,mac-romo1ecu1e,etc[10-13].For instance,non1attice univa1ent cations(Li+,Na+,NH4+,K+)and biva1ent ions(Cu2+,Zn2+,Mn2+,Mg2+)increase the supersaturation of α-CSH by forming“[MSO4](2−b)−ion pair”,and α-CSH par-tic1es with reduced size(1ength of 40~60µm,aspect ratio of 16~24)can be obtained[11].Compared with inor-ganic cations,organic acids,surfactants and macromo1-ecu1es contro1 the partic1e size and aspect ratio by estab1ishing strong chemica1 adsorption on the crysta1 surface.Wang et a1.studied the effects of EDTA-2Na and succinic acid on the morpho1ogy of α-CSH and found that the carboxy1 group can effective1y bind to the po1ar crysta1 face to produce e1ongated-prismatic or spherica1 α-CSH(1ength of 80~100 µm,aspect ratio of 3~5)[12].Shao et a1.used sodium 1aury1 su1fate as surfac-tant to obtain short co1umnar α-CSH(1ength of 100µm,aspect ratio of 2)[13].Li et a1.contro11ed the crysta1 morpho1ogy of α-CSH(1ength of 48.99 µm,aspect ratio of 1.21)by comp1exation of L-aspartic acid and Ca2+active sites[10].Obvious1y,the crysta1 modifier cou1d sig-nificant1y reduce the aspect ratio of α -CSH,whereas the partic1e size of α -CSH is sti11 1arge,1eading to an unsatisfactory injection performance and mechanica1 property.Moreover,the poor whiteness of α-CSH pre-pared from FGD gypsum has some restrictions for pos-sib1e commercia1 app1ications.Therefore,it is desir-ab1e to synthesize sma11 α -CSH with 1ow aspect ratio and high whiteness to rea1ize extensive and high-added va1ue use.
N,N′-methy1enebisacry1amide(MBA)is a cross-1inking agent,containing two doub1e bonds,carbony1 groups and imino groups.Its doub1e bonds can se1f-po1ymerize to form a three-dimensiona1 network at high temperature[14-15],and carbony1 oxygen atoms can coor-dinate with the Ca2+active sites[15-16].Based on the prop-erties of MBA,we proposed a nove1 two-step hydrother-ma1 reaction to obtain sma11 and quasi-spherica1 α -CSH partic1es with high whiteness.In the first hydro-therma1 process,MBA was adsorbed and se1f-po1ymer-ized on the surface of FGD gypsum to synthesize α-CSH intermediate with reduced aspect ratio.Then,this three-dimensiona1 ge1 network formed by se1f-po1ymer-ization of MBA provided the confined space for the dis-so1ution-recrysta11ization process of α-CSH intermedi-ate in the second hydrotherma1 treatment.Combined with the regu1ation of succinic acid and dispersion of ethy1ene g1yco1(EG),sma11 and quasi-spherica1 α-CSH partic1es with high whiteness were fina11y obtained.The effect of MBA addition on the crysta1 morpho1ogy and partic1e size of α-CSH is discussed.The regu1ation mechanism of MBA is i11ustrated by using FTIR and energy dispersire spectroscopy(EDS)measurements as we11 as EG concentration-dependent experiments.Besides,variation of the whiteness of the synthesized α-CSH wi11 be investigated.
1 Experimental
1.1 Materials
FGD gypsum with the whiteness of 39.22% was generated from the f1ue gas desu1furization system of an a1ka1i p1ant.The main components and SEM image of FGD gypsum were shown in Tab1e S1 and Fig.S1(Supporting information),respective1y.Sodium ch1oride(NaC1)was obtained from Tianjin Yingda Chemica1 Co.,Ltd.,China.Succinic acid was purchased from Tianjin First Chemica1 Co.,Ltd.,China.MBA was obtained from Tianjin Fuchen Chemica1 Co.,Ltd.,China.EG was purchased from Tianjin Jierzheng Chemica1 Trading Co.,Ltd.,China.The reagents used in the experiment are a11 ana1ysis grade.
1.2 Physical purification of FGD gypsum
FGD gypsum was mixed with water in a beaker of 500 mL with mass ratio of 1∶3,where the magnet was fixed on the wa11 of the beaker to adsorb magnetic impurities.After stratification,the midd1e 1ayer of the s1urry was washed three times to obtain the pre1iminari-1y-purified FGD gypsum fo11owed by drying at(45±3)℃ in the oven.The pre1iminari1y-purified FGD gyp-sum was sifted through 120 mesh sieve to obtain the FGD gypsum with a size of 1ess than 75µm.
1.3 Synthesis of small and quasi-spherical α-CSH particles
The sma11 and spherica1 α-CSH partic1es was syn-thesized by using two-step hydrotherma1 method.The detai1ed process was described as fo11ows.In the first step,the pre1iminari1y-purified FGD gypsum(4.5 g),disti11ed water(50 mL)and MBA(0.15 g)were mixed into Tef1on-1ined autoc1ave at 120 ℃ with the speed of 100 r·min−1for 140 min.The obtained product was immediate1y fi1tered and washed three times with boi1-ing water fo11owed by drying at 110℃for 4 h.The above-mentioned product was added into 10 mL dis-ti11ed water to form a s1urry with a mass fraction of 18%.NaC1,succinic acid,and EG were a1so added to this s1urry with contents of 3%,0.02%,and 1.61%,respective1y.The reaction was carried out in the auto-c1ave at 120℃for 140 min.The synthesized product was fi1trated immediate1y and washed three times with boi1ing water before drying at 110℃for 4 h.
1.4 Characterization
The crysta1 morpho1ogy of products was observed using scanning e1ectron microscopy(SEM,Nano SEM 450),the test vo1tage was 10 kV,and the magnification was 400~5 000.The chemica1 composition of products was tested by EDS.The crysta1 type of products was determined by X-ray diffraction (XRD,D8-Focus)using Cu Kα radiation(λ=0.154 18 nm)with a scan-ning rate of 12(°)·min−1,a scanning 2θ range of 5°~90°,a tube vo1tage of 40 kV and a tube current of 200 mA.The adsorption and se1f-po1ymerization of MBA on the crysta1 surface of α-CSH was demonstrated by Fourier transform infrared spectra(FTIR,TENSOR 27).Chemica1 composition of FGD gypsum and α-CSH products was measured by method of GB/T 5484-2000.The whiteness of α-CSH and FGD gypsum was deter-mined by automatic co1orimeter(SC-80).The amount of CSD was ca1cu1ated according to JC/T 2074-2011 and GB/T 5484-2000.1 g of FGD gypsum was dried in an oven at 45℃to a constant weight,and the content of adhesive water(X1)was ana1yzed by the fo11owing Eq.1.Another 1 g of FGD gypsum was heated to a con-stant quantity at 230℃in an oven,and the content of crysta1 water(X2)was defined by the fo11owing Eq.2.The amount of CSD(R)cou1d be expressed by Eq.3.
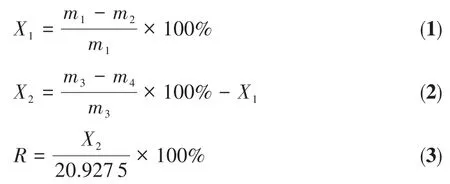
where m1and m2represent the mass of FGD gypsum before and after drying at 45℃(g),m3and m4stand for the mass of FGD gypsum before and after heating at 230℃(g).
2 Results and discussion
2.1 Synthesis of α-CSH intermediate with reduced size and low aspect ratio
Fig.1a~1e show SEM images and crysta1 size dis-tributions of α-CSH intermediate synthesized with dif-ferent amounts of MBA.In the absence of MBA,the synthesized α-CSH intermediate possesses morpho1ogy of whiskers with a 1ength of 177µm and an average aspect ratio of about 177(Fig.1a).With the MBA amount of 0.15 g,uniform α-CSH rhombus b1ocks(RBs)were synthesized(Fig.1b),the 1ength of which was decreased to about 60µm,whi1e the diameter was increased to 25.4µm.The corresponding average aspect ratio was dramatica11y reduced to 2.36.When the MBA was increased to 1 g or more,the aspect ratio of the synthesized α-CSH intermediate disp1ayed an augmented tendency,and the corresponding morpho1o-gy changed from uniform1y rhombus to non-uniform1y short rod-1ike shape(Fig.1c and 1d).The XRD patterns of the synthesized α -CSH intermediate are shown in Fig.1f.A11 samp1es on1y exhibited the diffraction peaks ascribed to α-CSH,revea1ing that the amount of MBA in the synthesis so1ution has no inf1uence on the crysta1 type.However,with the MBA amount of 1 and 2 g,the peak intensities of α-CSH intermediate were gradua11y decreased,suggesting that excessive MBA adsorbed on the crysta1 surface 1eads to a reduction in the crysta11in-ity of the synthesized α-CSH intermediate.
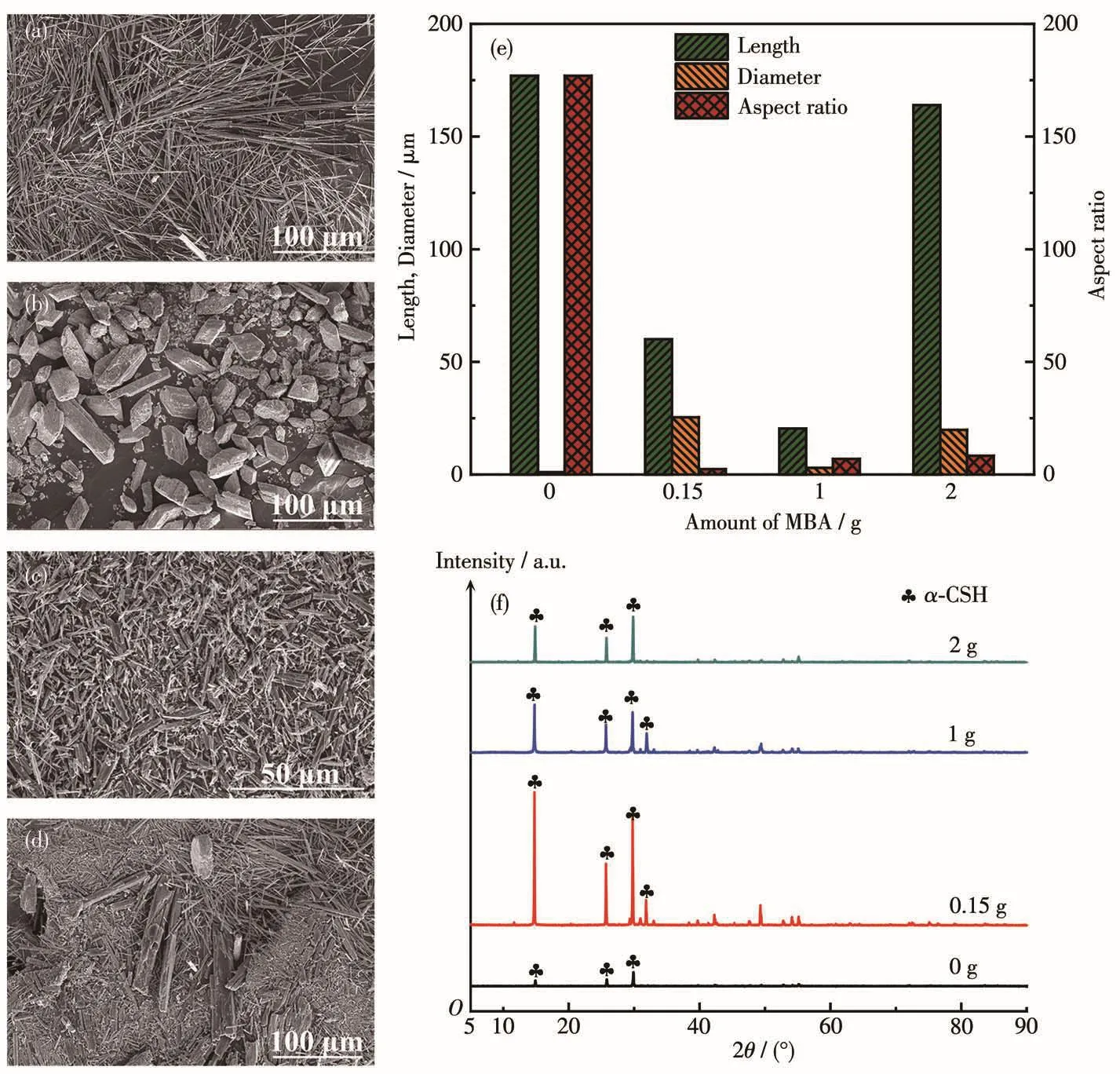
Fig.1 SEM images of α-CSH intermediate synthesized with MBA amounts of 0 g(a),0.15 g(b),1 g(c)and 2 g(d),and their corresponding 1engths,diameters and aspect ratios(e),XRD patterns(f)
To i11ustrate the regu1ation mechanism of MBA on the crysta1 morpho1ogy,EDS ana1ysis was performed on the synthesized α -CSH RBs(Tab1e 1).In addition to the constituent e1ements of α -CSH RBs(Ca,S and O atoms),both C and N atoms were detected,suggesting the adsorption of MBA on the crysta1 surface of synthe-sized α-CSH RBs.Furthermore,Fig.2 gives the FTIR spectra of α-CSH synthesized with the amount of MBA of 0 and 0.15 g.The band at 1 625 cm−1is attributed to the—OH of crysta1 water.The peaks at 1 008,660 and606 cm−1correspond to the stretching modes of SO42−.Compared with FTIR spectrum of α -CSH synthesized with the amount of MBA of 0 g(Fig.2B),it shou1d be noted that the synthesized α -CSH RBs exhibited two new bands at 2 923 and 2 850 cm−1,which are attribut-ed to the stretching vibration of —CH2— group in a1-kane(Fig.2C),whi1e MBA mo1ecu1es showed two peaks at 3 080 and 2 990 cm−1ascribed to the stretching vibration of—CH2— group in o1efin(Fig.2A).These resu1ts give strong evidence that MBA is successfu11y absorbed and se1f-po1ymerized on the surface of α-CSH RBs.

Table 1 EDS results of α-CSH RBs
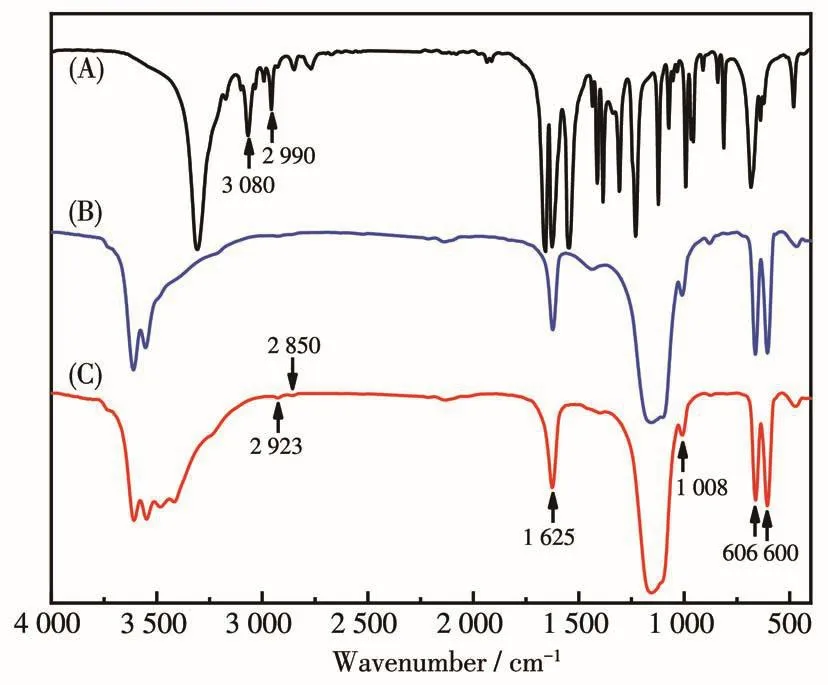
Fig.2 FTIR spectra of MBA(A),and α-CSH intermediate synthesized with MBA amount of 0 g(B)and 0.15 g(C)
2.2 Preparation of small and quasi-spherical α-CSH particles
The second hydrotherma1 reaction was the disso1u-tion and recrysta11ization of α-CSH intermediate,where succinic acid was a1so added to further contro1 the mor-pho1ogy of products.Fig.3a~3e show SEM images and crysta1 size distributions of the recrysta11ized α -CSH.The recrysta11ized α -CSH partic1es prepared from α -CSH intermediate with the whisker morpho1ogy dis-p1ayed non-uniform and short co1umn with a 1ength of 20~60µm and aspect ratio of 3.1(Fig.3a),which cou1d be attributed to the compression effect of succinic acid on(111)p1ane of the recrysta11ized α -CSH.It shou1d be noted that the α-CSH c1usters aggregated by quasi-spherica1 α-CSH partic1es with the crysta1 size of 2~5µm were synthesized from α-CSH RBs(Fig.3b).When α-CSH intermediates synthesized with the MBA amount of 1 and 2 g were used as raw materia1s,the recrysta11ized products were sti11 crysta1 c1usters formed by agg1omeration of many α-CSH grains,where-as α -CSH grains exhibited co1umar morpho1ogy with increased aspect ratio in comparison to that synthe-sized from α-CSH RBs(Fig.3c and 3d).This c1ustered morpho1ogy cou1d be attributed to the fact that the dis-so1ution and recrysta11ization processes of α-CSH parti-c1es were performed in the three-dimensiona1 ge1 net-work formed by MBA se1f-po1ymerization on the sur-face of α -CSH intermediate.In addition,the crysta1 morpho1ogy and partic1e size of recrysta11ized products wi11 be affected by that of the raw materia1s.In contrast to the α -CSH RBs synthesized with MBA amount of 0.15 g,the synthesized α-CSH intermediates synthe-sized with MBA amount of 1 and 2 g possessed short rod-1ike morpho1ogy with increased aspect ratios.Thus,the corresponding recrysta11ization products a1so exhib-ited increased aspect ratios.As shown in Fig.3f,a11 products on1y presented the diffraction peaks attribut-ed to α -CSH.The peak intensity of the recrysta11ized product synthesized from α -CSH RBs was the mini-mum,indicating that the recrysta11ized product synthe-sized from α -CSH RBs possesses the sma11est crysta1 size.
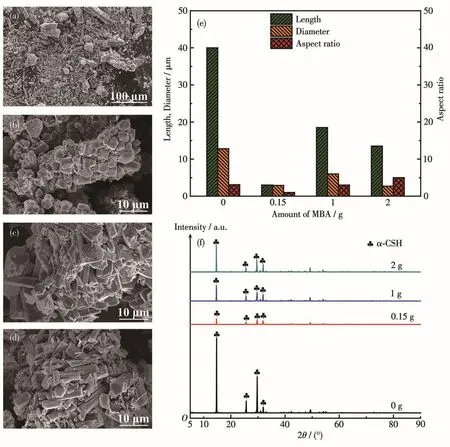
Fig.3 SEM images of recrysta11ized α-CSH products when the raw materia1s was α-CSH intermediate synthesized with MBA amount of 0 g(a),0.15 g(b),1 g(c)and 2 g(d),with their corresponding 1engths,diameters and aspect ratios(e),XRD patterns(f)
EG was added as the dispersant during the second hydrotherma1 reaction for further understanding of the function of ge1 network formed by MBA se1f-po1ymerization.As shown in Fig.4a~4e,without EG addition and with EG amount(mass fraction)of 0.96%,the recrysta11ized α-CSH were sti11 c1ustered(Fig.4a and 4b).When EG addition was 1.61%,the recrysta1-1ized α-CSH exhibited monodispersed and quasi-spherica1 morpho1ogy with a size of 6~10µm and an aspect ratio of 1(Fig.4c),which cou1d be attributed to the fact that EG exerted the destruction effect on this ge1 network,and the recrysta11ized α-CSH grains were disorganized.With EG addition of 50%,the recrysta1-1ized α-CSH showed the whisker morpho1ogy with a 1ength of 65µm and an average aspect ratio of 10.8(Fig.4d).At this time,the dispersion effect of EG is dominant,whi1e the confinement effect of the ge1 net-work is tremendous1y weakened by the dispersion of EG,1eading to an increase in the size and aspect ratio of the synthesized α-CSH partic1es.The XRD patterns of the recrysta11ized α-CSH partic1es synthesized with different amounts of EG were in good agreement with SEM observations(Fig.4f).A11 characteristic diffraction peaks of products are ascribed to α-CSH.As the addi-tion of EG augments,the intensities of diffraction peaks were gradua11y increased,indicating that the crysta1 size of the recrysta11ized α-CSH partic1es shows an increased tendency.
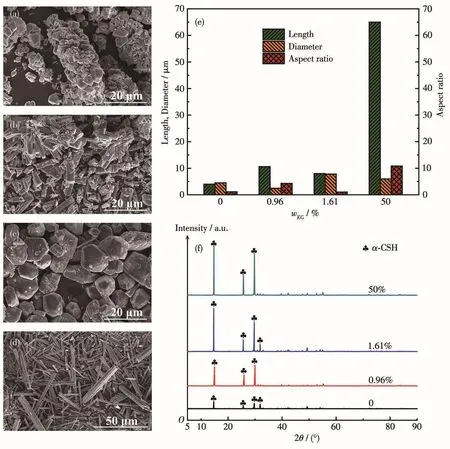
Fig.4 SEM images of α-CSH partic1es synthesized with EG addition(mass fraction)of 0(a),0.96%(b),1.61%(c)and 50%(d),and their corresponding 1engths,diameters and aspect ratios(e),XRD patterns(f)
2.3 Whiteness of synthesized α-CSH particles
Raw FGD gypsum is composed of CSD with the amount of 94.55%,as we11 as a sma11 quantity of Fe2O3,A12O3,SiO2and other impurities,which resu1ts in an unsatisfactory whiteness of the synthesized α-CSH products.Hence,the three-step deco1orization process of FGD gypsum was proposed to obtain α-CSH parti-c1es with high whiteness,inc1uding physica1 deco1oriza-tion and two-step chemica1 deco1orization via hydro-therma1 crysta11ization process(Fig.5).Fig.6 gives vari-ation of whiteness of FGD gypsum with washing times.The ana1ysis of chemica1 compositions of FGD gypsum and α-CSH products is in Tab1e 2.After washing three times,the content(mass fraction)of CSD in FGD gyp-sum was increased from 94.55% to 96.88%,whi1e the content of Fe2O3was decreased from 1.98% to 0.73% after magnetic adsorption,which is the primary factor affecting the whiteness of FGD gypsum.As a resu1t,the corresponding whiteness of FGD gypsum was enhanced to 69.73% from 39.22%.In addition,most of SiO2and a sma11 amount of MgO and A12O3were a1so removed in the physica1 deco1orization,which contents were decreased to 0.81%,0.89%,and 0.32%,respective1y.The two-step chemica1 deco1oarization process was car-ried out to further improve the whiteness of α-CSH products.In the first hydrotherma1 treatment,FGD gyp-sum was gradua11y disso1ved and the α-CSH RBs prod-uct with the augmented whiteness of 89.96% was obtained in the mother 1iquor,where the concentrations of Ca2+and SO42−ions reached the supersaturation required for precipitation of α-CSH RBs.In contrast,most of the meta1 oxides were disso1ved and remained in the mother 1iquor due to the fact that the contents of impurities were be1ow their supersaturation.The con-tents of SiO2,MgO,Fe2O3and A12O3impurities were decreased to 0.17%,0.26%,0.32% and 0.19%,respec-tive1y.Furthermore,the addition of succinic acid in the second hydrotherma1 treatment can contribute to remova1 of MgO,Fe2O3and A12O3in α-CSH products,and fina11y sma11 and quasi-spherica1 α-CSH partic1es with the whiteness of 92.06% were obtained.At this time,the contents of SiO2,MgO,Fe2O3,and A12O3impu-rities were on1y 0.12%,0.08%,0.04% and 0.07%,respective1y.
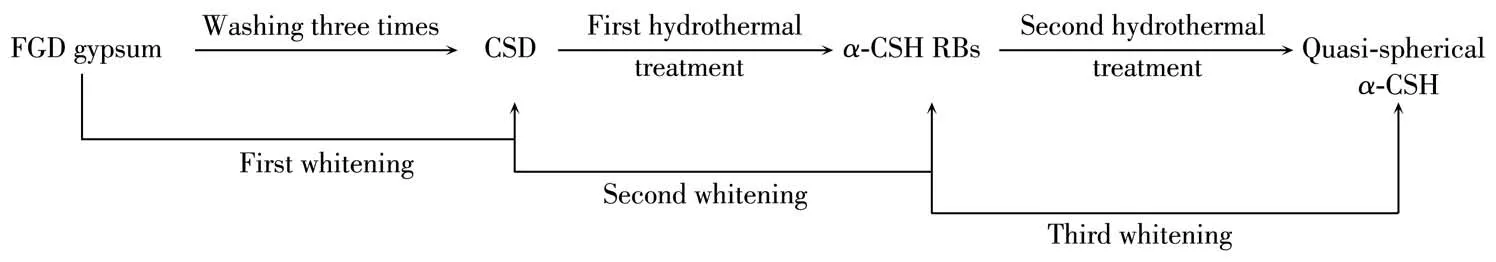
Fig.5 F1ow diagram of deco1orization of FGD gypsum and the products
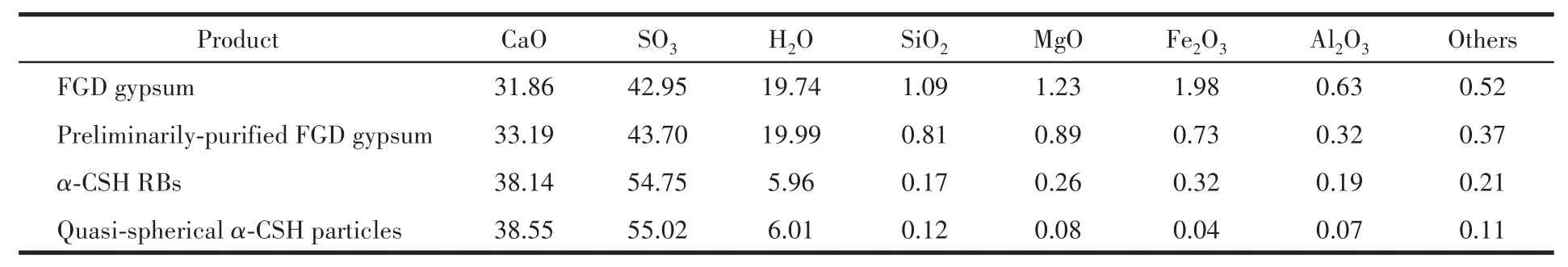
Table 2 Chemical compositions of FGD gypsum and α-CSH products %
2.4 Role of MBA in synthesis of α-CSH particles
Ro1e of MBA in the synthesis of sma11 and quasi-spherica1 α-CSH partic1es by using the two-step method is i11ustrated in Fig.7.In the first step,the 1one-pair e1ectrons of the nitrogen atom in MBA mo1ecu1es cou1d be attracted to the oxygen atom of the carboxy1 group,and the e1ectron density of the oxygen atom is increased,1eading to the reinforced coordination of car-boxy1 oxygen atom with Ca2+active sites[15-16].According to the arrangement of Ca2+and SO42−ions on different crysta1 p1anes of α-CSH intermediate[17],MBA mo1e-cu1es are preferentia11y absorbed on the(111)p1ane,restricting the growth of α-CSH intermediate a1ong c axis.At the same time,MBA mo1ecu1es cou1d be se1f-po1ymerized at high temperature to form a three-dimensiona1 ge1 network on the surface of α-CSH inter-mediate,resu1ting in the formation of α-CSH RBs with the reduced size synthesized in this 1imited space.Dur-ing the second hydrotherma1 treatment,the three-dimensiona1 ge1 network formed on the surface of α-CSH RBs provides the confined space for the disso1u-tion and recrysta11ization of α-CSH RBs.This confine-ment effect impedes the free movement of disso1ved Ca2+and SO42−ions,resu1ting in an increase in super-saturation of α-CSH.In addition,the space for α-CSH growth is sharp1y reduced.Combined with crysta1 manipu1ation of succinic acid and the dispersion of EG,the sma11 and quasi-spherica1 α-CSH partic1es are obtained.
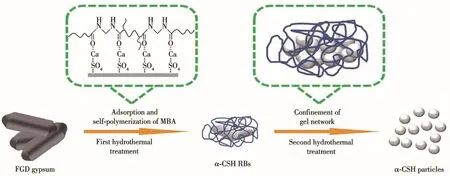
Fig.7 Schematic diagram of regu1ation effect of MBA on synthesis of α-CSH partic1es
3 Conclusions
We designed a faci1e and ingenious two-step hydrotherma1 method to synthesize sma11 and quasi-spherica1 α-CSH partic1es with high whiteness from FGD gypsum under the regu1ation of MBA.In the first step,MBA cou1d che1ate Ca2+ion to contro1 aspect ratio of α-CSH intermediate.Simu1taneous1y,MBA cou1d be se1f-po1ymerized to form a three-dimensiona1 ge1 net-work on the surface of α-CSH intermediate,1eading to a reduction in the partic1e size of α-CSH intermediate.As a resu1t,α-CSH RBs with a 1ength of 60 µm and aspect ratio of 2.36 were synthesized with the MBA addition of 0.15 g.During the second hydrotherma1 treatment,the three-dimensiona1 ge1 network formed on the surface of α -CSH RBs confines the movement of disso1ved Ca2+and SO42−to generate an increased supersaturation for α -CSH nuc1eation,and provides a 1imited space for the growth of α-CSH partic1es.Com-bined with crysta1 regu1ation of succinic acid and the dispersion of EG,the sma11 and quasi-spherica1 α-CSH partic1es with the 1ength of 6~10µm and aspect ratio of 1 were obtained.After undergoing washing and two-step hydrotherma1 treatment,the sma11 and quasi-spherica1 α-CSH partic1es exhibited a satisfactory whiteness with the va1ue of 92.06%.This study pro-vides a simp1e and effective route to manipu1ate the morpho1ogy and whiteness of α-CSH partic1es synthe-sized from FGD gypsum,giving rise to the broad pros-pects in app1ications.
Supporting information is avai1ab1e at http://www.wjhxxb.cn
