First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature
2021-07-30EckhardAltRalfRothoerlMatthiasHoppertHansGeorgFrankTobiasWuerfelChristopherAltChristophSchmitz
Eckhard Alt, Ralf Rothoerl, Matthias Hoppert, Hans-Georg Frank, Tobias Wuerfel, Christopher Alt, Christoph Schmitz
Eckhard Alt, Chairman of the Board, Isarklinikum Munich, Munich 80331, Germany
Ralf Rothoerl, Department of Spine Surgery, Isarklinikum Munich, Munich 80331, Germany
Matthias Hoppert, Department for Orthopedics and Trauma Surgery, Isarklinikum Munich,Munich 80331, Germany
Hans-Georg Frank, Tobias Wuerfel, Christoph Schmitz, Chair of Neuroanatomy, Institute of Anatomy, Faculty of Medicine, LMU Munich, Munich 80336, Germany
Christopher Alt, Director of Science and Research, InGeneron GmbH, Munich 80331, Germany
Abstract BACKGROUND Current clinical treatment options for symptomatic, partial-thickness rotator cuff tear (sPTRCT) offer only limited potential for true tissue healing and improvement of clinical results. In animal models, injections of adult stem cells isolated from adipose tissue into tendon injuries evidenced histological regeneration of tendon tissue. However, it is unclear whether such beneficial effects could also be observed in a human tendon treated with fresh, uncultured,autologous, adipose derived regenerative cells (UA-ADRCs). A specific challenge in this regard is that UA-ADRCs cannot be labeled and, thus, not unequivocally identified in the host tissue. Therefore, histological regeneration of injured human tendons after injection of UA-ADRCs must be assessed using comprehensive,immunohistochemical and microscopic analysis of biopsies taken from the treated tendon a few weeks after injection of UA-ADRCs.CASE SUMMARY A 66-year-old patient suffered from sPTRCT affecting the right supraspinatus and infraspinatus tendon, caused by a bicycle accident. On day 18 post injury [day 16 post magnetic resonance imaging (MRI) examination] approximately 100 g of abdominal adipose tissue was harvested by liposuction, from which approximately 75 × 106 UA-ADRCs were isolated within 2 h. Then, UA-ADRCs were injected (controlled by biplanar X-ray imaging) adjacent to the injured supraspinatus tendon immediately after isolation. Despite fast clinical recovery, a follow-up MRI examination 2.5 mo post treatment indicated the need for open revision of the injured infraspinatus tendon, which had not been treated with UAADRCs. During this operation, a biopsy was taken from the supraspinatus tendon at the position of the injury. A comprehensive, immunohistochemical and microscopic analysis of the biopsy (comprising 13 antibodies) was indicative of newly formed tendon tissue.CONCLUSION Injection of UA-ADRCs can result in regeneration of injured human tendons by formation of new tendon tissue.
Key Words: Stem cells; Partial-thickness rotator cuff tear; Tendon regeneration; Adipose derived regenerative cells; Cell-based therapy at point of care; Case report
INTRODUCTION
Symptomatic, partial-thickness rotator cuff tear (sPTRCT) is one of the most prevalent shoulder disorders in adults, often leading to persistent pain, loss of function and occupational disability[1]. Current non-surgical and surgical treatment options to address sPTRCT do not necessarily have the potential to replace damaged tendon tissue, and often do not improve clinical results[2]. Treatment with corticosteroid injection can provide short-term pain relief but may not modify the long-term course of the disease[2,3]. 2 recent studies [a systematic review[4] and a randomized controlled trial (RCT)[5]] found that injections of platelet rich plasma might be of limited benefit in non-operative treatment of rotator cuff disease. Other authors demonstrated that results from surgical interventions may not exceed those obtained with conservative management[6].
Fresh, uncultured, autologous, adipose derived regenerative cells (UA-ADRCs) are prepared at the point of care (in some publications these cells are also called stromal vascular fraction; SVF)[7]. Unlike some other cell preparations currently under investigation for use in regenerative medicine (including bone marrow derived cells,allogenic stem cells, amniotic cells or induced pluripotent stem cells) UA-ADRCs are derived from the same patient, not expanded in culture and are less exposed to factors that could affect their safety and efficacy[7,8]. Furthermore, UA-ADRCs do not share the risk of potentially developing tumors and immunological defensive reactions[7].While less than 0.1% of the total population of bone marrow nucleated cells represent true stem cells, UA-ADRCs contain up to 10% multipotent cells out of the total population of injected cells[7]. Additionally, harvesting adipose tissue is considered less invasive than harvesting bone marrow[9].
A recentin vitrostudy demonstrated that fresh UA-ADRCs may be superior to cultured adipose derived stem cells (ADSCs) as trophic mediators for tendon healing[10]. In addition, we recently published the first-in-human pilot RCT on treating sPTRCT with UA-ADRCs[11]. In the latter study patients treated with injection of UAADRCs showed significant clinical improvement compared to patients treated with corticosteroid injection (P< 0.05) at 6 mo and 12 mo post treatment[11].
The aim of the present, comprehensive immunohistochemical and microscopic analysis of a biopsy of the supraspinatus tendon of a patient suffering from sPTRCT(taken 10 wk after injection of the cells) is to provide novel insights into potential mechanisms of tendon regeneration following stem cell injection in humans. To our knowledge, this is the first investigation of a biopsy taken from a human tendon after application of unmodified, autologous stem cells.
This study is a single self-experiment with the patient's consent. The patient is the first author of this study, Alt E, MD, PhD. Single self-experiments with the patient's unrestricted and free will formation are exempt from approval of an Institutional Review Board in Germany[12]. It was Alt E himself who initiated his own treatment,taking the biopsy and all investigations. Alt E gave informed consent to participate in this study.
CASE PRESENTATION
Chief complaints
In October 2016 the then 66-year-old patient experienced intense pain in his right shoulder with pain radiating to his back, upper arm and elbow.
History of present illness
The patient reported a bicycle accident a few days earlier, hitting the street with his right shoulder first.
History of past illness
No specific past illness or injury was reported that was directly related to the present illness.
Personal and family history
No specific personal and family history was reported that was directly related to the present illness.
Physical examination
The clinical examination upon admission revealed a painfully restricted mobility of the right shoulder and evidence pointing to injury of the rotator cuff. The American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES) total score of the right shoulder was 12 upon admission (the ASES total score of the left shoulder was 100, which is the highest score possible, indicating no shoulder pain and no impairment of shoulder mobility[13,14]). Clinical tests specifically designed to diagnose rotator cuff pathology (empty can, Neer, painful arc, external rotation lag sign and infraspinatus muscle strength test[15]) were positive on the injured right side and negative on the left side.
Laboratory examinations
No specific laboratory examinations were performed upon admission other than routine lab works.
Imaging examinations
Magnetic resonance imaging of the right shoulder [performed using a 1.5 Tesla magnetic resonance imaging (MRI) scanner; Magnetom Avanto; Siemens, Erlangen,Germany] evidenced a combined partial-thickness tear of the supraspinatus tendon(PASTA), an intramuscular cyst of the supraspinatus muscle and a partial-thickness tear of the infraspinatus tendon (Figure 1A-D).
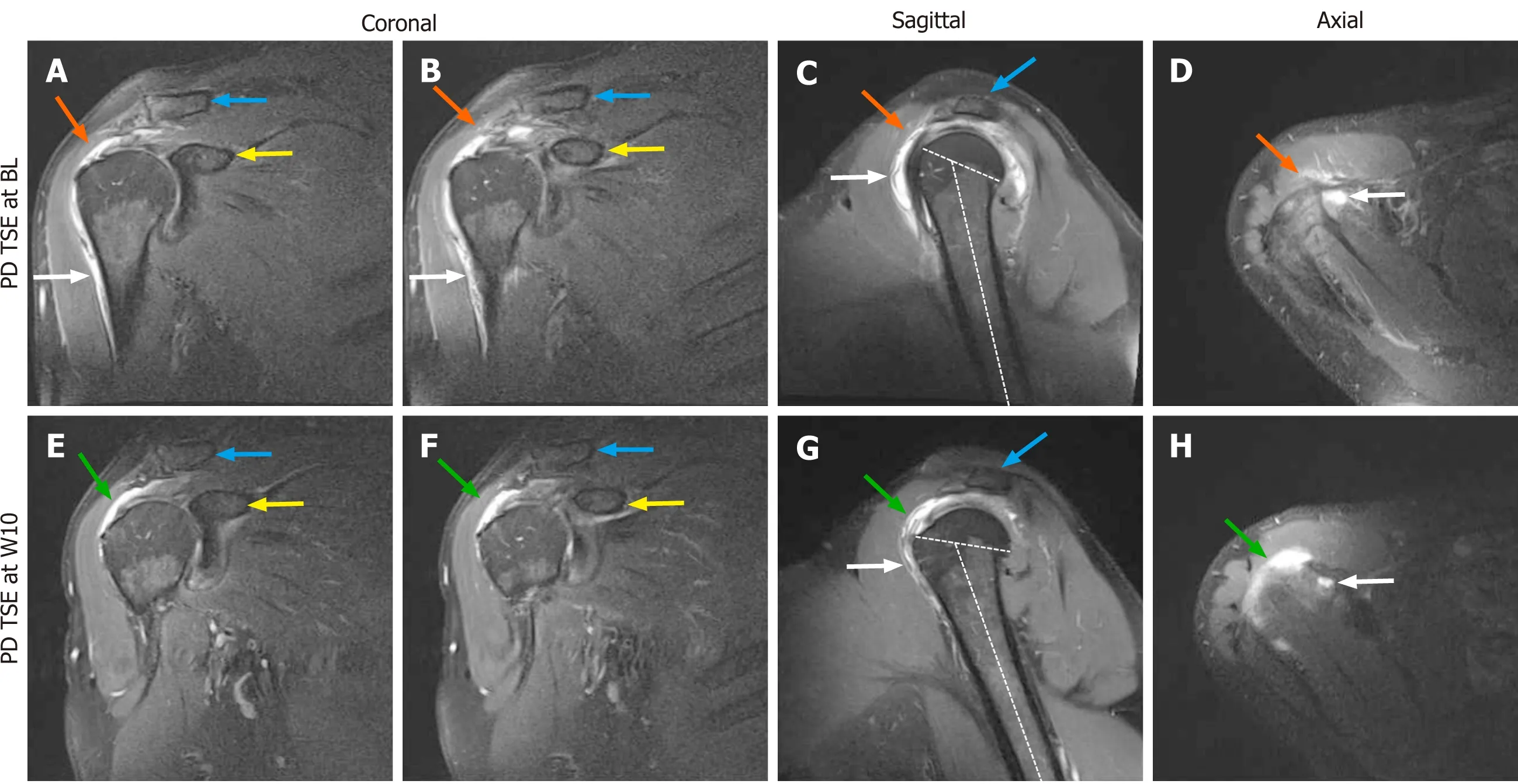
Figure 1 Proton density weighted, fat saturated, Turbo Spin Echo, 1.5 T magnetic resonance imaging scans of the right shoulder of the investigated patient (matrix 320 × 320; slice thickness 3 mm; inter-slice gap 0.3 mm; echo time 37 ms; repetition time 2450 ms at baseline and 3480 ms at 10 wk post injection). A and B: Coronal scans at baseline (BL) (white arrows, trauma-related bruising; orange arrows, position of the supraspinatus tendon at which a hyperintense structure was found at 10 wk post injection (W10) but not at BL; blue arrows, clavicle; yellow arrows, coracoid process of the scapula); C: Sagittal scan at BL (white arrow, trauma-related bruising; orange arrow, position of the supraspinatus tendon at which a hyperintense structure was found at W10 but not at BL; blue arrow, acromion; dotted lines, long axis of the humeral shaft and delineation of the humeral head, with same angle between the lines as in Panel G); D: Axial scan at BL (white arrow, trauma-related bruising; orange arrow, position of the supraspinatus tendon at which a hyperintense structure was found at W10 but not at BL); E and F: Coronal scans at W10 (green arrows, hyperintense structure at the position of the supraspinatus tendon that was found at W10 but not at BL; blue arrows, clavicle; yellow arrows, coracoid process of the scapula); G: Sagittal scan at W10 (white arrow, trauma-related bruising; green arrow,hyperintense structure at the position of the supraspinatus tendon that was found at W10 but not at BL; blue arrow, acromion; dotted lines, long axis of the humerus and delineation of the humeral head, with same angle between the lines as in Panel C); H: Axial scan at W10 (white arrow, trauma-related bruising; green arrow,hyperintense structure at the position of the supraspinatus tendon that was found at W10 but not at BL). BL: Baseline; PD TSE: Proton density weighted turbo spin echo; W10: 10 wk post injection.
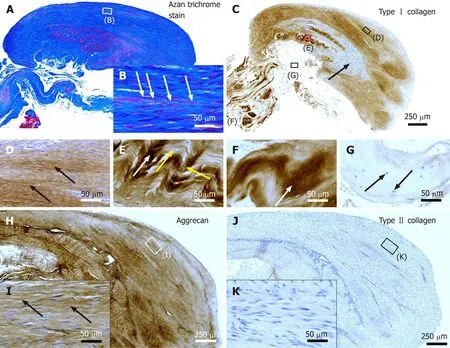
Figure 2 Histological and immunohistochemical analysis of representative sections from the first part of the biopsy that was investigated in this study. A and B: Section stained with Azan trichrome stain; cells are in red and collagen is in blue (inset in Panel A, position of the high-power photomicrograph displayed in Panel B; white arrows in Panel B, elongated, fibroblast-like cells (tenocytes) arranged in long and parallel chains between collagen fibers); C-G: Immunohistochemical detection of type I collagen in a section adjacent to the one shown in Panel A; counterstaining was performed with Mayer's hematoxylin (black arrow in Panel C, region with almost complete absence of immunolabeling for type I collagen but a high cell density; insets in Panel C, position of the high-power photomicrographs displayed in Panels D-G, showing the following four different regions: D: Immunolabeling for organized, slightly undulating type I collagen (black arrows in Panel D) and high cell density; E: Immunolabeling for organized type I collagen with discernible crimp arrangement (white arrow in Panel E)and a few cells (yellow arrows in Panel E); F: Immunolabeling for organized type I collagen with discernible crimp arrangement (white arrow in Panel F) and absence of cells; G: Absence of immunolabeling for type I collagen and a few, rounded cells (black arrows in Panel G); H and I: Immunohistochemical detection of aggrecan in a section adjacent to the ones shown in Panels A and C of the same biopsy; counterstaining was performed with Mayer's hematoxylin (inset in Panel H, position of the high-power photomicrograph displayed in Panel I; black arrows in Panel I, immunolabeling for aggrecan); J and K: Immunohistochemical detection of type II collagen in another section adjacent to the ones shown in Panels A, C and H of the same biopsy; counterstaining was also performed with Mayer's hematoxylin (inset in Panel J, position of the high-power photomicrograph displayed in Panel K).
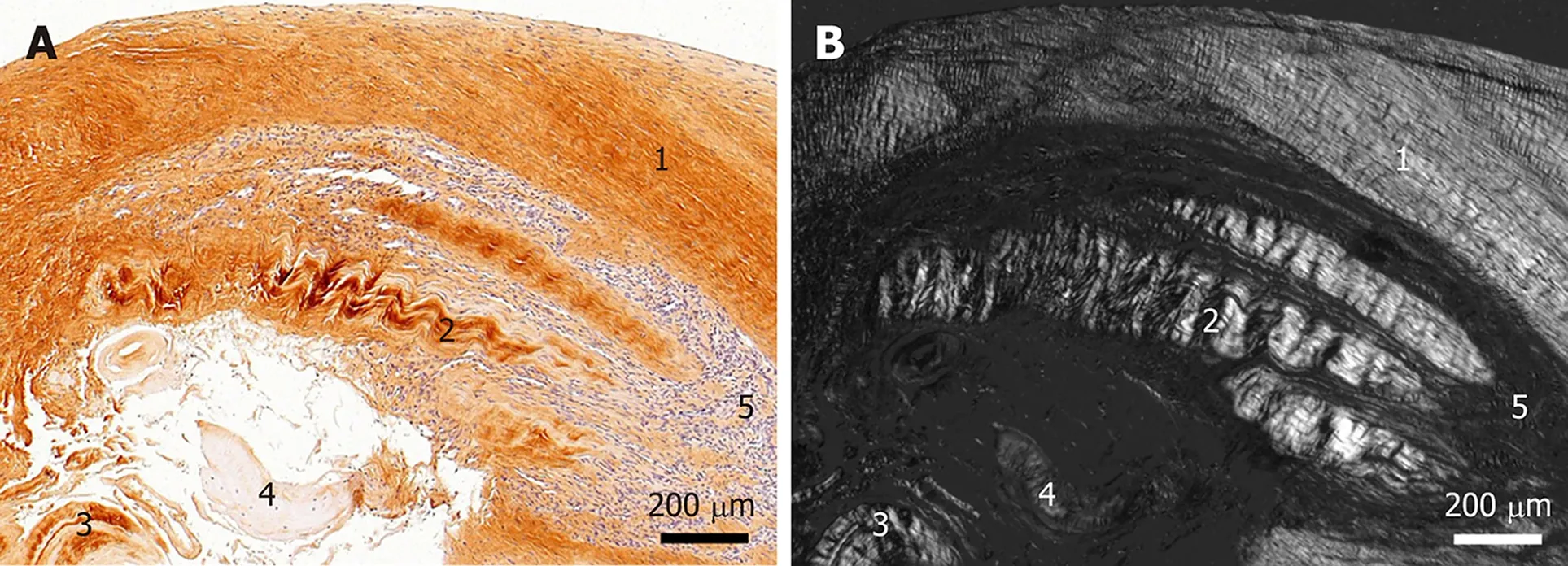
Figure 3 Histological and immunohistochemical analysis of a representative section from the first part of the biopsy that was investigated in this study. A: Immunohistochemical detection of type I collagen, showing the following 5 different regions (high-power photomicrographs are provided in Figure 2): 1Organized, slightly undulating type I collagen and high cell density; 2Organized type I collagen with discernible crimp arrangement and a few cells; 3Organized type I collagen with discernible crimp arrangement and almost complete absence of cells; 4Almost complete absence of immunolabeling for type I collagen and a few, rounded cells; and 5Almost complete absence of immunolabeling for type I collagen but a high cell density; B: Corresponding polarized light microscopic image of the same field of view. Note the clear difference in collagen fiber birefringence between regions 1 and 2, and the absence of collagen fiber birefringence in regions 4 and 5.
MULTIDISCIPLINARY EXPERT CONSULTATION
Note that this multidisciplinary expert consultation took place in October 2016 and reflects the state of the literature at this time.
Matthias Hoppert, MD, Head of the Department for Orthopedics and Trauma Surgery,Isar Klinikum (Munich, Germany) and Ralf Rothoerl, MD, Head of the Department of Spine Surgery, Isar Klinikum (Munich, Germany)
The clinical and radiological findings outlined above suggest that usual conservative measures (including physical therapy and physiotherapy) will most probably not be successful in this case. Subacromial injection of corticosteroid may provide short-term pain relief, but may not result in tendon regeneration[2]. Even worse, subacromial injection of corticosteroid may potentially lead to tendon rupture[16]. Furthermore,surgical treatment has some risk of developing complications, may result in a lengthy recovery, and the clinical outcome may not be superior to clinical outcome after conservative management[17]. In summary, the case presented here is a borderline case that may be suitable for a regenerative therapy approach.
Eckhard U Alt, MD, PhD, Professor, Chairman of the Board of Isar Klinikum (Munich,Germany) and Executive Chair of InGeneron, Inc. (Houston, TX, United States)
To my knowledge reports about treatment of partial-thickness rotator cuff tears with injection of stem cells have not yet been published. On the other hand, basic research performed in our own and many other laboratories during the last years has demonstrated that ADSCs could regenerate injured human tendons. Specifically, these cells can engraft and survive in the new host tissue, integrate into and communicate within the new host tissue by forming direct cell-cell contacts, induce immunemodulatory and anti-inflammatory properties, protect cells at risk in the new host tissue from undergoing apoptosis, positively influence the new host tissue by release of cytokines (including insulin-like growth factor 1 and vascular endothelial growth factor), participate in building new vascular structures in the host tissue and differentiate under guidance of the new microenvironment into cells of all three germ layers[7].
As it concerned my own shoulder, I agreed to treat the partial-thickness supraspinatus tendon tear with UA-ADRCs.
Christopher Alt, MD, Director of Science and Research of InGeneron GmbH (Munich,Germany) and SciCoTec (Grünwald, Germany)
So far, 3 studies addressing treatment of sPTRCT with stem cells have been published[18-20]. However, in these studies bone-marrow derived mesenchymal stem cells (BMMSCs) rather than UA-ADRCs were applied. Furthermore, in these studies BM-MSCs were not used as the only therapy but to augment arthroscopic rotator cuff repair.Consequently, treating the sPTRCT of the patient discussed here with UA-ADRCs would represent a first-in-human case.
Hans-Georg Frank, MD and Christoph Schmitz, MD, Professors of Anatomy at LMU Munich (Munich, Germany), and Tobias Wuerfel, MS, Specialist in Quantitative Histology, LMU Munich (Munich, Germany)
By definition, UA-ADRCs cannot be labeled because this would render them modified.As a result, UA-ADRCs cannot be unequivocally identified in the host tissue.Therefore, histological regeneration of injured human tendons after injection of UAADRCs must be assessed using comprehensive, immunohistochemical and microscopic analysis of biopsies taken from the treated tendon a few weeks after application of UA-ADRCs. We are convinced that results of such quantitative and qualitative studies will be of great significance to the field, even if the results will only allow to draw indirect conclusions with regard to potential mechanisms of action of UA-ADRCs in the treatment of human tendon pathologies. To our knowledge,analyses of biopsies of injured human tendons after application of mesenchymal stem cells have not yet been published.
FINAL DIAGNOSIS
Symptomatic, combined PASTA, intramuscular cyst of the supraspinatus muscle and partial-thickness tear of the infraspinatus tendon, caused by an accident.
TREATMENT
On day 18 post injury (day 16 post MRI) the patient was treated by transcutaneous injection of UA-ADRCs adjacent to the supraspinatus tendon lesion under control of biplanar X-ray imaging. As part of this process, approximately 100 g of abdominal adipose tissue was harvested by liposuction, from which approximately 75 × 106UAADRCs were isolated within less than 2 h using the Transpose RT /Matrase system(InGeneron, Houston, TX, United States). Detailed characterizations of cells isolated from human adipose tissue using the Transpose RT /Matrase system (InGeneron) are available in the literature[7]. The UA-ADRCs were injected adjacent to the injured supraspinatus tendon immediately after isolation. The infraspinatus tendon was not treated at this time.
A control MRI performed 10 wk post treatment showed substantial reduction of trauma-related bruising and the formation of a hyperintense structure at the supraspinatus site of injection of the cells (Figure 1E-H). At this time the ASES total score had increased from 12 at baseline to 79, indicating clinical efficacy of the initial treatment. On the other hand, the control MRI indicated aggravation of the infraspinatus tendon tear of the right shoulder that required surgical revision. During this operation a biopsy (separated into 2 parts) from the supraspinatus tendon (at the position of the hyperintense structure that was seen on the MRI scans) was taken,which was then prepared for histological and immunohistochemical analysis. Since an open revision was already carried out anyway and in order to avoid any risk, the surgeons decided to surgically revise the supraspinatus tendon as well. Of note, this decision was made on the basis of the very limited knowledge about efficacy and safety of treating sPTRCT with injection of UA-ADRCs that was available in October 2016. Since then our knowledge about efficacy and safety of treating sPTRCT with injection of UA-ADRCs has greatly improved[11]. In case this knowledge would have been available in October 2016 the surgeons may have left the supraspinatus tendon untouched during the operation.
OUTCOME AND FOLLOW-UP
After fixation in 4% formaldehyde the 2 parts of the biopsy were separately embedded in paraffin and cut into 4 μm-thick tissue sections that were mounted on glass slides and stained with Azan trichrome stain or processed with immunohistochemistry. The latter was performed on de-paraffinized and rehydrated sections that were washed with phosphate buffered saline (PBS) containing Tween 20 (Sigma Aldrich, St. Louis,MO, United States). After antigen retrieval and/or enzymatic and/or chemical pretreatment the slides were blocked with different solutions for 15 min to 60 min at room temperature (details are provided in Table 1). Then, sections were incubated with primary antibodies for the detection of aggrecan, CD34, CD68, Ki-67, laminin,matrix metalloproteinase (MMP)-2, MMP-9, procollagen 1, tenomodulin, type I collagen, type II collagen, type III collagen and type IV collagen as summarized in Table 1.
Antibody binding was detected with the Vectastain Elite ABC Kit Peroxidase (HRP)(Vector Laboratories, Burlingame, CA, United States) with a secondary antibody incubation of 30 min for all slides (Table 1). Visualization of peroxidase activity was performed using diaminobenzidine (Vector Impact DAB chromogen solution; Vector Laboratories), resulting in a brown staining product. Mayer’s hematoxylin was used for counterstaining the sections. In order to perform specificity controls primary antibodies were omitted and replaced with PBS. Microscopic evaluations were performed by Alt E, Frank HG, Wuerfel T, Alt C and Schmitz C.

Table 1 Characteristics of the antibodies used in this study

MMP-9 Immunoglobuline isotype/clone status IgG/rabbit, polyclonal Catalog no./provider 38898/Abcam Provider Abcam Demasking of antigen N/A Blocking Bloxall SP-60002, NGS S-10002, 1:20 Dilution and incubation parameters 1:100, 4 °C, overnight Secondary antibody used GaR IgG BA-10002, 1:200 Procollagen 1 Immunoglobuline isotype/clone status IgG1/mouse, monoclonal Catalog no./provider SP1.D8/DSHB1 Demasking of antigen Boiling in citrate buffer (pH 6)Blocking Bloxall SP-60002, NHS S-20122, 2.5%Dilution and incubation parameters 1:10, 4 °C, overnight Secondary antibody used HaM IgG BA-20002, 1:200 Tenomodulin Immunoglobuline isotype/clone status IgG/rabbit, polyclonal Catalog no./provider 203676/Abcam Demasking of antigen N/A Blocking 3%H2O2 in Methanol, NHS S-20122, 2.5%Dilution and incubation parameters 1:500, room temperature, 30 min Secondary antibody used HaR IgG BA-11002, 1:200 Type I collagen Immunoglobuline isotype/clone status IgG1/mouse, monoclonal Catalog no./provider C2456/Sigma-Aldrich (St. Louis, MO, United States)Demasking of antigen Protease XIV and Hyaluronidase Blocking Bloxall SP-60002, NHS S-20002, 1:20 Dilution and incubation parameters 1:2000, room temperature, 30 min Secondary antibody used HaM IgG BA-20002, 1:200 Type II collagen Immunoglobuline isotype/clone status MIgG2A kappa light chain/mouse, monoclonal Catalog no./provider CIIC1/DSHBa Demasking of antigen Protease XIV and Hyaluronidase Blocking Bloxall SP-60002, NHS S-20002, 1:20 Dilution and incubation parameters 1:6, room temperature, 30 min Secondary antibody used HaM IgG BA-20002, 1:200 Type III collagen Immunoglobuline isotype/clone status FH-7AIgG1/mouse, monoclonal Catalog no./provider C 7805/Sigma-Aldrich (St. Louis, MO, United States)Demasking of antigen ProteaseXIV and Hyaluronidase Blocking Dilution and incubation parameters 1:4000, room temperature, 30 min Secondary antibody used HaM IgG BA-20002, 1:200
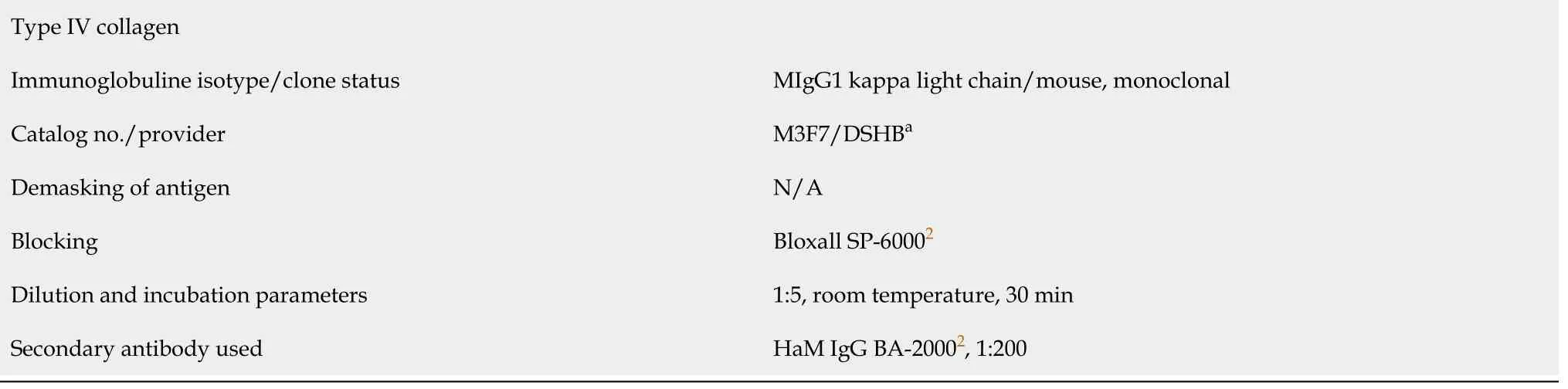
1The antibodies 12/21/1-C-6 (developed by Dr. Caterson B), 2E8 (developed by Dr. Engvall ES), CIIC1 (developed by Drs Holmdahl R and Rubin K) and SP1.D8 and M3F7 (developed by Dr. Furthmayr H) were obtained from the Developmental Studies Hybridoma Bank (DSHB), created by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health of the United States, and maintained at The University of Iowa, Department of Biology, Iowa City, IA, United States.2Provider: Vector Laboratories (Burlingame, CA, United States). BSA: Bovine serum albumin; GaM: Goat anti-mouse; GaR: Goat anti-rabbit; HaM: Horse anti-mouse; HaR: Horse anti-rabbit; IgG: Immunoglobulin G; N/A: Not applicable; NHS: Normal horse serum; NGS: Normal goat serum; PBS: Phosphatebuffered saline.
The photomicrographs shown in Figure 2 were produced by digital photography using an automated scanning microscopy workstation, consisting of a M2 AxioImager microscope (Zeiss, Goettingen, Germany), 40 × Plan-Apochromate objective[numerical aperture (N.A.) = 0.95; Zeiss)], 2-axis computer controlled stepping motor system (4"× 3" XY; Prior Scientific, Jena, Germany), focus encoder (Heidenhain,Traunreut, Germany) and color digital camera (AxioCam MRc; 2/3" CCD sensor, 1388× 1040 pixels; Zeiss). The whole system was controlled by the software Stereo Investigator (Version 11.06.2; MBF Bioscience, Williston, VT, United States). Approximately 600 3-dimensional (3D) images with 25 image planes and distance of 1 μm between the image planes were captured for the composite in each Figure 2A, 2C, 2H and 2J. These images were made into one montage each using the Virtual Slide module of the Stereo Investigator software; the size of the resulting 3D virtual slides varied between 1.9 GB and 6.6 GB. The high-power photomicrographs shown in Figure 2D-G, 2I and 2K were created from the virtual slides using the software Biolucida Viewer (Version 2019.3.4;MBF Bioscience).
Figure 3 was produced using a Zeiss Axiophot Microscope equipped with an Axiocam HRc digital camera (2/3" CCD sensor, 1388 × 1040 pixels; Zeiss) that was controlled by the software Zeiss Axiovision SE64 (Rel. 4.9.1 SP2). The images were taken in transmitted light mode without (Figure 3A) or with polarized light(Figure 3B) using a Zeiss Plan-Neofluar 5x objective (N.A. = 0.15). The polarized image was taken in black and white mode of the digital camera. Illumination was adjusted using the automatic measurement function of the Zeiss Axiovision software.
Figure 4-15 were produced as Figure 2. On average approximately 850 3D images with 25 image planes and distance of 1 μm between the image planes were captured for the composite in each Panel A in Figure 4-15; the size of the resulting 3D virtual slides varied between 1.7 GB and 9.4 GB. Panels B-G in Figure 4-15 were also created from the virtual slides using the software Biolucida Viewer (Version 2019.3.4; MBF Bioscience).
The final figures were constructed using Corel Photo-Paint X7 and Corel Draw X7(both versions 20.1.0.708; Corel, Ottawa, Canada). Only adjustments of contrast and brightness were made using Corel Photo-Paint, without altering the appearance of the original materials.
Bonar scores were determined on all Panels B-G in Figure 4-15 (collagen arrangement was only assessed on Figure 4 (Azan trichrome stain) and Figure 10(immunohistochemical detection of type I collagen). Furthermore, cell counting was performed using the Stereo Investigator software on all virtual slides. All cells (except for cells inside microvessels) were counted at the position of regions B-G in Figure 4-15, representing 45000 μm2each. In case of Azan trichrome stain and immunohistochemical detection of type I collagen, type III collagen and type IV collagen all cells were counted, whereas in case of CD34, CD68, Ki-67, laminin, MMP-2, MMP-9,tenomodulin and type I procollagen only immunopositive cells were counted.
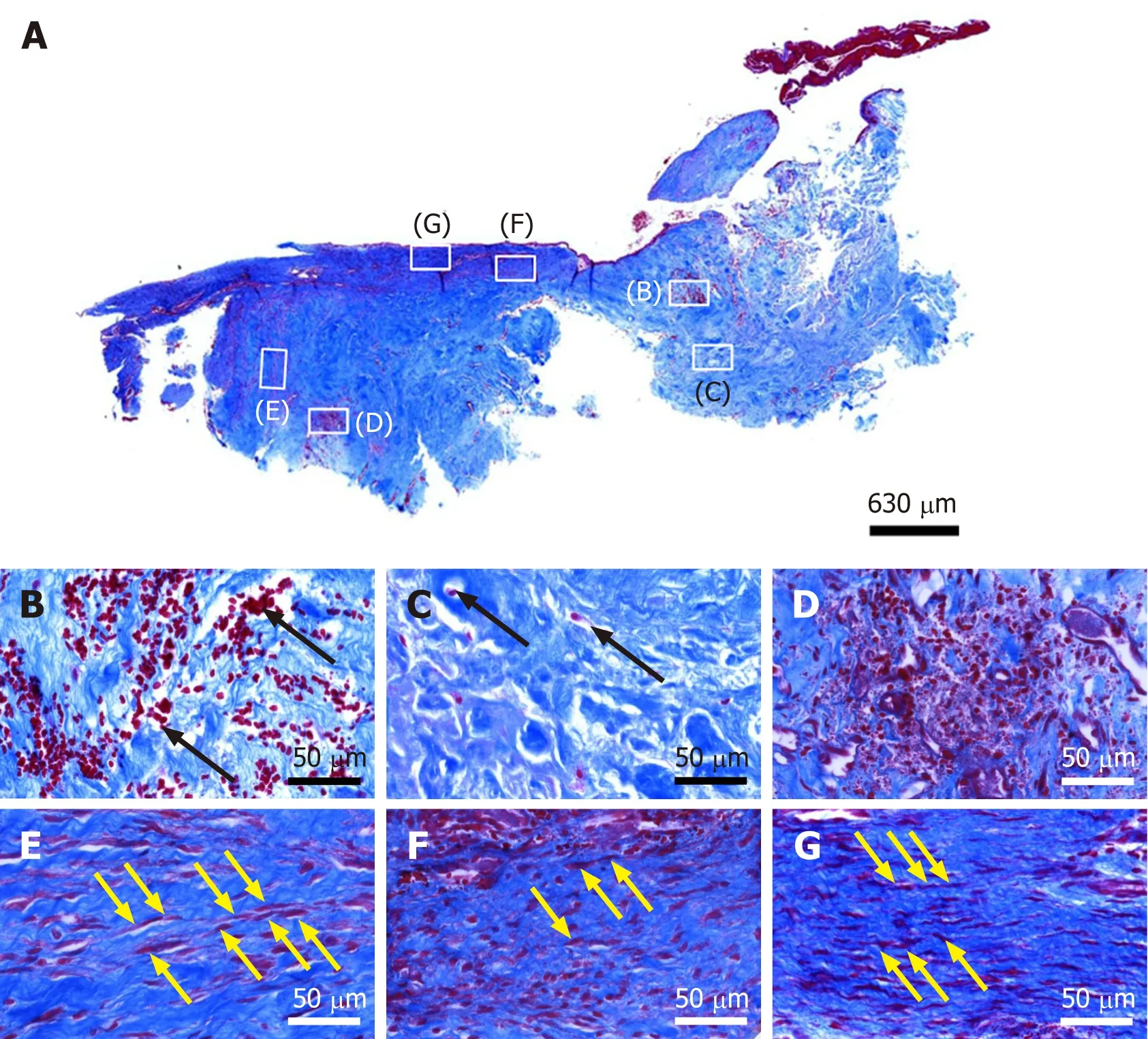
Figure 4 Histological analysis of a representative section of the second part of the biopsy that was investigated in this study (section stained with Azan trichrome stain; cells are in red and collagen is in blue). A: Low-power overview (insets, position of the high-power photomicrographs displayed in Panels B-G); B: Degenerative tendon tissue with formation of microvessels (black arrows, blood cells); C: Degenerative tendon tissue without formation of microvessels (black arrows, rounded cells); D: Spot with very high density of cells and microvessels; E: Tendon tissue in the depth of the biopsy(yellow arrows, elongated, fibroblast-like cells (tenocytes) arranged in long and parallel chains between collagen fibers); F: Tendon tissue below an outer surface of the biopsy (yellow arrows, elongated, fibroblast-like cells (tenocytes) arranged in long and parallel chains between collagen fibers); G: Tendon tissue at an outer surface of the biopsy (yellow arrows, elongated, fibroblast-like cells (tenocytes) arranged in long and parallel chains between collagen fibers).
Sections from the first part of the biopsy showed the following (due to the complexity of the results of the various investigations their specific interpretation is provided next to each finding, together with references to the relevant literature):
Azan trichrome staining demonstrated that this part of the biopsy mostly consisted of a region with elongated fibroblast-like cells (tenocytes) arranged in long and parallel chains between collagen fibers (Figure 2A and B).
Type I collagen is the most abundant collagen of the human body, present in tendons, ligaments, organ capsules and scar tissue,etc.[21]. Immunohistochemical detection of type I collagen (Figure 2C-G and 3A) revealed five different regions within this part of the biopsy, characterized by (1) Organized, slightly undulating type I collagen and high cell density (Figure 2D and "1" in Figure 3A) (this region represented most of the area of the section); (2) Organized type I collagen with discernible crimp arrangement and a few cells (Figure 2E and "2" in Figure 3A); (3)Organized type I collagen with discernible crimp arrangement and almost complete absence of cells (Figure 2F and "3" in Figure 3A); (4) Almost complete absence of immunolabeling for type I collagen and a few, rounded cells (Figure 2G and "4" in Figure 3A); and (5) almost complete absence of immunolabeling for type I collagen but a high cell density ("5" in Figure 3A).
Additional immunohistochemical analysis showed immunolabeling for aggrecan(Figure 2H and I) but not for type II collagen (Figure 2J and K). Aggrecan and type II collagen are markers of tissues with a fibrocartilaginous phenotype and thus,intermittent compressive load acting on the tendinous tissue[22]. Specifically, the presence of aggrecan in a tendon increases its capacity to imbibe water and, thus, to withstand compression; type II collagen is the typical collagen found in hyaline and various fibrous cartilages[22].
Collectively, the presence of immunolabeling for type I collagen and aggrecan and the absence of immunolabeling for type II collagen in the region of regenerative tendon tissue indicate that the latter was exposed to intermittent tensile load and probably slight intermittent compressive load, which is characteristic for the supraspinatus tendon (tensile load during contraction of the supraspinatus muscle;slight compressive load during adduction of the arm and wrapping the supraspinatus tendon around the humeral head).
Investigation of the section shown in Figure 3A with polarization microscopy demonstrated a clear difference in collagen fiber birefringence between regions 1 and 2, as well as absence of collagen fiber birefringence in regions 4 and 5 (Figure 3B).
A section from the second part of the biopsy that was stained with Azan trichrome stain also showed regions with elongated fibroblast-like cells arranged in long and parallel chains between collagen fibers (Figure 4A and E-G).
In addition, regions with unorganized collagen with (Figure 4B) or without(Figure 4C) formation of microvessels were found, as well as a spot with very high density of cells and microvessels (Figure 4D). Of note, no formation of any adipocytes was observed, indicating that the differentiation of stem cells (initially derived from adipose tissue) was guided by the new location and microenvironment. In the absence of adipose tissue the cells did not form adipose tissue but apparently followed the signaling coming from the injured tendon.
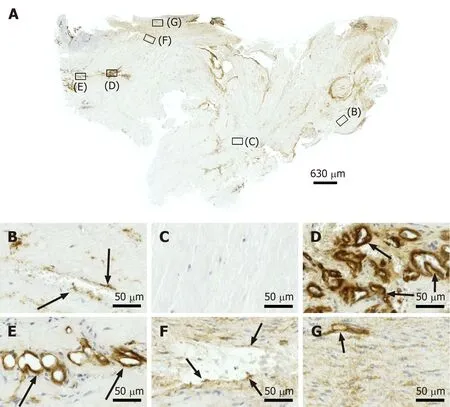
Figure 6 Immunohistochemical detection of type IV collagen in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview(insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (black arrows, immunolabeling for type IV collagen in the basement membrane of microvessels); C: position of degenerative tendon tissue without formation of microvessels; D: Position of a spot with very high density of cells and microvessels (black arrows, immunolabeling for type IV collagen in the basement membrane of microvessels); E: Position of tendon tissue in the depth of the biopsy (black arrows, immunolabeling for type IV collagen in the basement membrane of microvessels);F: Position of tendon tissue below an outer surface of the biopsy (black arrows, immunolabeling for type IV collagen in the basement membrane of microvessels); G:Position of tendon tissue at an outer surface of the biopsy (black arrow, immunolabeling for type IV collagen in the basement membrane of microvessels).
Based on the findings shown in Figure 4, sections from the second part of the biopsy were exposed to antibodies for the detection of CD34 (Figure 5), type IV collagen(Figure 6), Ki-67 (Figure 7), tenomodulin (Figure 8), type I procollagen (Figure 9), type I collagen (Figure 10), type III collagen (Figure 11), laminin (Figure 12), MMP-2(Figure 13), MMP-9 (Figure 14) and CD68 (Figure 15), revealing for each of the regions shown in Figure 4A-G a different, complex pattern of immunolabeling (summarized in Table 2). Weakest immunolabeling was found in the regions with unorganized collagen (Figure 4B and C), whereas strongest immunolabeling was observed in the spot with very high density of cells and microvessels (Figure 4D).
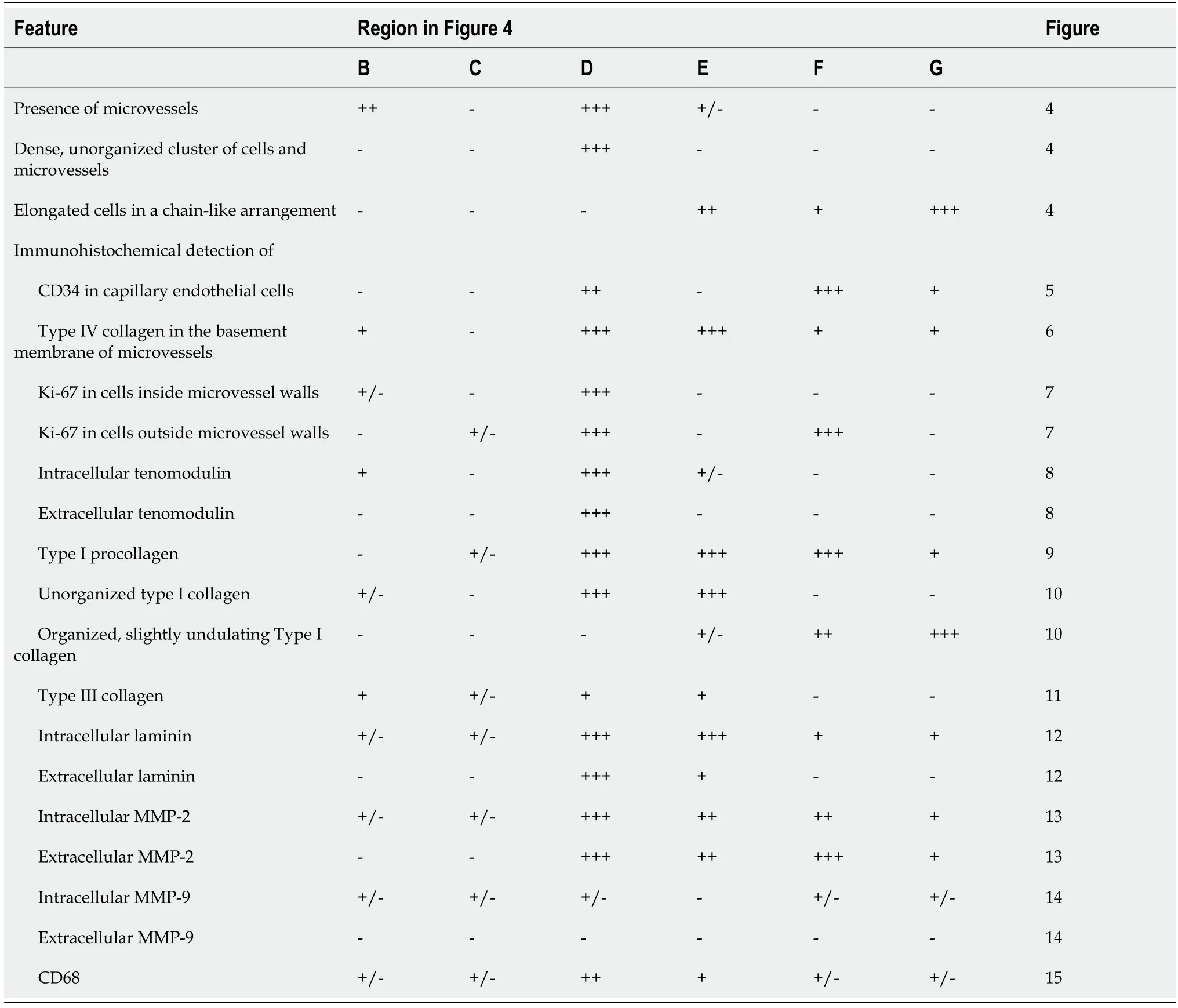
Table 2 Histological and immunohistochemical features of different regions within the second part of the biopsy that was investigated in this study
CD34 is considered a common progenitor cell marker and is expressed by a wide range of cell types, including bone marrow hematopoietic stem cells, MSCs and endothelial progenitor cells[23]. CD34 is primarily expressed by small or newly formed vessels, while endothelial cells of the placenta, lymphatic tissue and larger veins have been reported to be CD34 negative[24,25]. More specifically, CD34 is widely regarded as a marker of vascular endothelial progenitor cells (note that a subset of adult endothelial cells within smaller blood vessels was also reported to be CD34 positive[25]. More than 50% of freshly isolated MSCs express the CD34 cell marker; however, human cultured MSCs are commonly immunonegative for CD34[26]. Accordingly, the presence of CD34+ immunolabeling in endothelial cells of microvessels in regions D and F of the second part of the investigated biopsy(Figure 5D and F) may indicate ongoing angiogenesis in highly specific regions of the investigated biopsy 10 wk post injection of UA-ADRCs. On the other hand, anti CD34 immunohistochemistry could not be used to assess the potential presence of injected UA-ADRCs and their non-endothelial descendants in the investigated biopsy.
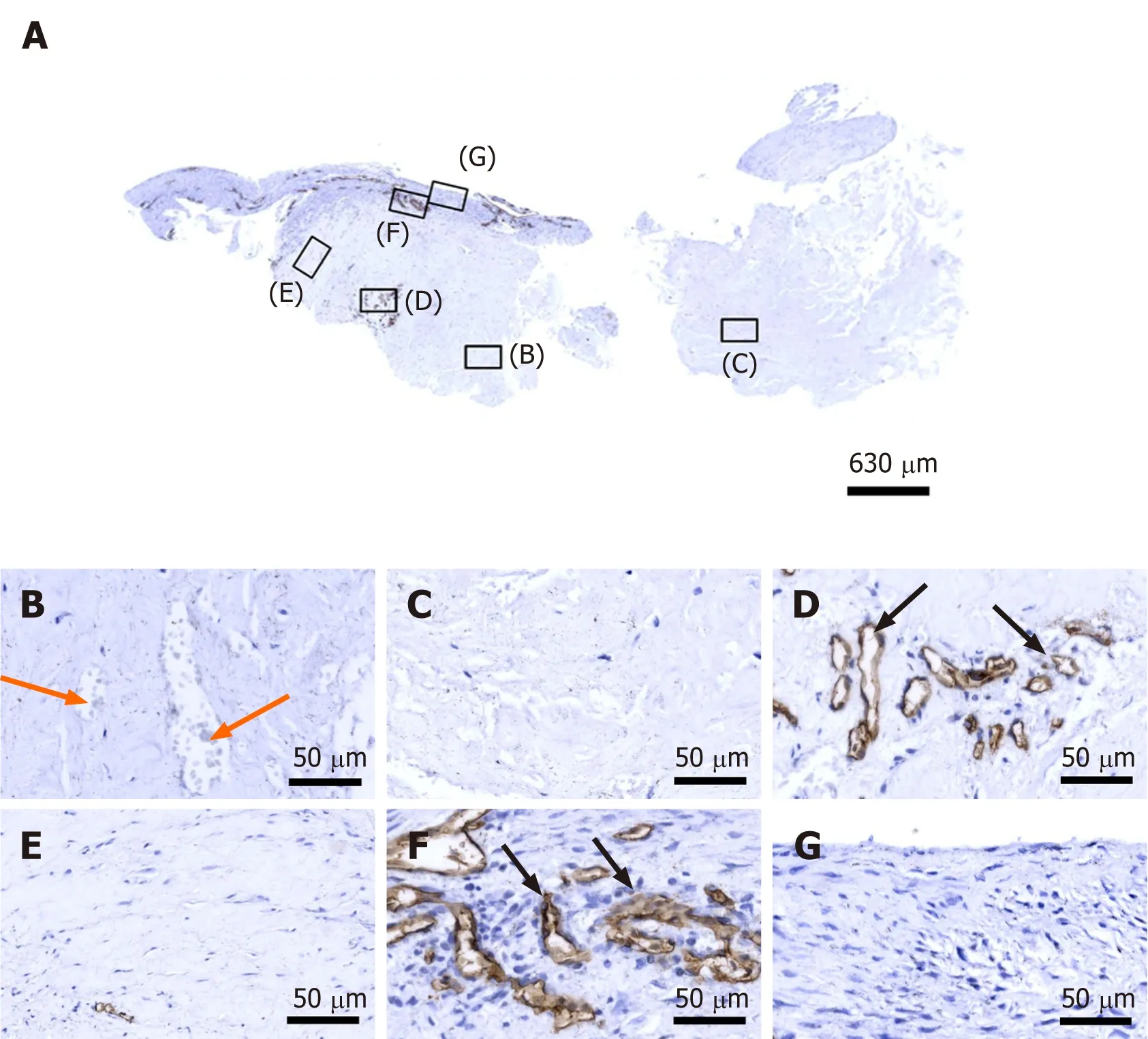
Figure 5 Immunohistochemical detection of cluster of differentiation 34 in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A:Low-power overview (insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (orange arrows, cells inside a microvessel); C: Position of degenerative tendon tissue without formation of microvessels; D: Position of a spot with very high density of cells and microvessels (black arrows, immunolabeling for cluster of differentiation (CD) 34 in endothelial cells of microvessels); E: Position of tendon tissue in the depth of the biopsy; F: Position of tendon tissue below an outer surface of the biopsy (black arrows, immunolabeling for CD34 in endothelial cells of microvessels); G: Position of tendon tissue at an outer surface of the biopsy.
The macromolecular network of type IV collagen provides the scaffold for basement membranes[27]; type IV collagen is the most abundant member of the basement membrane[28]. Thus, immunohistochemical detection of type IV collagen is suitable for detecting vessels in connective tissue independent of endothelial markers.Immunolabeling for type IV collagen was found in the basement membrane ofmicrovessels in all regions of the investigated biopsy 10 wk post injection of UAADRCs, except for regions in which no microvessels were found (Figure 6). Of note, by immunohistochemical detection of type IV collagen many vessels were found in the degenerative tendon tissue (i.e.those areas that included regions B and C in Figure 4),whereas no immunolabeling for CD34 was found in the degenerative tendon tissue.This finding demonstrates that the majority of endothelial cells in the second part of the biopsy that was investigated in this study was not immunopositive for CD34,which is in line with reports in the literature[24,25].
Ki-67 is a cellular marker for proliferation[29]. The presence of immunolabeling for Ki-67 in cells inside microvessel walls in region D of the second part of the investigated biopsy (Figure 7D) is in line with the presence of immunolabeling for CD34 in endothelial cells of microvessels in this region (Figure 5D). Furthermore, it appears reasonable to hypothesize that immunolabeling for Ki-67 in cells outside microvessel walls in region D (Figure 7D) could indicate the presence of injected UA-ADRCs and their non-endothelial descendants in this region 10 wk post injection of UA-ADRCs(note that this hypothesis cannot be tested because UA-ADRCs can in principle not be labeled); and immunolabeling for Ki-67 in cells with the characteristic morphology of tenocytes in region F indicates tendon regeneration (Figure 7F).
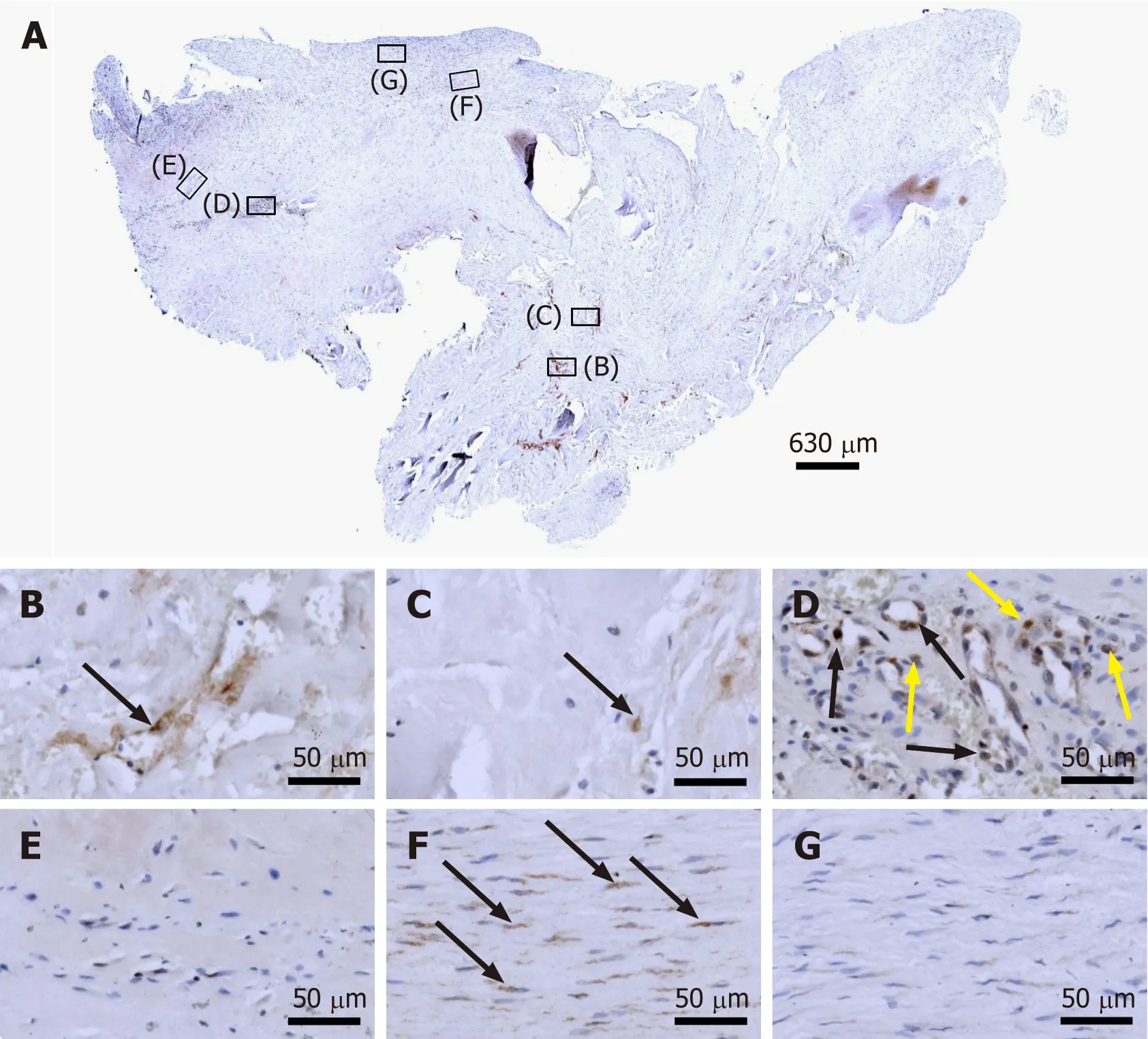
Figure 7 Immunohistochemical detection of Ki-67 in a section of the second part of the biopsy that was investigated in this study(section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview (insets,position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (black arrow,intracellular immunolabeling for Ki-67); C: Position of degenerative tendon tissue without formation of microvessels (black arrow, intracellular immunolabeling for Ki-67); D: Position of a spot with very high density of cells and microvessels (black arrows, intracellular immunolabeling for Ki-67 inside microvessel walls; yellow arrows,intracellular immunolabeling for Ki-67 outside microvessel walls); E: Position of tendon tissue in the depth of the biopsy; F: Position of tendon tissue below an outer surface of the biopsy (black arrows, intracellular immunolabeling for Ki-67 in elongated cells in a chain-like arrangement); G: Position of tendon tissue at an outer surface of the biopsy.
Tenomodulin is a tendon-specific marker important for tendon maturation, with key implications for residing tendon stem/progenitor cells and the regulation of endothelial cell migration[30-32]. The abundant intracellular and extracellular presence of tenomodulin in region D of the second part of the biopsy (Figure 8D) is in line with the hypothesis that tendon regeneration observed in the investigated biopsy was 'orchestrated' from this region, further supporting the hypothesis that region D in the second part of the investigated biopsy hosted injected UA-ADRCs and their descendants (see also Figure 4D). On the other hand, the presence of tenomoduline immunopositive cells inside microvessels in region B of the second part of the biopsy(Figure 8B) may indicate an unsuccessful attempt of the body to endogenously initiate tendon regeneration by transferring corresponding cellsviathe blood stream into the injured/degenerative tissue
Type I procollagen is a triple-stranded, rope-like molecule that is processed by enzymes outside the cell, followed by self-arrangement of the processed molecules into long, thin collagen fibrils that cross-link to one another in the extracellular space[33,34]. Accordingly, immunolabeling for type I procollagen in cells outside microvessel walls in region D as well as in regions E and F of the second part of the investigated biopsy (Figure 9D-F) indicate that these regions were involved in tendon regeneration in the investigated biopsy.
The finding of unorganized type I collagen found in regions D and E as well as of slightly undulating type I collagen in regions F and G of the second part of the investigated biopsy (Figure 10D-E) indicates that these regions as well were involved in tendon regeneration, with region G representing fully regenerated tendon tissue.
Unorganized type III collagen is present in scar tissue and is considered representing higher hardness but lower strength than organized type I collagen[35,36]. Type III collagen was found in the degenerative tissue but not the regenerative tissue in the second part of the investigated biopsy (Figure 11). Of note, the patterns of type I procollagen and type I collagen detection (Figure 9 and 10) were inverse compared to the pattern of type III collagen detection (Figure 11). This finding indicated that treatment of sPTRCT with injection of UA-ADRCs may result in histological regeneration without scar formation.
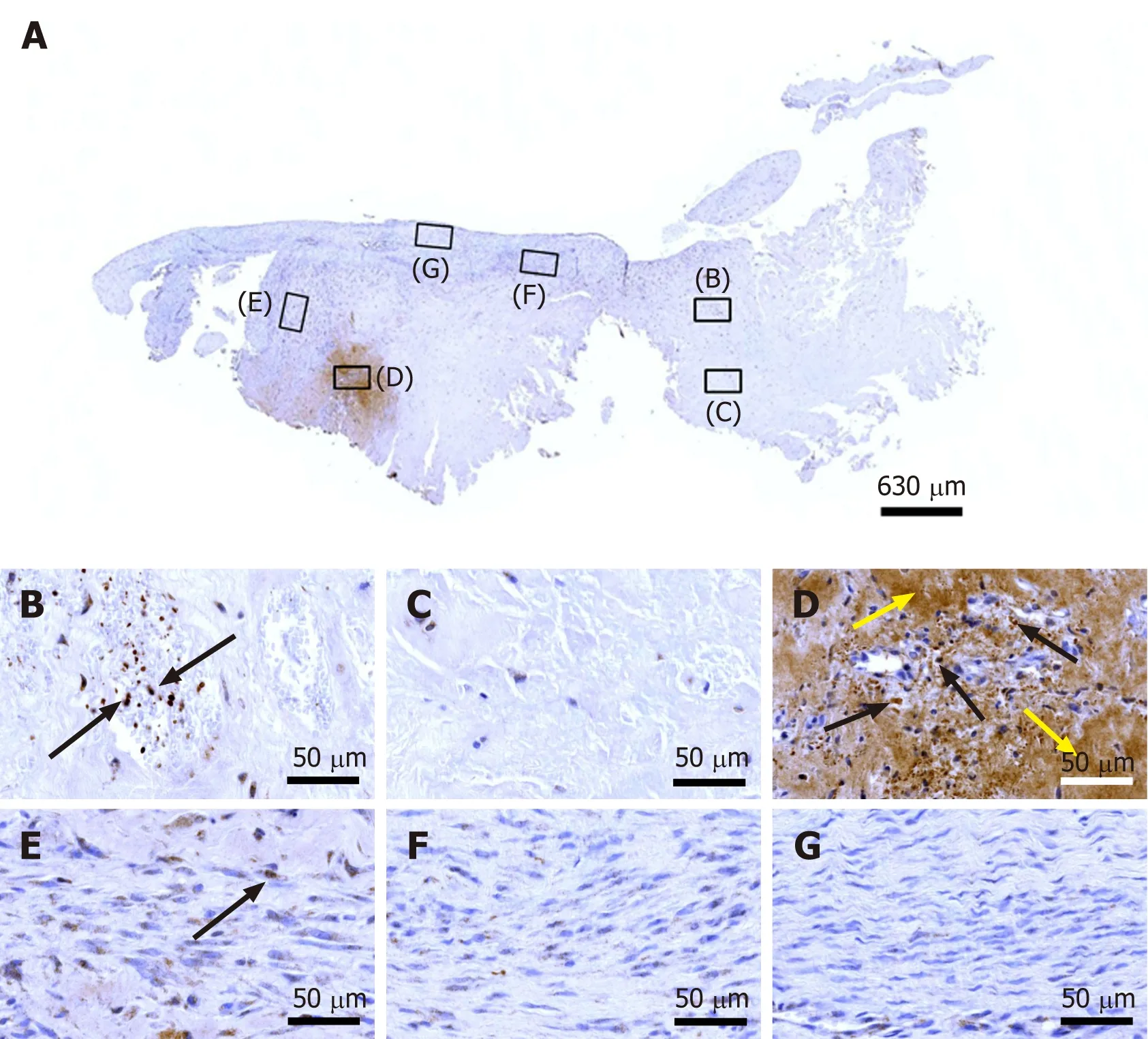
Figure 8 Immunohistochemical detection of tenomodulin in a section of the second part of the biopsy that was investigated in this study(section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview (insets,position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (black arrows,intracellular immunolabeling for tenomodulin in cells inside microvessels); C: Position of degenerative tendon tissue without formation of microvessels; D: Position of a spot with very high density of cells and microvessels (black arrows, intracellular immunolabeling for tenomodulin; yellow arrows, extracellular immunolabeling for tenomodulin); E: Position of tendon tissue in the depth of the biopsy (black arrows, intracellular immunolabeling for tenomodulin); F: Position of tendon tissue below an outer surface of the biopsy; G: Position of tendon tissue at an outer surface of the biopsy.
Laminin is an essential component of the extracellular matrix in soft tissues and modulates a number of cellular functions, including adhesion, differentiation and migration[37]. Anin vitrostudy on mouse embryonic stem cell migration found that laminin decreased cell aggregation and increased migration[38]. Anotherin vitrostudy on tenocytes isolated from injured rotator cuff tendons of human patients concluded that an early increase in the expression of laminin could be beneficial to tendon healing by expediting cellular migration, attachment and growth[39]. Thus, the presence of intracellular and extracellular immunolabeling for laminin in regions D and E of the second part of the investigated biopsy (Figure 12D and E) are in line with the hypothesis that precursors of endothelial cells and tenocytes migrated from region D (where they were generated)viaregion E to region F where tendon regeneration took place.
The interstitial collagenase MMP-2 catalyzes cleavage of collagen fibrils and soluble native type I collagen[40]. A number of recent studies demonstrated that MMP-2 plays an important role in proliferation, migration and angiogenesis of MSCs[41]. Thus, the presence of intracellular and extracellular immunolabeling for MMP-2 in regions D-F of the second part of the investigated biopsy (Figure 13D-F) is in line with the hypothesis that precursors of endothelial cells and tenocytes migrated from region D(where they were generated)viaregion E to region F where tendon regeneration took place. Of note, MMP-2 was also positively associated with adipogenic and chondrogenic differentiation of MSCs[42], which was not observed in this study. This was most probably due to the fact that the corresponding studies were performedin vitrowith single (or only a few) stimulating and inhibiting markers, whereasin vivounder the conditions of tissue injury the fate of MSCs depends on constant induction of differentiation and re-confirmation by complex signals released and communicated from the local microenvironment[43,44].
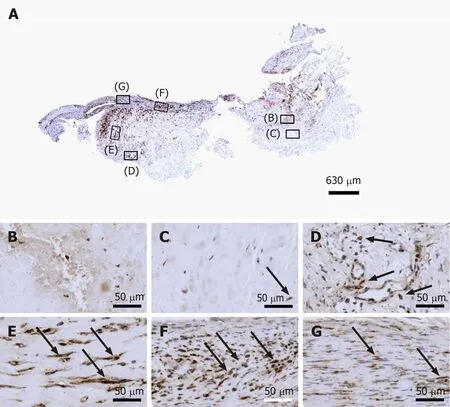
Figure 9 Immunohistochemical detection of type I procollagen in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview(insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels; C: Position of degenerative tendon tissue without formation of microvessels (black arrow, intracellular immunolabeling for type I procollagen); D: Position of a spot with very high density of cells and microvessels (black arrows, intracellular immunolabeling for type I procollagen outside microvessels); E: Position of tendon tissue in the depth of the biopsy (black arrows, intracellular immunolabeling for type I procollagen); F: Position of tendon tissue below an outer surface of the biopsy (black arrows,intracellular immunolabeling for type I procollagen); G: Position of tendon tissue at an outer surface of the biopsy (black arrows, intracellular immunolabeling for type I procollagen).
A recent study suggested that in tendons, MMP-9 is specifically involved in debridement of individual collagen fibrils following tendon overload injury, and prior to deposition of new collagen[45]. This is in line with an earlier study that demonstrated that MMP-9 is involved in tissue degradation during the early phase of healing, whereas MMP-2 contributes to tissue degradation and later remodeling[46].Another recent study on tenocyte-like cells isolated from biopsies of torn supraspinatus tendons from donors undergoing arthroscopic or open shoulder surgery found an approximately 20000 times higher mean relative mRNA level of MMP-2 than of MMP-9[47]. However, neither individual nor average time intervals between occurrence/diagnosis of tendon tear and surgery were reported in this study[47]. Collectively, these data can explain why almost no MMP-9 immunolabeling was found in the second part of the investigated biopsy (Figure 14).
CD68 is highly expressed by circulating macrophages and tissue macrophages[48].By producing a variety of growth factors (including IGF-1, VEGF-α, TGF-β and Wnt proteins) that regulate, among others, differentiation of stem and tissue progenitor cells, proliferation of endothelial cells, activation of myofibroblasts and angiogenesis,macrophages play an important role in regulating tissue regeneration following injury[48]. Thus, the presence of CD68 immunopositive cells particularly in region D (and, to a lesser extent, in region E) of the second part of the investigated biopsy (Figure 15D and E) support the hypothesis that region D represented the site of injection of UAADRCs from where tendon regeneration began and was orchestrated.
Bonar scores and results of cell counting are summarized in Table 3 and 4.
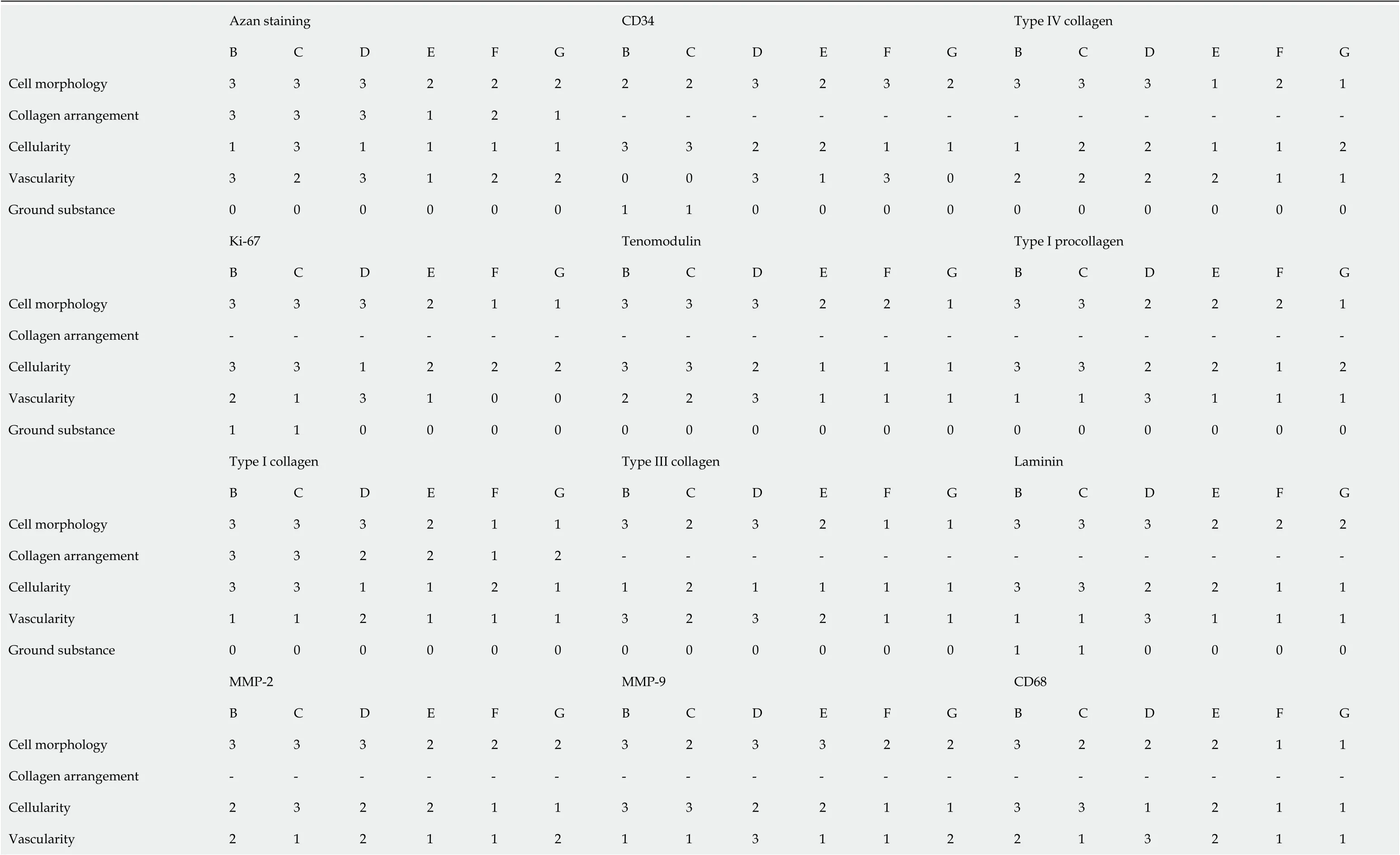
Table 3 Bonar scores of all regions B-G in Figures 4-15 of the second part of the investigated biopsy
DISCUSSION
To our knowledge this is the very first report demonstrating true regenerative healing of a partial-thickness tear in a human tendon following injection of UA-ADRCs. This is evidenced by comprehensive histological and immunohistochemical analysis of the biopsy taken from this tendon 10 wk post stem cell treatment.
With respect to the latter, the morphological appearance of regions 3 and 4 in Figure 3 and regions B and C in Figure 4-15 is in line with descriptions of the morphological appearance of degenerative supraspinatus tendon tissue at various degrees inthe literature[49-51]. Additionally, to our knowledge, spots within tendons with morphological appearance and immunohistochemical characterization as the one observed in the second part of the biopsy (Panels D in Figure 4-15) have not previously been described in the scientific literature. Besides this, in both parts of the biopsy the morphological appearance of those regions is characterized by elongated fibroblastlike cells arranged in long and parallel chains between collagen fibers (Figure 2B;Panels E-G in Figure 4-15). Organized, slightly undulating type I collagen (region 1 in Figure 3) is consistent with descriptions of the morphological appearance of a process that is known as "regenerative healing in tendons without scar formation" in the literature[52-54]. Of note, this process does not occur in spontaneous tendon healing which typically results in a localized scar defect adjacent to intact enthesis[52,53,55].Rather, regenerative healing without scar formation so far has been attributed to fetal tendons[52-54].

Note that collagen arrangement was only assessed on sections that showed type I collagen (Azan staining, type I collagen). CD: Cluster of differentiation; MMP: Matrix metalloproteinase.
The Bonar score is a well-established scoring system to classify the histopathological findings of tendinopathy and tendon degeneration, focusing on cell morphology,collagen arrangement, cellularity, vascularity and ground substance (Grade 0-Grade 4 each)[56-58]. Bonar scores determined on regions B and C (and, to a lesser extent,region E) indicated substantial tendon degeneration (Table 3), which was in line with the detailed immunohistochemical analysis of the investigated biopsy. On the other hand, Bonar scores determined on regions D, F and G would also have indicated tendon degeneration (or tendinopathy, respectively), despite the fact that these regions showed different aspects of histological regeneration of injured human tendon tissue.In addition, Bonar scores did not adequately reflect the substantial differences in cell numbers among different regions that were found by cell counting (Table 4).Accordingly, the Bonar score appears unsuitable for characterizing histological regeneration of injured human tendons after injection of UA-ADRCs.
In animal models, injections of adult stem cells isolated from adipose tissue into pathologic tendon tissue has produced a positive biological response[59-63]. Reported beneficial effects include a decreased number of inflammatory cells, improved regeneration of tendons with less scarred healing, improved collagen fiber arrangement, higher load-to-failure and higher tensile strength of the treated tendons[59-63]. These findings support the results we obtained by investigating a biopsy of a human tendon 10 wk post injection of UA-ADRCs. Our comprehensive immunohistochemical analyses of the biopsy with a broad number of antibodies (Table 1 and 2)allow the conclusion that region D in the second part of the biopsy (Panels D in Figure 4-15) represented the site of injection of UA-ADRCs from where tendon regeneration started. It further indicates that endothelial precursors and tenocytes might have migrated from region D (where they were generated) via region E to regionF, in which tendon regeneration took place. Induction of differentiation and reconfirmation were most probably guided by complex signals released and communicated from the local microenvironment[43,44].
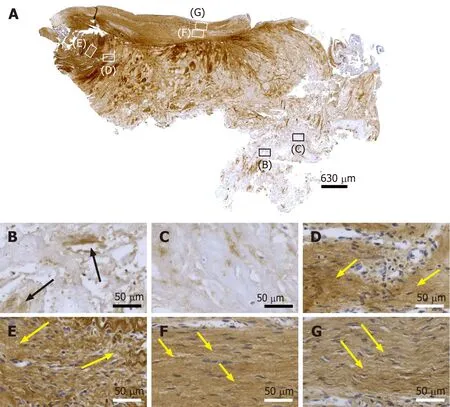
Figure 10 Immunohistochemical detection of type I collagen in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview(insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (black arrows, immunolabeling for unorganized type I collagen); C: Position of degenerative tendon tissue without formation of microvessels; D: Position of a spot with very high density of cells and microvessels (yellow arrows, immunolabeling for unorganized type I collagen); E: Position of tendon tissue in the depth of the biopsy (yellow arrows, immunolabeling for unorganized type I collagen); F: Position of tendon tissue below an outer surface of the biopsy (yellow arrows, immunolabeling for organized, slightly undulating type I collagen); G: Position of tendon tissue at an outer surface of the biopsy (yellow arrows, immunolabeling for organized, slightly undulating type I collagen).
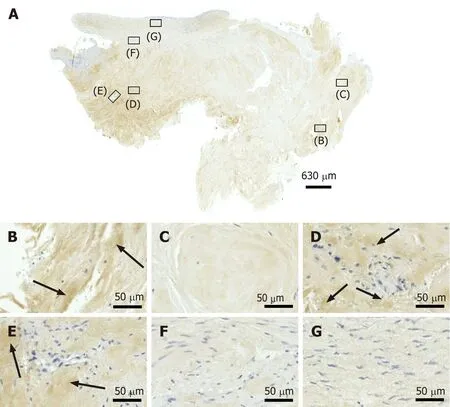
Figure 11 Immunohistochemical detection of type III collagen in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Low-power overview(insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels (black arrows, extracellular immunolabeling for type III collagen); C: Position of degenerative tendon tissue without formation of microvessels; D: Position of a spot with very high density of cells and microvessels (black arrows, extracellular immunolabeling for type III collagen); E: Position of tendon tissue in the depth of the biopsy (black arrows, extracellular immunolabeling for type III collagen); F: Position of tendon tissue below an outer surface of the biopsy; G: Position of tendon tissue at an outer surface of the biopsy.
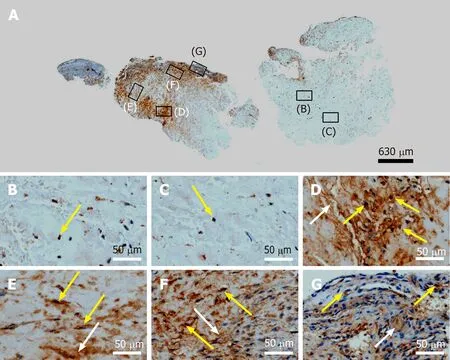
Figure 13 Immunohistochemical detection of matrix metalloproteinase 2 (MMP-2) in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A:Low-power overview (insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels [yellow arrow, intracellular immunolabeling for matrix metalloproteinase 2 (MMP-2)]; C: Position of degenerative tendon tissue without formation of microvessels (yellow arrow, intracellular immunolabeling for MMP-2); D: Position of a spot with very high density of cells and microvessels (yellow arrows, intracellular immunolabeling for MMP-2; white arrow, extracellular immunolabeling for MMP-2); E: Position of tendon tissue in the depth of the biopsy (yellow arrows, intracellular immunolabeling for MMP-2; white arrow, extracellular immunolabeling for MMP-2); F: Position of tendon tissue below an outer surface of the biopsy (yellow arrows,intracellular immunolabeling for MMP-2; white arrow, extracellular immunolabeling for MMP-2); G: Position of tendon tissue at an outer surface of the biopsy (yellow arrows, intracellular immunolabeling for MMP-2; white arrow, extracellular immunolabeling for MMP-2).
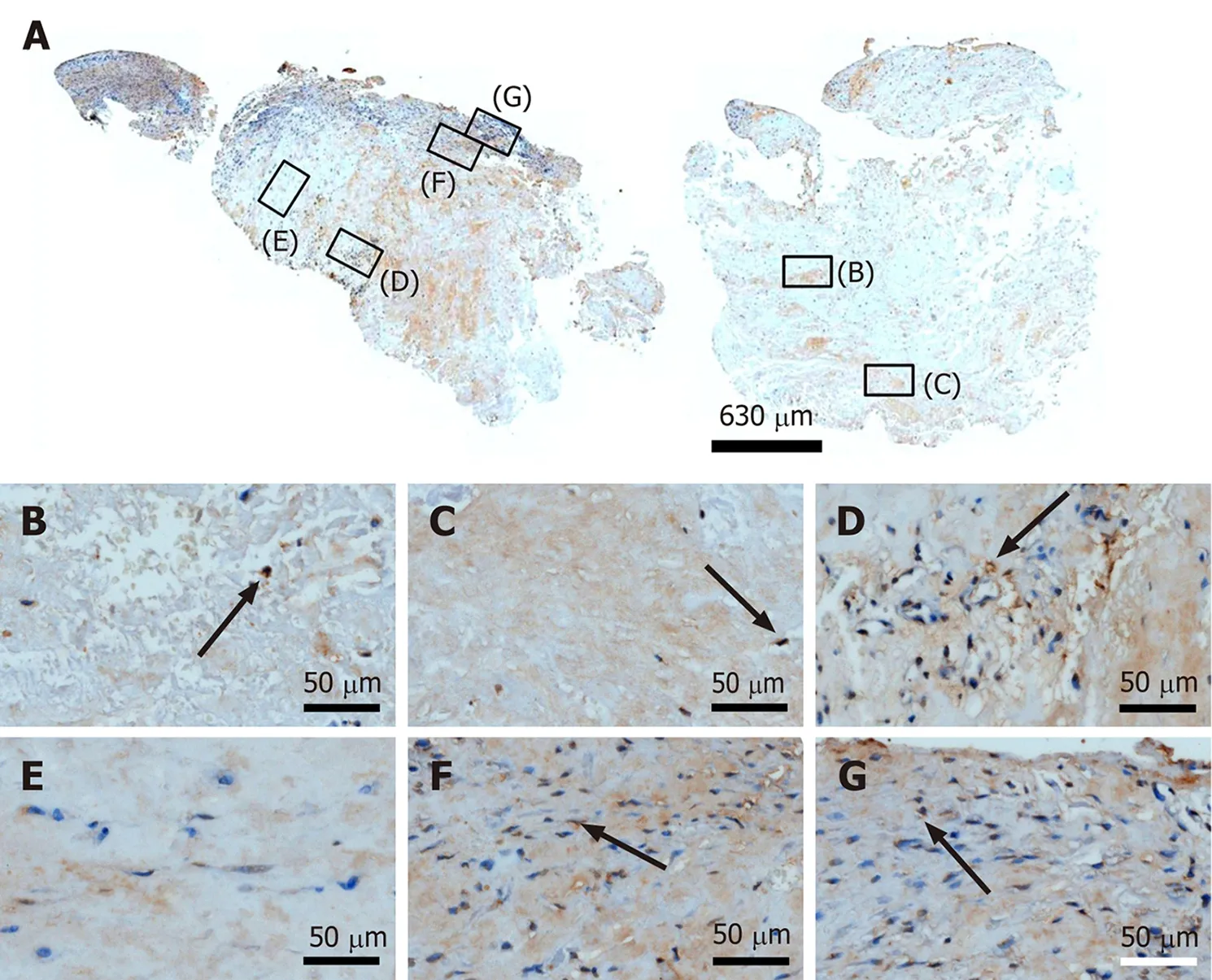
Figure 14 Immunohistochemical detection of matrix metalloproteinase 9 in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A: Lowpower overview (insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels [black arrow, intracellular immunolabeling for matrix metalloproteinase 9 (MMP-9)]; C: Position of degenerative tendon tissue without formation of microvessels (black arrow, intracellular immunolabeling for MMP-9); D: Position of a spot with very high density of cells and microvessels (black arrow, intracellular immunolabeling for MMP-9); E: Position of tendon tissue in the depth of the biopsy; F: Position of tendon tissue below an outer surface of the biopsy (black arrow,intracellular immunolabeling for MMP-9); G: Position of tendon tissue at an outer surface of the biopsy (black arrow, intracellular immunolabeling for MMP-9).
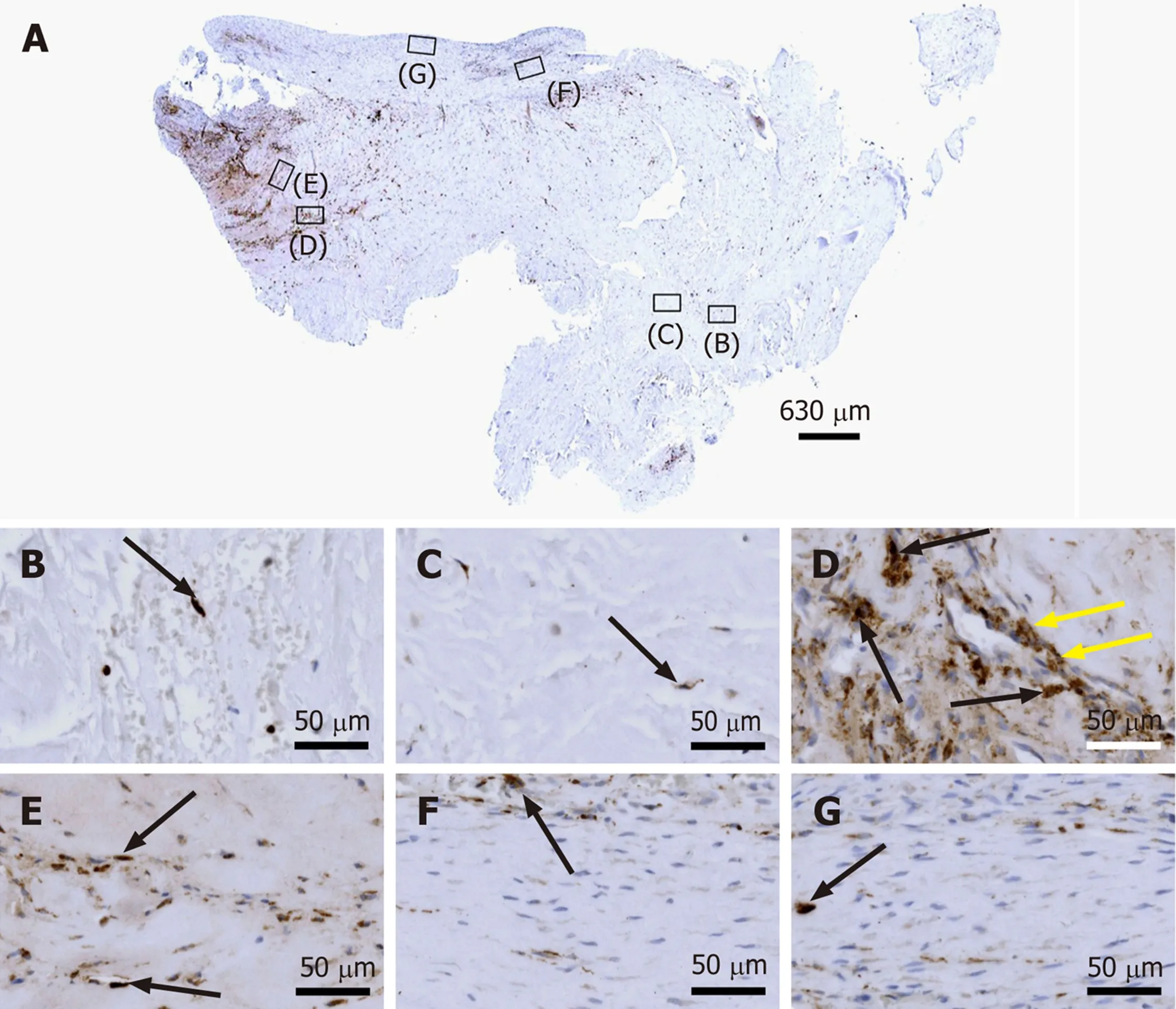
Figure 15 Immunohistochemical detection of cluster of differentiation 68 in a section of the second part of the biopsy that was investigated in this study (section adjacent to the one shown in Figure 4; counterstaining was performed with Mayer's hematoxylin). A:Low-power overview (insets, position of the high-power photomicrographs displayed in Panels B-G); B: Position of degenerative tendon tissue with formation of microvessels [black arrow, intracellular labeling for cluster of differentiation (CD68)]; C: Position of degenerative tendon tissue without formation of microvessels(black arrow, intracellular labeling for CD68); D: Position of a spot with very high density of cells and microvessels (black arrows, intracellular labeling for CD68 outside microvessel walls; yellow arrows, intracellular immunolabeling for CD68 inside microvessel walls); E: Position of tendon tissue in the depth of the biopsy(black arrows, intracellular labeling for CD68); F: Position of tendon tissue below an outer surface of the biopsy (black arrow, intracellular labeling for CD68); G:Position of tendon tissue at an outer surface of the biopsy (black arrow, intracellular labeling for CD68).
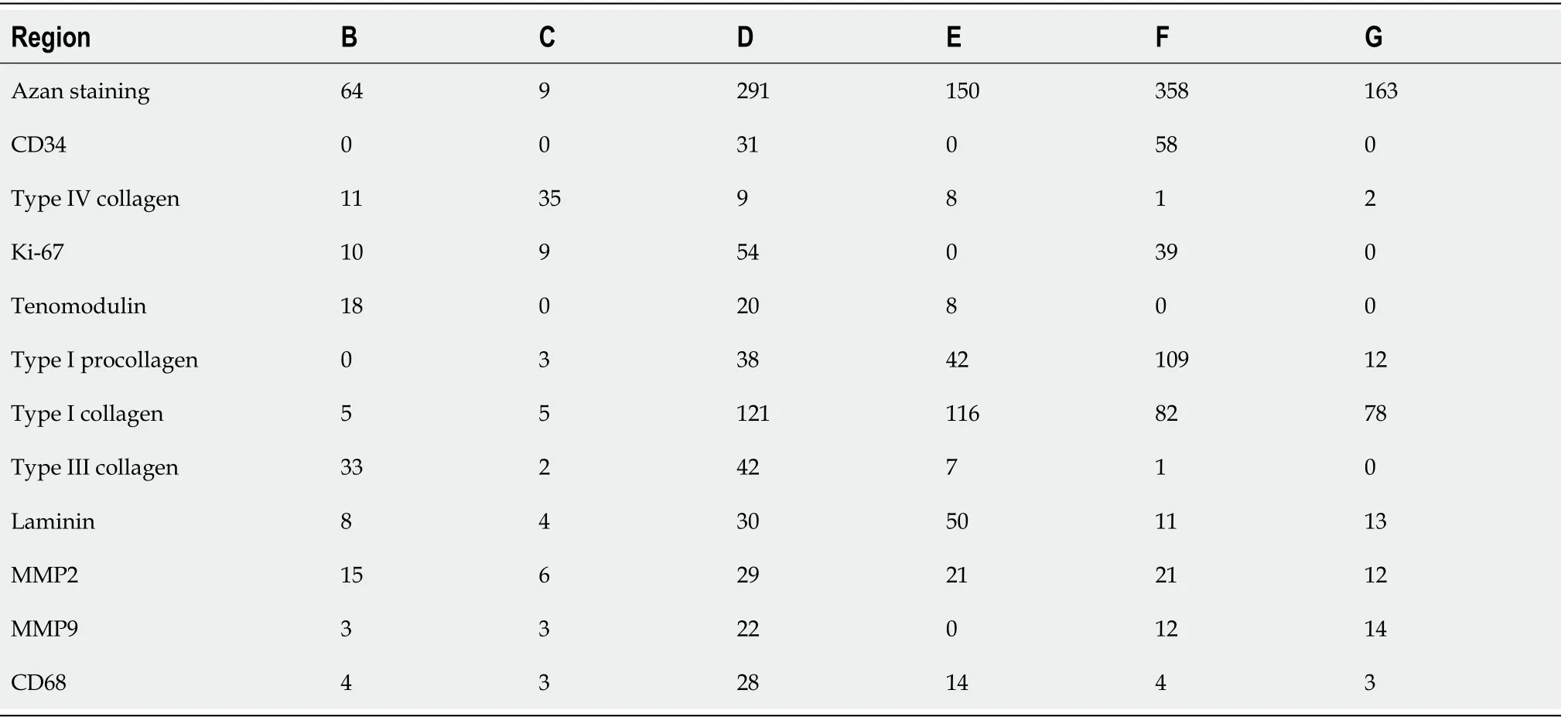
Table 4 Number of cells per 45000 μm2 area each in all regions B-G in Figures 4-15 of the second part of the investigated biopsy
Some authors raised concerns about the use of ADSCs/ADRCs in tendon regeneration due to their assumed “indigenous” preference towards forming adipocytes'[64,65], albeit without reference to corresponding findings in the literature.We did not observe formation of adipocytes in the investigated biopsy. The latter result is in line with an earlier finding of our group clearly demonstrating that UAADRCs do not form adipocytes when used for treating chronic myocardial infarction[66]. Furthermore, in guided bone regeneration using UA-ADRCs we found substantially less formation of adipocytes (only 2% with stem cells) than without the use of UA-ADRCs (18%)[67]. Collectively, these findings do not only support the role of the local microenvironment for induction and guidance of differentiation of stem cells in the target tissue, but also underscore the need to exercise caution in drawing incorrect conclusions absent of convincing scientific evidence, or without a correct understanding of stem cell biology.
It should be pointed out again that UA-ADRCs can in principle not be labeled because this would render them modified. In this regard UA-ADRCs fundamentally differ from ADSCs that can be derived from UA-ADRCs by culturing[7]. In contrast to UA-ADRCs, ADSCs can be intracellularly labeled with fluorescent probes, as demonstrated by ourselves[7] and many others. Furthermore, ADRCs have been demonstrated to survive, proliferate and differentiate for a long time in the host tissue(discussed in detail in[7]). Because UA-ADRCs cannot be labeled, it is in principle not possible to provide the same evidence for UA-ADRCs as has be done in the literature for ADCS.
For the sake of completeness it should be mentioned that mesenchymal stem cellderived exosomes have been shown to promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem and progenitor cells[68-70].Thus, one cannot exclude that paracrine effects may play a certain role in histological regeneration of injured human tendon tissue after injection of UA-ADRCs. On the other hand, the latter would only limit the significance of our results if histological regeneration of injured human tendon tissue after injection of UA-ADRCs would be fully due to paracrine effects (only in this case one could replace injection of UAADRCs by injection of exosomes or secretomes of mesenchymal stem cells). However,there is no evidence in the literature supporting this view. In this regard a recent systematic review[71] summarized 5 studies in which the potential of stem cells conditioned medium (secretome) in ligament and tendon healing was investigated. Of note, in none of these studies injection of stem cells conditioned medium was compared to injection of UA-ADRCs or ADSCs. In summary, the question about potential contribution of paracrine effects to the histological regeneration of injured human tendon tissue after injection of UA-ADRCs as described in this study cannot be answered at this time. Clarifying the role of paracrine effects in tendon repair after injection of UA-ADRCs would require controlled trials with repeated taking of biopsies. This may be achieved in animal studies in the future.
Conclusions of this study are presented on the basis of a single time point analysis of molecular and cellular events. As such, limitations consist in the fact that only a single patient was investigated; no control biopsy was analyzed, and the scientists who analyzed the biopsy were not blinded. It further was not the aim of the present study to establish a clinical treatment. To more conclusively evaluate clinical results with UA-ADRCs for incomplete tendon tears, a respective pivotal RCT is now recruiting[72], which is based on the encouraging clinical results of the pilot study[11].
CONCLUSION
In summary, the results of this study indicate, for the first time, that treatment of an injured human tendon with unmodified, autologous regenerative cells can enable true regenerative healing. This process has previously been attributed only to fetal tendon
development. It is of special importance that this success was achieved without prior manipulation, stimulation and/or (genetic) reprogramming of the cells injected.
ACKNOWLEDGEMENTS
We thank Aschauer B, Haderer A, Harbauer C and Tost S for excellent and highly valuable technical support.
杂志排行
World Journal of Stem Cells的其它文章
- Epigenetic modulators for brain cancer stem cells: Implications for anticancer treatment
- Mechanisms involved in selecting and maintaining neuroblastoma cancer stem cell populations, and perspectives for therapeutic targeting
- Roles of mitochondrial unfolded protein response in mammalian stem cells
- Stem cell therapies in tendon-bone healing
- Exosomal microRNAs from mesenchymal stem/stromal cells:Biology and applications in neuroprotection
- Immunotherapy against programmed death-1/programmed death ligand 1 in hepatocellular carcinoma: Importance of molecular variations, cellular heterogeneity, and cancer stem cells
