Roles of mitochondrial unfolded protein response in mammalian stem cells
2021-07-30LiFangGuJiaQiChenQingYinLinYanZhouYang
Li-Fang Gu, Jia-Qi Chen, Qing-Yin Lin, Yan-Zhou Yang
Li-Fang Gu, Jia-Qi Chen, Qing-Yin Lin, Key Laboratory of Fertility Preservation and Maintenance, Ministry of Education, Key Laboratory of Reproduction and Genetics in Ningxia,Department of Histology and Embryology, School of Basic Medicine, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
Yan-Zhou Yang, Key Laboratory of Fertility Preservation and Maintenance, Ministry of Education, Key Laboratory of Reproduction and Genetics in Ningxia, Department of Histology and Embryology, School of Basic Medicine, Ningxia Medical University, Yinchuan 750001,Ningxia Hui Autonomous Region, China,
Abstract The mitochondrial unfolded protein response (UPRmt) is an evolutionarily conserved adaptive mechanism for improving cell survival under mitochondrial stress. Under physiological and pathological conditions, the UPRmt is the key to maintaining intracellular homeostasis and proteostasis. Important roles of the UPRmt have been demonstrated in a variety of cell types and in cell development,metabolism, and immune processes. UPRmt dysfunction leads to a variety of pathologies, including cancer, inflammation, neurodegenerative disease,metabolic disease, and immune disease. Stem cells have a special ability to selfrenew and differentiate into a variety of somatic cells and have been shown to exist in a variety of tissues. These cells are involved in development, tissue renewal, and some disease processes. Although the roles and regulatory mechanisms of the UPRmt in somatic cells have been widely reported, the roles of the UPRmt in stem cells are not fully understood. The roles and functions of the UPRmt depend on stem cell type. Therefore, this paper summarizes the potential significance of the UPRmt in embryonic stem cells, tissue stem cells, tumor stem cells, and induced pluripotent stem cells. The purpose of this review is to provide new insights into stem cell differentiation and tumor pathogenesis.
Key Words: Mitochondrial unfolded protein response; Mammals; Stem cells; Cancer
INTRODUCTION
Mitochondria are double-membrane-bound organelles composed of four compartments: The outer and inner membranes, the intermembrane space, and the matrix[1]. While mitochondria provide some metabolic advantages for eukaryotic cells, they may be detrimental in other ways[2]. For example, reactive oxygen species(ROS) produced by mitochondria damage the nucleus, cytoplasm, and mitochondria,leading to mutations, aging, and diseases[3]. The implication is that mitochondria may be significant to mammalian stem cell functions[4].
The normal functions of mitochondria (mitochondrial homeostasis) are very important for cells and individuals. Therefore, to maintain mitochondrial homeostasis,organisms have evolved a series of mitochondrial quality control pathways, one of which is the mitochondrial unfolded protein response (UPRmt)[5]. UPRmtis an adaptive transcriptional response that was initially described as a mechanism by which cells maintain mitochondrial protein homeostasis during mitochondrial dysfunction. The program includes genes that promote mitochondrial protein homeostasis and defective organelle recovery[6]. It transfers signals that are helpful for maintaining the homeostasis of mitochondria-related proteins and cell survival from the mitochondrion to the nucleus[7]. It can directly affect the occurrence and development of aging,neurodegenerative diseases, cancer, and other diseases[8].
The mitochondrial UPRmtpathways in mammalian cells are largely unknown. The following information on UPRmtpathways has been discovered thus far[9-11]:(1) The UPRmtpathway is induced by protein accumulation in the mitochondrial matrix; (2)The UPRmtpathway is induced by protein accumulation in the mitochondrial membrane gap; (3) The UPRmtpathway is regulated by the activating transcription factor associated with stress-1 (ATFS-1) homologous geneATF5; and (4) The UPRmtpathway is regulated by the heat shock factor 1-single-stranded DNA-binding protein 1 (HSF1-SSBP1) complex under heat stress.
Through these four pathways, the mitochondrial load is ameliorated by the following four methods: (1) When a large number of misfolded proteins accumulate in mitochondria, c-Jun N-terminal kinase 2 (JNK2) is activated, which promotes the phosphorylation of c-Jun[7]. Activated c-Jun binding at an AP-1 binding site induces CCAAT-enhancer binding protein (C/EBP) homologous protein (CHOP) and C/EBP expression. CHOP and C/EBP proteins form dimers that act as transcription factors by binding the promoter of UPRmt-related genes, thereby inducing the expression of mitochondrial heat shock proteins and proteases[12,13]; (2)When a large number of misfolded proteins accumulate in the mitochondrial membrane gap, a large number of ROS produced by mitochondria can activate AKT kinase. Phosphorylated AKT kinase promotes the estrogen receptor (ER)activity, induces theNRF1gene to promote biosynthesis in mitochondria, and induces the expression of mitochondrial protease high temperature requirement protein A2 to restore mitochondrial function[11]; (3)ATF5 functions similarly to the ATFS-1 pathway induced in nematodes[14]. ATF5 induces high expression of mitochondrial heat shock proteins (HSP60 and mtHSP70),the mitochondrial protease Lon, and the antimicrobial peptide HD-5 in mammalian cells and promotes cell proliferation and mitochondrial function recovery under stress[14]; and (4) Under the condition of heat stress, the mitochondrial single-stranded DNA-binding proteins SSBP1 and HSF1 jointly regulate the UPRmtpathway. Under the condition of heat stress or other protein-induced toxicity, mitochondrial membrane potential is decreased, and SSBP1 is discharged from the mitochondria by means of the ANT- VDAC1 complex. After binding with HSF1, SSBP1 is transported to the nucleus,and through the recruitment of chromatin regulators, open chromatin is formed to drive high levels of transcription. The HSF1-SSBP1 complex is associated with the expression of nuclear and cytoplasmic molecular chaperones (HSP70,etc.) and mitochondrial molecular chaperones (HSP60 and HSP10)[15].
Although the precise pathways and marker molecules of the UPRmtare not known,ATF4, CHOP, HSP60, HSP10, caseinolytic protease proteolytic subunit (CLPP), LON peptidase 1, mitochondrial (Lonp1), JNK2, c-Jun, C/EBP protein, Sirtuin3 (SIRT3), and ATF5 have been found to be important regulators of the UPRmtin mammals[16-19].Most of these proteins are molecular chaperones and proteolytic enzymes that promote the correct folding of mitochondrial proteins, and the UPRmtis a protective program activated by the transcriptome of gene groups that include molecular chaperone and proteolytic enzyme genes with transcription initiated by mitochondria to maintain the homeostasis of their internal proteins[20].
Stem cells produce all the cells and build tissue structure in the body, and they are critical for maintaining tissue homeostasis. The characteristics of stem cells are heterogeneity and plasticity. Understanding the properties of stem cells improves our ability to maintain tissue homeostasis[21].
However, it is not clear whether the UPRmtis associated with mitochondrial dysfunction, protein homeostasis, oxidative stress, and/or autophagy in stem cells.Therefore, studying the link between the UPRmtand stem cells is crucial to understanding the development of human beings. UPRmtactivation in stem cells may act as a sentinel against mitochondrial damage[22].
In this review, we discuss the roles of the UPRmtin stem cell proliferation, differentiation, and aging in mammals (Figure 1), and the role of UPRmt-regulated genes in stem cells is summarized (Table 1). This work provides a novel perspective on the maintenance of cell homeostasis, life extension, stem cell therapy, and cancer.
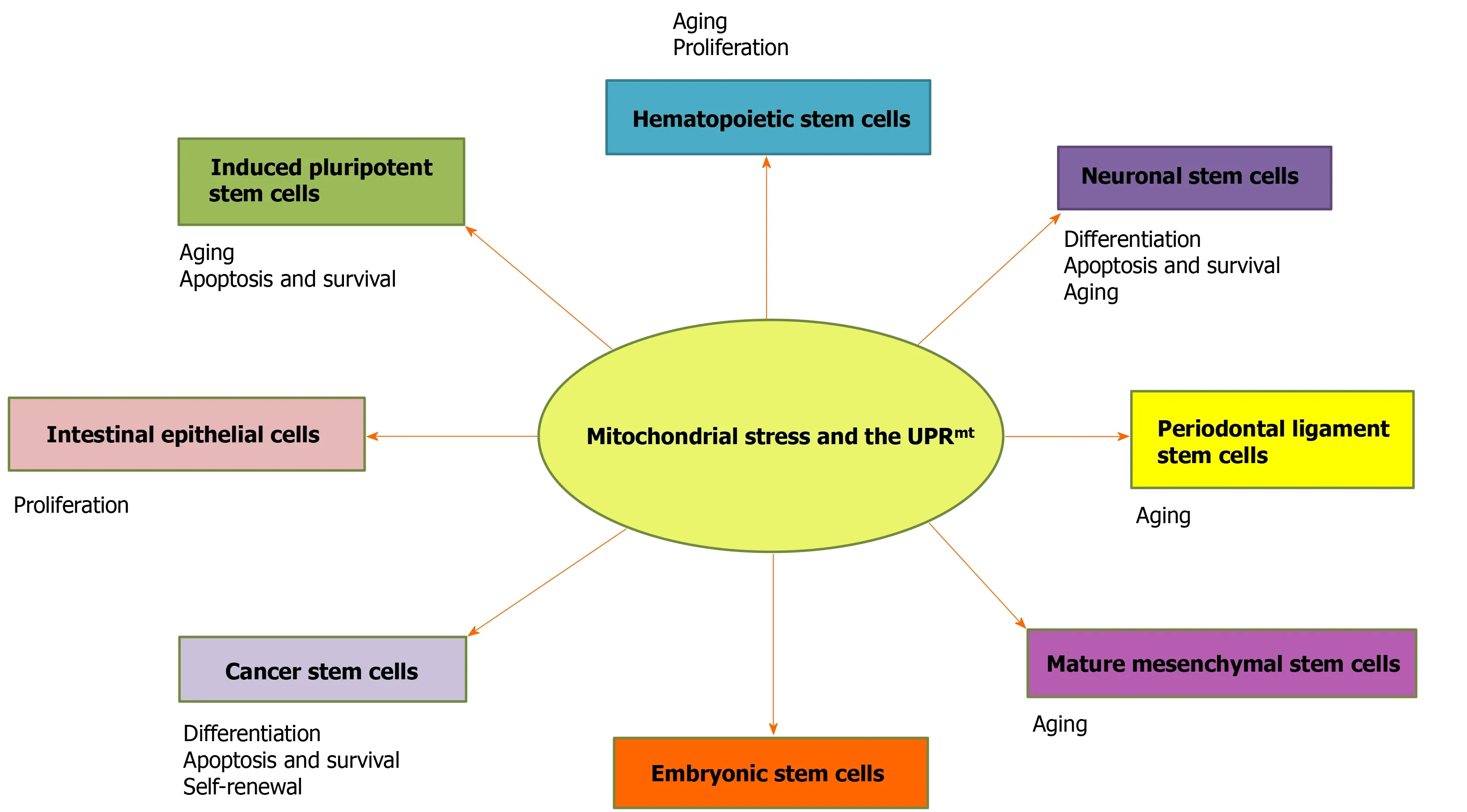
Figure 1 Roles of the UPRmt in stem cells. The activation of the mitochondrial unfolded protein response is involved in stem cell survival, self-renewal,proliferation, differentiation, and apoptosis to maintain multipotency.
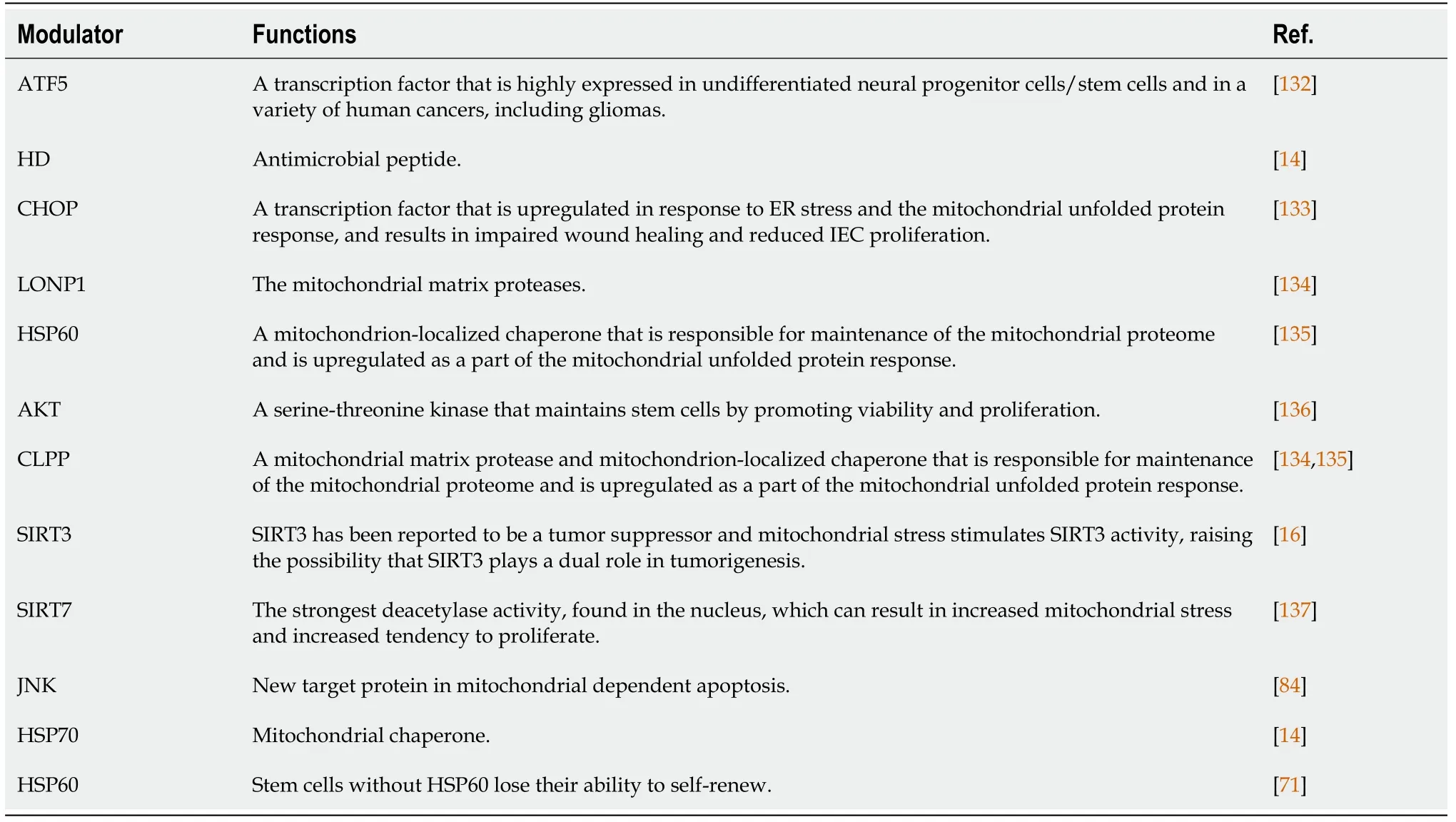
Table 1 List of mitochondrial stress and mitochondrial unfolded protein response modulators in stem cells
MITOCHONDRIA UNFOLDED PROTEIN RESPONSE AND STEM CELLS
Embryonic stem cells and mitochondrial unfolded protein response
Embryonic stem cells (ESCs) are derived from the blastocyst inner cell mass (ICM).In vivo, ESCs differentiate into three primary germ layers: The ectoderm, endoderm, and mesoderm.In vitrodifferentiation of human ESCs can help with research into certain diseases, screenings for drug discovery, and identifying cells for regenerative use[22].Mitochondria are essential for maintaining the properties of ESCs and regulate their subsequent differentiation into different cell lineages[23,24]. Studies have shown that the proliferation and differentiation of ESCs depend on the normal function of mitochondria[25].
AKT1 is activated and transferred to mitochondria after stimulation by growth factors in ESCs. Genes that promote the proliferation and survival of ESCs are upregulated, while genes that promote differentiation are downregulated[26]. CHOP activates UPRmt-related genes, and its expression is inducedviaactivation of Jun,which is mediated by c-Jun N-terminal kinase 2[12,13]. Interestingly, the growth of ESCs was not affected by c-Jun deficiency, but c-Jun-deficient fibroblasts was arrested in the G1 phase of the cell cycle, suggesting that the effect of c-Jun on cell proliferation is cell type-dependent[27].Does the absence of c-Jun in ESCs affect fibroblast differentiation? Pharmacological and genetic evidence supports the supposition that c-Jun plays an important role in neural induction of ESCs[28]. The effect of UPRmt-induced c-Jun in intestinal epithelial cells (IECs) depends on the activity of the mitochondrial protease CLPP and cytoplasmic kinase PKR[29]. Indeed, c-Jun is a vital regulatory factor of the UPRmt[16-19].
Studies have shown that ESCs have only a small number of immature mitochondria,and additional mitochondria with mature characteristics, such as fully developed cristae, dense matrix, and higher oxidation capacity, are evident during the process of differentiation[30,31]. It is speculated that the UPRmtmay be involved in the differentiation process of ESCs.
HSP60 is a mitochondrial protein important for folding key proteins after its introduction into the mitochondrion. In the heart, the binding of HSP60 to Bax in the cytoplasm plays a key role in the regulation of apoptosis[32]. Overexpression of HSP60 increased the expression of the antiapoptoticBcl-2gene and decreased the level of theproapoptotic Bax protein[33]. The apoptosis induced by mycotoxin citrinin in ESCs was induced by ROS production, which also increased the cytoplasmic free calcium level, intracellular nitric oxide production, the Bax/Bcl-2 ratio, the loss of mitochondrial membrane potential, the release of cytochrome c, the activation of caspase-9 and caspase-3[34], and the levels of p21-activated protein kinase 2 and c-Jun N-terminal protein kinase. The UPRmtcan protect ESCs against apoptosis. Other studies with ESCs suggested that SIRT1 downregulation can lead to the acetylation/ phosphorylation of forkhead transcription factor pathways such as FOXO1 and, in association with PTEN and JNK, block oxidative stress-induced apoptosis[35]. Mitochondrial HSP60 plays an important role in maintaining cell viability.HSPD1encodes HSP60, and embryos with homologousHSPD1mutations die after implantation[36]. Changes in key UPRmtrelated genes may be the reason for the failure of ESCs to survive.
However, evidence showing that mitochondrial homeostasis regulates the pluripotency of ESCs remains to be seen[37], and the exact role of the UPRmtneeds to be explored in future studies.
GERMLINE STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
Germline stem cells (GSCs) were categorized into female GSCs (FGSCs) and spermatogonial stem cells (SSCs). Research on human GSCs was originally on SSCs. The role of the UPRmtin SSCs has rarely been reported, but the role of the UPRmtin sperm has been discussed. Male mammals are able to continuously produce sperm due to the self-renewal and differentiation abilities of SSCs[38]. The conventional wisdom is that the number of follicles in mammals ceases to increase once a primal follicular pool is formed after birth. With development, maturation, and atresia, follicles are constantly depleted, ovarian function gradually declines, and women gradually enter menopause[39]. However, the discovery of FGSCs, which are derived from primitive germline cells and have the ability to differentiate into oocytes in a directional manner, is expected to lead to the replenishment of depleting primal follicular pools[40].Although the role of the UPRmtin FGSCs has rarely been reported, the roles of the UPRmtin follicular development and atresia have been discussed.
The differentiation of GSCs is characterized by gradual changes in the structure of multiple organelles, among which the mitochondrion plays unique roles[41]. During oocyte maturation and senescence, mitochondrial aggregation is related to germ cell formation and epigenetic regulation[42].
More recently, the absence ofCLPP, a mitochondrial stress response gene, has been shown to cause female infertility and accelerate ovarian follicular failure[43].CLPPmutation was observed in Perrault syndrome, which is associated with defects in human ovaries[44]. The study ofCLPP-null mice (usingCLPPgene ablation) showed complete female and male infertility[45].CLPPdeletion caused selective profound vulnerability of specific cells in testes and ovaries. Therefore, mutations inCLPPmay be related to the survival of GSCs. C/EBPβ is a transcriptional activator of PGC-1α in developing embryos, whereas CHOP blocks the DNA-binding ability of C/EBPβ by forming a CHOP/C/EBP heterodimer and repressing PGC-1α expression[46]. In mouse GC culture, upregulated CHOP expression was induced, leading to apoptosis.ATF4 and CHOP expression was higher in the GCs of goats with follicular atresia[46].This suggests that the UPRmtmay be related to GC apoptosis, which damages the viability of oocytes and embryos.
The UPRmtmarker c-Jun is involved in a number of mammalian male reproductive processes, including spermatogenesis, sperm maturation and activation, and acrosomal responses prior to oocyte fertilization[47]. Therefore, the role of the UPRmtin GSCs in infancy might be explored in future studies.
HEMATOPOIETIC STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
Hematopoietic stem cells (HSCs) are adult stem cells in the blood system. HSCs form a heterogeneous population with the capacity for long-term self-renewal and the potential to differentiate into various mature blood cells. HSCs, similar to many types of stem cells, are particularly vulnerable to damage from ROS, and the damage may be transmitted to progenitor cells, leading to various pathological conditions[48,49]. The main sources of ROS in cells are mitochondria[50].
A study showed that the UPRmtis activated upon HSC transition from quiescence to proliferation[51]. Remodeling the activity of SIRT7, a component of the UPRmt,translates into a reduction in quiescence, and higher SIRT7 activation can rescue the reduced regenerative capacity of aged HSCs[17]. These findings suggest that inhibition of SIRT7 can enhance the biological generation of mitochondria and activate the UPRmt, thereby reducing its quiescence and differentiation ability[4]. Thus, SIRT7-mediated UPRmtmay be important for cells that experience bursts of mitochondrial biogenesis and transition between growth states with markedly different bioenergetic demands and proliferative potentials, such as stem cells[52]. During osteogenic differentiation, SIRT7 is downregulated, and Wnt/β-catenin signaling is activated[53].Growth factor independence 1 (GFI1) may prevent the proliferation of HSCs, and GFI1 is a downstream target of C/EBPα, which prevents cell proliferation when GFI1 levels are low; however, not all cells will become hematopoietic[54]. The differentiation of HSCs is controlled by a series of transcription factors (GATA-1, PU.1, and C/EBP)[55],and C/EBP is closely related to the UPRmt.
The interaction between SIRT7 and NRF1 is a regulatory branch of the UPRmtand is related to cell energy metabolism and proliferation. The expression of SIRT7 declines during the aging of HSCs, and its downregulation induces mitochondrial protein folding stress and contributes to the dysfunction of HSCs. SIRT7 inactivation reduces quiescence, increases mitochondrial protein folding stress, and compromises the regenerative capacity of HSCs. These findings characterize the deregulation of the UPRmt-mediated metabolic checkpoint as a reversible contributing factor to HSC aging[52]. SIRT7 binds to the promoter of NRF1 target genes and thus represses transcription of these genes to impair mitochondrial biogenesis and respiration[56].Inhibition of CLPP was found to kill human leukemia cells because CLPP has a greater mitochondrial presence and is more dependent on oxidative phosphorylation[57].Mice withSIRT7knocked out showed hematopoietic stem cell aging[58].
SIRT3 is important in maintaining metabolic regulation, stem cell regeneration, and neuroprotection[59]. SIRT3 protects HSCs from the oxidative damage associated with stress or aging[60]. SIRT3-mediated mitochondrial homeostasis inhibition leads to increased oxidative stress in elderly HSCs, and upregulation of SIRT3 restores the vigor of elderly HSCs, suggesting that high oxidative stress may lead to stem cell senescence, which is reversible in stem cells[60]. Studies have shown that the plasticity of mitochondrial homeostasis controls the aging of HSCs, and the expression of SIRT3 can rejuvenate aging HSCs[60].
Although it is unclear whether the specific loss of UPRmtregulators affects the maintenance of HSCs, studies have shown the importance of mitochondrial protein homeostasis to stem cell viability[61].
Nevertheless, several studies have shown that c-Jun is involved in the quiescence and self-renewal of HSCs[62].
Hematopoietic cells are precursors of a variety of non-hematopoietic tissues that can be differentiated laterally in a specific environment, such as liver cells. Enhancement of the UPRmtor restoration of CLPP levels not only reduces cellular senescence by preventing oxidative stress but also enhances hepatocyte function to prevent the functional decompensation associated with cirrhosis[63].
Although the roles of the UPRmtand UPRmtmarker genes in HSCs have been investigated, especially those involved in proliferation, differentiation, and aging, the mechanism by which the UPRmtaffects the proliferation, differentiation, and aging of HSCs needs to be discovered, especially the interaction between the UPRmtandSIRTgenes.
NEURONAL STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
Neural stem cells (NSCs) reside in the nervous system and have the potential to differentiate into nerve neurons, astrocytes, and oligodendrocytes to produce a large number of brain cells that can self-renew and produce a large number of brain tissues[64]. Mitochondria are central regulators of the fate of NSCs and are critical to both neurodevelopment and adult neurogenesis[65].
FBW7 is highly expressed in the nervous system and controls neural stem cell differentiation and apoptosisviaNotch and c-Jun during embryonic development[66]. The AP1-binding site plays an indispensable role in the UPRmt, and c-Jun, a member of the AP1 family of transcription factors, plays an important role in the regulation of the UPRmt.
In the brain, high levels of ATF5 are found in neuronal stem cells, the number of which needs to be reducedviatheir differentiation into mature neurons or glial cells[67]. ATF5 heterotopic expression in NSCs induces the expression of several olfactory sensory neuron (OSN)-specific genes. ATF5 is expressed in immature OSNs and promotes their maturation into OSNs[68]. Therefore, ATF5 is important for the differentiation of NSCs. In mammals, ATF5 has considerable homology with ATFS-1 in the bZIP domain. ATF5 knockdown impairs cell proliferation, especially in cells expressing an ornithine transcarbamylase-deficient mutant (ΔOTC)[14]. And ΔOTC causes changes in neurocognitive function. Therefore, the UPRmtis important for the differentiation of NSCs.
Wnt, an important developmental regulator, is involved in mediating the UPRmtin nerve cells and intestinal cells[69]. Moreover, the proliferation/differentiation of NSCs is affected by glucocorticoids because of the functions of intracellular signaling pathways such as Wnt[70]. In IEC-specific mouse models, loss of HSP60 chaperone activated the UPRmtand led to mitochondrial dysfunction. The release of Wnt-related paracrine factors from the affected IECs is controlled by factors involved in stem cell proliferation[71].
The genetic and idiopathic forms of Parkinson's disease (PD) are characterized by the loss of dopamine neurons, and the protein levels of CLPP are selectively reduced in the dopaminergic neurons in the brain of PD patients, as determined by postmortem examination[72]. CLPP, a marker of the UPRmt, may be a useful therapeutic target for PD.
Furthermore, the roles ofWntgenes and the UPRmtin NSCs are largely unknown and should be studied because the Wnt pathway and the UPRmtplay vital roles in nervous system development and diseases.
INTESTINAL STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
IECs are the most active metabolic site in the body of mammals. IECs constantly renew themselves throughout the life cycle because the stem cells located in intestinal crypts maintain vigorous proliferation and differentiation abilities. Intestinal stem cells(ISCs), located near the base of crypts, terminally differentiate near the crypt opening and produce a variety of intestinal epithelial cell types[73]. ISCs play important roles in maintaining the structural and functional integrity of the intestinal barrier and repair after injury[74]. Under different stress or diet conditions, the proliferation capacity of ISCs is very important for maintaining intestinal integrity. Mitochondrial dysfunction leads to tissue degradation and aging by affecting the homeostasis of somatic cells[75,76]. Therefore, it is very important to understand the biological characteristics of ISCs.
In an IEC-specific mouse model, the deletion of HSP60 activated the UPRmt,resulting in mitochondrial dysfunction, stem cell loss, and impaired intestinal epithelial cell proliferation through CHOP-independent signaling pathways[71].However, overexpression of epithelial-specific CHOP induced cell cycle arrest in the mice, resulting in impaired wound healing and reduced proliferation of IECs[77].UPRmtsignaling is important to the localization of intestinal epithelial stem cells and their differentiation and lineage commitment[78].
PKR integrates the UPRmtin inflammatory bowel disease (IBD). The endoplasmic reticulum (ER) UPR is initiatedviaeIF2α phosphorylation and AP1 activation[29].There may be a connection between mitochondria and the ER with respect to the UPR in IBD.
Hence, the role of the UPRmtin ISCs is poorly understood, and the roles of the UPRmtin the proliferation, differentiation, and aging of ISCs need to be further explored.
PERIODONTAL LIGAMENT STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
Periodontal ligament stem cells (PDLSCs) are undifferentiated mesenchymal cells that remain in the periodontal membrane after the development of periodontal tissue[79].
ROS generated by mitochondria are produced as byproducts of normal oxidative metabolism[80]. In mammals, ROS are also invoked as agents important in processes triggered in cells undergoing apoptosis. Increases in the levels of ROS activate the CHOP branch of the UPRmtand increase the levels of CLPP and HSP10[81]. A study found that the JNK/mitochondrial pathway regulates glycation end products, causing damage and inducing the apoptosis of periodontal membrane stem cells. This pathway is activated by excessive ROS as induced by JNK, known as a stress-activated protein kinase[82]. The phosphorylation activation of JNK induces a decrease in mitochondrial membrane potential, which changes the permeability of the mitochondrial membrane and causes the small-molecule solutes in the cytoplasm to flood into the mitochondrial matrix, resulting in mitochondrial swelling and rupture,triggering the mitochondria-mediated endogenous cell apoptosis pathway, regulating the expression of Bax and Bcl-2, and inducing the apoptosis of PDLSCs. The JNK signaling pathway can activate the proapoptotic protein Bax, inhibit the activity of the antiapoptotic protein Bcl-2, activate c-Jun/AP1 to upregulate proapoptotic proteins,and activate P53 family proteins, thus inducing apoptosis of different stem cells[83,84].
Therefore, many more roles for mitochondria and the UPRmthave been discovered in PDLSCs and are interesting and worthy of further exploration.
CANCER STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
Cancer stem cells (CSCs) represent a highly tumorigenic subset of cells in primary tumors[85]. They play important roles in tumorigenesis and tumor progression and recurrence[86]. Mitochondrial changes in CSCs, including morphological changes,abnormal activation of signaling pathways, dysfunction, production of ROS and mitochondrial autophagy, and the UPRmt, are key to the regulation of CSC proliferation and apoptosis and are also among the reasons for the failure of tumor treatment[87]. Therefore, targeting CSCs is crucial for the effective treatment of cancer[88] and finding an attractive target for the development of therapeutics for CSCs.
c-Myc is an important transcriptional regulator in cancer, somatic cell reprogramming, and ESCs[89]. A previous study found that Myc was located in the mitochondrion[90]. ATF4 is the main coordinating factor for cell survival under nucleolar stress and is generally overexpressed in cancer[91]. The mechanism by which Myc sensitizes cells to apoptosis involves an ATF4 agonist, which may be a potential Myc-selective cancer treatment[92].
In addition, the UPRmtis thought to improve the survival rate of cancer cells and thus promote tumor growth[93,94]. ATF5 is highly expressed in undifferentiated NSCs and in a variety of human cancers, including gliomas[95]. Similarities in the expression of ATF5 in rodent, dog, and human tumors and the cross-species efficacy of the CPd/n ATF5 peptide support the development of an ATF5-targeting approach as a novel and translational therapy for dog gliomas[94]. Analysis of human glioblastoma samples showed that ATF5 expression is negatively correlated with disease prognosis,and interference with ATF5 function can lead to glioma cell death in primary tumors without affecting normal cells surrounding the tumor, indicating that ATF5 is a therapeutic target for glioblastoma[96]. ATF5 may also be a potential therapeutic target for CSC treatments.
HSP70 elimination can lead to depletion of tumor stem cells[97]. Compared with its level in the non-neoplastic prostatic epithelium, HSP60 expression is significantly increased in both early and advanced prostate cancers and in malignant prostate cancer cell lines[98]. The HSP10 pathway is a very active cell signaling network that affects the cell cycle, nuclear and cytoplasmic molecule transport and metabolism, and is an important cause of cancer[99]. HSP10 is highly expressed in a variety of cancers,including lung, pancreatic, and bladder cancers[100]. HSP70, HSP60, and HSP10 play important roles in the UPRmt, maintaining mitochondrial function and quality control;therefore, the modification of HSPs may become a new target for tumor therapy[101].
LONP1 is a UPRmteffector. In mouse models of colorectal cancer and skin cancer,heterozygousLONP1deficiency attenuated tumor formation[102]. Additionally, in human specimens, elevated LONP1 was associated with a poor cancer prognosis[103].
Mitochondrial CLPP is overexpressed in human cancer cells, which promotes metastasis, and inhibiting CLPP may bring hope for cancer treatment[104].
As a downstream target of the EPHA2 receptor in NSCLCs and in conjunction with EPHA2 in tumor stem cell-like cells, the JNK/C-Jun pathway provides an opportunity for CSC-targeted therapy[105].
Mitochondrial redox homeostasis plays a key role in many biological processes,including biosynthesis and apoptosis, and is therefore a potential target for cancer therapy[106].
Thapsigargin (TG) limits the accumulation of CSCs. The cytoskeleton is rearranged in the presence of TG, and cytoskeleton rearrangement is related to the regulation of the cytoplasm and the UPRmt[107], thus the UPRmthas the potential to treat cancer.
Importantly, discovering the roles and pathways of the UPRmtin CSCs will be very significant for cancer prevention and treatment; thus, the UPRmtmight be a novel drug target for cancer treatment.
OTHER STEM CELLS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
The UPRmtinduces many stem cells, such as induced pluripotent stem cells,mesenchymal stem cells (MSCs), muscle stem cells, and skeletal muscle stem cells.
Compared with somatic cells, induced pluripotent stem cells have fewer mitochondria and undergo less oxidative phosphorylation[31]. There is increasing evidence that during somatic reprogramming, mitochondrial mass is significantly reduced and energy metabolism is switched from oxidative phosphorylation to glycolysis, but the exact molecular mechanisms for these changes remain unclear[108].They are most likely related to the UPRmt.
For mature MSCs, reducing the level of NAMPT led to a decrease in the intracellular NAD+concentration, thereby downregulating the expression of SIRT1 after exposure to the NAMPT inhibitor FK866. Young MSCs were induced to become senescent cells.This was mainly caused by the depletion of NAD+and reduction in SIRT1 activity.NAMPT overexpression can delay the senescence of MSCs during aging[109]. The UPRmtwas activated in primary mouse hepatocytes with increased or absent SIRT1 expression[110]. Increasing NAMPT requires a complete mitochondrial NAD salvage pathway and UPRmt-associated protein deacetylase SIRT3[111].
Improving the level of NAD+ cells in mice not only enhances the function of mitochondria but also induces the expression of UPRmt-related genes and inhibition of proteins, thereby preventing skeletal muscle stem cells from aging and prolonging the life of treated mice[112]. In addition to skeletal muscle stem cells, increasing NAD+can also delay the aging of pigment stem cells[112]. Gastrocnemius muscle differentiated from muscle cells showed low expression of the UPRmtmarker CLPP during aging[113]. The UPRmtcan delay aging. Additionally, at the cellular level, reduced CLPP impairs myoblast differentiation and cell proliferation and increases eukaryotic initiation factor 2α phosphorylation, thus inhibiting translation[114].
SIRT7knockout enhanced osteogenic differentiation of bone marrow MSCs[115]. In addition, miR-152 can promote the aging of human dental pulp stem cells by targeting SIRT7 expression[116].
HEAT SHOCK PROTEINS AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
The UPRmtplays a key role in modulating corals' ability to adapt to a changing world,including the production of HSPs and antioxidants[117]. The same may be true of human evolution. Recent studies have found that the UPRmtofC. elegansis very similar to that of mammals[118]. The UPRmttranscription factor ATFS-1 has been shown to regulate HSP70 and other mitochondrial chaperons[10].ATF5, a homologous gene ofATFS-1, may also regulate HSPs. UBL-5 is a highly conserved protein and is abundant in mitochondria-rich human tissues such as the heart, skeletal muscle, liver, and kidneys[119]. Two UPRmtreporter genes (HSP60andHSP70) were attenuated by inactivation ofUBL-5 gene encodingC. elegansand animal ubiquitin like small protein[120]. The HSP pathway of the UPRmtmay be related to UBL-5. The UPRmtwas discovered by the modulation of nuclear genes encoding mitochondrial chaperone proteins by perturbations of the folding environment in mitochondria[121]. In mammalian cells, truncated folding defects of OTC upregulate mitochondrial chaperone proteins HSP60/10, HSP40, and the protease CLPP[122]. This signal transduction pathway may involve the transduction of mitochondrial matrix UPRmtpathway into the nucleus. mtHSP90 inhibitors can induce the UPRmtrapidly[123].
SIRT GENES AND MITOCHONDRIA UNFOLDED PROTEIN RESPONSE
The sirtuin family is critical to the mitochondrial stress response;in particular, SIRT1,SIRT3, and SIRT7 are involved in the UPRmton different axes[124]. In addition to SIRT3 described above, SIRT7 is involved in the UPRmt. Nicotinamide riboside prevents and reverses non-alcoholic fatty liver disease by inducing the SIRT1 and SIRT3 dependent UPRmt, triggering an adaptive mitotic pathway to increase liver β-oxidation and mitochondrial complex content and activity[125]. There are seven sirtuins in mammals: Sirt1, Sirt2, Sirt6, and Sirt7 are located in the nucleus; Sirt1 and Sirt2 in the cytoplasm; and Sirt3, Sirt4, and Sirt5 in the mitochondrion[126]. This phenomenon may involve the transduction of the mitochondrial UPRmtpathway into the nucleus.
CONCLUSION
The UPRmtis a double-edged sword with dual effects. The UPRmtinitiated by shortterm and mild mitochondrial stress, as an intracellular defensive response system, can resist mitochondrial damage and maintain and promote the function of mitochondria.Prolonged and repeated mitochondrial stress may aggravate the irreversible damage to cells by mediating apoptosis[127]. Therefore, controlling the UPRmteffectively is a current challenge, and the role of the UPRmtin stem cells is still unclear and deserves further attention.
The role of the UPRmtin longevity has primarily been examined inC. elegans, an organism that lacks somatic stem cells[128]. Recent reports have shown that activation of the UPRmt, through the administration of an NAD-increasing compound, can rejuvenate stem cells and extend the lifespan of mice[129].It remains to be seen whether the longevity of human stem cells is similar to that ofC. elegansand mice.
Despite recent reports of the potential existence of stem cells that might be used to restore the primordial follicle and thereby the oocyte pool, therapeutic interventions during female reproductive aging currently remain limited[130]. The UPRmthas been used to find ways to prolong female reproduction. Study into the relationship between stem cells and the UPRmtin the field of regenerative medicine is ongoing.
Mitochondria are key factors of environmental stability in the body. During this homeostasis, the regulation of mitochondria in stem cells becomes increasingly important[131]. In addition, the UPRmtis inextricably linked to mitochondrial homeostasis.
However, the self-renewal, differentiation, aging, and apoptosis of stem cells are dependent on cell type, and some of the mechanisms need to be further investigated.
杂志排行
World Journal of Stem Cells的其它文章
- Epigenetic modulators for brain cancer stem cells: Implications for anticancer treatment
- Mechanisms involved in selecting and maintaining neuroblastoma cancer stem cell populations, and perspectives for therapeutic targeting
- Stem cell therapies in tendon-bone healing
- Exosomal microRNAs from mesenchymal stem/stromal cells:Biology and applications in neuroprotection
- Immunotherapy against programmed death-1/programmed death ligand 1 in hepatocellular carcinoma: Importance of molecular variations, cellular heterogeneity, and cancer stem cells
- Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair
