Current advances of stem cell-based therapy for kidney diseases
2021-07-30CheeYinWong
Chee-Yin Wong
Chee-Yin Wong, Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman,Kajang 43000, Selangor, Malaysia
Chee-Yin Wong, Research Department, Cytopeutics, Cyberjaya 63000, Selangor, Malaysia
Abstract Kidney diseases are a prevalent health problem around the world. Multidrug therapy used in the current routine treatment for kidney diseases can only delay disease progression. None of these drugs or treatments can reverse the progression to an end-stage of the disease. Therefore, it is crucial to explore novel therapeutics to improve patients’ quality of life and possibly cure, reverse, or alleviate the kidney disease. Stem cells have promising potentials as a form of regenerative medicine for kidney diseases due to their unlimited replication and their ability to differentiate into kidney cells in vitro. Mounting evidences from the administration of stem cells in an experimental kidney disease model suggested that stem cell-based therapy has therapeutic or renoprotective effects to attenuate kidney damage while improving the function and structure of both glomerular and tubular compartments. This review summarises the current stem cell-based therapeutic approaches to treat kidney diseases, including the various cell sources, animal models or in vitro studies. The challenges of progressing from proof-of-principle in the laboratory to widespread clinical application and the human clinical trial outcomes reported to date are also highlighted. The success of cell-based therapy could widen the scope of regenerative medicine in the future.
Key Words: Stem cells; Kidney regeneration; Kidney disease; Mesenchymal stem cells;Embryonic stem cells; Induced pluripotent stem cells
INTRODUCTION
Kidney disease is a prevalent global health problem. A new analysis suggested that the global prevalence of chronic kidney disease (CKD) in the year 2017, was 9.1% (697.5 million cases)[1,2]. The World Health Organization has estimated that as many as 5 to 10 million people die annually from kidney diseases worldwide[3]. By 2040, CKD is projected to be the fifth leading cause of death worldwide[4].
To date, there has been no significant breakthrough in the medical treatment of kidney diseases, whereas the current routine treatment consisting of multidrug therapies can only delay the disease’s progression. These drugs cannot reverse the progression into the end-stage kidney disease (ESKD). The current therapeutic repertoire to prolong the lifespan of ESKD patients is limited to kidney replacement therapy, dialysis or organ transplantation[5]. Due to the high medical cost involved in dialysis therapy, which also compromises the patients’ quality of life, dialysis is not an ideal solution. This is primarily because dialysis does not restore or substitute all kidney functions[6]. Meanwhile, the severe shortage of organ donors and potential organ rejection risks limit the practice of kidney transplantations[7]. Therefore, it is crucial that medical researchers explore novel therapeutics to improve the quality of life for patients with kidney diseases and to potentially cure, reverse, or alleviate the kidney disease.
Stem cells are defined as cells capable of self-renewal and can differentiate into a variety of cell types. Moreover, stem cells possess cellular plasticity and easily expandin vitro, which are the beneficial properties of stem cell therapy. Stem cells have been extensively explored in treating cardiac, neuron, vascular, immunological, and kidney diseases[8,9]. In some countries, there are stem cell therapies based on mesenchymal stem cells (MSCs), which are available as commercial products approved by local regulatory agencies for specific diseases or health indications[10]. Thus, this form of intervention can pave the way as the next regenerative medicine for human diseases.Figure 1 showed an overview of stem cell-based strategies to treat kidney disease.
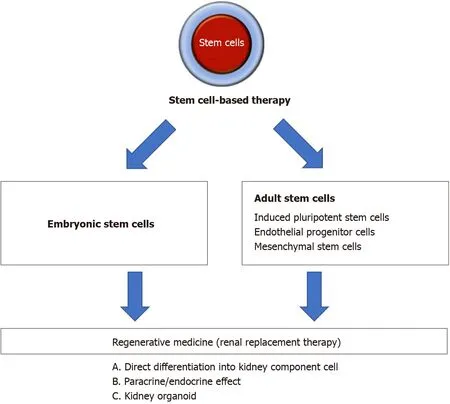
Figure 1 Overview of stem cell-based strategies to treat kidney disease. Different types of stem cell were explored for their ability to treat kidney disease. One strategy was to direct the differentiation of stem cells into kidney component cells in vitro or in vivo to replace the injured or damaged cells. Other strategies were to transplant stem cells or stem cell-derived extracellular vesicles to generate paracrine effect/endocrine effect, and to use kidney organoid formation to replace whole kidney organ.
This present article reviews stem cell-based therapy in kidney regeneration based on animal models andin vitrostudies, as well as discusses its potential for clinical application and the challenges in translating from animal models to clinical application.
EMBRYONIC STEM CELLS
Embryonic stem cells (ESCs) are pluripotent cells with unlimited differentiation potentials. Several research groups have demonstrated that mouse ESCs can integrate into kidney compartments, suggesting the potential value of stem cells in kidney repair. Implantation of ESCs directly into mouse embryonic kidney culture resulted in ESC-derived tubules and proximal tubular cells[11]. Exposing ESCs to specific inducers or factors also caused the induction of cells to differentiate into kidney lineage cellsin vitro[12,13]. Kim and Dressler[14] induced mouse ESCs to differentiate into renal progenitor cells and then incorporated these cells' into the tubular epithelial by injection into embryonic kidney cultures. In another study, when intact rat kidneys were decellularised in this manner, the kidney’s intricate architecture was preserved,and the seeded ESCs could proliferate within the glomerular, vascular, and tubular structures[15]. Vazquez-Zapienet al[16] further reported that after mice with cisplatininduced kidney failure received mouse ESCsviainjection, the mortality rate decreased significantly and prevented further disease-related histological deterioration.
The kidney, on another hand, is a very complex organ made of several cell types(Figure 2). Therefore, it is an intricate organ to reconstruct. Many researchers have worked on a protocol to induce ESCs into generating complex structures to resemble kidneys with multiple renal cell types and capable of self-organization, termed as organoids[17,18]. Tanet al[19] reported that mouse ESCs-derived nephron progenitors,aggregated with primary ureteric bud, formed kidney organoids with full nephron structures.
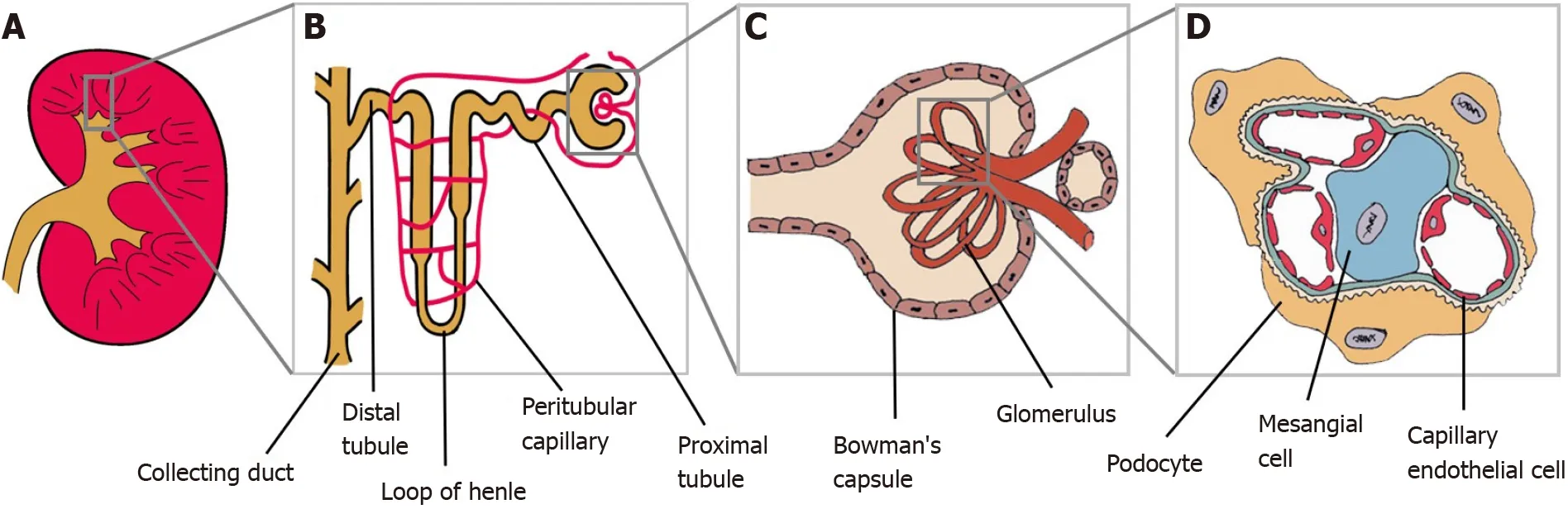
Figure 2 Kidney and its main components. A: Kidney; B: Magnification of the nephron; C: Renal corpuscle; D: Glomerulus. Podocytes, mesangial cells and capillary endothelial cells are parts of the glomerulus structure.
Despite its clinical potentials, the risk of tumorigenicity, together with legal and ethical concerns, continue to hinder the development of ESC-based therapies.Additionally, ESC-derived differentiated cells are allogeneic in nature and can therefore express specific surface proteins to trigger the recipient’s immune system.Therefore, acute and chronic rejection, or graftvshost disease, can occur due to the use of allografts if they fail to achieve immunocompatibility with the recipient[20,21].
INDUCED PLURIPOTENT STEM CELLS
Induced pluripotent stem cells (iPSCs) share many regenerative properties to ESCs. In 2006, Takahashi and Yamanaka showed that mouse adult fibroblasts can be reprogrammed into iPSCs, by introducing four transcription factors (OCT4, SOX2,KLF4 and c-MYC)[22]. This was a breakthrough finding that became a landmark in stem cell research. The development of iPSCs-based therapies could overcome the specific issues related to the use of ESCs, such as ethical concerns due to the cells’source and the potential of cell rejection by the recipient patient.
The use of ESCs has its controversies due to certain parties who are in the opinion that destroying an embryo for its ESCs is akin to killing an unborn child. Hence, iPSCs are an attractive alternative to ESC-like stem cells as the iPSCs can be generated from adult cells. These cells also retain the genetic background and peculiar epigeneticmemory of their parent cell, thus possibly avoiding any strong immune response. To date, iPSCs have been generated from fibroblasts[23], umbilical cord blood[24],peripheral blood[25,26], and keratinocytes[27]. Researchers have also successfully generated iPSCs from mesangial cells[28], renal tubular cells[29], and renal epithelial cells[30,31]. Reprogrammed iPSCs from kidney cells are believed to potentially aid in the study of genetic kidney diseases, that may lead to the development of novel therapies.
The potential of iPSCs in kidney regeneration have been explored, including establishing unique methods to stimulate human iPSCs to differentiate into kidney lineages[32-34] or three-dimensional structures of the kidney[35]. Toyoharaet al[36]had established a multistep differentiation protocol to induce human iPSCs to differentiate into renal progenitors capable of constructing three-dimensional proximal renal tubule-like structuresin vitro. The same group subsequently discovered that renal subcapsular transplantation of these human iPSC-derived renal progenitors ameliorated the acute kidney injury (AKI) in the animal model.
In addition to relying on the differentiation ability of iPSCs, researchers have also used the renotropic factors produced by the iPSCs in kidney regeneration. Transplantation of iPSCs in a murine model with ischemic AKI, reduced the expression of oxidative substances, pro-inflammatory cytokines, and apoptotic factors, resulting in eventual improvement in survival[37]. Additionally, Tarnget al[38] demonstrated that iPSCs-derived conditioned medium attenuated AKI and significantly improved survival in an animal model.
From recent developments, iPSCs can now be directed to differentiate and generate kidney organoids resembling the human kidneyin vitro.An artificially created human kidney can be applied in regenerative medicine, and in developmental, toxicity, and disease models[39-41]. Furthermore, using patients' own iPSCs to generate highquality kidney organoids enables drug validation in a patient-specific manner. This is contributed by the tight correlation between the patients’ individual genetic background and drug responsiveness[42,43]. Melissa H Little’s group had successfully generated human iPSCs-derived kidney organoids that had all the anticipated kidney cell types. These organoids possessed nephrons segmented into the glomerulus,proximal tubule, loop of Henle, and distal tubule along with the collecting duct,endothelial network, and renal interstitium[44-46]. Meanwhile similarly, the Izpisua-Belmonte’s group had also generated a kidney organoid containing glomeruli with podocytes, proximal and distal tubule cells, and endothelial cells[42]. Morizaneet al[47] reported several differentiation protocols for creating kidney organoids with epithelial nephron-like structures. These organoids expressed the markers for podocytes, proximal tubules, loops of Henle, and distal tubules.
Other research groups reported that iPSCs derived from various cell types are not identical in their differentiation capacity[48,49]. This is likely to happen because iPSCs maintain the epigenetic memory of their parental cells[50]. Therefore, renal parenchymal cells may be a better candidate than cells from other tissues for reprogramming to treat kidney diseases.
ENDOTHELIAL PROGENITOR CELLS
Endothelial progenitor cells (EPCs) have essential roles in maintaining vascular integrity and in repairing any form of endothelial damage[51]. EPCs can be isolated from different cell sources, mainly from the readily available bone marrow, cord blood, and peripheral blood[52]. The beneficial effects of EPCs-based therapies have been shown in studies performed using different models of kidney diseases such as AKI, CKD, and renal artery stenosis. In an animal model of renal ischemia/reperfusion (I/R)-induced AKI, renal artery-derived EPC-like cells integrated into the endothelium after AKI, led to decreased levels of serum creatinine (SCr) and albuminuria while blood flow improved[53]. Patschanet al[54] further demonstrated that the systemic injection of peripheral blood-derived early EPCs decreased SCr,ameliorated interstitial fibrosis, and subsequently reduced the progression to CKD after AKI.
The possible effect of EPC treatment on CKD progression was studied in an animal model[55]. In this study, bone marrow-derived EPCs that were homed into the injured kidney, prevented the inflammatory condition from adversely affecting the kidney,and successfully preserved kidney function and structure. Huanget al[56]demonstrated a rodent model injected with peripheral blood-derived EPCs and observed effective inhibition of the propagation of CKD and the deterioration of kidney function. The injected cells enhanced angiogenesis and blood flow, and had anti-oxidative capacity while suppressing inflammation, oxidative stress, apoptosis,and fibrosis[56]. Meanwhile, in renal artery stenosis models, peripheral blood derived-EPCs demonstrated renoprotective effects after injection into the stenotic kidney, by improving microvascular density and kidney functions along with diminishing fibrosis[57-59].
MSCs
MSCs, or as recently referred to as mesenchymal stromal cells, were first discovered by Friedenstein and his colleagues from bone marrow[60]. Over the years, researchers have found that MSCs can be isolated from various organs or tissues, such as adipose tissue[61], umbilical cord[62,63], placenta[64], peripheral blood[65], amniotic fluid[66],and skeletal muscles[67].
Bone marrow is the most commonly used source for MSCs in clinical treatments,including treating kidney diseases. However, the use of bone marrow derived-MSCs(BM-MSCs) became limited because of a high degree of viral exposure, and that the cell proliferation/differentiation capability significantly decreases as the donor’s age increases[68]. Therefore, researchers began exploring other types of MSCs for kidney regeneration. Among the many sources, adipose tissue-derived MSCs (AD-MSCs) and umbilical cord-derived MSCs (UC-MSCs) have become desirable candidates because a large amount of the MSCs can be obtained using relatively minimal invasive procedures[69].
In the field of kidney disease, MSCs are among the most efficient type of cell population for activating regeneration in a damaged kidney[70]. Pre-clinical reports have demonstrated the therapeutic potential of MSCs in animal models of AKI and CKD[71,72]. According to a systematic review of more than 70 articles, MSCs are among the most effective cell populations to treat experimental CKD[73]. Meanwhile,in a meta-analysis involving animal models of chronic and AKI, MSCs led to kidney regeneration despite the variable modes of administration (arterial, venous or renal)[71]. There is evidence suggesting the beneficial effects of MSCs in blocking AKI-CKD transition, a term used to describe an incomplete recovery from AKI resulting in longterm functional deficits, such as CKD[74].
In an experiment performed by Brasileet al[75], when ischemically damaged human kidneys were perfusedex vivowith MSCs for 24 h, kidney regeneration was documented. The MSCs-based treatment caused the kidneys to synthesize significantly lower levels of inflammatory cytokines. Compared to exsanguinous metabolic support perfusion alone, there was a significant increase in the number of renal cells undergoing mitosis in the MSCs-treated kidneys[75].
Numerous studies have demonstrated that MSCs can either differentiate into renal cells in general[76] or specifically into kidney component cells such as renal epithelial cells[77-80], mesangial cells[81,82], and endothelial cells[83,84].
BM-MSCs
Many studies have demonstrated the efficacy of BM-MSCs in the treatment of kidney disease using animal models of AKI[85,86], podocyte injury[87], and glomerulonephropathy[88,89]. Morigiet al[90,91] is among the first groups to demonstrate the renoprotective role of BM-MSCs and documented the therapeutic potential of human BM-MSCs in the treatment of kidney diseases, leading to survival in animal models.Transplantation of human BM-MSCs into cisplatin-induced AKI mice resulted in markedly improved kidney function and recovery by accelerating tubular proliferation and reducing the number of tubules affected by apoptosis, necrosis and tubular lesions. A similar form of protection was conferred by injected BM-MSCs in a glycerol-induced pigment nephropathy model[77,92] and I/R-induced AKI[86,93].More importantly, infused BM-MSCs have shown to enhance kidney functional recovery even when administered 24 h after the injury[94]. Furthermore, BM-MSCs were more effective in treating AKI in the animal model compared to candesartan,which is an angiotensin II blocker[95]. In essence, there is good evidence that when MSCs are transplanted in toxic and ischemic animal models, the cells protect the animals against AKI and accelerate the recovery phase.
BM-MSCs have also shown promises in the treatment of CKD in animal models.BM-MSCs prevented the loss of peritubular capillaries and slowed down the progression of proteinuria (protein in the urine)[96]. During the initial phase of the immune response before the onset of CKD, these cells also reduced kidney fibrosis[97]. According to a histological analysis of a rat model with CKD, BM-MSCs reduced glomerulosclerosis, resulting in preservation of kidney function and attenuation of kidney injury[98]. Additionally, when animal models with CKD were treated with BM-MSCs, there were reduced progression of proteinuria and scarce engraftment of these cells in the kidneys. These observations suggested that these beneficial effects were probably caused by cytokines or growth factors, which are also known as the paracrine secretion of mediators[99].
In addition to the ability of BM-MSCs to differentiate into renal cells, more recent reports suggested that BM-MSCs exert protective and regenerative effects on kidneys by their paracrine anti-inflammatory, anti-fibrotic, and vascularisation properties[93,100]. According to reports, BM-MSCs can transfer biological cuesviathe secretion of extracellular vesicles (EVs) to promote regenerative processes in injured renal cells[101-103].
Several studies investigated the effects of BM-MSCs in experimental models of kidney organ transplantation[104,105]. Most studies focused on the intervention’s efficacy through prolonged graft survival and inhibition of the rejection process[106-108]. Given the advantage of BM-MSCs having low immunogenicity and immunoregulatory properties, BM-MSCs can reduce alloimmune injury and immune suppressionrelated side effects to optimise preservation of the transplanted kidney’s functions[109,110].
AD-MSCs
AD-MSCs are highly abundant in adipose tissues and can be easily extractedvialiposuction, a method which is widely used in the clinical setting. Adipose tissue may become the preferred source of MSCs due to its less invasive procurement and higher MSCs concentration than those found in the bone marrow[111]. Their allergenic transplantationviathe intra-renal route contributed to a low degree of necrosis, but caused higher vascularisation of the renal parenchyma in Wister rats[112]. There are reports on the therapeutic effect of AD-MSCs in AKI-induced animal models. Kimet al[113] have demonstrated that AD-MSCs reduced apoptotic cell death while simultaneously reducing the activation of p53, c-Jun NH2-terminal kinase and extracellular signal-regulated kinase, which are inflammation-related molecules. These effects resulted in increased survival rate of the AKI-induced animals. Katsunoet al[114]further discovered that human AD-MSCs cultured in low serum secreted high levels of hepatocyte and vascular endothelial growth factors. When these cells were transplanted into AKI-induced rats, they enhanced the attenuation of kidney damage[114]. Even though adipose tissue is a good source of MSCs, AD-MSCs have been less effective in proliferative and kidney regenerative activities compared to BM-MSCs[112].
UC-MSCs
The easy collection of UC-MSCs provides a new abundant source of MSCs. The usage of UC-MSCs, transforms a medical waste into a beneficial product with clinical applications[115]. Compared to MSCs from other sources, UC-MSCs have low immunogenicity, thus preventing the occurrence of immune rejection in allogeneic transplantation[68,116]. Moreover, UC-MSCs have a greater proliferation capacity compared to BM-MSCs and AD-MSCs[117,118]. UC-MSCs also show no sign of senescence over several passages[119], so mass cell production of UC-MSCs is highly possible without causing the loss of cell potency.
More studies are being performed to demonstrate that human MSCs isolated from the umbilical cord exert superior therapeutics effects compared to other sources of MSCs. Researchers also found that when UC-MSCs were implanted into AKI-induced mice, the cells exerted renoprotective effects by inducing tubular cell proliferation[120]and promoting glomerular filtration that prolonged the animals’ survival[121].Meanwhile, in a CKD-induced rodent model, transplanted UC-MSCs inhibited inflammation and fibrosis while the expression of growth factors was promoted. These effects protected the injured kidney tissues and prevented disease progression[122,123].
Other sources of MSCs
In addition to BM-MSCs, AD-MSCs and UC-MSCs, researchers have also used other types of MSCs that are less commonly studied for kidney regeneration studies. The efficacy of cord blood-derived MSCs (CB-MSCs) administration in the restoration of kidney function had been reported in animal AKI models[124]. This study demonstrated that CB-MSCs promoted kidney regeneration and prolonged the survival of the animal. Based on this study, the paracrine action of CB-MSCs on the tubular cells may have been mediated by the reduction of oxidative stress, apoptosis,and inflammation. Hauseret al[125] and Georgeet al[126] found that amniotic fluidderived MSCs (AF-MSCs) possess the same characterisation as BM-MCSs, facilitating functional and structural improvement in a rat model of CKD. Sedrakyanet al[127]showed the injection of AF-MSCs in mice delays the progression of renal fibrosis.Meanwhile, in a CKD-induced rat model in a study by Cetinkayaet al[128],transplanted placenta-derived MSCs (PL-MSCs) alleviated kidney damage and inhibited fibrosis-induced apoptosis. PL-MSCs were also used to treat kidney injury and inflammation in lupus nephritis (LN) mice[129].
STEM CELL-DERIVED EVs
EVs are small membrane vesicles secreted by various cells and found in most body fluids[130]. This review uses the general term, EVs, because there is no method to precisely identify the vesicles[131]. EVs can be classified into three major categories,which are exosomes, microvesicles, and apoptotic bodies. In the current context,researchers suggest that EVs could be transferred to injured cells to restrain tissue injury, reduce inflammation, inhibit apoptosis, and induce cell cycle re-entry of resident cells, all of which lead to cell proliferation, tissue self-repair and regeneration[132]. Upon administration with a therapeutic regimen, EVs will mimic stem cells'effects in various experimental models.
The use of the stem cell-derived EVs could have multiple advantages in clinical application including bypassing most of the safety concerns related to stem cell therapy, such as cellular contamination with oncogenic cells, tumorigenicity, and emboli formation after transplantation[133]. Similarly, EVs also enable a wide range of potential manipulations to carrier molecules for improvements in delivery and desired effects[134]. EVs can also be safely stored in medical facilities without losing any of their functions[135].
Several studies had provided convincing evidence on the regenerative potential of EVs released by stem cells, specifically MSCs, in different models of kidney injury[136,137].In vitrostudies have demonstrated the potential of MSCs-derived EVs to transfer mRNA, miRNA, and proteins to renal cells[132]. Currently, this cell-free therapy is being studied in animal models of AKI[138-140] and CKD[141,142]. Intravenous MSCderived EVs exert renoprotective effects by reducing renal cell damage and apoptosis while enhancing proliferation of the renal cells. These effects lead to improved kidney function, similar to those induced by MSCs, as reported in rats injected with EVs from EPCs[143,144] and iPSCs[145,146].
When a patient living with ESKD undergoes a kidney transplantation, the patient’s quality of life significantly improves[5]. Nevertheless, chronic allograft nephropathy limits organ survival, eventuating in the patient having to undergo kidney transplantation more than once in a lifespan[147]. The administration of EVs after kidney transplantation was found to ameliorate I/R injury in both the acute and chronic stages, favour tolerance and prolong allograft survival[148].
The preconditioning of a kidney with stem cells-derived EVs may also conveniently limit tissue damage caused by chronic allograft nephropathy. Evidence showed that the MSCs-derived EVs delivered in the perfusate during organ cold perfusion for 4 h protected the kidney from reperfusion damage and can preserve the organ’s enzymatic machinery, which is essential for cell viability[149].
TRANSLATION FROM ANIMAL MODEL TO HUMAN CLINICAL APPLICATION: THE CHALLENGES
Despite numerous animal experiments to demonstrate the effectiveness of stem cell therapy in kidney disease, outcomes from those animal models were unsuccessfully reproduced in human clinical studies in entirety[150]. The failure to translate the promising results from an animal experiment, which has a sound and standardised design and conduct, to a clinical application happens due to variations in the animal model and the human physiology[151].
Human kidney diseases are typically artificially induced in the animal model. The induced injury is generally acute, unphysiological, and cannot epitomise the human kidney diseases' complex pathological features[152,153]. It is thus difficult to precisely simulate or predict the response of the human kidney disease to treatment by using an animal model. CKD animal experiments are insufficient to reflect the disease’s conditions because other factors such as age, sex, and comorbidities are not represented[154,155]. CKD patients may also have comorbidities involving multiple organs and functions that further aggravate the pathological processes underlying CKD[156]. In the animal model, however, these complications were not taken into account.
Both humans and animals may have the same protein functions but there are species-specific differences in the molecular regulation of genes. This causes difficulty in extrapolating an outcome from an animal gene analysis to the physiological conditions in the human body[157]. The differences between the animals' and humans'immune systems is another reason for the failure to translate therapeutics with promising outcome inin vivostudies into a clinical application with potential[158].
ADVANCING CLINICAL TRIALS OF STEM CELLS FOR KIDNEY DISEASE
Up until March 2021, more than 40 clinical trials, either on-going or completed,involving the use of stem cell-based therapy in the treatment of kidney diseases have been registered in the United States National Library of Medicine (ClinicalTrials.gov).Most of these stem cell clinical trials for kidney disease use MSCs in their approach.Table 1 showed the completed clinical trials of MSC- and EPC-based therapies in kidney diseases.
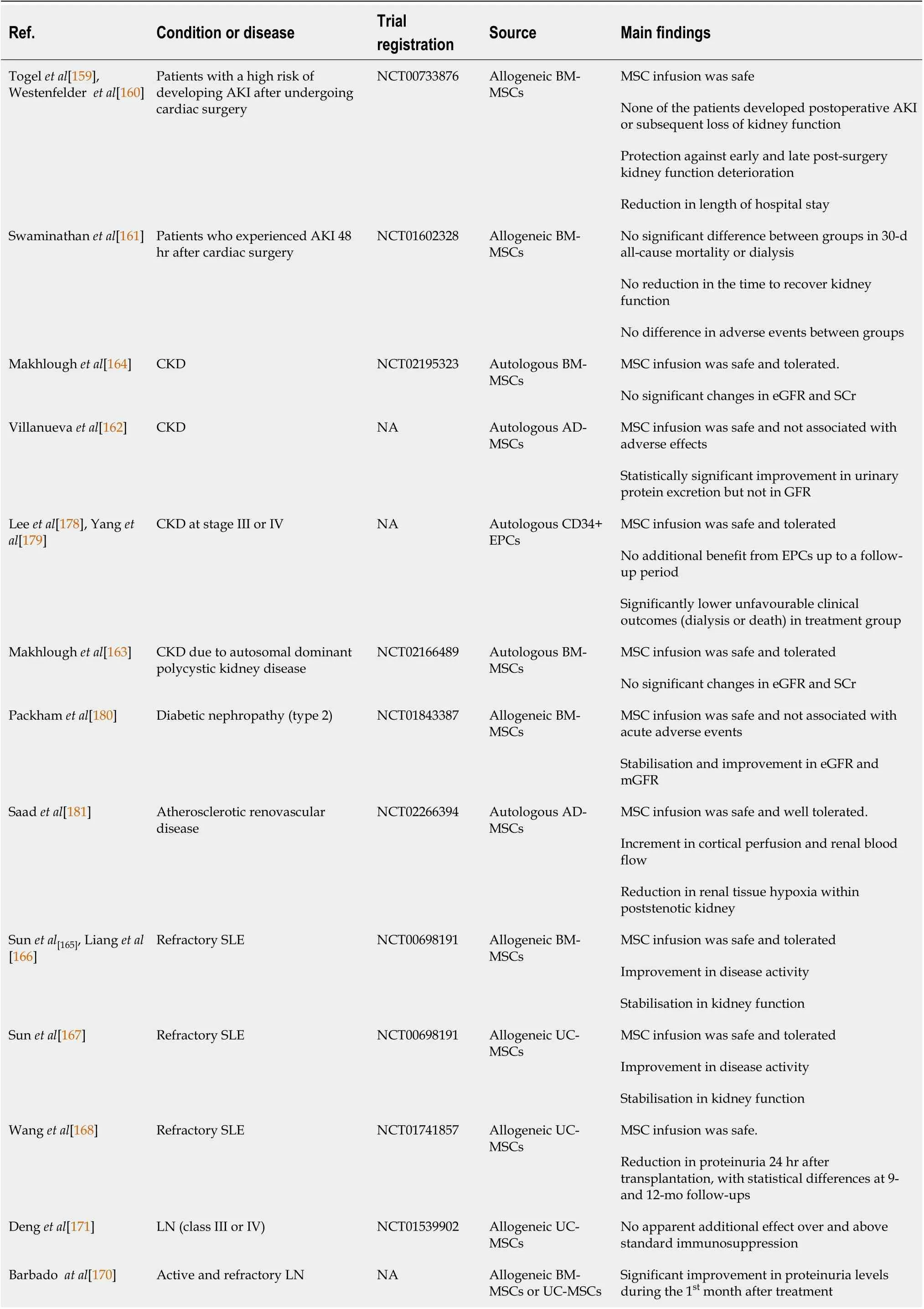
Table 1 Completed clinical trials of mesenchymal stem cell- and endothelial progenitor cells -based therapies in kidney diseases
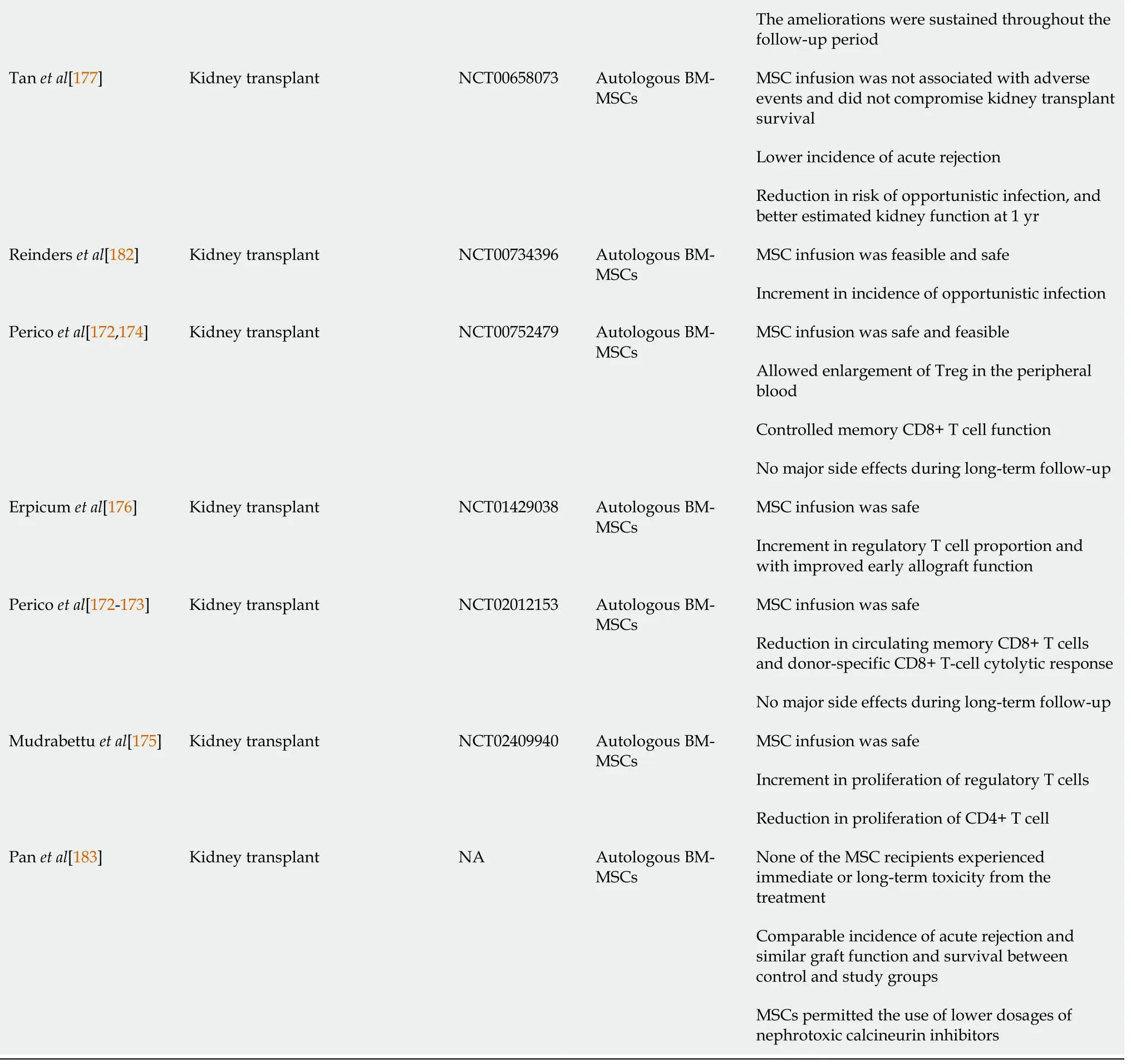
MSC: Mesenchymal stem cells; EPC: Endothelial progenitor cells; BM-MSCs: Bone marrow derived- mesenchymal stem cells; AKI: Acute kidney injury;CKD: Chronic kidney disease; SCr: Serum creatinine; SLE: Systemic lupus erythematosus UC-MSCs: Umbilical cord-derived mesenchymal stem cells; NA:Not available.
The first few trials using MSCs from different tissue sources (bone marrow, adipose,umbilical cord,etc.) either autologously or allogeneically suggested that these cells can be given safely to humans. However, the efficacies of MSCs to treat kidney diseases have mixed results. In one study, patients who had a high risk of post-operative AKI and underwent cardiac surgery, concurrently received allogeneic BM-MSCs. The patients had a shorter hospital stay and did not need readmission. The administered BM-MSCs did not have adverse effects and protected the patients against early and late post-surgery kidney function deterioration[159,160]. However, Swaminathanet al[161] reported a contrasting finding, whereby the administration of allogeneic MSCs did not decrease the time to recovery of kidney function in patients with a developed stage of AKI after cardiac surgery. They also did not detect any significant differences between the group treated with allogeneic MSCs and the group treated with placebo in the 30-day all-cause mortality study. While the rates of adverse events did not differ between groups, the MSC infusion was safe and well-tolerated[161].
More human trials have been conducted using MSCs to treat CKD. A pilot study assessing the safety and clinical feasibility of autologous administration of AD-MSCs for patients with CKD reported that the cells were safe and did not exert any adverse effects[162]. At the same time, improvement in urinary protein excretion was observed[162]. However, Makhloughet al[163,164] reported no significant changes in estimated glomerular filtration rate and SCr during the 18 mo of follow-up after autologous BMMSCs were transplanted in these CKD patients.
In studies involving the treatment of autoimmune diseases such as systemic lupus erythematosus (SLE) with MSCs, the cells were investigated for their therapeutic benefits. Sun L and team conducted phase I/II clinical trials to examine the effects of allogeneic BM-MSCs and UC-MSCs infusions in patients with primary and refractory SLE[165-167]. They found that the infusion of either allogeneic BM-MSCs or UC-MSCs was safe and well-tolerated. Besides improving the SLE Disease Activity Index and kidney function, the level of proteinuria declined 24 h after the MSCs transplantation[168]. Furthermore, among the SLE patients, allogeneic MSCs transplantation resulted in renal remission for active LN patients within a 12-mo follow-up period[169].Barbadoet al[170] in their pilot study also reported a dramatic improvement to proteinuria level during the first month post-treatment. The ameliorations were sustained throughout the follow-up period of nine months[170]. Conversely, a recent multicentre randomised, double-blind controlled trial showed that UC-MSCs have no apparent additional effects over and above standard immunosuppression therapy[171].
Given the immunomodulatory properties of MSCs, these cells have been transplanted in patients who received kidney transplants to promote immune tolerance to kidney transplantation in the setting of peri-transplant T cell-depleting induction therapy. Reports showed that transplanted MSCs were safe and without major side effects even over a long-term follow-up[172]. Additionally, there was increased proliferation of Treg noted[173,174], increment in regulatory T cell proportion[175], and improved early allograft function[176]. Simultaneously, the transplanted MSCs also controlled memory CD8+ T cells’ functions and reduced donor-specific CD8+ T cell cytolitic response[173]. Furthermore, MSCs infusion showed a lower incidence of acute rejection leading to a decreased risk of opportunistic infections and faster kidney function recovery than the controls[177]. These preliminary data suggest that transplanted MSCs are safe, well-tolerated, and can suppress host immune responses after kidney transplants with the combination of the appropriate immunosuppressive regimen.
A recent phase I trial using autologous CD34+ EPCs in treating CKD at stage III or IV has been reported. Yip HK and team demonstrated that intra-renal arterial transfusion of CD34+ EPCs was safe and well-tolerated[178]. However, when the efficacy in a phase II randomised controlled trial was further investigated, the infused EPCs did not offer additional benefit to patients with CKD up to a follow-up period of 12 months. Despite the less encouraging outcome, it is worth noting that the unfavourable clinical outcomes, such as dialysis or death, were significantly lower in the treatment group than those in the control group[179]. Currently, many clinical trials are still on-going and will provide more insights into and possibly further support these achievements with cell-based therapy for kidney diseases.
CONCLUSION
Although stem cell therapies in kidney regeneration fromin vitroand preclinical studies are promising, and an encouraging safety profile have been demonstrated in early-phased human clinical trials, these cell-based therapies are yet to be translated into more significant proof of clinical efficacy. The side effects of stem cell therapies on kidney diseases still need further investigation, as the preliminary results available still lack long-term follow-up data. Some concerns about the use of live stem cells should be kept in consideration. EVs should also be evaluated as a possible alternative to live stem cells. The use of stem cells-derived EVs that can mimic its parental cells' effects in renoprotection could be pursued. Nevertheless, it appears that stem cell therapy will have a great future in the field of kidney regeneration. Further clarification will be gained on the stem cells' protective mechanisms in the treatment of kidney diseases through further understanding of the mechanisms of stem cells’ actionsin vivo. The success of this new cell-based therapy could genuinely change the scope of the future of regenerative medicine.
杂志排行
World Journal of Stem Cells的其它文章
- Epigenetic modulators for brain cancer stem cells: Implications for anticancer treatment
- Mechanisms involved in selecting and maintaining neuroblastoma cancer stem cell populations, and perspectives for therapeutic targeting
- Roles of mitochondrial unfolded protein response in mammalian stem cells
- Stem cell therapies in tendon-bone healing
- Exosomal microRNAs from mesenchymal stem/stromal cells:Biology and applications in neuroprotection
- Immunotherapy against programmed death-1/programmed death ligand 1 in hepatocellular carcinoma: Importance of molecular variations, cellular heterogeneity, and cancer stem cells
