Role and mechanism of neural stem cells of the subventricular zone in glioblastoma
2021-07-30GuiLongZhangChuanFangWangChengQianYunXiangJiYeZhongWang
Gui-Long Zhang, Chuan-Fang Wang, Cheng Qian, Yun-Xiang Ji, Ye-Zhong Wang
Gui-Long Zhang, Chuan-Fang Wang, Cheng Qian, Yun-Xiang Ji, Ye-Zhong Wang, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China
Abstract Glioblastoma multiforme (GBM), the most frequently occurring malignant brain tumor in adults, remains mostly untreatable. Because of the heterogeneity of invasive gliomas and drug resistance associated with the tumor microenvironment, the prognosis is poor, and the survival rate of patients is low. Communication between GBMs and non-glioma cells in the tumor microenvironment plays a vital role in tumor growth and recurrence. Emerging data have suggested that neural stem cells (NSCs) in the subventricular zone (SVZ) are the cells-of-origin of gliomas, and SVZ NSC involvement is associated with the progression and recurrence of GBM. This review highlights the interaction between SVZ NSCs and gliomas, summarizes current findings on the crosstalk between gliomas and other non-glioma cells, and describes the links between SVZ NSCs and gliomas. We also discuss the role and mechanism of SVZ NSCs in glioblastoma, as well as the interventions targeting the SVZ and their therapeutic implications in glioblastoma. Taken together, understanding the biological mechanism of glioma-NSC interactions can lead to new therapeutic strategies for GBM.
Key Words: Neural stem cells; Glioma; Tumor microenvironment; Communication;Exosomes
INTRODUCTION
Glioblastoma multiforme (GBM) is the most frequently occurring malignant brain tumor in adults, for which no effective therapy is currently available. Current conventional therapies, such as a combination of surgery and radio- or chemo-therapy, yield poor prognosis and low median survival times of patients[1,2]. In addition, the recurrence of GBM is common or inevitable, and there are no standardized therapeutic approaches for such cases[3]. To improve clinical outcomes, immunotherapy has been successfully performed by activating the immune systems of patients[4], employing chimeric antigen receptor T cells, oncolytic viruses (OV), anti-cytotoxic-T-lymphocyteassociated protein 4, and anti-programmed cell death protein 1, among others[5-8].However, the therapeutic efficacy remains limited in GBM due to the effects of the tumor microenvironment (TME), which leads to immunosuppression or immune tolerance[1,5,9]. Recent advances integrating metabolomics with genomics or proteomics have provided new insight into the mechanisms that drive the origin and development of tumors, including GBM[10-12], especially the interactions between the tumor and TME, and provide important clues for new therapeutic strategies. Neural stem cells (NSCs), as unique stem cell type in the brain, have the abilities of selfrenewal and multi-directional differentiation, and can differentiate into neurons,astrocytes, and oligodendrocytes[13]. NSCs mainly exist in the subventricular zone(SVZ) of the lateral ventricle and dentate gyrus [subgranular zone (SGZ)] of the hippocampus. Furthermore, NSCs create a unique stem cell microenvironment in the SVZ or SGZ region that maintains stem cell homeostasis and stemness and inhibits differentiation[14,15]. Recent studies[16-20] have found that NSCs located in the SVZ might be the cells-of-origin of gliomas, and that SVZ involvement is associated with GBM recurrence in patients. Further, GBMs contacting the SVZ significantly decrease the overall survival (OS) and progression-free survival (PFS) of patients[16-19]. Thus,crosstalk between the oncogenic signaling of tumors and SVZ NSCs might be important in GBM. In this review, we focus on recent advances of the origin and development of GBM and explore novel strategies for GBM treatment. First, we summarize current findings on the crosstalk between gliomas and other non-glioma cells in the tumor niche. Then, we address the recently identified links between NSCs and gliomas and discuss the role and mechanism of SVZ NSCs in glioblastoma.Finally, we provide insight into the interventions targeting the SVZ and their therapeutic implications in glioblastoma. This review provides an overview of current opinions on gliomas.
TUMOR NICHE IN GLIOBLASTOMA
Emerging evidence[5,21-25] suggests that the unique TME involved by different nonglioma cells is critical for glioma growth, invasion, recurrence, and tumor angiogenesis. In particular, the communication or crosstalk between glioblastoma and other non-glioma cells in the TME mediates tumor progression and therapeutic drug resistance[5,25]. The non-glioma cells in the TME or glioma niche contain neurons,normal and reactive astrocytes (RAs), glioma-associated microglia/macrophages,endothelial cells (ECs), neural stem cells,etc.[23-25]. These non-cancer cells secrete proteins or non-protein biomolecules (including nucleic acids, lipids, and nitric oxide)within the TME to regulate glioma growth. Furthermore, glioblastoma cells can recruit non-tumor cells to alter their phenotype to regulate the TME[23-25]. Neurons are the main cell type in the glioma niche. Venkateshet al[26] found that excitatory neuronal activity affects the growth of glioblastomas, and neurons can mediate the interaction with gliomas mainlyviathe cytokine NLGN3 secreted by activated neurons.Furthermore, the specific interaction between neurons and gliomas occurs mainlyviathebona fideα-amino-3-hydroxy-5-methyl-4 isoxazole propionic acid receptordependent neuron–glioma synapses[27]. These findings[26-28] describe the vital role of neurons in the glioma niche and crosstalk between these neurons. Astrocytes,especially reactive astrocytes, are involved in brain injury, tumors, and inflammatory and degenerative diseases[29]. The glioma-astrocyte interaction also plays a vital role in the TME[30,31]. Yuet al[32] showed that glioma cells can stimulate the transformation of normal human astrocytes into RAs in the absence of direct contact, and RAs deliver exosomal O6-alkylguanine DNA alkyltransferase to glioma cells, resulting in temozolomide (TMZ) resistance of gliomas. Furthermore, tumor-associated astrocytes exhibit immunomodulatory properties within the TME[33]. This indicates that reactive astrocytes or glioma-associated astrocytes in the TME mediate the potential interaction with gliomas. The TME of gliomas normally contains infiltrated microglia/macrophages, and the ability of immunoregulation in glioma is positively correlated with the number of tumor-associated microglia/macrophages[34,35]. Chenet al[34] found that a complex composed of circadian regulator, circadian locomotor output cycles kaput, and its heterodimeric partner, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1, contributes to the interaction between the glioma and microglia. In addition, the authors[36] found that phosphatase and tensin homolog (PTEN) deficiency in glioblastomas significantly increased macrophage infiltration to sustain GBM survival and stimulate tumor angiogenesis. A review by Poonet al[37] summarized the biology of glioblastoma-associated microglia/macrophages.These findings indicate the vital crosstalk of microglia/ macrophages with GBM in the TME. Gliomas have a high metabolic level, and the vasculature or angiogenesis is always abundant in the TME. Griveauet al[38] investigated glioma-vascular interactions by focusing on tumor-stromal and vascular regulation to explore the role of the glial phenotype associated with anti-angiogenic therapy escape[38,39]. Thus, the biological interaction between glioblastomas and non-glioma cells in the tumor niche is important in the development and progression of gliomas and exploration of new therapeutic opportunities. First, we describe the potential crosstalk between gliomas and glioma-associated non-glioma cells, such as neurons, astrocytes, microglia/macrophages, and ECs (Figure 1); next, we carefully review current findings on the interactions between gliomas and NSCs.
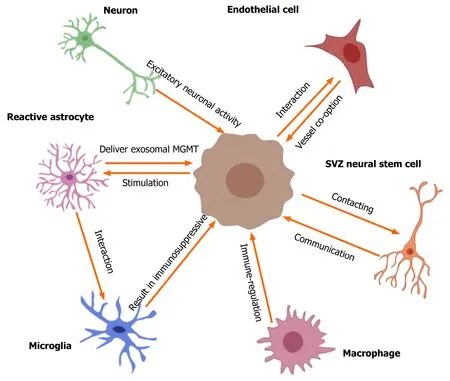
Figure 1 Crosstalk between gliomas and non-glioma cells in tumor microenvironment. The tumor microenvironment of glioma contains many nonglioma cells around the tumor, such as neurons, astrocytes, oligodendrocytes, immune cells (microglia and macrophages), endothelial cells, stem cells, etc. Active neurons promote the growth of glioblastomas through secreted cytokine NLGN3. Excitatory neuronal activity mediates the neuron-glioma interactions mainly via the bona fide AMPA receptor-dependent neuron–glioma synapse. Glioma cells stimulate normal astrocytes into reactive astrocytes and then reactive astrocytes induce temozolomide-resistance of glioma cells via delivering exosomal MGMT. The interactions of astrocyte-microglia can regulate transcriptional re-programming of microglia, and microglia contribute to sustaining the immunosuppressive microenvironment in glioblastoma multiforme (GBM) for promoting tumor progression.Glioblastomas increase macrophage infiltration, and then infiltrated macrophages promote GBM survival and tumor angiogenesis via secreted cytokines. Olig2-positive glioma cells can be invaded by vessel co-option; the glioma-EC interactions restrict the anti-angiogenic therapy of gliomas. In addition, GBM contacting neural stem cells in the subventricular zone of the lateral ventricles result in a shorter overall survival of patients and increase early recurrence of gliomas. SVZ:Subventricular zone.
ROLE OF SVZ NSCS IN GLIOMAGENESIS
Emerging data[16-19,40] have revealed that SVZ NSCs are closely related to glioblastoma development and progression, and GBM may arise from the accrual of gene mutations in NSCs. Jianget al[41] found that GBMs associated with glial fibrillary acidic protein-expressing SVZ NSCs in mice showed accelerated tumor development,higher malignancy, and lesser drug resistance in comparison to those in the control group. In addition,TERTpromoter mutation can permit the protracted self-renewal of cells and may induce gliomagenesis of NSCs[20,42,43]. Currently, NSCs in the SVZ are considered as potential cells-of-origin in gliomas[44-47]. In the next sections, we address the possible gene mutations in SVZ NSCs inducing gliomagenesis.
P53 or IDH1 mutation in SVZ NSCs
Recent findings[20,46,48-50] showed that SVZ NSCs that have acquired mutations in the tumor proteinp53orIDH1gene can result in uncontrolled proliferation and tumorigenesis. Furthermore, p53 deficiency can induce the accumulation of oncogenic alterations[51,52]. Wanget al[53] showed the presence of mutant p53 proteins in SVZ NSCs, and that subsequent expression of mutant p53-expressing Olig2+ transitamplifying progenitor-like cells was associated with the initiation of glioma formation.Modreket al[54] introduced IDH1R132H, P53 short hairpin (shRNA), and αthalassemia/mental retardation syndrome X-linked shRNA into human NSCs and found that these oncogenic hits blocked NSC differentiation and increased invasiveness, thus representing early drivers of gliomagenesis. Pirozziet al[55] reported that mouse NSCs expressing IDH1R132H displayed reduced proliferation within the SVZ due to p53-mediated cell-cycle arrest and underwent neuronal differentiation. Bardellaet al[56] conditionally expressed IDH1R132H in the SVZ of the adult mouse brain, and the mice developed hydrocephalus and dilated lateral ventricles (LVs), with the accumulation of 2-hydroxyglutarate and reduced α-ketoglutarate. Besides, stem cell populations were expanded, and mutant SVZ cells displayed features similar to those of gliomas[56]. Leeet al[20] sought direct molecular genetic evidence associated with GBMs that had originated from SVZ NSCs in clinical patients. They utilized brain tissue from 28 patients with IDH wild-type GBM or other types of brain tumors to perform deep sequencing and found low-level GBM driver mutations in healthy SVZ tissue away from the tumor in 56.3% of patients with IDH wild-type GBM. Moreover,astrocyte-like NSCs carrying the driver mutations led to high-grade malignant gliomas in a genome-edited mouse model[20]. These results show that P53 or IDH1 mutation in SVZ NSCs drives gliomagenesis by disrupting the characteristics and phenotypes of SVZ NSCs.
Other gene mutations in SVZ NSCs
Many genes or molecules such as tumor oncogenes and transcription factors involved in biological functions may affect the development of glioblastoma[46,47]. Abelet al[57] found that infiltrating glioma cells may be derived from SVZ NSCs that are transformed by activation of the oncogenic K-Ras. Danielet al[58] showed that PI3K activation in NSCs can drive the initiation of tumorigenesis. Liuet al[50] found that overexpression of the nuclear receptor, tailless, inhibited age-dependent exhaustion of NSCs in mice, induced migration of stem cells from the SVZ niche, and led to the development of gliomas. Yanget al[59] found that loss of the transcriptional repression factor, Capicua, promoted gliomagenesisviaaberrant NSC proliferation and differentiation. The transcription factors Forkhead Box G1 (FOXG1) and sex-determining region Y-box 2 (SOX2) are frequently overexpressed in GBMs. Bulstrodeet al[60]demonstrated that FOXG1-null cells showed increased astrocyte differentiation and SOX2 ablation attenuated NSC proliferation, which suggests that FOXG1 and SOX2 play complementary, but distinct, roles in GBM self-renewal. The Y-box binding protein 1 (YB-1) is vital gene in brain development and is upregulated in glioblastomas[61,62]. Fotovatiet al[63] showed that YB-1 was also overexpressed in the SVZ region of the mouse fetal brain; indeed, YB-1 knockout mice displayed reduced expression of NSC markers in the SVZ, as well as reduced neurosphere growth, but showed enhanced NSC differentiation[63]. These data indicate the importance of oncogenes or cancer-associated transcription factors in SVZ NSCs involved in the genesis of GBM.
Undifferentiated NSCs
Recently, undifferentiated NSCs, especially intermediate progenitor cells, rather than NSCs, have been considered as the cells-of-origin of glioma tumors[64]. Llagunoet al[47] edited glioblastoma-relevant tumor suppressors, neufibromatosis type 1 (Nf1),transformation-related protein p53, and PTEN by a tamoxifen-inducible Crerecombinase in late-stage neuronal progenitors, neuroblasts, and differentiated neurons, respectively, but found no evidence of glioma formation. They showed that mainly early neural progenitor cells were responsible for gliomagenesis[47]. Liuet al[65] mutated concurrent p53/Nf1 in NSCs to establish gliomagenesis in mice by using mosaic analysis with double markers (MADM). The results showed that only oligodendrocyte precursor cells expressed aberrant/malignant growth and led to gliomagenesis, determined by tracing in MADM-based lineage analysis[65]. This suggests that undifferentiated stem cells, or oligodendrocyte precursor cells, are susceptible to tumorigenesis.
Thus, taken together, although many glioma-associated oncogene or transcription factor mutations in SVZ NSCs are responsible for the development of glioblastoma,understanding the role and potential mechanisms of SVZ NSCs driving GBM genesis or progression will be very meaningful for developing novel therapeutic interventions.
MECHANISM OF SVZ NSCS IN GLIOBLASTOMA
Patients with GBM with high isotropic p values in the SVZ region with high fluidattenuated inversion recovery indicated tumor infiltration involving the SVZ region[66]. Emerging data[67,68] confirmed that GBMs in close contact with the SVZ possessed aggressive characteristics, furthermore, the SVZ region may be an independent predictor of lower OS and PFS and early recurrence in patients with GBM. Therefore, the mechanism of the interaction between GBMs and SVZ NSCs should be carefully evaluated.
Evidence for SVZ involved in GBM progression
Recent studies suggested that patients with GBMs in contact with the lateral ventricle-SVZ region have lower survival rates than those with GBMs contacting the subgranular zone, corpus callosum, or cortex[16,17]. Furthermore, Şuşmanet al[69]found a significant difference in the PFS of patients with GBM who were administered with high radiotherapy doses within the LV-SVZ region. Chenet al[70] investigated 102 patients with GBM who had undergone surgical resection followed by adjuvant intensity-modulated radiation therapy and concomitant TMZ, and found that the recurrence of GBM was significantly related to the proximity to neurogenic regions(SVZ)[70]. To identify the potential molecules in the SVZ associated with GBM progression, Gollapalliet al[71] used proteomics techniques (two-dimensional difference gel electrophoresis and liquid chromatography-tandem mass spectrometry)to investigate the differences between SVZ+ (contacting) and SVZ− (non-contacting)GBM subtypes. Both serum and tissue proteomic analyses revealed significant alterations in various proteins associated with disease pathobiology, including lipid proteins, cytoskeletal, lipid binding, and cell-cycle-regulating proteins[71]. In addition,because of the similarities between tumor-initiating, GBM-derived neural stem (GNS)cells and genetically normal NSCsin vitro[72], Okawaet al[73] performed quantitative proteomics to compare total proteome and secreted proteome between GNS cells and NSCs. They identified 447 proteins in the total proteome and 138 proteins in the secreted proteome that were differentially expressed in GNSs and NSCs. Gene enrichment analysis mainly included extracellular matrix interactions, focal adhesion,cell motility, and cell signaling. They suggested that cell-matrix and cell-cell adhesion molecules play crucial roles in tumor infiltration[73]. Thus, these findings provide clinical and molecular evidence for SVZ NSCs in the regulation of GBM progression.
Gliomas invade SVZ region via chemoattractants secreted by NSCs
However, the mechanism of SVZ NSCs in glioma progression remains unclear,specifically the interactive biological functions between SVZ NSCs and GBMs. Qinet al[74] focused on the role or action of NSCs/neural progenitor cells (NPCs) on glioma cells, and found that the CM from SVZ NPCs had a chemoattractant effect on glioma cells. Through proteomic and functional analyses, they identified a chemoattractant complex secreted by SVZ NPCs, which included the neurite outgrowth-promoting factor, pleiotrophin (PTN), and its binding partners, secreted protein acidic and rich in cysteine (SPARC)/SPARC-like protein 1 and heat shock protein 90-beta. The chemoattractant complex promoted tumor invasion by activating Rho/Rho-associated protein kinase signaling in gliomas. Furthermore, PTN was expressed at high levels in the SVZ, and its knockdown by shRNAin vivoremarkably reduced the ability of glioma to invade the SVZ[74]. This study mainly proposed that NSCs in the SVZ induced highgrade gliomas to invade the SVZ region by secreting specific chemoattractant factors,and considered that the cytokine PTN is a potential target for glioma therapy. These results provide an experimental basis for glioma invasion of the SVZ region. Thus,targeting the interaction process between GBM and SVZ NSCs can represent a novel strategy to curtail the malignant potential of SVZ NSCs and restrict the progression of gliomas.
Gliomas promote tumor transformation of NSCs by extracellular vesicles delivery
Many studies[75-78] have shown that extracellular vesicles (EVs)/exosomes play an important role in intercellular communication. In addition, EVs/exosomes derived from gliomas or non-glioma cells in the TME are involved in tumor cell proliferation,invasion, malignancy, and drug resistance owing to their functions delivering mRNA,microRNAs, or proteins[79,80]. Wanget al[81] added glioblastoma-derived EVs to culturing with NSCs and found that NSCs de-differentiated into tumor-promoting cells. They found that these transformed cells had higher proliferative, migratory, and clonogenic activities than naïve cells, and accelerated tumor formationin vivo. Using single-cell transcriptome sequencing analysis, they identified several key genes in the transformed NSCs, includingS100B, CXCL14, EFEMP1, SCRG1, GLIPR1, HMGA1, andCD44[81]. This study preliminarily shows that EVs secreted by gliomas can regulate and promote tumor transformation of SVZ NSCs by gene delivery, suggesting an origin for glioma recurrence. However, the targets or potential links between SVZ NSCs and gliomas are unclear and require further investigation and experimental validation.
INTERVENTIONS TARGETING THE SVZ (NSCS) FOR GLIOBLASTOMA TREATMENT
Recent data revealed that GBM contacting the SVZ region presented highly aggressive characteristics, and radiotherapy received within the SVZ region increased the PFS of patients with GBM. Furthermore, SVZ NSCs not only contribute to neurogenesis and play an important role in nerve regeneration[13,82], but also have a tumor-homing property and can be used to deliver drugs for tumor treatment[83-86]. The pre-clinical and clinical studies of interventions using SVZ/NSCs for glioblastoma treatment are shown in Table 1.
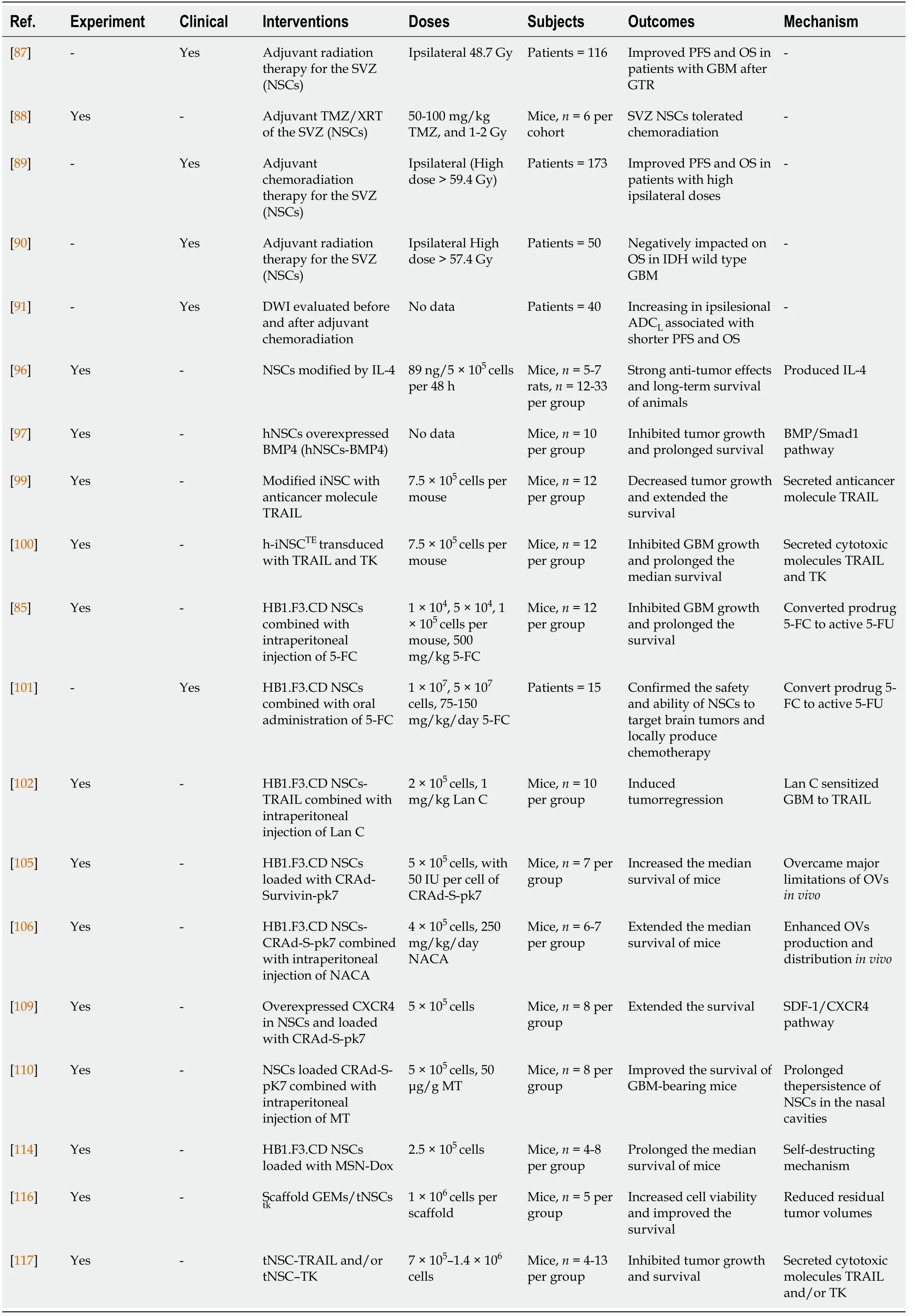
Table 1 Pre-clinical and clinical studies of interventions using subventricular zone/neural stem cells for glioblastoma treatment
Radiotherapy for the SVZ region in glioma intervention
Currently, in addition to surgery and chemotherapy, radiation therapy has been used as a standard treatment strategy for patients with GBM in the clinic, and can be used to target the SVZ region. Chenet al[87] retrospectively analyzed 116 patients with surgically resected glioblastoma and found that the PFS and OS of patients significantly improved with a mean radiation dose of 40 Gy to the ipsilateral SVZ. This result suggests that targeting the SVZ region was necessary for treating GBM. To determine whether SVZ NSCs can tolerate radiation therapy, Cameronet al[88]combined the chemotherapy drug TMZ with X-irradiation in mice, and found that chemoradiation resulted in type A neuroblast apoptosis, but not NSC death.Furthermore, type A cells can be repopulated within the V-SVZin vivoby sufficient recovery time[88]. Animal experiments suggested that SVZ NSCs could tolerant standard chemoradiation therapy. However, high radiation therapy doses to the ipsilateral SVZ may not be effective in patients with GBM[89]. Muraccioleet al[90]found that high radiation doses > 57.4 Gy to ipsilateral NSCs and > 35 Gy to contralateral SVZ negatively impacted the OS of IDH-wild-type glioblastoma patients[90]. Moreover, Choet al[91] found that the apparent diffusion coefficient with lower Gaussian distribution values of ipsilesional SVZ increased after chemoradiation,leading to a poor PFS and OS of patients.
Therefore, although radiotherapy can be used to target the SVZ area, some problems warrant further consideration. First, the SVZ area is very small, making it difficult to accurately control the dose to the targeted SVZ. In particular, a high dose of radiation therapy may result in adverse effects. Second, due to the fact that SVZ NSCs are physiologically involved in the replenishment and repair of injured nerve tissue,radiation therapy-induced damage to NSCs in the SVZ may affect the repair capability of neurological functions. Therefore, it is necessary to develop novel gene-targeted therapeutic methods to precisely target glioma and avoid potential side effects.
NSCs loading anticancer molecules for targeted therapy of gliomas
The tumor-homing ability of NSCs has been confirmed to enable NSCs to migrate toward and co-localize within the tumor isletsin vivo[85,92-94]. Glasset al[95] reported that endogenous NSCs in mice migrated from the SVZ toward gliomas and surrounded them. They injected red fluorescent protein-labeled GL261 cells into transgenic mice with a promoter for nestin (nestin-GFP) to explore the association between endogenous NSCs and gliomas. They found that nestin-GFP cells surrounded the tumors and expressed early precursor markers; furthermore, the tumor-associated precursor cells originated from the SVZ[95].
Because current gene therapies are unable to infiltrate the brain parenchyma and hard-to-reach glioblastoma core site, NSCs have been used to load therapeutic molecules for targeted treatment of gliomas. Benedettiet al[96] transferred IL-4 to C57BL6J mouse NSCs and injected them into the brains of mice to establish a glioblastoma model. They found that the survival of tumor-bearing mice was significantly extended, which was also observed in Sprague-Dawley rats with C6 glioblastomas[96]. Liuet al[97] overexpressed bone morphogenetic protein 4 (BMP4) in hNSCs (hNSCs-BMP4) and found that the cells inhibited gliomasin vitroandin vivoby secreting BMP4. These findings suggest effective approaches based on loading of NSCs with therapeutically effective molecules for glioma treatment. In recent years,transdifferentiation (TD) has been successfully used in somatic cell reprogramming[98]. Bagóet al[99] generated TD-derived induced NSCs (iNSCs) by transdifferentiating fibroblasts in mice, and found that the iNSCs not only rapidly homed and migrated to glioblastomasin vitroandin vivobut also successfully delivered the anticancer molecule, tumor necrosis factor α–related apoptosis-inducing ligand(TRAIL), leading to a significant decrease in the growth of xenograft glioblastoma and prolongation of the median survival times of mice[99]. Next, they[100] also engineered human iNSCs by TD of human fibroblasts to deliver the cytotoxic agents TRAIL and TK (thymidine kinase). The cytotoxic h-iNSCs rapidly migrated to human GBM cells and penetrated GBM spheroids, significantly reducing the size of solid human GBM xenografts and prolonging the median survival of mice[100]. These results suggest that NSCs can be used as a cell platform for glioma-homing cytotoxic therapy.
Pre-clinical and clinical applications of human NSCs for GBM treatment
HB1.F3.CD, a cytosine deaminase (CD)–expressing clonal human NSC line that can convert the prodrug 5-fluorocytosine (5-FC) to active chemotherapeutic 5-fluorouracil(5-FU), has been approved by the United States Food and Drug Administration for use in human clinical trials. Aboodyet al[85] used HB1.F3.CD and 5-FC to treat tumorbearing mice and showed that the average tumor volume of mice was significantly decreased, with no difference in toxicity. This result confirmed the efficacy of an allogeneic NSC-mediated enzyme/prodrug-targeted therapy in high-grade glioma.Portnowet al[101] reported the first-in-human study (NCT01172964) in patients with recurrent, high-grade glioma by retrovirally transducing HB1.F3.CD.C21 (CD-NSCs)to express cytosine deaminase stably. Fifteen patients with recurrent, high-grade glioma underwent intracranial administration of CD-NSCs during tumor resection or biopsy. After oral administration of 5-FC, CD-NSCs produced 5-FU locally in the brain in a 5-FC-dose-dependent manner by intracerebral microdialysis with no dose-limiting toxicity. Furthermore, autopsy results revealed that CD-NSCs that had migrated to distant tumor sites were non-tumorigenic[101]. These findings demonstrate the initial safety and proof-of-concept of NSCs in targeting brain tumors. In addition, the cardiac glycoside lanatoside C (Lan C) sensitizes glioma cells to the anticancer agent, TRAIL.Tenget al[102] showed that HB1.F3.CD engineered to express TRAIL migrated towards tumors in mice and induced tumor regression in combination with Lan C.Oncolytic adenoviral virotherapy exhibits limitations, such as a poor viral distribution and infiltration throughout tumors[103,104]. Ahmedet al[105] used HB1.F3.CD loaded with the oncolytic adenovirus, CRAd-Survivin-pk7 (CRAd-S-pk7), and found that OVloaded HB1.F3.CD cells effectively migrated to the contralateral hemisphere of mice,inhibited the progression of clinically relevant human-derived glioma models, and prolonged the median survival times of mice compared to OV alone[105]. Furthermore, Kimet al[106] used the novel anti-oxidant thiol, N-acetylcysteine amide(NACA), to prevent OV-mediated potential toxicity, and found that NACA combined with CRAd-S-pk7 significantly increased NSC activity, enhanced CRAd-S-pk7 production, and improved the therapeutic efficacyin vivo[106]. Currently, NSCs loaded with CRAd-S-pk7 have been used in a clinical trial in patients with GBM(NCT03072134).
Other pre-clinical strategies of NSCs
Intranasal delivery of therapeutics to the brain is a novel strategy[107,108]. Deyet al[109] utilized hypoxic preconditioning or overexpression of CXCR4 to enhance the tumor-targeting ability of NSCs. They found that NSCs intranasally delivered oncolytic virus into glioma efficiently and extended the survival of mice. Spenceret al[110] found that methimazole (MT), a US-FDA-approved compound, effectively disrupted the olfactory epithelium, delayed clearance, and kept cells in the nasal cavity. After MT injection, oncolytic virus-loaded NSCs delivered intranasally significantly improved the survival of GBM-bearing mice[110-112]. Thus, intranasal delivery as a novel pharmacologic strategy can employ the non-invasive NSCs-based therapeutic platform to optimize the treatment.
Mesoporous silica nanoparticles (MSNs) have controlled-release capabilities and non-toxic features. Chenget al[113] conjugated MSNs with 111In and administered 111In-MSN labeled NSCs into glioma-bearing miceviaeither intracranial or systemic injection. Their results revealed that 111In-MSN-NSCs actively migrated toward glioma xenografts[113]. Chenget al[114] employed a pH-sensitive, MSN-doxorubicin(Dox)-loaded NSC delivery system for delaying drug release and non-invasively trigger programmed cell death. They found that MSN-Dox-loaded HB1.F3.CD cells efficiently preserved their migratory function and released MSN-Dox conjugates,causing significant toxicity to glioma cells, glioma apoptosis, and animal survival[114]. These results suggest a multimodal, controlled-release, therapeutic strategy.
Engineered tumoricidal neural stem cells (tNSCs) show potential for treating aggressive brain glioblastoma[94,99-101,115]. Sheetset al[116] optimized and used HB1.F3.CD cells to prepare a polymeric scaffold [nanofibrous electrospun poly (Llactic acid) scaffolds]. They found that the polymeric scaffold significantly extended tNSC persistence in the cavity of a mouse model of human GBM resection/recurrence as the tNSCs migrated from the scaffolds into the tumors, bothin vitroandin vivo.After engineering tNSCs with the prodrug/enzyme TK and transplanting them into the post-operative cavity of mice, the researchers found that the residual tumor volume of mice was markedly reduced, and the median survival times were extended[116]. Satterleeet al[117] used organotypic brain slice explants and distinct human glioma types to create a novel hybrid tumor model and then evaluated the efficacy of iNSCs loaded with TRAIL or enzyme-prodrug therapy. They found that tNSC-TRAIL significantly decreased tumor growth and promoted the survival of the animals[117].These findings suggest a new strategy and model for testing targeted GBM therapy.Overall, as an effective drug delivery platform, NSCs can be modified for delivering various anti-tumor agents, including apoptotic agents, oncolytic viruses, or prodrug-activating enzymes, and optimized to improve their therapeutic benefits in glioblastomas.
CONCLUSION
With the development of gene-targeted therapy and further studies demonstrating the role of the TME in tumor progression, crosstalk between glioma and its microenvironment has been recognized, especially the communication of glioma and non-glioma cells. Recently, pre-clinical and clinical experiments confirmed that SVZ NSCs are closely related to glioma origin and progression through gene mutation and factor delivery. In general, gliomas are separated by a long distance from the SVZ region and may interact paracrine pathways, such as secreted cytokines and EVs. However,studies demonstrating an interaction between gliomas and SVZ are only preliminary,and the crosstalk mechanism remains unclear. In particular, SVZ NSCs are generally in a resting state but can be activated by brain disease or nerve damage. When gliomas occur, which can induce a state of intracranial stress, SVZ NSCs may be activated through the TME. However, how the microenvironment of glioma stimulates SVZ NSCs, and how SVZ NSCs react to the glioma, as well as the potential mechanisms,need further exploration (Figure 2). Thus, the genetic mutations or secreted factors associated with both GBMs and SVZ NSCs should be further examined.
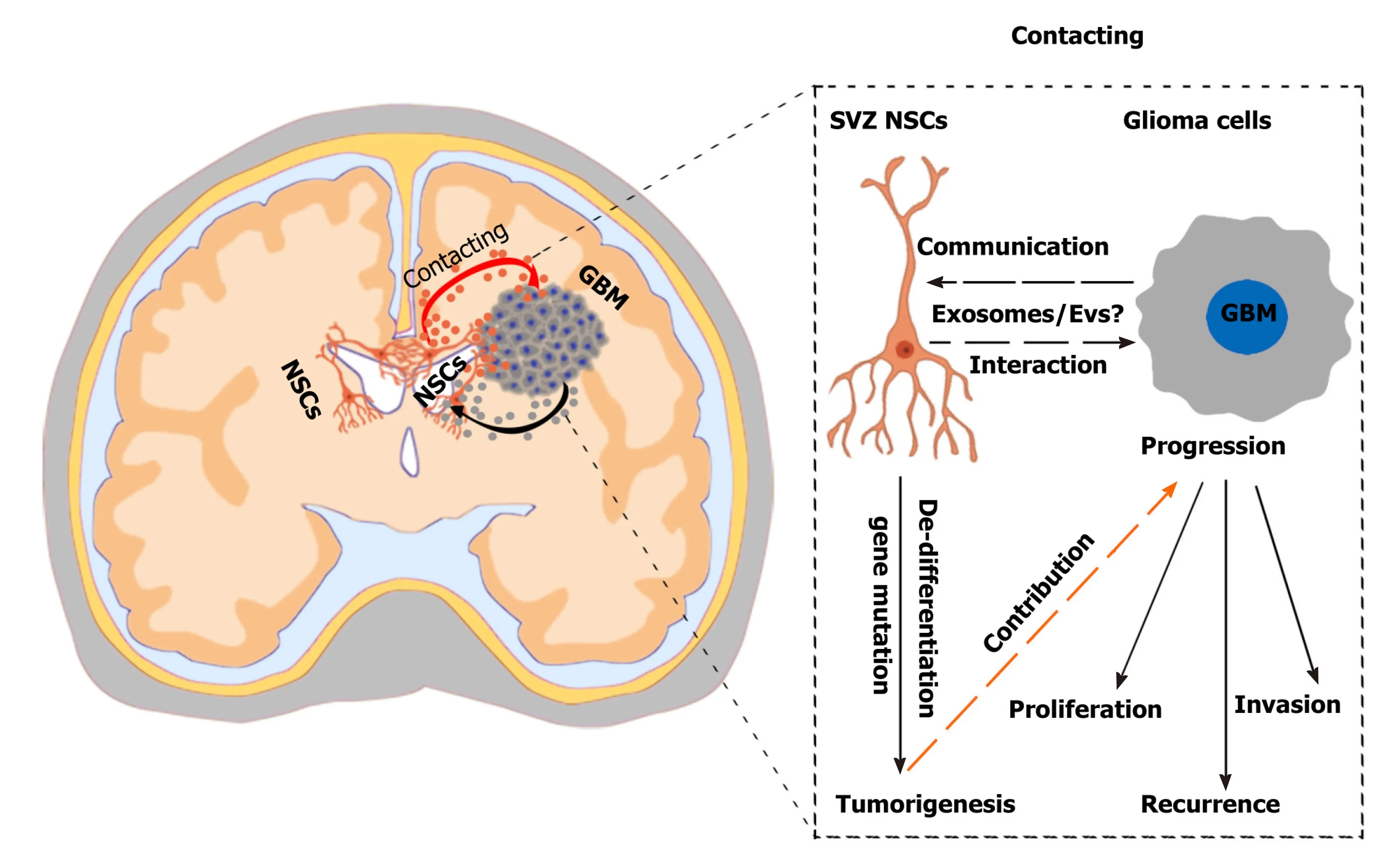
Figure 2 Challenges and effects of neural stem cells in the subventricular zone in glioma progression. A hypothetical scenario shows the crosstalk between neural stem cells (NSCs) in the subventricular zone (SVZ) and glioblastoma multiformes and the effects of SVZ NSCs in glioma progression. SVZ NSCs can exert chemoattractant effects on glioma cells through secretion of chemoattractant complex factors [such as pleiotrophin, HSP90B, and secreted protein acidic and rich in cysteine (SPARC)/SPARC-like protein 1], and glioblastomas can induce SVZ NSCs to de-differentiate into tumor-promoting cells via glioblastomaderived extracellular vesicles (EVs). The SVZ NSC-glioma interactions are mainly mediated by the secreted factors, especially EVs/exosomes. Understanding the biological mechanisms mediated by cytokines and EVs/exosomes will help to discover new therapeutic strategy. NSCs: Neural stem cells; SVZ: Subventricular zone;GBM: Glioblastoma multiforme.
In summary, as specialized stem cells in the nervous system, NSCs play vital roles in regulating physiopathological functions of the brain, including glioma development and progression. Studies of the interaction between SVZ NSCs and GBMs may reveal new molecular, epigenetic, and genetic characteristics that can be employed for combination therapy. Further research is needed to verify the mechanisms and advantages of SVZ NSCs in glioma progression and discover specific gene target treatment to increase the survival of patients with GBM. By exploring how gliomas stimulate the activation of SVZ NSCs, and how SVZ NSCs regulate the development and progress of gliomas, particularly the interaction mechanism of glioma-NSC mediated by secreted EVs/exosomes or factors, potential therapeutic strategies can be developed to treat gliomas.
ACKNOWLEDGEMENTS
We really appreciate Dr. Ouyang SS for assistance with the valuable suggestions and image editing.
杂志排行
World Journal of Stem Cells的其它文章
- Epigenetic modulators for brain cancer stem cells: Implications for anticancer treatment
- Mechanisms involved in selecting and maintaining neuroblastoma cancer stem cell populations, and perspectives for therapeutic targeting
- Roles of mitochondrial unfolded protein response in mammalian stem cells
- Stem cell therapies in tendon-bone healing
- Exosomal microRNAs from mesenchymal stem/stromal cells:Biology and applications in neuroprotection
- Immunotherapy against programmed death-1/programmed death ligand 1 in hepatocellular carcinoma: Importance of molecular variations, cellular heterogeneity, and cancer stem cells
