Antibacterial resistance patterns of Acinetobacter baumannii complex: The results of Isfahan Antimicrobial Resistance Surveillance-1 Program
2021-07-20SayedNassereddinMostafaviSoodabehRostamiZaryNokhodianBehroozAtaeiAzamCheraghiParisaAtaabadiNaserAlmasiZohrehNorouziRoyaKelishadi
Sayed Nassereddin Mostafavi, Soodabeh Rostami, Zary Nokhodian, Behrooz Ataei, Azam Cheraghi, Parisa Ataabadi, Naser Almasi, Zohreh Norouzi, Roya Kelishadi
1Infectious Diseases and Tropical Medicine Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
2Child Growth and Development Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
3Nosocomial Infection Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
4Department of Hospitals Supervision and Accreditation, Vice-Chancellery for Clinical Affairs, Isfahan University of Medical Sciences, Isfahan, Iran 5Department of Infection Control, Alzahra Medical Center, Isfahan University of Medical Sciences, Isfahan, Iran
6Department of Microbiology, Clinical Laboratory of Dr. Shariati Hospital, Isfahan Social Security Organization, Isfahan, Iran
ABSTRACT
KEYWORDS: Acinetobacter baumannii; Acinetobacter infections;Anti-bacterial agents; Drug resistance; Iran
1. Introduction
Acinetobacter species are Gram-negative, oxidase negative,non-motile coccobacilli that are ubiquitous in the environment.The Acinetobacter (A.) baumannii complex consists of several genospecies. They are genetically related and phenotypically indistinguishable by using phenotypic methods and molecular methods for accurate identification[1]. Almost 80% of the isolates which cause human diseases belong to A. baumannii[2], who can cause both community-acquired infections (CAIs) and healthcareassociated infections (HAIs)[3,4]. According to the World Health Organization and Centers for Disease Control and Prevention definitions, HAIs are the infections occurring in patients during the process of care in a care facility such as hospitals which was not present or incubating at the time of admission[5,6].
Infections caused by A. baumannii are frequently reported from intensive care units where they are implicated as the cause of ventilator-associated pneumonia. However, it can cause other infections including bacteremia, septicemia, endocarditis, urinary tract infection, secondary meningitis, and infections of the skin,soft tissue, and prosthetic devices[2,7-9]. Since contamination with transient or normal flora can occur during clinical sample collection procedures, the distinction between contamination and confirmed infection could be a problem and false-positive culture results in increased length of patients with antibacterial use and costs of preclinical investigation[10].
The bacterium can use different mechanisms of resistance,leading to the emergence of strains that are resistant to all classes of antibiotics[8]. Because of the high-level resistance to various antimicrobial agents and the easily spread from one patient to another and also persisting in the environment for a long time, it has become a global health threat. Up-to-date data on the local susceptibility pattern of isolates is necessary for appropriate antimicrobial therapy and control of hospital outbreaks. Several studies have assessed on the antibiotic susceptibility pattern of A.baumannii strains isolated from Iranian patients[11]. In most of these studies, the power of the study was low due to the small sample size and inclusion of contaminated samples in the analysis of the results. Besides, there is no plan to differentiate between CAI from HAI. Due to these limitations, we decided to study the prevalence and pattern of antibiotic resistance of A. baumannii complex isolated from patients with conformed infections admitted to three referral hospitals in Isfahan province in the central region of Iran who participate in the Isfahan Antimicrobial Resistance Surveillance-1(IAS-1) Program.
2. Materials and methods
2.1. Study design
This study is a part of a local surveillance survey entitled “IAS-1”which was performed for two years (March 2016 to March 2018)in Isfahan, Iran. In the IAS-1 Program, in addition to registering antibiotic susceptibility of clinical isolates, it was targeted to exclude contaminated samples, determine hospital/community source of infection and site of the infection by collaboration with trained infectious control nurses and physicians in the participant medical centers[12]. Three major referral hospitals that enrolled in the study were Al-Zahra, Dr. Shariati, and Dr. Gharazi hospitals. They had more than 1 300 beds totally and their laboratories had achieved approved quality credit from the Iranian Ministry of Health for the microbiological report and were collaborators of the World Health Organization in the Global Antimicrobial Resistance Surveillance System Program. They receive more than 21 500 samples per year and all of the microbiological data was gathered in WHONET software version 5.6.
2.2. Bacterial isolation, phenotypic tests, and antibiotic susceptibility testing
From March 2016 to March 2018, all clinical samples (such as blood, urine, and other urinary tract samples, central nervous system fluid, upper and lower respiratory tract secretions, skin and soft tissue, etc.) from hospitalized patients suspected to infection in three participate hospitals were cultured. These samples were received from different wards; however, were considered as intensive care units and non-ICU wards in this study. Non-ICU wards included emergency rooms, surgeries, medicines, and pediatrics.Identification of A. baumannii was made by routine conventional methods such as oxidase test, API 20 NE kit (version 6.0,bioMerieux, Marcy L Etoile, France), and growth in 42 ℃[13]. Since routine phenotypic identification tests were not able to differentiate between baumannii and non-baumannii, so all isolates were considered A. baumannii complex.Antibiotic susceptibility testing was performed by disk diffusion in Mueller-Hinton agar and was interpreted according to the Clinical Laboratory Standard Institute guidelines (CLSI 2016 and 2017[14,15].Laboratories have assessed the sensitivity of isolates to the following class of antibiotics: Penicillin-penicillinase inhibitors (ampicillinsulbactam 10/10 µg), aminoglycosides (gentamicin 10 µg or amikacin 30 µg), cephalosporins (cefotaxime 30 µg or ceftriaxone 30 µg or cefepime 30 µg, and ceftazidime 30 µg), fluoroquinolones(ciprofloxacin 5 µg or levofloxacin 5 µg), folate inhibitors(trimethoprim-sulfamethoxazole 1.25/23.75 µg), carbapenems(imipenem 10 µg or meropenem 10 µg) and tetracycline (minocycline 30 µg). Dehydrated antibiotic discs were commercially prepared from MAST, Merseyside, UK. Minimum inhibitory concentrations of colistin were determined by the E-test strips (Liofilchem, Roseto Degli Abruzzi, Italy) according to the manufacturer’s instructions.The methods and kits were checked repeatedly (every three months)to be the same in all participant microbiology laboratories. Reference strains, including Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for quality control testing.Isolates resistant to at least one agent in three or more antimicrobial categories were recognized as multidrug-resistant (MDR)[16,17].
2.3. Identification of confirmed infection, CAI, and HAI
If A. baumannii complex strains were isolated from a patient with clinical symptoms and para clinical findings of infection, it was considered as a confirmed pathogen. Otherwise, the isolate was considered as a contaminant or colonizer isolate. Clinical symptoms and para clinical findings of infection are presented in the supplementary table. If the patient showed a new clinical symptom of infection (such as fever, erythema/swelling of the surgical site,or any change in the general condition of the patient) after 48 hours or more after hospitalization or within 30 days after having surgery infection is considered as an HAI[18,19]. In other cases, infection is assumed as a CAI.
2.4. Statistical analysis
Data on antibiotic susceptibility testing, age group, admission ward, site of infection, and hospital/community acquisition were extracted from WHONET software Version 5.6, and analysis was done with SPSS Version 20.0. Continuous variables were compared using a one-way analysis of variance. Variables were analyzed by the Chi-square test. A P-value of <0.05 was considered statistically significant.
3. Results
Totally 539 A. baumannii complex isolates were enrolled in the study. Two hundred and eighty-five (52.87%) isolates were determined as a contaminant or colonizer isolates and excluded fromthe study. Of 254 patients who had conformed A. baumannii complex infection, 158 (62.20%) cases were male, 27 (10.63%) were under 20 years old, and 172 (67.72%) had HAIs. Ninety-six (37.79%) patients were admitted to ICU wards, and 86 of them (89.58%) have HAIs.The most frequent diagnosis was bloodstream infections (sepsis or bacteremia) (111, 43.70%), pneumonia/pulmonary empyema (41,16.14%), urinary tract infection (39, 15.35%), surgical site infections(31, 12.20%), meningitis (21, 8.26%) and other infections (11,4.33%), respectively (Table 1).According to disk diffusion method for detection of antibioticresistant pattern of A. baumannii complex isolates, the results showed high level resistance to ceftazidime (94.21%), ceftriaxone(94.20%), meropenem (94.14%), cefepime (91.06%), trimethoprim/sulfamethoxazole (85.34%), ciprofloxacin (84.87%), amikacin(76.85%), ampicillin-sulbactam (76.79%), levofloxacin (76.29%)and imipenem (67.97%). A lower rate of non-susceptibility was observed against minocycline (44.44%). No resistance to colistin was detected by the E-test method. The rate of MDR isolates was 88.97% (Table 2).The resistance to levofloxacin, minocycline, meropenem, amikacin,imipenem, ciprofloxacin, trimethoprim/sulfamethoxazole, and rate of MDR was significantly more frequent in patients with HAI in comparison to those with CAI (Table 2).
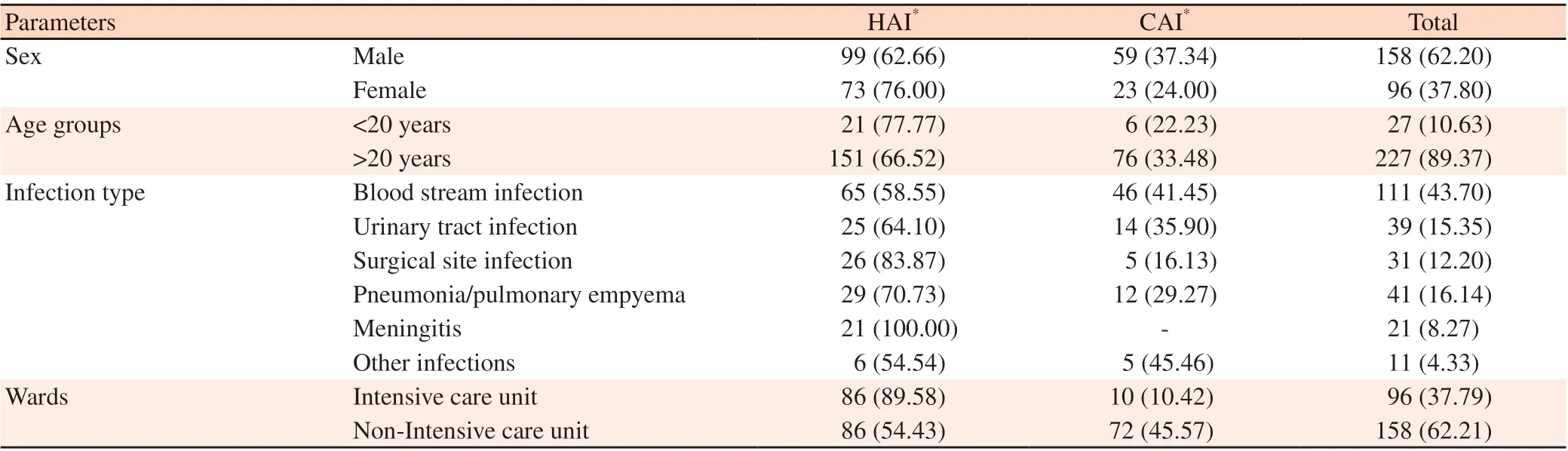
Table 1. The characteristic of healthcare-association infections and community-association infection [n (%)].

Table 2. Antibacterial resistance of Acinetobacter baumannii in accordance to the source of the infection in Isfahan Iran 2016-2018: Results of IAS-1 study [%(NRI/TES)].

Table 3. Antibacterial resistance of Acinetobacter baumannii in accordance to the age and sex of the patients in Isfahan Iran 2016-2018: Results of IAS-1 study [% (NRI/TES)].

Table 4. Antibacterial resistance of Acinetobacter baumannii in patients with HAIs in accordance to admission ward in Isfahan Iran 2016-2018: Results of IAS-1 study [% (NRI/TES)].

Table 5. Antibacterial resistance of Acinetobacter baumannii complex accordance to diagnosis in Isfahan Iran 2016-2018: Results of IAS-1 study [% (NRI/TES)].
There was no significant difference between resistance of A.baumannii complex isolates according to the age of patients(<20 versus >20 years). However, the resistance to amikacin and minocycline and the rate of MDR were significantly different between males and females (Table 3). In patients with HAI, MDR isolates was significantly different regarding admission in ICU ward(ICU versus non-ICU) (Table 4).
Resistance to levofloxacin and ciprofloxacin and resistance to minocycline were lower in isolates from patients with bloodstream infections and urinary tract infections, respectively, compared to patients admitted with other diagnoses (Table 5).
4. Discussion
Our study revealed that A. baumannii complex strains in our region are highly resistant to many antibiotic agents. Resistance is more frequent in strains isolated from patients with HAIs. Previous studies were conducted in mixed clinical specimens from patients with both CAI and HAI. In addition, contaminant/colonizer isolates were not recognized and excluded from the analysis. Thus the results could have sampling biases. In this study, we excluded these kinds of isolates by simple practical guidelines prepared by the scientific committee of the IAS-1 study and contribution with infection control nurses and physicians in enrolled hospitals[12].About 53% of A. baumannii complex strains in our study were recognized as contaminant or colonizer organisms. A. baumannii was not considered as a common source of contamination of culture media. However, some previous reports suggested that the isolate would be specimen contamination[20,21].
More than 90% of isolates in our study were resistant to cephalosporins and meropenem and these agents may be unsuitable for empiric treatment of A. baumannii infections in our region. Also,in the sub-analysis of the antibiotic susceptibility pattern resistance to most of the antibiotic classes was significantly more frequent in patients with HAI in comparison to those with CAI. In a report of 647 clinical isolates from China, the resistance of A. baumannii isolates to cefepime, ceftazidime, and imipenem was 74.5%, 76%,and 71.4%, respectively[22]. In another study from India, more than 90% of A. baumannii isolates were resistant to ceftazidime,cefepime, meropenem, and imipenem[23]. It seems that the resistance of A. baumannii strains to cephalosporins and carbapenems in Iran is alarmingly high. As discussed in other studies, most of the carbapenem-resistant A. baumannii strains are MDR and even pandrug resistant. Restriction of inappropriate usage of these antibiotics in hospital settings by appropriate stewardship programs may help to decrease the production and spread of resistance in A. baumannii strains in the area.
In our investigation, the resistance rate of the A. baumannii complex to ampicillin/sulbactam was 76.79%. Un-susceptibility to this agent was similar in CAIs and HAIs. In three different studies from our city Isfahan and Shiraz, Iran, the rate of un-susceptibility to this agent was 33.9%, 92%, and 94.9%, respectively[7,24,25].Differences in target population and inclusion of contaminant and/or colonizer isolates may be the cause of this significant dissimilarity.These high-level un-susceptibility of A. baumannii may be a sign of inappropriate advice of the antibiotic in clinical settings.
In our study, 76.79% of the isolates were resistant to amikacin.Aminoglycoside resistance was significantly more prevalent in isolates from HAI in comparison to CAI. Similar results were reported from other studies in Iran during recent years[7,24-28]. In other recent A. baumannii antimicrobial resistance surveys in China,and the USA, un-susceptibility was reported to be 66.3% and 45.7%,respectively[22,29]. This agent could be a choice for the treatment of A. baumannii infections after documentation of susceptibility of the strain to it.Our research showed that 85.34% of A. baumannii isolates were resistant to trimethoprim-sulfamethoxazole. The resistance rate was significantly higher in patients with HAIs in comparison to those with CAIs. In two reports from India and one study in China resistance to this antibiotic was 80%, 73.3%, and 61.1%,respectively[22,23,30]. This agent should be considered in the treatment of A. baumannii infections especially CAIs.In the present study resistance to ciprofloxacin and levofloxacin was 84.87% and 76.30%, respectively. The susceptibility of isolated A.baumannii strains to these agents was significantly higher in CAI. In recent reports from Iran, more than 90% of A. baumannii strains were resistant to ciprofloxacin[7,24,25,27]. Resistance rates of 75.3% and 96% to ciprofloxacin were recognized in one study in China during 2013-2014 and one report in India in 2012-2016, respectively[22,23].Exclusion of contaminant isolates and differentiation of CAIs from HAIs in our study revealed that fluoroquinolones could be a good choice for the treatment of A. baumannii infections acquired from community settings in our hospitals.In our study, all isolated A. baumannii strains showed susceptibility to colistin. In a recent systematic review on the susceptibility of A. baumannii to colistin in Iran, a resistance rate of 4% had been estimated[11]. In China and India resistance rate of 3% and 0%to colistin had been reported, respectively[22,30]. Susceptibility of all A. baumannii isolates to colistin necessitates the meticulous implementation of stewardship programs in hospitals that use this agent logically and appropriately. This can save the agent for the treatment of pan-drug resistant Gram negatives. Our results showed that 89.0% of Acinetobacter isolates in accordance to the source of the infection were MDR. In two previous reports from Iran, MDR rate of 95% and 100% was detected in A. baumannii strain[7,24].Continuous surveillance of A. baumannii strains is necessary and essential.In conclusion, high-level resistance to antibiotics was detected in both community-acquired and healthcare-associated A. baumannii isolates. More sensitivity to colistin, minocycline, and levofloxacin was detected in CAIs; respectively. In HAIs colistin was the single acceptable agent for empiric treatment of the infections. Appropriate antibiotic prescription in a clinical setting is an essential need for the control and prevention of A. baumannii associated infections.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
We appreciate the excellent support provided at each center by numerous technologists and managers. Also, the following individuals contributed to the development of the protocol and the draft manuscript.
Authors’ contributions
SN.M. was the main investigator and proposed the main idea,designed the study, wrote the protocols and guidelines and revised the manuscript. S.R. was project manager and collected the data. ZN analyzed the data and wrote the first draft of the manuscript. B.A.,AC, and RK were members of the scientific committee. P.A., N.A.,and Z.N. were members of the executive committee.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of short- and long-term immunization of recombinant disorganized muscle protein-1 (rDIM-1) against human filarial parasite Brugia malayi in rodents
- Plasmid DNA encoding neutralizing human monoclonal antibody without enhancing activity protects against dengue virus infection in mice
- Spatio-temporal history of H9N2 viruses in Iran and neighbor countries by Bayesian analysis and molecular characterization
- Phylogeny of Brucella abortus strains isolated in the Russian Federation
- ACE2 downregulation promotes thrombosis and cardiac injury in COVID-19 patients
