Effect of short- and long-term immunization of recombinant disorganized muscle protein-1 (rDIM-1) against human filarial parasite Brugia malayi in rodents
2021-07-20VikasKushwahaPuvvadaKalpanaMurthy
Vikas Kushwaha, Puvvada Kalpana Murthy
Division of Parasitology, CSIR-Central Drug Research Institute, New Campus, BS 10/1, Sector 10, Jankipuram Extension, Sitapur Road, Lucknow 226031, India
ABSTRACT
KEYWORDS: Brugia malayi; Disorganized muscle protein-1;Th1/Th2 cytokines; Macrophage activity
1. Introduction
Lymphatic filariasis also called elephantiasis, a painful, disfiguring neglected tropical disease, causes permanent or temporary disability worldwide. The causative agents are Wuchereria bancrofti, Brugia(B.) malayi and B. timori, and different mosquito species like Culex,Anopheles, Aedes, and Mansonia are responsible for the transmission of the disease. For prevention of disease transmission, the World Health Organization (WHO) has recommended preventive chemotherapy strategy (MDA: mass drug administration) of three
drugs (albendazole, ivermectin and diethylcarbamazine citrate)annually to individuals living in endemic areas[1]. A diverse array of parasite antigens elicit the host immune responses in a host which may be protective and non-protective. Researchers have identified various filarial antigens and their responses that help in the development of vaccine candidates. While adequate efforts have been made but to date, no acceptable antifilarial vaccine exists. Till date, various potential vaccine candidates have been identified and characterized and their vaccine potentials in rodent models have been evaluated. Previously, we have reported that recombinant DIM-1bm (rDIM-1bm) partially protects the host from B. malayi infection by modulating the host immune response and can be explored as a vaccine candidate[2].
Often it was observed that in an endemic population almost all individuals are exposed to the mosquito bites, so an equal probability prevails for individuals to be exposed to inoculation of larval stage 3 of B. malayi (L3). Continuous immune stimulation with the helminth infection in the host produces anti-inflammatory responses that favor long-term survival of parasite, enhance the cellular specific hyporesponsiveness[3], and shifting of cytokine balance toward a Th2/Th3 response[4]. With this background in the present study, we used short-term (3-dose) and long-term (3- and 6-week repeated administration for a prolonged period) immunization schedules for vaccination of Mastomys (M.) coucha with rDIM-1bm to understand the role of a stimulatory protein ‘DIM-1’ molecule in hostparasite interactions and their effects on subsequent invasion of the parasites. Animals were immunized with rDIM-1bm by 3 different immunization protocols, afterward inoculated with L3 of B. malayi.Further, effects on the various responses (parasitological, humoral and cell-mediated responses) of the host were assessed.
2. Materials and methods
2.1. Host and parasite
Male M. coucha (45-50 g or 9-10 weeks old) were received from National Laboratory Animal Centre and handling was done after taking the permission of the Institutional Animal Ethics Committee(IAEC) of CSIR-CDRI, Government of India (approval No: 48/IAEC/2012). Animals were housed in the controlled environment[Temp.: (23±2) ℃; relative humidity: 60% and photoperiod: 12 h light-dark cycles] and fed on standard rodent maintenance diet(carbohydrates 58.30%, protein 21.1%, fat 7.2%, crude fiber 6.6%,moisture 6.8%) supplemented with dried shrimps, ad libitum, and water[2]. The experimental animals were monitored daily until the endpoint of the study and were killed by cervical dislocation. The procedure followed was approved by regulatory authorities.
B. malayi parasite infection was periodically cultivated through the susceptible mosquito strain Aedes (Ae.) aegypti in host M. coucha[5].L3 stage of B. malayi parasite were isolated from 9-10 days old blood-fed Ae. aegypti mosquitoes[6].
2.2. Cloning and purified recombinant protein production
rDIM-1bm protein of ~40 kDa was cloned, expressed, and endotoxin-free recombinant protein was prepared using a Ni-NTA affinity column chromatography[2]. Briefly, the amplified and purified PCR product of DIM-1bm was cloned in competent Escherichia (E.) coli DH5ααand subcloned in the expression vector(pTriEx-4), and transformed into E. coli BL21-DE3 cells. Cells having recombinant DIM-1bm gene were induced by 1 mM of IPTG (isopropylβ-D-galactopyranoside; Sigma, USA) for gene expression. His-tagged rDIM-1bm protein was purified using Ni-NTA affinity column chromatography (Thermo, USA). After purification, the contaminating endotoxins in recombinant proteins were removed through polymyxin B columns (Thermo Fisher,Rockford, IL). Finally, endotoxin levels in purified recombinant protein were checked by E-toxate kit (Sigma-Aldrich, USA) and were found to be below 1 IU/mL.
2.3. Immunization and challenge
In this study, 12 groups of animals were divided into 3 different immunization schedules. Each schedule consisted of 4 groups, and each group comprised of 5-6 animals in two experiments. For shortterm (3-dose) immunization schedule, two groups of M. coucha received 3 injections of the rDIM-1bm or PBS through subcutaneous(s.c.) route. The first injection (50 μg/animal) was given in Freund's complete adjuvant (FCA) on day 0. The two booster doses (25 μg/animal) were administered on day 14 and 21 in Freund’s incomplete adjuvant (FIA). To see effect of rDIM-1bm immunization on subsequently introduced infection, two groups of animals,immunized (rDIM-1bm+FCA/FIA) and non-immunized/control(PBS+FCA/FIA) received 100 L3 on day 28 post first immunization(p.f.i.) through s.c. route. For long-term (3 week) immunization schedule, four groups of M. coucha were included. Two groups received rDIM-1bm (10 μg r-protein) or PBS at 3 or 4 days intervals(i.e. twice a week) for 3 weeks. The first and subsequent doses were administered as described above. One group each from immunized(rDIM-1bm+FCA/FIA) and non-immunized/control (PBS+FCA/FIA) were kept as uninfected controls. The 6-week immunization schedule followed the same protocol as described above for the 3-week immunization schedule except that the repeated injections were given for 6-weeks (Figure 1).
2.4. Evaluation of protective immunity
2.4.1. Lymphocyte proliferative response
Spleen cells from all animals were cultured in complete RPMI-1640 medium [Penicillin: 100 U/mL; Streptomycin: 100 μg/mL(Sigma-Aldrich, USA); 10% fetal bovine serum (FBS; Gibco-Thermoscientific, USA)] at 37 ℃ in 5% CO. Lymphocyte transformation test (LTT) was performed using 72 h splenocytes culture treated with rDIM-1 (1 μg protein/mL) and Concanavalin A (ConA; 10 μg/mL) followed by incubation with pulse-labeled with [3H] thymidine (BARC, India) at the above environment. After 16-18 h of incubation, cells were harvested, counted by the liquid scintillation counter (LS Analyzer, Beckman)[7].

Figure 1. Schematic diagram of the immunization and challenge with third-stage larvae of filarial parasite Brugia malayi (L3) infection. Mastomys coucha were immunized with rDIM-1bm for short term (3-dose schedule) and long term (3-and 6-week schedule) and subsequently challenged with L3. Non-Im: Non-immunized; rDIM-1bm-Im: rDIM-1bm immunized; Non-Im+L3: Non-immunized+L3 infected; rDIM-1bm-Im+L3: rDIM-1bm-immunized+L3 infected. PBS: Phosphate bufferred saline, FCA: Freund's complete adjuvant, FIA: Freund's incomplete adjuvant. p.l.a.: post last administration of antigen;p.l.i.: post L3 inoculation.
2.4.2. Phagocytosis assay
Phagocytosis activity was performed using peritoneal macrophages of all the animals. The cells were stimulated for 48 h with rDIM-1bm (1 μg/mL) or lipopolysaccharide (LPS; 1 μg/mL).Fluorescent cells were measured at 543 nm (excitation) and 610 nm(emission) and total phagocytic activity was assessed by % positive cells×mean channel fluorescence[2].2.4.3. Nitric oxide determination
NO release was estimated in rDIM-1bm (1 μg/mL) and LPS (1 μg/mL) stimulated 48 h macrophage-culture supernatant according to the protocol described by Sahoo et al.[7].
2.4.4. Cytokine release assay
Th1 (IFN-γ: Pierce Endogen, Rockford, USA, TNF-α: Biosciences,USA) and Th2 cytokine (IL-4, IL-6, IL-10, and IL-13; BD Biosciences, USA) release were quantified in rDIM-1bm (1 μg/mL)and LPS (1 μg/mL) stimulated 48 h splenocyte culture supernatants by sandwich ELISA[8]. Each sample was used in triplicate. Cytokine concentration was measured using OD readings of standards cytokines.2.4.5. Antigen-specific antibody response
Estimation of various DIM-1bm specific IgG, IgG1, IgG2a, IgG2b and IgG3, IgE, IgM, and IgA in animal sera were performed by indirect ELISA[2,9,10]. Briefly, rDIM-1bm (1 μg protein/mL for IgG and its subtypes, 0.5 μg protein/mL for IgA and IgM, 2 μg protein/mL for IgE)was prepared in carbonate buffer (0.06 M; pH 9.6) and incubated overnight at 4 ℃. Optimally diluted sera (IgG and its subtypes at 1:100; IgE at 1:4; IgM and IgA at 1:50) were added and further incubated with rabbit anti-mouse IgG (1:50 000), IgG1, IgG2a, IgG2b and IgG3 (1:1 000), IgE, IgM and IgA (1:1 000) conjugated with streptavidin HRP (AbD Serotec, USA). Orthophenylenediamine dihydrochloride (OPD; Sigma, USA) was used as a substrate and OD were read in ELISA reader (PowerWaveX, BioTek, USA) at 492 nm.2.4.6. Assessment of infection status and worm burden
Microfilaraemia was assessed in L3 exposed groups on day 90 post larval (L3) inoculation (p.l.i.) and continued till day 205 p.l.i.Percent reduction in microfilarial count was calculated. Parasites were isolated from tissues like heart, lung, and testes of euthanized animals on day 205 p.l.i. and examined for physical status like death or calcification, motility, cell adherence on their surface. Percent reduction in worm burden and sterilization of female worms was calculated[2].
2.5. Statistical analysis
The experiment was performed twice using 5-6 animals/group.Results were analyzed by GraphPad Prism 6.01 (GraphPad Software,San Diego, CA, USA) and expressed as mean ± S.D. Student's ‘t’test and one-way ANOVA test followed by Tukey’s tests were used for analysis of the results. The minimum level of significance was set at P<0.05.
3. Results
3.1. Immunization enhanced cellular proliferation
Immunized and immunized+L3 infected animals of all the schedules (3-dose, 3-week, and 6-week) except immunized animals of 3-week schedule showed increased cell proliferative response to rDIM-1bm in vitro (P<0.01-0.001; Figure 2A, 2D and 2G). However,cells from immunized and immunized+L3 infected animals of 3-dose schedule proliferated >8-10 times higher than animals of 3-week and 6-week schedule in response to rDIM-1bm as compared to their counterpart controls. ConA stimulation induced increased cellular proliferation in immunized+L3 infected animals of 3-week schedule(P<0.001; Figure 2D), both immunized and immunized+L3 infected animals of 6-week schedule (P<0.001; Figure 2G) while there was decreased cellular proliferation in immunized+L3 infected animals of 3-dose schedule (P<0.01; Figure 2A) and immunized animals of 3-week schedule (P<0.001; Figure 2D) in response to ConA.Immunized and immunized+L3 animals of all the schedules without stimulation (unstimulated) didn’t show any changes in proliferation response.
3.2. Recombinant protein induced increased phagocytic activity
When macrophages were challenged with rDIM-1bm or LPS in vitro or without stimulation, 3-dose, 3-week, and 6-week schedules immunized alone and immunized+L3 infected animals showed higher phagocytic activity (P<0.05-0.001; Figure 2B, 2E, 2H)over their counterpart controls except immunized animals without stimulation in 6-week schedule.
3.3. Immunization trigger increased NO production
NO release from macrophages of immunized and immunized+L3 infected animals was found to be increased (P<0.05-P<0.001, Figure 2C, 2F and 2I) in response to rDIM-1bm stimulation in vitro in all three schedules, while NO release from 3-dose schedule immunized and immunized+L3 infected animals was greater than that of other schedules. LPS induced NO release from cells of immunized+L3 infecetd animals in 3-dose and 6-week schedules (P<0.05, Figure 2C and 2I), as well as both immunized and immunized+L3 infected animals of the 3-week schedule (P<0.001, Figure 2F). Immunized animals of 3-dose schedule showed a greater increase in NO release than 3-week (Figure 2F) and 6-week (Figure 2I) schedules (P<0.001;Figure 2C), immunized animals and immunized+L3 infected animals of 3-week schedule (P<0.05 and P<0001; Figure 2F) also demonstrated higher NO release without any stimulation.
3.4. Cytokines levels in immunized and challenged animals
In 3-dose schedule, immunization of M. coucha was immunized with rDIM-1bm, rDIM-1bm induced increasedαIFN-γ, TNF-α,IL-4, IL-6, and IL-13 release (P<0.05-0.001; Figure 3A, 3B, 3C,3D and 3F) compared to the non-immunized control. While rDIM-1bm immunized animals produced TNF-α, IL-4, IL-6, and IL-10 in response to LPS (P<0.05-0.001; Figure 3B, 3C, 3D and 3E)compared to non-immunized controls, TNF-α, IL-6, IL-10, and IL-13 release were found without any stimulation (P<0.05-0.001;Figure 3B, 3D, 3E and 3F). In comparison to non-immunized+L3 infected animals, immunized+L3 infected animals displayed elevated levels of IFN-γ, TNF-α, IL-4, IL-6, IL-10 and IL-13(P<0.001; Figure 3A, 3B, 3C, 3D, 3E and 3F) in response to rDIM-1bm, while LPS induced release of IFN-γ, TNF-α, IL-4, IL-6, and IL-10 in immunized+L3 infected animals compared to the nonimmunized+L3 infected animals (P<0.05 or P<0.001; Figure 3A,3B, 3C, 3D, and 3E). Without stimulation these animals produced IL-6, IL-10, and IL-13 (P<0.05-0.001; Figure 3D, 3E, and 3F).
In the 3-week immunization schedule, cells of immunized M. couch stimulated with rDIM-1bm in vitro demonstrated increased IFN-γ,TNF-αα, IL-4, IL-6, and IL-10 production (P<0.001; Figure 3G, 3H,3I, 3J and 3K) compared to non-immunized control. These animals also produced IFN-γ, TNF-α, IL-4, IL-6, and IL-10 (P<0.05-0.001;Figure 3G, 3H, 3I, 3J and 3K) in response to LPS and IFN-γ, TNFαα, IL-4, IL-6, and IL-10 (P<0.05-0.001; Figure 3G, 3H, 3I, 3J and 3K) in the absence of stimulation (P<0.05-0.001; Figure 3G, 3H,3I and 3K). In response to rDIM-1bm, immunized+L3 challenged animals induced increased release of IFN-γ, TNF-αα, IL-4, and IL-6 (P<0.05-0.001; Figure 3G, 3H, 3I, 3J and 3K), whereas LPS stimulated increased IL-4 and IL-10 release (P<0.05-0.001;Figure 3I and 3K) in immunized+L3 infected animals comparing with that in non-immunized+L3 infected animals. In absence of stimulant, the release of IFN-γ, TNF-α, IL-6, IL-10, and IL-13 production (P<0.001; Figure 3G, 3H, 3J, 3K and 3L) was increased in immunized+L3 challenged animals in comparison to nonimmunized+L3 infected animals.

Figure 2. Cellular immune responses of rDIM-1bm-immunized animals challenged with third-stage larvae of filarial parasite Brugia malayi (L3) infection.(A-C) proliferative response of splenocytes by LTT; (D-F) phagocytic activity of peritoneal macrophages by MAT and (G-I) NO production by peritoneal macrophages of Mastomys coucha immunized with rDIM-1bm for 3-dose, 3- and 6-week shedules and subsequently challenged with L3. Non-Im: Nonimmunized; rDIM-1bm-Im: rDIM-1bm immunized; Non-Im+L3: Non-immunized+L3 infected; rDIM-1bm-Im: rDIM-1bm immunized+L3 infected. The cells of animals were stimulated with LPS (1 μg/mL) and rDIM-1bm (1 μg/mL) in vitro. Tukey’s multiples comparison test was used. *P<0.05, **P<0.01,***P<0.001. The experiment was repeated twice on each group of 5-6 animals. ConA: Concanavalin A, Unst: Unstimulated, LPS: Lipopolysaccharide, p.f.i:Post first immunization; CMP: Count per minute; LTT: Lymphocyte transformation test; MAT: Macrophage activation test; NO: Nitric oxide.
Immunization of M. coucha for 6 weeks significantly enhanced IFN-γ, TNF-α, IL-4, and IL-6 release when cells were stimulated with rDIM-1bm in vitro (P<0.05-0.001; Figure 3M, 3N, 3O and 3P)compared to non-immunized controls. LPS activation of immunized animals resulted in increased release of IFN-γ, TNF-α, IL-4, IL-6,and IL-10 (P<0.05-0.001; Figure 3M, 3N, 3O, 3P and 3Q), whereas cells of immunized animals released more IFN-γ, TNF-α, and IL-6(P<0.01; Figure 3M, 3N, 3P) without stimulation. In comparison to non-immunized+L3 infected animals, immunized+L3 infected animals showed increased release of IFN-γ, TNF-α, IL-4, IL-6(P<0.05-0.001; Figure 3M, 3N, 3O and 3P) in response to rDIM-1bm. Immunized+L3 infected animals also showed increased release of IFN-γ, TNF-α, IL-6, IL-10 and IL-13 (P<0.05-0.001; Figure 3M, 3N, 3P, 3Q and 3R) in response to LPS compared with nonimmunzed+L3 infected animals.
Immunized and immunized+L3 infected animals of all three schedules showed increased release of IFN-γ, TNF-αα in response to rDIM-1bm stimulation but levels of IFN-γ, TNF-αα in 3-dose schedule were higher than 3-week and 6-week schedule. 3-dose immunization also resulted in increased IL-4, IL-6, IL-10 and IL-13 and L3 exposure to these animals further increased the IL-4, IL-6,IL-10 and IL-13 release in response to rDIM-1bm in comparison to their counterpart controls. In comparison to 3-dose schedule,immunized animals of 3-week schedule showed increased release of IL-4, IL-6, and IL-10 and immunized+L3 infected animal showed increased release of IL-4 and IL-6 in response to rDIM-1bm, while immunized animals of 6-week schedule showed increased levels of IL-6 and IL-10 and immunized+L3 infected animals showed increased release of IL-4 and IL-6 in response to rDIM-1bm.
3.5. Humoral responses in immunized and challenged animals

Figure 3. Effect of immunization and third-stage larvae of filarial parasite Brugia malayi (L3) infection on cytokine production. Th1 (IFN-γ: A, G, M;TNF-α: B, H, N) and Th 2 (IL-4: C, I, O; IL-6: D, J, P; IL-10: E, K, Q; IL-13: F, L, R) cytokine release by splenocytes of Mastomys coucha immunized with rDIM-1bm for 3-dose, 3- and 6-week shedule and then challenged with L3 infection. Non-Im: Non-immunized; rDIM-1bm-Im: rDIM-1bm immunized;Non-Im+L3: Non-immunized+L3 infected; rDIM-1bm-Im: rDIM-1bm immunized+L3 infected. The cells of animals were stimulated with LPS (1 μg/mL)and rDIM-1bm (1 μg/mL) in vitro; concentration of released cytokines were calculated by using values of standard cytokines with absorbance of released cytokines during experiment (expressed as pg protein/mL); Tukey’s multiples comparison test was used, *P<0.05, **P<0.01, ***P<0.001; The experiment was repeated twice on each group of 5-6 animals. Unst: Unstimulated, LPS: Lipopolysaccharide, p.f.i: post first immunization.

Figure 4. Humoral immune responses of immunized animals. (A, E, I) IgG and its subclasses, (B, F, J) IgE, (C, G, K) IgM and (D, H, L) IgA in sera of Mastomys coucha immunized with rDIM-1bm for 3 doses, 3 and 6 weeks and subsequently challenged with third-stage larvae of filarial parasite Brugia malayi (L3) infection. Non-Im: Non-immunized; rDIM-1bm-Im: rDIM-1bm immunized; Non-Im+L3: Non-immunized+L3 infected; rDIM-1bm-Im: rDIM-1bm immunized+L3 infected. Tukey’s multiple comparison test was used. *P<0.05, **P<0.01, ***P<0.001. Concentration of released cytokines are calculated by using values of standard cytokines with absorbance of released cytokines during experiment.The experiment was repeated twice on each group of 5-6 animals. Immunoglobulins G, A, M, E and IgG isotypes were measured using indirect ELISA; the concentrations were expressed as OD readings at 492 nm.
In 3-dose schedule, immunization of M. coucha with rDIM-1bm caused increase in IgG and its subclasses, IgE, and IgM levels(P<0.05-0.001; Figure 4A-4C) as compared to non-immunized animals but not IgG3 and IgA. Elevated level of IgG and its subclasses (except IgG3), IgE and IgM were found increased in immunized + L3 animals as compare to non-immunized+L3 animals(P<0.05-0.001; Figure 4A-4C). In 3-week schedule, filaria specific IgG and its subclasses, IgM and IgA were increased (IgG: P<0.01-0.001, Figure 4E-4H) but not IgE in rDIM-1bm immunized animals as compared to non-immunized animals. Immunized+L3 infected animals showed increased IgG, IgG2b, IgE and IgM (P<0.001;Figure 4E-4G). However, in animals immunized for 6-weeks,increase in levels of IgG and its subclasses (except IgG3), IgE, IgM and IgA (P<0.05-0.001; Figure 4I-4L) were observed in immunized animals as compared to non-immunized animals; immunized+L3 animals showed increase in IgG, IgG1, and IgM (P<0.05-0.01; Figure 4I and 4K) levels as compared to non-immunized+L3 infected animals.
3.6. Protective immunity in immunized and L3-challenged animals
Microfilaria count of all animals was initiated on day 90 (post L3 inoculation) p.l.i till day 205 p.l.i. Animals of each group were euthanized on day 205 p.l.i. and adult worms were recovered from heart, lung, testes, and lymph nodes, then counted. Animals of 3-dose, 3-week, and 6-week immunization schedules showed 36%-63%, 23%-58% and 9%-27% reduction in microfilarial burden with 52%, 9% and 12.5% adulticidal effect (Figure 5A-5F), respectively.In summary, the short-term immunization schedule (3-dose) showed the best result on the removal and reduction of B. malayi infection.
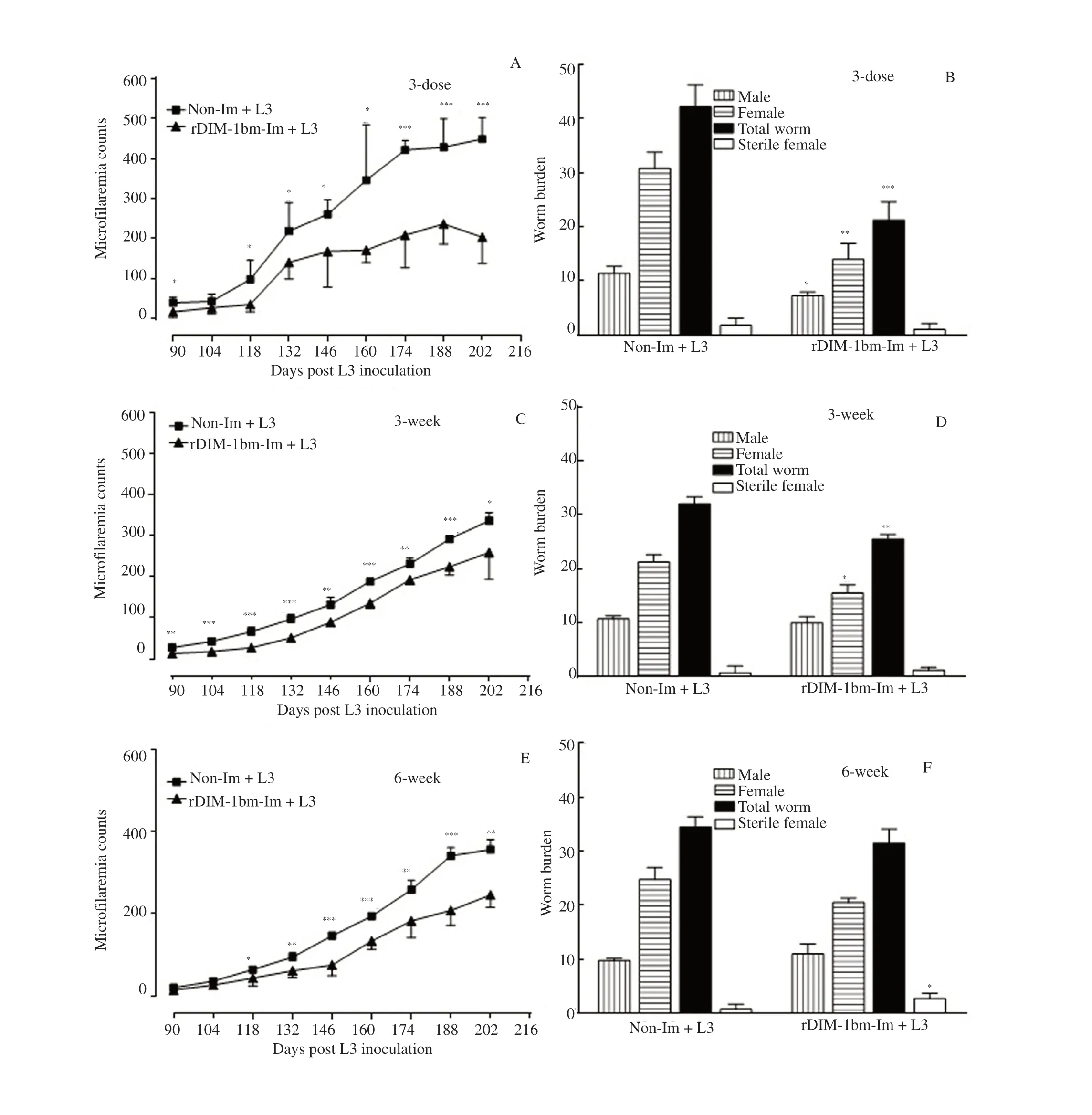
Figure 5. Parasite load in rDIM-1bm-immunized Mastomys coucha subsequently challenged with third-stage larvae of filarial parasite Brugia malayi (L3)infection. (A, C & E) Status of microfilaremia in 10 μL tail blood of Mastomys coucha immunized with rDIM-1bm for 3-dose, 3-week and 6-week shedule and subsequently inoculated with L3 of Brugia malayi. (B, D & F) Non-Im: Non-immunized; rDIM-1bm-Im: rDIM-1bm immunized; Non-Im+L3: Nonimmunized+L3 infected; rDIM-1bm-Im: rDIM-1bm immunized+L3 infected. Parasites were recovered from all the animals and analyzed. Student’s t test was used. *P<0.05, **P<0.01, ***P<0.001 vs. Non- Im+L3. The experiment was repeated twice on each group of 5-6 animals.
4. Discussion
B. malayi, a lymphatic filariid, is a well-studied pathogen. There are many proteins of the filarial parasite that were reported to be immunogenic[2] or prophylactic[11] or some are immunogenic but not prophylactic[12]. It has been hypothesized that in the host the filarial proteins are chopped into short peptides, which get associated with MHC classⅡmolecules and presented to CD4T cells to initiate host cellular immune responses. The proteins between 21 and 84 kDa of B. malayi adult worm BmAFⅡfraction were protective against B.malayi in M. coucha[11]. Later on, immunization with recombinant mitochondrial HSP60 of B. malayi modulated the host’s immune responses for parasite survival without any pathology[13]. We have earlier demonstrated that the DIM-1 has immunoprophylactic potential which partially protects the host from B. malayi infection[2].This structural muscle protein is the part of the muscle cell membrane that stabilizes the thin filament of the sarcomere and it is also essential for muscle contraction[14].
In endemic areas, it was observed that continuous biting of mosquito and helminth infection modulate the immune responses of the host. Therefore, on that basis in the present study rodent host were immunized with rDIM-1bm in 2 different immunization schedules; short-term (3-dose) and long-term (3- and 6-weeks)and subsequently exposed to L3 of B. malayi to assess the immunostimulatory role of rDIM-1bm in host-parasite interactions and their effects on subsequent invasion by the parasites in different immunization schedules.
Findings of the current protection studies against L3 challenge showed that out of these immunization schedules, short-term (3-dose)immunization offered a better reduction in microfilariae burden(36%-63%) in peripheral circulation and adult worm load (52%),whereas long-term (3- and 6-week) immunization exerted less effect on peripheral microfilariae count (23%-58%), adult worm burden(9%-12.5%). Our results of cell-mediated and humoral responses showed that cellular proliferation, macrophages activity, NO release,specific IgG, IgG1, IgG2a, IgG2b, IgE and IgA antibodies levels,both Th1 (IFN-γ, TNF-αα) and Th2 (IL-4, IL-6, IL-10 and IL-13) cytokine release were remarkably much higher in M. coucha immunized with short-term (3-dose) immunization schedule whereas long-term (repeated administration of rDIM-1bm for 3- and 6-week)exerted lesser effect on parasite burden and mixed immunological responses.
IgG antibody mediate filarial parasite removal by antibodydependent cell-mediated cytotoxicity[15]. IgG1 and IgG2b are Th2 type whereas IgG2a is indicative of Th1 type responses[16].Filarial parasite-specific IgG1, IgG2, and IgG3 are shown to provide admittable protection against lymphatic filariasis in mice and humans[17,18]. Earlier, immunization with a proinflammatory B. malayi adult worm fraction F6 intensely enhanced the IgG1,IgG2a, IgG2b in mouse model[7]. Such results were also observed in other nematodes like Ascaris suum[19] and Trichostrongylus colubriformis[20]. In this study, high IgG and IgG1, IgG2b and IgG2a antibody responses with high cellular responses might have helped in significant parasite reduction in 3-dose immunized host indicating that rDIM-1bm had the potency to stimulate the host immune response. It also provides evidence that clearance of pathogen depends on the synergetic work of antibody-mediated responses and cell-mediated Th1 responses. Various reports are demonstrating that parasite-specific IgE has an effective role in protection against helminth infection by promoting parasite killing and deletion of IgE gene resulted in raised worm burdens[21]. Corresponding to this, our study demonstrated that different immunization schedules produced different status of IgE levels in animals and this situation could have modulated the specific IgE effector mechanisms as evidenced by the different status of parasite load in animals receiving different doses of the rDIM-1bm. Elevated levels of filaria-specific IgA in endemic controls than chronic patients suggested the protective role of IgA in human filariasis[9]. Similar results were reportedly associated with a reduced percentage of L3 for Ostertagia ostertagi in calves[22].However in this study, IgA levels were significantly increased in 3- and 6-week immunized animals but not in 3-dose rDIM-1bmimmunized animals, the reason for this is not clear at present. During filarial infection, a display of the high level of IgM on activated macrophage surface suggested the protective role of IgM against the Strongyloides stercoralis[23]. In this study, we observed an elevated level of IgM in both immunized and immunized L3 infected animals suggesting the protective role of anti-rDIM-1bm IgM antibodies against B. malayi.
Parasite survival also depends on the CMI response of the host[24].In the present study, we found marked host’s cell-mediated immunity responses in all schedules which suggested that rDIM-1bm is a potent stimulator of cell proliferation and may be implicated in protection against B. malayi infection. Macrophages are one of the important mediators that regulate the immune responses during filarial infection[25]. Reactive oxygen intermediates or reactive nitrogen intermediates released from macrophages after activation by IFN-γ leads to parasite killing[26,27]. It was noticed that NO release from macrophages and other cells play a crucial role in microfilariae killing and L3 elimination[7]. In vitro microfilaria and in vivo L3 killing occurs due to nitric oxide mediated mechanism, while Th1 type cytokine IFN-γ also protect the host. The above reports substantiate our findings that macrophage activity, NO and IFN-γ release was increased in cells of all the immunized and immunized L3 infected animals and reduced worm burdens (both microfilariae and adult). This finding is in line with earlier findings[7,28] which have reported that Th1 cytokine IFN-γ and NO protect the host against B. malayi. Recently, NO generation and IFN-γ mediated iNOS induction by F8 (45.24-48.64 kDa) fraction of B. malayi protected the host from filarial infection[29]. In endemic normal and chronic filarial subjects higher IFN-γ was reported which suggested that L3 killing was also performed by IFN-γ mediated type 1 inflammatory responses[29].
It was observed that in vitro stimulation of immunized (short and long-term schedules) animals, and L3 infected animals, splenocytes with rDIM-1bm caused higher release of Th1 cytokines (IFN-γ and TNF-α). Earlier reports indicate that T cell has prominent role in immunity against filarial infection. Filarial infection occurs due to impaired T cell responses which persist even after eviction of the parasite[30]. During helminth infection, Th1 type immunity mediated by IL-2, IFN-γ (produced by T cells) and IL-12 (Produced by NK cells and monocytes) cytokines modulate the antibody production by cross-regulating the Th2 type immunity[31]. IFN-γ was found to be involved in the protection of the rodent host M. coucha against B. malayi[7,28] while TNF-ααwas found to be required for parasite elimination or persistence[32]. Dixit et al.[28] demonstrated that a pro-inflammatory fraction of B. malayi (BmAFⅡ) eliminates intraperitoneally transplanted adult worms in M. coucha through TNF-ααcytokine. The above reports substantiate our findings that rDIM-1bm specific robust T cell response may protect the vaccinated groups against filarial infection.
Various stages of helminths increased the production of Th2 type cytokines from CD4T cells and other cells in humans and experimental models. Th2 response involves the production of cytokines: IL-4, IL-6, IL-9, IL-10 and IL-13, the antibody isotypes:IgG1, IgG4, IgE, and alternatively activated macrophages. IL-4(Th2 type cytokine) has been implicated in protective responses to Litomosoides sigmodontis in BALB/c mice as IL-4 knockout mice have shown greater worm burdens and circulating microfilariae[33].Other studies have revealed that expression of IL-4 receptors and release of IL-4 from T cells protect the host[34]. In the present study,it has been observed, that increased IL-4 release was relatable to a significant reduction in parasite load in short-term schedule (3-dose immunized animals) but not in long-term schedule (3- or 6-week immunized animals) where IL-4 release was not elevated indicating non-association of IL-4 in the parasite elimination. In the present study, IL-6 release from cells of animals immunized for long-term(3-dose or 6-week) and subsequently given L3 to these animals was observed to be increased but not in animals receiving immunization for 3-weeks. Investigators[7,11] reported that proinflammatory fractions protect the host against B. malayi infection via proinflammatory cytokine (TNF-α, IL-6, and IL-1β) release.Muhsin et al.[35] reported that IL-6 deficient mice challenged with filarial parasite Litomosoides sigmodontis showed increased worm recovery which suggested a protective nature of IL-6 against filarial infection. Anti-inflammatory cytokine (IL-10 and TGF-β) together with regulatory T cells get elicited during helminth infection. These cytokines modulate host immune system against long standing parasite survival/invasion in filarial infection. IL-10 also regulates filarial parasite infection and prevents pathology[36]. IL-10 deficient mice fail to control pathological reactions, with fatal results in infections with Trichuris muris[37] and higher mortality in Schistosoma mansoni[38]. In the current investigation, immunization of animals with rDIM-1bm for long-term (3- and 6-week schedules) resulted in upregulation of IL-10 release but not in animals receiving short-term(3-dose) of rDIM-1bm; these findings provided evidence of poor protection against L3 induced infection in repeated immunization for prolonged period. In various helminth infection, IL-13 protects the host in the absence of IL-4[39]. B. malayi infected IL-4 knock out mice showed no difference in recovery of L3 which suggests that IL-4 is not necessary for the survival of primary infection[40].However, B. pahangi L3 infected IL-4 KO mice showed very high levels of IL-13[41]. In this study, upregulated IL-13 in animals of short-term schedule (3-dose) seemed effective in parasite removal via this mechanism. On the contrary in long-term schedule (3- and 6-week) IL-13 response was not observed and perhaps no significant inhibition in parasite recovery was observed. Nevertheless, Joseph et al.[42] reported that anti-inflammatory BmAFⅠfraction derived from B. malayi adult worm extract facilitated parasite development and survival via downregulation of IL-13 response. Thus, in the present study elevated level of IL-13 (Th2) mediated protective responses was due to vaccination of M. coucha with 3 doses immunization schedule of rDIM-1bm.
In this context, the present study’s data described here indicated that, out of the both short-term schedule (3-dose) and long-term(3-and 6-weeks) immunization schedules, short-term (3-dose)immunization schedule was effective in triggering protection against filarial infection in an experimental murine model and this protection correlates with a skewing towards a robust Th1 and Th2 type of immune responses. Thus, in conclusion, findings of the present study suggest that short-term immunization with rDIM-1bm (3-dose)protects the host M. coucha against the parasites via a robust mixed Th1 and Th2 immune responses.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
The authors thank the Director of the CSIR-Central Drug Research Institute, Lucknow, India, for keen interest and encouragement. Thanks are due to CSIR, New Delhi, India, for award of Emeritus Scientist (scheme No. 21 (0963)/13/EMRII grant; 29-10-2014) to P.K.M. Thanks are also due to the Honourable Vice Chancellor, Lucknow University, Lucknow,India, for providing laboratory space and facilities for manuscript preparation at Department of Zoology. Thanks are also due to Indian Council of Medical Research, New Delhi, India, for a Senior Research Fellowship to V.K.
Funding
This work was supported by Indian council of Medical Research,New Delhi, India (ICMR approval no. F/802/2010-ECD-11). The funding agency had no role in the study design, data collection and interpretation.
Authors’ contributions
P.K.M. conceived the hypothesis, planned the experiments,supervised the project and contributed to the final version of the manuscript. V.K. performed the experiments, analyzed data and wrote the manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Plasmid DNA encoding neutralizing human monoclonal antibody without enhancing activity protects against dengue virus infection in mice
- Spatio-temporal history of H9N2 viruses in Iran and neighbor countries by Bayesian analysis and molecular characterization
- Antibacterial resistance patterns of Acinetobacter baumannii complex: The results of Isfahan Antimicrobial Resistance Surveillance-1 Program
- Phylogeny of Brucella abortus strains isolated in the Russian Federation
- ACE2 downregulation promotes thrombosis and cardiac injury in COVID-19 patients
