Plasmid DNA encoding neutralizing human monoclonal antibody without enhancing activity protects against dengue virus infection in mice
2021-07-20SurachetBenjathummarakAtsushiYamanakaThanyalukKrasaeChonlatipPipattanaboonSubenyaInjampaPannamthipPitaksajjakulPongramaRamasoota
Surachet Benjathummarak, Atsushi Yamanaka, Thanyaluk Krasae, Chonlatip Pipattanaboon, Subenya Injampa,Pannamthip Pitaksajjakul, Pongrama Ramasoota✉
ABSTRACT
KEYWORDS: Dengue virus; LALA mutation; Antibodydependent enhancement (ADE); Antibody plasmid; Electroporation
1. Introduction
Dengue is one of the most important mosquito-borne viral diseases. Dengue fever and its severe form, including dengue hemorrhagic fever and dengue shock syndrome, are significant public health concerns especially in tropical and subtropical countries[1,2]. This disease is caused by dengue virus (DENV) of the family Flaviviridae. There are four distinct serotypes (DENV-1-4), and DENV is a single-stranded positive-sense RNA virus.The 11 kb genome encodes for three structural proteins (capsid,C; premembrane/membrane, prM/M; and envelope, E), and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5)[3]. The E protein plays an important role in virus attachment to cells and membrane fusion with the target cells. It is the major antigenic protein of DENV virion for neutralizing antibodies[4].
The antigenic difference among the four DENV serotypes results in the stimulation of a host immune response, producing serotypespecific neutralizing antibodies against the infecting serotype,with only a short-period protective response against other DENV serotypes in primary infection[5,6]. However, the low levels of sub-neutralizing or non-neutralizing antibodies from previous DENV infection form immune complexes with heterologous serotypes that enhance the access to Fc gamma receptor (FcγR)-expressing target cells. This consequence leads to an increase in viral replication, which may cause a more severe disease. This infection-enhancing phenomenon is known as antibody-dependent enhancement (ADE) [7-9]. Currently, there is no specific antiviral drug available for the treatment of DENV infection[10]. Also, the ideal dengue vaccine should elicit equal immune response to all serotypes. Nevertheless, a vaccine alone might not be adequate in the prevention of dengue infection. Cross-reactive and potent neutralizing antibodies are considered as the core therapy along with passive immunoprophylaxis in the protection against DENV infection[11-13].
The antibody vector-mediated gene transfer is one alternative approach that produces therapeutic antibody directly in vivo, which uses simplified technology and low production cost. Several studies have demonstrated the application of electroporation in vivo with a single intramuscular injection of an antibody-expressing plasmid could produce neutralizing antibodies against viral infection[12,14,15].Recent studies have evaluated the DNA-encoded anti-DENV antibodies gene transfer in vitro or in vivo[12,16,17].
Previously, HuMAb-1G7C2 from hybridoma cells has been generated with strong cross-neutralizing activity with enhancing activities for some DENV serotypes[18,19]. Hence, this study is aimed at describing the construction, Fc modification, and in vivo delivery of antibody-expressing plasmids containing heavy- or light-chain genes of HuMAb-1G7C2 to produce human IgG against DENV. The production of biological activities of a neutralizing antibody without enhancing activity was evaluated in vitro and in vivo.
2. Materials and methods
2.1. Virus and cells
Four laboratory strains of DENV were used in this study: DENV-1(Mochizuki strain), DENV-2 [New Guinea C strain (NGC)],DENV-3 (H87 strain), and DENV-4 (H241 strain). Vero cells were cultured in MEM/EBSS medium (Hyclone, USA) with 10% fetal bovine serum (FBS). Chinese hamster ovary (CHO-K1) cells were cultured in MEM/EBSS (Hyclone, USA) with 10% FBS and 1%nonessential amino acid. K562 cell was cultured in RPMI 1640 medium (Hyclone, USA) with 10% FBS.
2.2. Construction of 1G7C2 DNA plasmid and modified LALA vector
The variable domains of the heavy (VH) and light (VL) chains of HuMAb-1G7C2 were separately amplified by PCR from parental 1G7C2-pQC plasmids[20]. The transgene encoding VH or VL were flanked with restriction sites for EcoRI and XhoI in the VH gene, and AgeI and NcoI in the VL gene, and a Kozak sequence(GCCACC) was added at upstream of the start codon. The VH amplicon was inserted into the EcoRI-XhoI sites of pFUSE-CHIghG1 vector, as pFUSE_1G7C2_hVH plasmid. Meanwhile, the VL amplicon was inserted into AgeI-NcoI sites of pFUSE2-CLIg-hl2, as pFUSE_1G7C2_hVL plasmid (InvivoGen, San Diego, CA, USA)using In-FusionHD Cloning kit (Takara Bio USA, CA, USA). The pFUSE_1G7C2_hVH plasmid was genetically modified 2 amino acids at positions 234 and 235 on the CH2 domain from leucine (L)to alanine (A) by site direct mutagenesis, as pFUSE_1G7C2_hVH_LALA plasmid. All plasmids for transfection in CHO-K1 cells and injection in mice were extracted using Presto™ Midi Plasmid Kit Endotoxin Free (Geneaid Biotech Ltd., China), in accordance with the manufacturer’s instructions. The primers in this study were listed in Table S1.
2.3. Antibody expression in vitro
The heavy- and light-chain plasmids were co-transfected into CHOK1 cells. Briefly, 7×10CHO-K1 cells were seeded in a 6-well plate a day before transfection. Subsequently, 4 µg of pFUSE plasmid,both heavy and light chains (2 µg each), was mixed with 10 µL of lipofectamine 2000 transfection reagent (Invitrogen, USA) according to the manufacturer’s instruction. The culture supernatant was harvested on days 2, 3, 4, 5, and 6 after transfection to determine IgG expression.
2.4. In vivo assessment of 1G7C2_hG1-LALA expression
Six-week-old female BALB/c mice (25 g-30 g body weight) were housed in the animal facilities at the Faculty of Tropical Medicine.This study was approved by the Faculty of Tropical Medicine Animal Care and Use Committee (FTM-ACUC), Mahidol University, with approval number FTM-ACUC 005/2017. Thirty minutes before DNA plasmid electroporation, a group of BALB/c mice (n=8) were pre-treated with 20 µL of 80 IU/mL Hyaluronidase Solution (Irvine Scientific, USA) in anterior tibialis muscles. Subsequently, 50 µg of each heavy- and light-chain plasmid was injected intramuscularly at a single dose, followed by electroporation using an electroporator(Nepa Gene, Chiba, Japan) with a poring pulse (voltage, 100 V;pulse interval, 50 ms; pulse length, 30 ms; number of pulses, 3; 10%decay rate with + polarity) and a transfer pulse (voltage, 20 V; pulse interval, 50 ms; pulse length, 50 ms; number of pulses, 5; 40% decay rate with ± polarity). Blood was collected from the submandibular vein of each mice 3 days prior injection and 3, 5, 7, 9, 15, and 30 days after inoculation. Mouse serums were pooled for the assay.
2.5. Western blot analysis
A total of 100 ng of culture supernatant containing antibodies was loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 3% bovine serum albumin in 0.1%Tris-buffered saline with Tween 20 (TBST) at room temperature for 2 h. After washing, the membrane was incubated with 1:5 000 horseradish peroxidase-conjugated goat anti-human IgG (H+L)(Merck KGaA, Darmstadt, Germany) for 1 h at room temperature,and the signal was allowed to develop with ECL Prime (GE Bioscience, USA). The immunoreactive bands were visualized under a chemiluminescence LAS 4000 mini (GE Bioscience).
2.6. Quantitative human IgG ELISA
A microtiter plate was coated with 1 µg of goat anti-human IgG(Fc) (EMD Millipore Corporation, CA, USA) in 100 µL coating buffer (0.2 M NaHCO+ 0.2 M NaCO) per well and incubated at 4 ℃ overnight. After washing the plate with 0.05% TBST, the coated wells were blocked with 1% bovine serum albumin in TBST at room temperature for 1 h. Subsequently, culture supernatant (diluted 1:40 in TBST with 1% bovine serum albumin) or mouse serum and human reference serum (Bethyl, USA), as a standard, were added to each well and incubated at room temperature for 1 h. Thereafter,horseradish peroxidase-conjugated goat anti-human IgG-Fc at dilution 1:150 000 were added and incubated at room temperature for 1 h. The plate was developed with tetramethylbenzidine microwell peroxidase substrate (KPL, Gaithersburg, MD, USA) and stopped the reaction with 1 M phosphoric acid, H3PO4. The absorbance was measured at 450 nm under the ELISA reader (Tecan, Switzerland).
2.7. Immunofluorescence assay
Immunofluorescence assay plates of each dengue serotype were prepared to determine the reactivity of expressing antibodies against DENV. Briefly, 50 µL of culture supernatant or mouse serum was added to each well and incubated at 37 ℃ for 1 h. Then, the well was washed with 100 µL of PBS for 3 times prior adding with 50 µL of goat anti-human IgG conjugated with Alexa Fluor488 dye (diluted 1:1 000) and incubated at 37 ℃ for 1 h. The fluorescent signal on infected cells was observed under fluorescence microscope (IX71,Olympus, Japan).
2.8. Focus reduction neutralization test (FRNT)
FRNT assay has been previously described[20]. Briefly, 2.5×10vero cells were seeded in a 96-well plate one day before viral infection.Purified antibodies, starting with concentration at 64 µg/ mL, were serially diluted two-fold up to 11 dilutions. Likewise, the mouse serum was serially diluted two-fold up to 11 dilutions. Then, each dilution of purified antibodies or mouse serum was mixed with an equal volume of DENV (MOI 0.01) and incubated at 37 ℃ for 1 h.Then, 50 µL of the virus-antibody mixture was added to vero cells and incubated for 2 h. Next, 100 µL of overlaid medium (2X MEM,2% carboxymethyl cellulose, and 3% FBS) was added to the wells and incubated for 2 days for DENV-4 and 3 days for DENV-1, -2 and-3. After that, the infected cells were fixed with 3.7% formaldehyde/PBS and permeabilized with 0.1% Triton X-100/PBS. The anti-DENV HuMAb was added into the infected cell and incubated at 37 ℃ for 1 h, followed by 1:1 000 dilution of goat anti-human IgGAlexa Fluor 488 (Invitrogen, USA). The foci numbers were counted under a fluorescence microscope. The percentage of reduction was calculated by comparing the foci number for each antibody concentration with the number of foci obtained from a virus-PBS mixture, as negative control.
2.9. Neutralizing and enhancing activities in K562 cells
The balance between antibody neutralizing and DENV infectionenhancing has been previously described[16,21]. In sum, the four-fold serial dilution of purified antibodies (starting with a concentration at 100 µg/mL) or the two-fold serial dilution of mouse serum (starting with undiluted serum) were mixed with DENV in a poly-L-lysinetreated plate (BD Bioscience, USA.) and incubated at 37 ℃ for 2 h.Thereafter, 50 µL of 2.5×10cells/mL of K562 cells were added to the antibody-virus mixture and incubated at 37 ℃ for 2 days. After that, the plate was washed with PBS, and the cells were fixed with acetone/methanol (1:1 ratio) solution and placed at −20 ℃ for at least 30 min. The plate was stained with anti-dengue HuMAb at 4 ℃ overnight. The infected cells were probed with horseradish peroxidase-conjugated goat anti-human IgG (H+L) (1:250), 0.05% Tween, and 1% FBS and incubated at 37 ℃ for 1 h. Immunostaining was further developed with a 3,3ʹ-diaminobenzidine substrate solution (KPL, USA) to observe interaction signal. The infected cell numbers were counted under a light microscope.
2.10. Statistical analysis
All data were represented as mean ± standard deviation (SD) for statistical analysis. Independent t-test and one-way ANOVA were performed by GraphPad Prism 6 software. FRNTvalues were calculated using a non-linear regression of log (reciprocal antibody dilution) vs. response. P<0.05 was considered significant.
3. Results
3.1. In vitro expression of HuMAb-1G7C2_hG1 and 1G7C2_hG1-LALA in CHO-K1 cells
The expressing plasmids pFUSE_1G7C2_hVH and pFUSE_1G7C2_hVL for 1G7C2_hG1 were co-transfected into CHO-K1 cells. Meanwhile, the modified plasmid pFUSE_1G7C2_hVH_LALA and pFUSE_1G7C2_hVL were co-transfected to produce 1G7C2_hG1-LALA, as illustrated in Figure S1. The culture supernatants were harvested at indicated time points for antibody production analysis. The results showed that both 1G7C2_hG1 and 1G7C2_hG1-LALA could specifically bind to all four DENV serotypes-infected vero cells, as illustrated in Figure 1A. The amount of 1G7C2_hG1 and 1G7C2_hG1-LALA antibodies was detected on day 2 post-transfection, and the antibody concentrations were continuously increasing every day. In this time course, the antibody yield showed the highest concentration on day 6, which was estimated to be 3 900 ng/mL for 1G7C2_hG1 and 3 700 ng/mL for 1G7C2_hG1-LALA. In contrast, no expression was observed in cells transfected with the parental pFUSE vector, as illustrated in Figure 1B. The western blot analysis also demonstrated that the heavyand light-chain proteins were observed at their expected molecular weights (approximate size of heavy- and light-chain protein was 50 kDa and 25-28 kDa, respectively), as described in Figure 1C.This result confirms that the expression plasmids were capable of producing anti-DENV human IgG, and the modified LALA in the CH2 domain of the Fc region did not disrupt IgG expression in CHO-K1 cells.
3.2. Cross-neutralizing activity of 1G7C2_hG1 and 1G7C2_hG1-LALA against four DENV serotypes
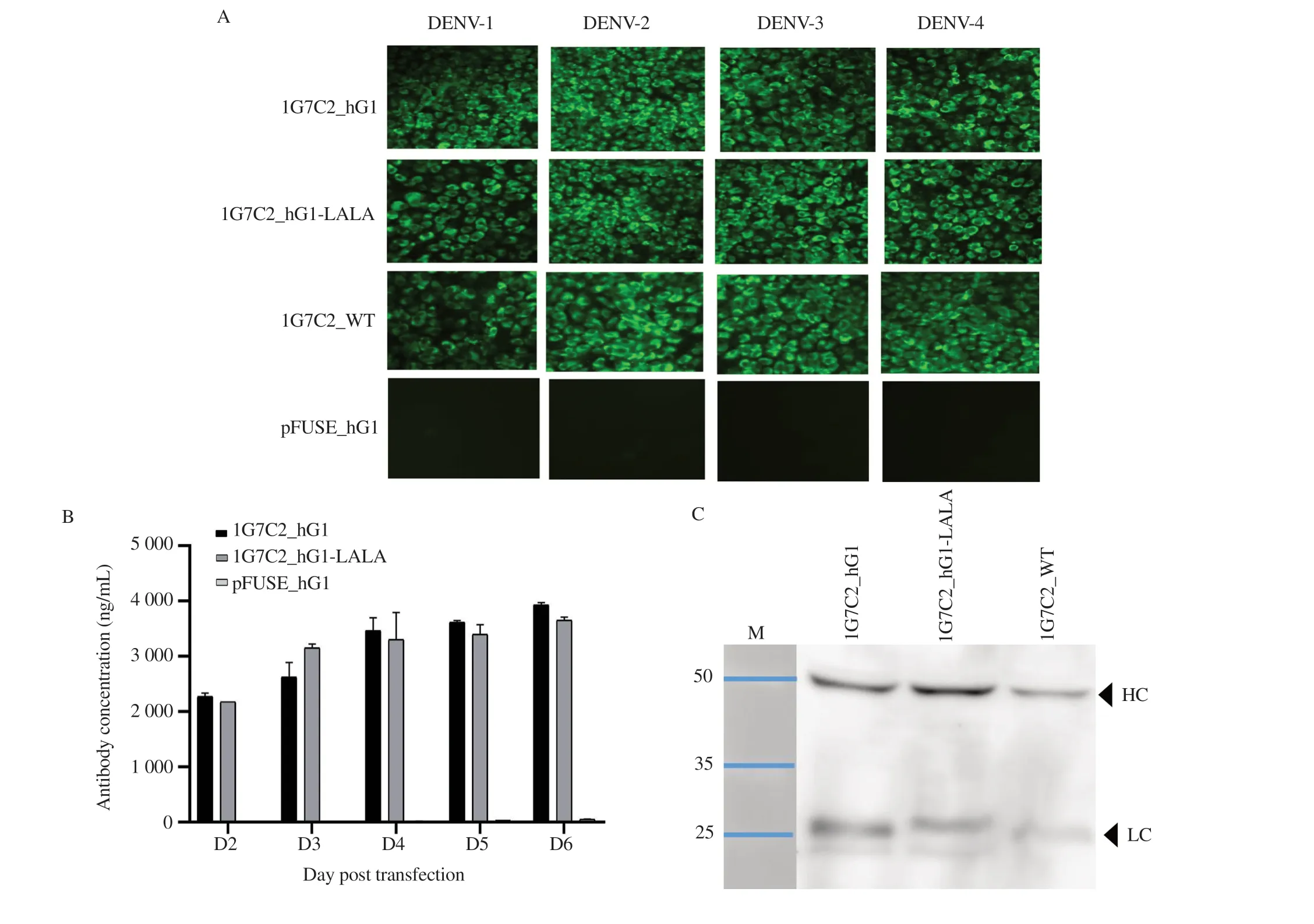
Figure 1. In vitro expression analysis of the secreted HuMAb against DENV. (A) Immunofluorescence assay demonstrated that the secreted 1G7C2_hG1 and 1G7C2_hG1-LALA reacted to the envelope protein of four DENV serotypes. pFUSE_hG1 was used as negative control. (B) Human IgG in culture supernatant was quantified by ELISA. The values are presented as mean ± SD. (C) Western blot analysis of human IgG heavy- and light-chain peptides from culture supernatant 1G7C2_hG1 and 1G7C2_hG1-LALA under reducing condition. The original photograph is shown in Supplementary Figure 1C.
The efficiency of neutralizing activities of purified 1G7C2_hG1 and 1G7C2_hG1-LALA antibodies was compared to parental 1G7C2 wild-type (1G7C2-WT) antibodies, as described in Figure 2.At the highest concentration of 64 µg/mL, all antibodies exhibited a 100% reduction in DENV-2 and as high as 98%-100% reduction was observed in DENV-3 and -4. Meanwhile, the neutralizing activities of the three HuMAbs to DENV-1 were determined as 90% reduction. The FRNTvalues comparing 1G7C2_hG1, 1G7C2_hG1-LALA, and 1G7C2-WT were summarized in Table 1. However,different degrees of neutralizing activities of those three antibodies were observed.
3.3. Antibody-dependent enhancement on K562 cells for 1G7C2_hG1 and 1G7C2_hG1-LALA
The level of neutralizing and enhancing activities was interpreted by the number of infected cells compared with the baseline number of the no-antibody control: the number of infected cells above the baseline indicated enhancing activities, whereas that under the baseline indicated neutralizing activities, as described in Figure 3.The results showed that 1G7C2_hG1 could not neutralize but induce the enhancement of infection to DENV-1 and DENV-3 even at the highest antibody concentration. Conversely, 1G7C2-WT could neutralize at a concentration of 100 µg/mL and initiate enhancement of infection at a concentration of 25 µg/mL in DENV-3 but could only enhance infection in DENV-1 at a range of antibody concentration 100-0.097 6 µg/mL. Both 1G7C2_hG1 and 1G7C2-WT were evaluated for their neutralizing activity at a high antibody concentration (100-25 µg/mL) and induced enhancing activity at a low concentration (6.25-1.562 5 µg/mL) in DENV-2 and DENV-4.The degree of antibody-enhanced infection indicated that 1G7C2-WT was induced at a level of ADE higher than 1G7C2_hG1 in terms of the infectivity of all DENV serotypes, as outlined in Table 2.Furthermore, the modified 1G7C2_hG1-LALA showed neutralizing activity at a high antibody concentration and a completely absent of enhancing activity to all DENV serotypes at every concentration.The findings of this study suggest that the double-point mutations of L234A and L235A on the CH2 domain of the Fc region were able to disrupt ADE activity.

Figure 2. Focus reduction neutralization test (FRNT) of 1G7C2_hG1, 1G7C2_hG1-LALA, and 1G7C2-WT against four DENV serotypes. The serial twofold dilution of each purified HuMAb was subjected to DENV-1 (A), DENV-2 (B), DENV-3 (C), and DENV-4 (D). The number of foci from three independent experiments was counted and calculated as percent reduction. The values are presented as mean ± SD.
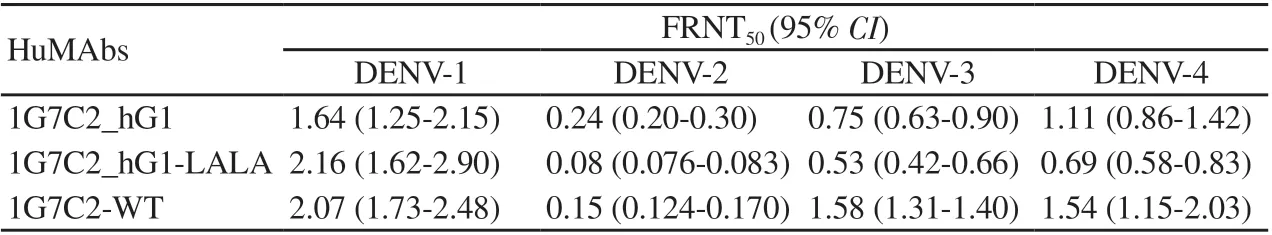
Table 1. FRNT50 of HuMAbs against four DENV serotypes (µg/mL).
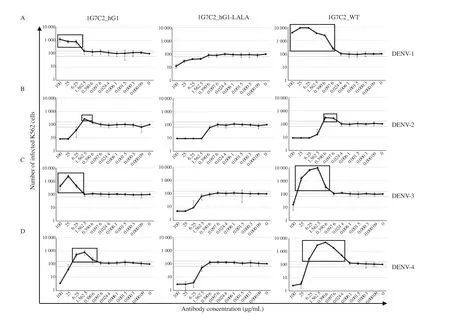
Figure 3. Antibody-dependent enhancement for 1G7C2_hG1, 1G7C2_hG1-LALA, and 1G7C2-WT individually infected with (A) DENV-1, (B) DENV-2,(C) DENV-3, and (D) DENV-4. The enhancing activities of each HuMAb are indicated in boxes. Dotted line represents the mean number of infected cells of no antibody control ± SD.

Table 2. The degree of antibody-enhanced infection of each HuMAb from ADE assay in vitro.
3.4. In vivo expression analysis of 1G7C2_hG1-LALA
A mixture of heavy- and light-chain expressing plasmids of 1G7C2_hG1-LALA or pFUSE vector (control) was administered at 100 µg via intramuscular in BALB/c mice, followed by electroporation. Blood samples were collected at each time point to determine the biological function of the secreted antibody
in the mouse serum. The results showed that the 1G7C2_hG1-LALA antibody rapidly generated within 3 days after inoculation at a concentration of 550 ng/mL. Then, the secreted IgG was drastically increased to the highest on day 5 and 7 at a concentration approximately 1 300 ng/ mL. The antibody concentration on day 9 rapidly decreased to 150 ng/mL and was undetectable after day 30(Figure 4A). The secreted 1G7C2_hG1-LALA antibody in mouse serum was able to show specific binding to all DENV serotypes(Figure 4B). Moreover, the pooled serum from each day was subjected to FRNT against DENV-2. The results indicated that the mouse serum on day 3 could neutralize DENV-2 at 80% reduction,and the highest reduction (>90% reduction) was observed on days 5 and 7, whereas no reduction was detected after day 30 (Figure 4C).Moreover, the FRNT level to DENV-1, -3, and -4 of the mouse serum on day 7 was detected at 72%, 88%, and 81% reduction,respectively (Figure 4D).
In addition, the balancing between the neutralizing and enhancing activities of secreted 1G7C2_hG1-LALA antibody in mouse serum demonstrated that it could neutralize all DENV serotypes without enhancing activities, as described in Figure 5. These results indicated that a single dose inoculation of 1G7C2_hG1-LALA-expressing plasmid could rapidly produce cross-neutralizing antibody to all DENV serotypes without enhancing activity.

Figure 4. In vivo antibody expression analysis of 1G7C2_hG1-LALA in BALB/c mice. (A) A total of 100 µg of pFUSE-1G7C2-hVH-LALA and pFUSE-1G7C2-hVL expressing plasmids were injected into each BALB/c mice (n=8). Injection of pFUSE vector was used as negative control (n=3). Serums were collected at each time point and measured by human IgG ELISA. ***P<0.001. (B) The pooled mouse serum on day 7 was investigated by immunofluorescence assay of four DENV serotypes. (C) Pooled mouse serum of each time point was subjected to FRNT against DENV-2. (D) The percent reduction by using the pooled mouse serum on day 7 against DENV-1, -3, and -4.

Figure 5. Antibody-dependent enhancement for the secreted 1G7C2_hG1-LALA in mouse serum. Pooled mouse serum on day 7 after inoculation was serially diluted two-fold and subjected to perform balancing between neutralizing and enhancing activity on four DENV serotypes. Dotted lines represent the mean numbers of infected cells of no antibody control ± SD.
4. Discussion
Dengue is a viral infectious disease with an immune response complex; thus, there is a possible risk of stimulating sub-neutralizing immune response to promote heterotypic serotype virus and enter the effector cells via Fcγ receptors. Therefore, the developed therapeutic antibodies for dengue treatment should show cross-neutralization to all four serotypes without infection enhancement, which might lead to severe manifestations[22]. In a previous study, we have reported the generation of cross-neutralizing HuMAb-1G7C2. This HuMAb is human IgG1 that targets the fusion loop domainⅡ(DⅡ) of DENV envelope glycoprotein, which is the main target epitope of human antibodies for neutralization and ADE activity. This antibody has been characterized for its ability to neutralize DENV-1-4, but at subneutralizing concentrations, it promoted ADE activity[19,20]. This limitation of HuMAb-1G7C2 was overcome by Fc modification of IgG. The modified Fc region of HuMAb-1G7C2 has been previously described to generate a recombinant antibody through the mutation of IgG-1 Fc region at amino acid position 297 from asparagine to alanine. However, the protection efficiency of 1G7C2-IgG1-N297A was lower when compared to that of the parental antibody after passive immunization in an infected mouse[11].In the present study, we have introduced LALA mutations on the Fc region of HuMAb-1G7C2 to eliminate enhancing activity. The amino acid substitution of leucine to alanine at position 234 and 235 on lower hinge region of the CH2 domain leads to abolishes binding between the Fc region and Fcγ receptor-bearing cell that have been observed to eliminate antibody-dependent enhancement in DENV[12,23]. The results of this study suggest that the 1G7C2_hG1-LALA antibody expressing plasmid could secrete 1G7C2_hG1-LALA antibodies from CHO-K1 cells with a high degree of cross-neutralizing activity against all four serotypes, similar to its parental HuMAb. However, we observed the differences of FRNTamong all four serotypes of those three antibodies used in this study(1G7C2_hG1, 1G7C2_hG1-LALA, and 1G7C2-WT). This might be due to those antibodies that were generated from different vectorbased platforms and also the constant presence of some amino acid modification on the region of heavy chain genes[11,20,21,24].The balance between the neutralizing and enhancing activities of anti-dengue antibodies was determined by K562 cells because these cells express FcγRIIa on the cell surface, which is the major receptor driving the ADE, and could represent as immune effector cells in vivo[25]. Interestingly, the result indicated that the 1G7C2_hG1 antibody-based pFUSE vector could reduce the enhancement of infection in all DENV serotypes when compared to the parental antibody 1G7C2-WT. Furthermore, the modified 1G7C2_hG1-LALA also showed cross-neutralization in all DENV serotypes and completely showed no enhancing activity.
Recently, DNA expressing plasmid-based gene delivery of monoclonal antibodies in vivo has been developed and found to be effective in neutralizing viruses, such as HIV, chikungunya,influenza and Zika[15,26-28]. DNA-encoded monoclonal antibody genes are delivered into skeletal muscle cells, which directly express immunoglobulins that enter the blood circulation system to neutralize the virus[29-31]. This study demonstrated the advantage of electroporation to deliver DNA expressing plasmids for in vivo rapid production of cross-neutralizing anti-dengue HuMAb. These BALB/c mice were pre-treated with hyaluronidase to break down the connective tissue barrier and facilitate higher IgG expression in muscle cells without other adverse effects in mice[27,32,33]. The highest human IgG level was obtained at more than 1 µg/mL on day 5 to day 7 but rapidly declined after day 9. This might be due to an immune response of BALB/c mice against the xenogeneic part of human antibody as non-self protein, which leads to rapid clearance,and short time persistence in blood circulation[34]. Moreover, the secreted 1G7C2_hG1-LALA in the mouse serum still exhibited a biological function similar to that exhibited by purified 1G7C2_hG1-LALA, as revealed by the in vitro study. Moreover, this amount of producing IgG in mouse serum at higher than 1 µg/mL was reported to be able protect mice from DENV infection with lethal doses[12].
This study has certain limitations. We did not investigate the duration of DNA plasmid-encoded human antibody gene expression in immune-deficient nude mice, indicating the lack of adaptive immune responses and appropriate use as a model for human antibody expression in an immune-accommodating host[12,15,27,31].Further studies are required to determine the protective activity of 1G7C2_hG1-LALA against all four DENV serotypes in AG129 mice, which were susceptible to DENV infection and presented some clinical symptoms similar to humans[35,36]. A singleplasmid antibody-encoding open reading frame of antibody gene is also considered as a target DNA antibody plasmid platform for injection to reduce the process and variation that might occur during preparation or delivery of heavy- and light-chain plasmids in vivo.In conclusion, this study demonstrated that the Fc modification of 1G7C2_hG1-LALA, which is able to cross-neutralize all DENV serotypes without enhancing activity, has a potential to be developed as dengue therapeutic. A single dose of this DNA-encoded antibody gene plasmids was able to express anti-dengue neutralizing antibodies during a short-term period in BALB/c mice. Therefore,this new antibody-expressing plasmid is a potential candidate of therapeutic antibody against DENV infection. Taken together, the DNA-encoded antibody gene transfer could represent a simple,rapid, and reproducible approach for evaluating the biological functions of MAb to inhibit viral infection.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding
This research was supported by the Faculty of Tropical Medicine,Mahidol University, Thailand, Research Fund through a Research and Researcher for Industry (RRi, Grant Number PHD59I0063 for SB), TRF Grant for New Researcher (TRG, Grant Number TRG5980015 for CP) and the Office of the National Research Council of Thailand-Japan Society for the Promotion of Science(JSPS) or NRCT-JSPS.
Authors’ contributions
SB and PR designed the study; SB, AY, TK, CP, SI and PP performed experiments; SB performed data analysis and wrote original draft manuscript; PR and PP reviewed and edited manuscript. All authors have read and approved the submitted version of the manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of short- and long-term immunization of recombinant disorganized muscle protein-1 (rDIM-1) against human filarial parasite Brugia malayi in rodents
- Spatio-temporal history of H9N2 viruses in Iran and neighbor countries by Bayesian analysis and molecular characterization
- Antibacterial resistance patterns of Acinetobacter baumannii complex: The results of Isfahan Antimicrobial Resistance Surveillance-1 Program
- Phylogeny of Brucella abortus strains isolated in the Russian Federation
- ACE2 downregulation promotes thrombosis and cardiac injury in COVID-19 patients
