Effects of inhibitors on the protease profiles and degradation of activated Cry toxins in larval midgut juices of Cnaphalocrocis medinalis (Lepidoptera:Pyralidae)
2021-06-24YANGYajunXUHongxingWUZhihongLUZhongxian
YANG Ya-jun,XU Hong-xing,WU Zhi-hong,LU Zhong-xian
State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products,Institute of Plant Protection and Microbiology,Zhejiang Academy of Agricultural Sciences,Hangzhou 310021,P.R.China
Abstract Midgut juice plays an important role in food digestion and detoxification in insects. In order to understand the potential of midgut juice of Cnaphalocrocis medinalis (Guenée) to degrade Bt proteins,the enzymatic activity of midgut juice and its degradation of Bt proteins (Cry2A,Cry1C,Cry1Aa,and Cry1Ac) were evaluated in this study through protease inhibitor treatments. The activities of total protease in midgut juices were significantly inhibited by phenylmethylsulfonyl fluoride (PMSF),tosyl-L-lysine chloromethyl ketone (TLCK),pepstatin A and leupeptin. The enzymatic activity of chymotrypsin was significantly inhibited by PMSF,and enzymatic activity of trypsin was significantly inhibited by ethylenediaminetetraacetic acid (EDTA),PMSF,tosyl phenylalanine chloromethyl ketone (TPCK),TLCK and trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64). EDTA could significantly inhibit the degradation of Cry2A by C.medinalis. EDTA,PMSF,TPCK,and TLCK could inhibit the degradation of Cry1C and Cry1Aa. EDTA,PMSF,TPCK,TLCK,and E-64 could inhibit the degradation of Cry1Ac. Our results indicated that some protease inhibitors hindered various enzymatic activities in the larval midgut of C.medinalis,which may reduce the insect’s ability to degrade Bt toxins. These findings may aid the application of protease inhibitors in the management of this insect pest in the future.
Keywords:Cnaphalocrocis medinalis,midgut juice,protease inhibitor,enzyme activity,degradation,Bt protein
1.Introduction
The midgut is an important insect organ,with midgut proteases playing a role in food digestion and utilization as well as detoxification (Linser and Dinglasan 2014),and the midgut proteases are always involved in these functions (Terra and Ferriera 2012;Linser and Dinglasan 2014). Midgut proteases are mainly comprised of serine proteases,aspartic proteases,cysteine proteases,and metalloproteinases,while among these proteases the serine proteases primarily function in digestion (Terra and Ferriera 2012). Serine proteases such as chymotrypsin and trypsin were found in the larval midgut of most lepidopteran pests (Srinivasanet al.2006). Lepidopteran proteases are often most active under alkaline conditions (Berenbaum 1980;Applebaum 1985;Teoet al.1990),and trypsins display a variety of optimal conditions for activity in different insects (Bighamet al.2013;Kipgen and Aggarwal 2014;Zhaoet al.2016). Midgut proteases also contribute to the insecticidal action ofBacillusthuringiensisBerlin (Bacillales:Bacillaceae) (Bt) proteins (Oppertet al.1997). The midgut juice of lepidopteran larvae can degrade the 130-140 kDa Cry protoxin into a 60-70 kDa activated protein (Pardo-Lópezet al.2013). Changes in midgut juice are associated with resistance to Bt proteins in many lepidopteran insects (Yamazakiet al.2011;Talaei-Hassanlouiet al.2014).Moreover,the stability of the activated Cry protein affects its toxicity to the target pests (Pang and Gringorten 1998). Further degradation of Cry protein could lead to the loss of its toxicity (Sugimuraet al.1997). The protease from a non-susceptible population ofMamestrabrassicaeL.(Lepidoptera:Noctuidae) could degrade Bt protein into small fragments (Lightwoodet al.2000). The midgut juice of Cry1Ab-resistantSpodopterafrugiperda(S.E.Smith) (Lepidoptera:Noctuidae) harbored a greater ability to produce a 30-kDa fragment of Cry protein than that from the Cry1Ab-susceptibleManducasexta(L.) (Lepidoptera:Sphingidae) (Mirandaet al.2001). Rapid degradation to Cry protein contributes to a loss of Cry1C susceptibility in fifth-instarSpodopteralittoralis(Boisduval) (Lepidoptera:Noctuidae) larvae (Kelleret al.1996),and the insecticidal activity of certain Cry proteins could be increased up to 20-fold by serine protease inhibitors (MacIntoshet al.1990).
Protease inhibitors are a class of compounds which target the active site of proteases to decrease or even abolish protease activity without affecting protease denaturation,and they are widely found in animals,plants,and microorganisms (Yuet al.2004). Protease inhibitors will bind to the protease in the intestinal tract of the insect to inhibit protease activity,which affects insect food digestion and zymogen activation and stimulates excessive synthesis and secretion of the digestive enzyme,resulting in deficiency of certain essential amino acids (methionine) and disruption of insect metabolism and ultimately,abnormal development or even death of the insect (Yuet al.2004;Wuet al.2013). Saadati and Bandani (2011) evaluated the effects of various protease inhibitors on the growth and development ofEurygasterintegricepsPuton (Hemiptera:Scutelleridae) as well as on its serine protease,and found that tosyl phenylalanyl chloromethyl ketone (TPCK) is the most effective for inhibiting the serine protease activity ofE.integriceps. The typsin inhibitor fromMadhucaindicaJ.F.Gmelin (Ebenales:Sapotaceae) (MiTI) could negatively affect growth and development ofHelicoverpaarmigera(Hübner) (Lepidoptera:Noctuidae) (Jamalet al.2014). Zhaoet al.(2019) found that protease inhibitors hindered the synthesis and secretion of protease,leading to inhibition of nutrient absorption,delaying of reproductive development,and reduction of the reproductive capacity inPlutellaxylostella(L.) (Lepidoptera:Plutellidae). Studies on the properties of proteases in insects through protease inhibitors will promote the development of pest control strategies based on proteases and/or protease inhibitors.
Rice leaffolder,Cnaphalocrocismedinalisis one of the most important agricultural pests in the paddy fields of China,and it damages rice plants through folding and feeding the leaves which affects photosynthesis (Cheng 1996;Yanget al.2015). Heavy occurrence of rice leaffolder could reduce rice yields,and rice leaffolder damaged 15.5451 million ha and caused 475 200 tons of actual loss in 2015 in China according to the statistics from the Chinese government (Yanget al.2015;Lu 2017). Bt agents are used in the“green control”ofC.medinalisdue to their safety to humans and livestock (Yanget al.2012,2015;Chenet al.2015). Rice expressingcrygene has been bred as a reserve measure for the prevention and control ofC.medinalisand other lepidopteran pests (Chenet al.2011;Yanget al.2011). A Bt rice expressing Cry1Ac/Cry1Ab protein targetingC.medinalisgained certificate of biosafety in China in 2009 (Chenet al.2011). T2A-1 and T1c-19 rice lines expressing Cry2A and Cry1C proteins could cause high mortality inC.medinalis(Zhenget al.2011). Yanget al.(2012) reported four Cry toxins,including Cry1Ac,Cry1Aa,Cry1Ab,and Cry1C,harbored high insecticidal activity againstC.medinalis. However,the development of insect resistance could seriously threaten Bt protein utilization,and a variety of insect pests have already developed resistance to Bt proteins (Yanget al.2007;Wu 2014;Jinet al.2015). The research on the interaction between Bt protein andC.medinalisis of great significance,while few studies have investigated on this topic. Karim and Dean (2000) reported that Bt protein binds to BBMV ofC.medinalis.Cnaphalocrocis medinalisshowed a potential adaptive ability to low amounts of Cry2A protein through a trypsin activity increase in the larvae (Wuet al.2015). The midgut acts an important target in the action of Cry protein (Bravoet al.2013;Xuet al.2016). The midgut juice functions in food digestion and detoxification,and it can be affected by many factors (Terra and Ferriera 2012). Different Cry toxins with different structures may result in different action mechanisms (Meloet al.2016). In this study,we selected a Cry2 toxin (Cry2A) and three Cry1 toxins (Cry1C,Cry1Aa,and Cry1Ac) as materials and analyzed the effects of protease inhibitors on the profiles of midgut juices and their ability to degrade activated Cry proteins to further understand the interaction between Bt protein andC.medinalis. Our findings will provide a technical basis for the safe use of Bt proteins and establish a foundation for developing pest control tactics based on proteases and/or protease inhibitors.
2.Materials and methods
2.1.Insects
Cnaphalocrocismedinalisadults were collected from rice fields (30.06°N,120.20°E) of Hangzhou,Zhejiang Province of China in August,2015. The adults were cultured with 10% honey solution in plastic cups covered with nylon mesh for oviposition according to Zhenget al.(2010). After hatching of eggs,the newly emerged larvae were reared with leaves of 45-d-old Taichung Native 1 (TN1) in the box and fifth-instar larvae were used in the experiments. All insect rearing steps were under a condition of (27±1)°C,70-80% RH and a photoperiod of 14 h L:10 h D.
2.2.Preparation of midgut juices of C.medinalis
Fifth-instar larvae ofC.medinaliswere sampled as three replicates,and each replicate had 15 larvae. Midgut tissues of each replicate were dissected after chilling of theC.medinalislarvae on ice for 30 min. The midgut juices were centrifuged for 20 min at 10 000×g and filtered through 0.22-μm filters to remove the solid materials. The determination of total protein was conducted with the microplate reader (Tecan Group Ltd.,Switzerland) following Bradford’s method (Bradford 1976). Then,midgut juices were aliquoted and stored at -70°C prior to use.
2.3.Midgut juice treatments with different inhibitors
Seven protease inhibitors,including ethylenediaminetetraacetic acid (EDTA) (5 mmol L-1),phenylmethylsulfonyl fluoride (PMSF) (5 mmol L-1),tosyl phenylalanine chloromethyl ketone (TPCK) (2 mmol L-1),tosyl-Llysine chloromethyl ketone (TLCK) (2 mmol L-1),transepoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64) (20 μmol L-1),pepstatin A (20 μmol L-1),and leupeptin (20 μmol L-1) (Sigma-Aldrich®,Millipore Sigma Canada Co.,Canada) were used in the experiment. The mixture samples were generated by mixing the midgut juices (2 μg μL-1) and each of the different protease inhibitors (v/v=1:1) separately.The mixture samples were incubated at 25°C for 20 min,then subjected to the experiments below.
2.4.Effects of different inhibitors on the activity of proteases in midgut juices of C.medinalis
Total protease activity was determined by the method described in Yanget al.(2020). The reaction system includes 100 μL casein (20 mg mL-1in 0.15 mol L-1NaCl),10 μL mixture samples (5 μg/reaction),and 40 μL glycine-NaOH solution (0.1 mol L-1,pH 10.5). The reaction system was incubated at 30°C for 3 h,and then the reaction was stopped by adding 10% (v/v) pre-cooled trichloroacetic acid solution (150 μL). The mixture was centrifugated with 12 000×g at 4°C for 15 min,and then 200 μL of supernatants were used to read absorbance values at 415 nm using a microplate reader (Tecan Group Ltd.,Switzerland). There were three replicates for the determination of total protease activity.
Chymotrypsin-like enzyme activity was determined by the methods described in Yanget al.(2020). The reaction system includes 40 μL N-succinyl-alanine-alanine-prolinephenylalanine-p-nitroanilide (SAAPPpNA;Sigma-Aldrich®,Millipore Sigma Canada Co.,Canada) (2 mg mL-1,soluted with 0.15 mol L-1NaCl) as the substrate,90 μL Glycine-NaOH solution (0.1 mol L-1,pH 10.5) and 10 μL mixture samples (10 μg/reaction). Absorbance values were read at 405 nm for 25 min at intervals of 30 s using a microplate reader (Tecan Group Ltd.,Switzerland).Three replicates were used for measuring chymotrypsin-like enzyme activity.
Trypsin-like enzyme activity was determined by the method described in Yanget al.(2020). The reaction system includes 100 μL Nα-benzoyl-L-arginine-p-nitroanilide (BApNA;Sigma-Aldrich®) (20 mg mL-1,dissolved in dimethyl sulfoxide),40 μL Glycine-NaOH solution (0.1 mol L-1,pH 10.5) and 10 μL mixture samples (10 μg/reaction). Absorbance values were read at 405 nm for 20 min at intervals of 10 s using a microplate reader (Tecan Group Ltd.,Switzerland). There were three replicates for the determination of trypsin-like enzyme activity.
2.5.In vitro processing of activated Cry toxins by larval midgut juices of C.medinalis treated with protease inhibitors
In order to detect the degradation of activated Cry toxin under seven inhibitors,including EDTA,PMSF,TPCK,TLCK,pepstatin A,leupeptin and E-64,1.0 μg activated Cry toxin was mixed with midgut juices (Cry toxin:midgut juices ratio of 10:1,w/w) with the addition of different inhibitors and then incubated at 30°C for 8 h. The activated toxins of Cry1Ac,Cry1Aa,Cry2A and Cry1C (purchased from Shanghai Youlong Biotech Co.,Ltd.,China) were used in the experiments. Protein was run on 8-10% SDS-PAGE,and stained using Coomasie brilliant blue.
2.6.Statistical analysis
Data for enzymatic activities are shown as mean±SE and subjected to analysis of variance (ANOVA),using Tukey’s test at a significant level ofP<0.05 in the software SPSS18.0 (SPSS Inc.,Chicago,IL).
3.Results
3.1.Effects of different inhibitors on the activities of proteases in the midgut juices of C.medinalis
Total protease activities ofC.medinalismidgut juices treated with PMSF,TLCK,E-64,pepstatin A and leupeptin were significantly lower than the untreated control (P<0.05),while total protease activities in the treatments of EDTA and TPCK did not significantly differ with that of the control (Fig.1-A). PMSF treatment decreased the activity of chymotrypsin compared with the control (P<0.05),while the other six protease inhibitors did not affect the activity of chymotrypsin in the midgut juices ofC.medinalis(Fig.1-B). Trypsin activity of midgut juices fromC.medinalisin the treatments of EDTA,PMSF,TPCK,TLCK,and E-64 were lower than that of the control (P<0.05),while trypsin activities in the treatments of pepstatin A and leupeptin did not differ from that of the control (Fig.1-C).
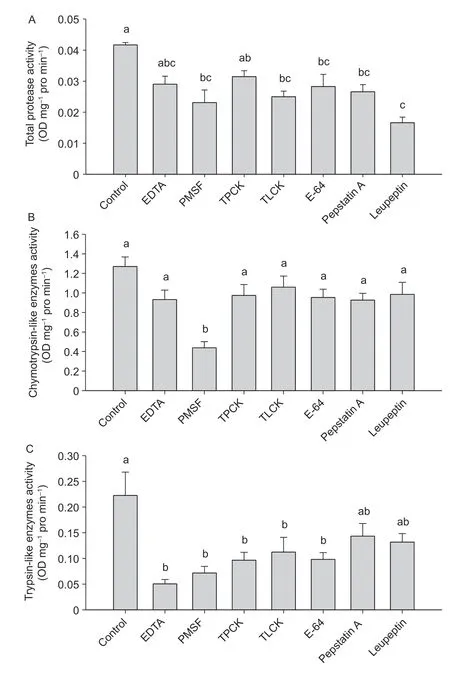
Fig.1 Enzymatic activities in the midgut juices of Cnaphlacrocis medinalis treated with different inhibitors. A,total protease. B,chymotrypsin. C,trypsin. EDTA,ethylenediaminetetraacetic acid;PMSF,phenylmethylsulfonyl fluoride;TPCK,tosyl phenylalanine chloromethyl ketone;TLCK,tosyl-L-lysine chloromethyl ketone;E-64,trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane. Data are mean±SE (n=3).Different lowercase letters above the bars indicate significant differences among the treatments at a P<0.05 level.
3.2.Degradation of Cry toxins by midgut juices treated with different inhibitors
The degradation of Cry toxins by midgut juices treated with different inhibitors were investigated in this study. EDTA exerted the strongest inhibitory effect on the degradation of Cry2A byC.medinalis,followed by PMSF and E-64,while the other inhibitors exhibited weaker inhibition of the degradation of Cry2A (Fig.2).
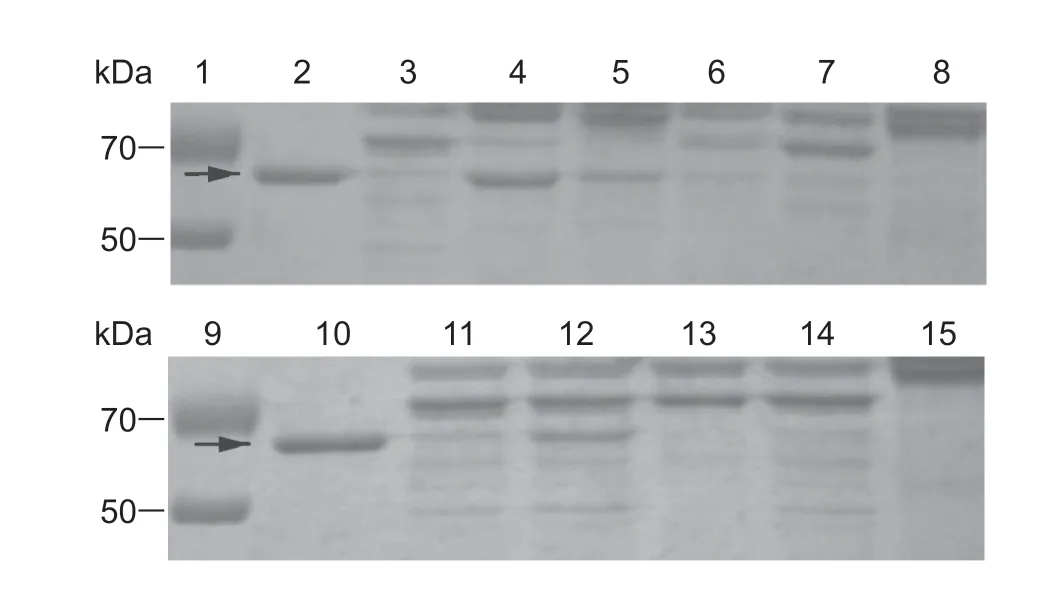
Fig.2 Degradation of Cry2A toxin in midgut juice treated with different inhibitors in Cnaphalocrocis medinalis larvae. Lanes 1 and 9,marker;lanes 2 and 10,Cry2A protein;lanes 3 and 11,midgut juice plus Cry2A protein (without inhibitor);lanes 8 and 15,the midgut juice;lanes 4-7,midgut juice plus Cry2A protein treated with ethylenediaminetetraacetic acid (EDTA),phenylmethylsulfonyl fluoride (PMSF),tosyl phenylalanine chloromethyl ketone (TPCK),tosyl-L-lysine chloromethyl ketone (TLCK);lanes 12-14,midgut juice plus Cry2A protein treated with trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64),pepstatin A and leupeptin,respectively. Arrows indicate the positions of activated Cry protein bands.
Degradation of Cry1C was inhibited by EDTA,PMSF,TPCK,and TLCK at different levels,while no inhibitory effects against Cry1C degradation were observed in the treatments of E-64,pepstatin A,and leupeptin (Fig.3).Cry1Aa degradation was inhibited by EDTA,PMSF,TPCK,and TLCK,while E-64,pepstatin A,and leupeptin displayed no inhibitory effects (Fig.4). EDTA,PMSF,TPCK,TLCK,and E-64 inhibited the degradation of Cry1Ac at different levels (Fig.5).
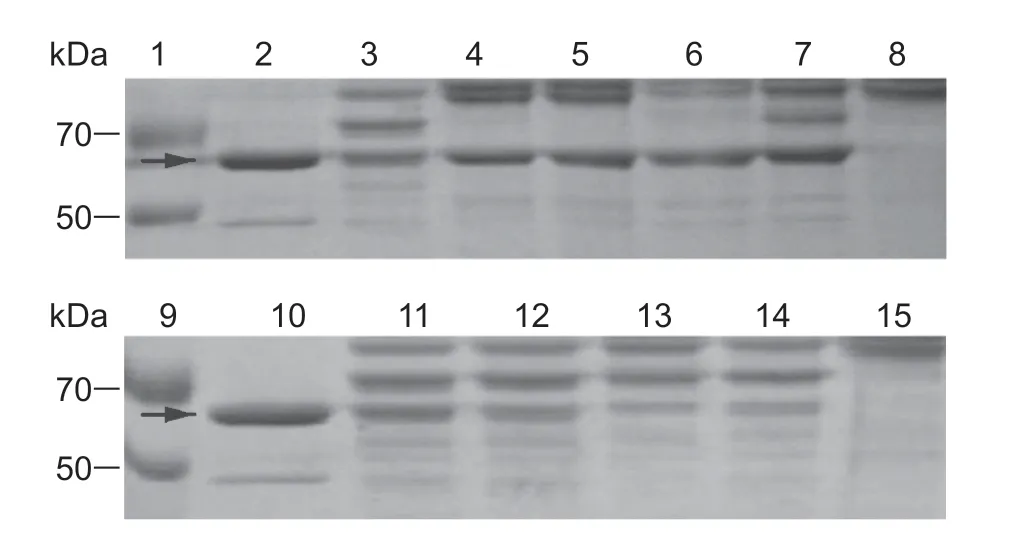
Fig.3 Degradation of Cry1C toxin in midgut juice treated with different inhibitors in Cnaphalocrocis medinalis larvae. Lanes 1 and 9,marker;lanes 2 and 10,Cry1C protein;lanes 3 and 11,midgut juice plus Cry1C protein (without inhibitor);lanes 8 and 15,the midgut juice;lanes 4-7,midgut juice plus Cry1C protein treated with ethylenediaminetetraacetic acid (EDTA),phenylmethylsulfonyl fluoride (PMSF),tosyl phenylalanine chloromethyl ketone (TPCK),tosyl-L-lysine chloromethyl ketone (TLCK);lanes 12-14,midgut juice plus Cry1C protein treated with trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64),pepstatin A and leupeptin,respectively. Arrows indicate the positions of Bt protein bands.
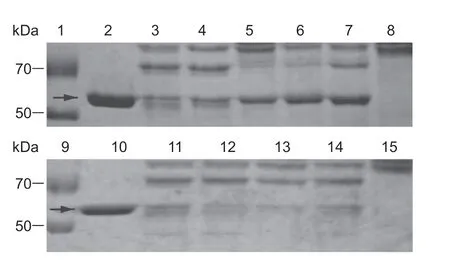
Fig.4 Degradation of Cry1Aa toxin in midgut juice treated with different inhibitors in Cnaphalocrocis medinalis larvae.Lanes 1 and 9,marker;lanes 2 and 10,Cry1Aa protein;lanes 3 and 11,midgut juice plus Cry1Aa protein (without inhibitor);lanes 8 and 15,the midgut juice;lanes 4-7,midgut juice plus Cry1Aa protein treated with ethylenediaminetetraacetic acid (EDTA),phenylmethylsulfonyl fluoride (PMSF),tosyl phenylalanine chloromethyl ketone (TPCK),tosyl-L-lysine chloromethyl ketone (TLCK);lanes 12-14,midgut juice plus Cry1Aa protein treated with trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64),pepstatin A and leupeptin,respectively. Arrows indicate the positions of activated Cry protein bands.
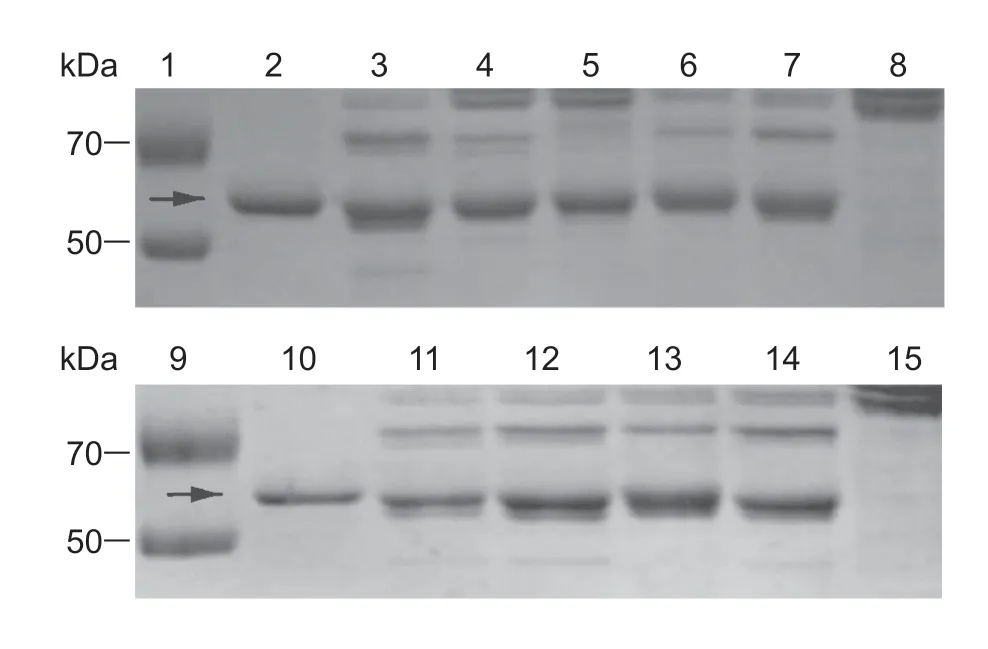
Fig.5 Degradation of Cry1Ac toxin in midgut juice treated with different inhibitors in Cnaphalocrocis medinalis larvae. Lanes 1 and 9,marker;lanes 2 and 10,Cry1Ac protein;lanes 3 and 11,midgut juice plus Cry1Ac protein (without inhibitor);lanes 8 and 15,the midgut juice;lanes 4-7,midgut juice plus Cry1Ac protein treated with ethylenediaminetetraacetic acid (EDTA),phenylmethylsulfonyl fluoride (PMSF),tosyl phenylalanine chloromethyl ketone (TPCK),tosyl-L-lysine chloromethyl ketone (TLCK);lanes 12-14,midgut juice plus Cry1Ac protein treated with trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64),pepstatin A and leupeptin,respectively. Arrows indicate the positions of activated Cry protein bands.
4.Discussion
The midgut is the primary site for digestion and utilization of food in insects,and there are many proteases in midgut juices of insects (Terra and Ferriera 2012;Linser and Dinglasan 2014). However,protease activity can be influenced by many factors including protease inhibitors. In this study,different protease inhibitors exerted different effects on the midgut proteases inC.medinalis. Total protease activity in the midgut juices ofC.medinaliswas inhibited by PMSF,TLCK,E-64,pepstatin A,and leupeptin;PMSF displayed strong inhibition of chymotrypsin activity;EDTA,PMSF,TPCK,TLCK,and E-64 strongly inhibited trypsin activity. Chymotrypsin activities inE.integricepslarvae fed with 1% soybean trypsin inhibitor (SBTI),1% TLCK,1% TPCK or 4% TPCK,decreased by 40,26,23 and 17%,respectively,while trypsin activities inE.integricepsfed with 1% SBTI,1% TLCK,or 4% TPCK decreased by 14,9,and 36%,respectively (Saadati and Bandani 2011).Soybean trypsin inhibitor kunitz (STI) could inhibit <25% of trypsin activity and 50% of chymotrypsin activity inPierisbrassicae(L.) (Lepidoptera:Pieridae),but TPCK did not affect chymotrypsin activity (Kumaret al.2015). Zhaoet al.(2019) observed that different protease inhibitors exerted different effects on the protease activity in the midgut ofP.xylostella. Differences in the protease composition among insect species could affect the efficacy of inhibitors. Many genes encoding trypsin,chymotrypsin and other proteases were found inC.medinalisaccording to a report on the midgut transcriptome of this insect (Yanget al.2018).In general,protease inhibitors could bind to specific sites of a particular protease and reduce or abolish protease activity.The nucleotide change in a gene encoding chymotrypsin induced insect resistance in aSpodopteraspecies to NaPI,a serine protease inhibitor derived fromNicotianaalataLink et Otto (Tubiflorae:Solanaceae) (Dunseet al.2010). It is crucial to investigate the profiles of midgut juice affected by protease inhibitors. Understanding the profiles of midgut juice will promote the development of new technologies for pest control based on proteases and/or protease inhibitors.
Insect midgut proteases secreted by midgut epithelial cells are important for the activation of Bt protoxin and the degradation of activated Bt toxin (Bravoet al.2013). Studies on the interaction of Bt toxin and midgut proteases will provide further understanding of the mechanism of Bt toxin action. Increased midgut protease activity in fifth-instar larvae ofS.ittoralisis related to its susceptibility to Cry1C (Kelleret al.1996). Yamazakiet al.(2013) found that the midgut juices inP.xylostellawith high resistance to Cry1Ac contained three times more glucosinolatesulfatase than a susceptible strain. Tetreauet al.(2013) demonstrated that gut proteolytic activity was increased in the larvae ofBti-resistant mosquitoes and one of the important reasons may be that midgut proteases may promote Bt toxin degradation. Previous studies indicated that Cry1C protein had higher insecticidal activity againstC.medinalisthan Cry2A protein (Yanget al.2016). Cry1Ac had the highest insecticidal activity againstC.medinalisamong four Cry1 toxins (Cry1Ab,Cry1Ac,Cry1Aa,and Cry1C) (Yanget al.2012). Wuet al.(2015) suggested thatC.medinaliscould develop adaptation to Cry2A protein by increasing trypsin activity when exposed to low amounts of Cry2A proteins over generations. Decreases or losses of function of Cry toxin caused by further degradation of Cry protein by midgut protease have been documented in many insects (Kelleret al.1996;Sugimuraet al.1997;Mirandaet al.2001),although proteases played an active role in the activation of Cry protoxin (Rukminiet al.2000;Pardo-Lópezet al.2013).Yanget al.(2020) reported the effect of pH on the profiles of midgut juices inC.medinalisand its degradation of activated Cry toxins. In this study,some protease inhibitors could inhibit the degradation of Cry toxin byC.medinalis. EDTA most strongly inhibited Cry2A degradation inC.medinalis,followed by PMSF and E-64,while other inhibitors displayed weaker inhibitory effects on Cry2A degradation. Cry1C degradation was inhibited by EDTA,PMSF,TPCK,TLCK,and E-64 at varying levels,while pepstatin A and leupeptin were found to show weak inhibitory effects. Degradation of Cry1Aa byC.medinaliswas inhibited by EDTA,PMSF,TPCK,and TLCK,while E-64,pepstatin A,and leupeptin exhibited weaker inhibition. Cry1Ac degradation was inhibited by EDTA,PMSF,TPCK,TLCK,and E-64. Most inhibitors do not affect the pore-forming function of Cry1Aa toxin inM.sexta,but a high concentration of PMSF was found to promote pore formation,while EDTA and ethylene glycol bis (2-aminoethyl ether)-N,N,N´,N´-tetraacetic acid (EGTA) decreased the pore-forming rate at pH 10.5 (Kirouacet al.2006). The effect of protease inhibitors on the insecticidal activities of the Cry proteins need to be further investigated inC.medinalis.
Protease inhibitors could be used in pest control through affecting the proteases of insect pests (Jamalet al.2014). More than four decades ago,Green and Ryan (1972) proposed that protease inhibitors could be a possible mechanism of plants resistance against insects. From then on,more protease inhibitors have been studied and utilized in pest control as successful insecticides (Gatehouse 2011).Cowpea trypsin inhibitor (CpTI),tomato inhibitor II,and potato inhibitor II expressed in the plants could enhance the plant resistance to insects. CpTI,potato proteinase inhibitor II and barley trypsin inhibitor were introduced into rice plants to confer the resistance to insect pests,respectively (Duanet al.1996;Xuet al.1996;Alfonso-Rubíet al.2003). Besides the conventional transgenic inserts for the utilization of protease inhibitors in insect pest control,modern transgenic inserts,such as gene stacking/multigene engineering and RNAi-based approaches,also could be used in the protease and/or protease inhibitor-based insect pest management (Singhet al.2020). Insect midgut juice is related to the action of Bt protein,and protease is involved in the interaction of insects and Cry proteins (Bravoet al.2013). In this study,some inhibitors were found to inhibit the insect’s midgut protease and reduce the insects’ ability to degrade Cry toxins which may reduce their chance to develop resistance. Furthermore,the inhibition toward Cry2A differed from the other Cry1 proteins,which means that the characteristics of the degradation of Cry2A by midgut juice inC.medinaliswere different than those of other Cry1 proteins. Interestingly,activated Cry2A protein could be fully degraded by midgut juice ofC.medinalisat pH 9.0-10.5,and other Cry1 toxins (Cry1C,Cry1Aa,and Cry1Ac) were only partially degraded (Yanget al.2020).Insecticidal activity of fragments of Cry toxins degraded byC.medinalismidgut juice should be evaluated in further investigations. Therefore,elucidating the effects of different inhibitors on the protease activity facilitates our understanding of the interactions between Bt proteins andC.medinalisand provides a technical basis for the safe use of Bt protein and a foundation for the development of pest control strategies based on proteases and/or protease inhibitors.
5.Conclusion
Based on our results,various protease inhibitors showed differential effects on the protease activity in midgut and the degradation of Cry proteins by midgut juice. Total protease activities in midgut juices were significantly inhibited by PMSF,TLCK,pepstatin A and leupeptin,chymotrypsin activity was significantly inhibited by PMSF,and trypsin activity was significantly inhibited by EDTA,PMSF,TPCK,TLCK and E-64. The degradation of Cry2A byC.medinaliswas significantly inhibited by EDTA;the degradation of Cry1C was significantly inhibited by EDTA,PMSF,TPCK,and TLCK;the degradation of Cry1Aa was significantly inhibited by EDTA,PMSF,TPCK and TLCK;the degradation of Cry1Ac was significantly inhibited by EDTA,PMSF,TPCK,TLCK and E-64. The results of this study will further our understanding of the interactions betweenC.medinalisand the Cry toxin,and aid the application of protease inhibitors in the management of this insect pest in the future.
Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LY20C140004),the National Science and Technology Major Project of China (2016ZX08001001),the National Natural Science Foundation of China (31501669) and the earmarked fund for China Agriculture Research System (CARS-01-36).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- Comparison of grain yield and quality of different types of japonica rice cultivars in the northern Jiangsu plain,China
- Natural nematicidal active compounds:Recent research progress and outlook
- lmproving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele
- Comparative transcriptome analysis of different nitrogen responses in low-nitrogen sensitive and tolerant maize genotypes
