Natural nematicidal active compounds:Recent research progress and outlook
2021-06-24CHENJixiangSONGBaoan
CHEN Ji-xiang,SONG Bao-an
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education/Guizhou University,Guiyang 550025,P.R.China
Abstract Plant-parasitic nematodes cause substantial economic losses to global agriculture yearly. The use of nematicides is an effective way of controlling plant-parasitic nematodes. However,the long-term use of traditional organophosphorus and carbamate chemical nematicides can lead to increased nematode resistance. With the increasing awareness of the necessity for the protection of the environment and human health,highly toxic nematicides no longer meet the developmental requirements of modern agriculture. Recently,many studies have been undertaken on the isolation and nematicidal activity of natural products against plant-parasitic nematodes and Caenorhabditis elegans. As an important model nematode,C.elegans plays a vital reference role in studying plant-parasitic nematodes regarding nematicidal activity,metabolic mechanism,and modes of action and target. We reviewed the latest research progress of natural nematicidal active compounds against plantparasitic nematodes and C.elegans over the past ten years,discussed the structure-activity relationship and mechanism of action,and examined the development and application of natural nematicidal active compounds.
Keywords:natural products,nematicides,research progress,plant-parasitic nematodes
1.Introduction
Plant-parasitic nematodes are important pathogenic nematodes,which seriously threatens the sustainable development of agriculture,causing approximately $157 billion in economic losses to world agriculture each year (Abadet al.2008). Plant-parasitic nematodes are difficult to see with the naked eye due to their good concealment ability. When the host plant is attacked,the above-ground parts of the plant will not show specific symptoms,such as yellowing and wilting leaves until the middle and late stages,and the symptoms are easily confused with lack of nutrients and water (Ntalliand Caboni 2012). It is difficult to control parasitic nematodes in plant roots without using systemic nematicides,which significantly increases the difficulty of root-knot nematode management (Mariaet al.2012). Based on their economic importance,the top 3 plant-parasitic nematodes worldwide are root-knot nematodes,cyst nematodes,and root lesion nematodes (Joneset al.2013). Root-knot nematodes are the most notorious obligate parasites,which are distributed worldwide and attack almost all vascular plants (Joneset al.2013). Among them,Meloidogyne incognita,Meloidogyne arenria,Meloidogyne hapla,andMeloidogyne javanicaare the four most important nematodes. Cyst nematodes are obligate biological vegetative bodies,and their eggs can survive in the soil for a long time even without a host. The most destructive cyst nematodes include the soybean cyst nematode,cereal cyst nematode and potato cyst nematode (Singhaet al.2015). Once the cyst nematode establishes an infection in the host plant,it is almost impossible to eradicate it. Therefore,it is extremely difficult to control cyst nematodes with crop rotation. Cyst nematodes can produce a peptide,which might be important for the induction of feeding sites (Tortoet al.2018).
With the increasing awareness of impacts to human health and environmental protection,highly toxic nematicides are no longer suitable for the sustainable development of modern agriculture. For example,the highly toxic nematicides methyl bromide and dibromochloropropane have been eliminated. Traditional nematicides such as fosthiazate and abamectin will be increasingly restricted in the future due to their environmental unfriendliness and effect on human health. Thus,nematicides face the problems of fewer types and higher regulatory pressures. However,the frequent occurrence of plant nematode damage worldwide has resulted in annual increase in the market value of nematicides,which is expected to increase to $1.4 billion by 2020 (Lahmet al.2017). Integrated management strategies for the control of plant-parasitic nematodes will become increasingly important,which rely on specific locations,sustainable management methods,and the correct selection and use of nematicides. The cultivation of new resistant varieties,soil disinfection before planting,crop rotation,changing of planting times,and fallow strategies are often used to manage nematodes. Many of these strategies are less expensive,but,sometimes they are not as effective as nematicides in controlling nematodes. Combining nonpesticide control methods and highly-efficient,low-toxic nematicides often manage nematode populations better.However,discovering new,highly-efficient,and low-toxic nematicides is a great challenge facing the current integrated management of nematodes.
Natural products are extensively present in animals,plants,and microorganisms. They have broad-spectrum biological activities and provide diverse chemical structure models. Many pesticides or lead compounds are natural products,including abamectin,rotenone,ethicin,vanillin,cinnamic acid,and eugenol. Certain natural products have unique scaffolds,good pharmacodynamic groups,and excellent biological activities. Drug developers may find inspiration from these. Owing to the many advantages of natural products in drug discovery,they have attracted significant attention recently. Many natural nematicidal active compounds have been isolated and identified from plants (such asSisalsp.,Annona crassiflora,andMentha spicata) or microorganisms (such asChlorella vulgaris,Stereum gausapatum,andAscomycetes),and nematicidal activity tests have been performed. Many natural products exhibit excellent nematicidal activity against plant-parasitic nematodes,such as aldehydes,ketones,acids,esters,and alkaloids (D’Addabboet al.2020). Grafting certain specific structures into these natural product scaffolds might improve the nematicidal activity of small molecular compounds. The separation of natural products with nematicidal activity,structure identification,and evaluation of nematicidal activity are particularly important. However,research on the mode of action and mechanism of natural nematicidal active compounds is limited and must be undertaken in the future.
Abamectin is a group of natural active compounds isolated from the fermentation product ofStreptomycesactinomycesin the soil,including 8 glycosylated macrolide homologs. Abamectin has a high efficiency and broadspectrum nematicidal activity and is one of the most widely used nematicides in market. It can selectively act on glutamate-gated chloride channels in nematode nerve and muscle cells,resulting in nematode paralysis or death (Cullyet al.1994). Although abamectin is a very representative commercial natural nematicide,its field performance is inconsistent with its excellent laboratory activity. Abamectin is insoluble in water and experiences lipophilicity and instability to light,which might be reasons behind its lack of persistence in the field (Lianget al.2018). Many studies have been undertaken on the adsorption,degradation,and fluidity of abamectin in soil and the improvement of its stability to light using nanoparticles as carriers (Guanet al.2011). The use of nanoparticles as carriers might further expand the application of abamectin.
Specific molecular scaffolds can be used as bridges connecting natural products and synthetic compounds to provide innovative and feasible solutions for meeting the sustainability challenges of modern agriculture. However,to the best of our knowledge,no previous study has examined the research progress regarding natural nematicidal products. We reviewed the research progress of natural nematicidal active compounds over the past ten years,and classified and discussed the structure-activity relationship,mode of action,mechanism of nematicidal natural products,and the future of natural product nematicides or nematicides based on natural products development and application. The aim of this review was to provide new ideas and inspiration for the discovery of novel nematicides.
2.Natural nematicidal active compounds
2.1.Alcoholic or phenolic natural nematicidal active compounds
Alcoholic or phenolic compounds are extensively present in plants and microorganisms and show a broad spectrum of biological activities. Some alkyl alcohols and enols have good nematicidal activity,with the strength of the nematicidal activity related to the chain length or position of the ethylenic bond (Fig.1). For example,geraniol has good nematicidal activity againstM.javanica,and has an inhibitory effect on egg hatching (Seoet al.2010). In pot experiments,geraniol decreased the number ofM.javanicafemales in tomato roots (Nasiouand Giannakou 2018). The eggshell structure of nematode eggs is different from the structure of the larval body wall,which might create differences in the permeability and dynamics of some natural products to the eggshell (Widmerand Abawi 2000). Falcarindiol isolated from the ethanol extract ofNotopterygium incisumrhizomes showed excellent nematicidal activity againstM.incognitaandBursaphelenchus xylophiluswith median lethal concentration (LC50) values of 0.56 and 0.95 mg L-1,respectively. Ultraviolet radiation enhanced the nematicidal activity of falcarindiol (Liu Get al.2016). Although the mechanism of falcarindiol against fungi is related to the destruction of cell membranes,it might provide a reference for the study of its nematicidal mechanism (Garrodet al.1979). The LC50values of compound 1 from the aerial parts ofMentha canadensisagainstHeterodera avenaeandM.incognitawere 190.3 and 115.2 mg L-1,respectively. Compounds 1 and 2 are the main chemical components inM.canadensisessential oil and they might be the main contributors to its nematicidal activity (Jiet al.2016). The essential oil fromMonarda didymashowed excellent nematicidal activity againstM.incognitaafter 24 h,with an LC50value of 1.0 mg L-1. The main compound in essential oils is carvacrol,which has an LC50value of 14.2 mg L-1againstM.incognita. However,carvacrol is not the only nematicidal active ingredient in essential oils,and the synergistic effect of several compounds might cause this difference in activity (Laqualeet al.2018). The nematicidal activity of carvacrol againstC.elegansmight be mediated by tyramine receptors (Leiet al.2010). The LC50value of eugenol isolated fromAgastache rugosaagainstM.incognitawas 66.6 mg L-1. However,when the hydroxyl group was replaced with a methoxy group,the nematicidal activity decreased. Thus,the nematicidal activity of simple phenolic compounds might be related to the number of hydroxyl groups. After eugenol was docked withC.elegansglutathioneS-transferase,the docking energy was small and with a strong hydrogen bond (Babuet al.2012). Kojic acid isolated fromAspergillus oryzaeshowed moderate inhibitory activity againstM.incognitaand eggs,with half effective concentration (EC50) values of 195.2 and 238.3 mg L-1,respectively (Kimet al.2016).
Tannin compounds have good inhibitory activity againstB.xylophilus,Globodera rostochiensis,M.javanica,andM.incognitain vivoor in field trials. For example,the LC50value of punicalagin 1 isolated fromPunica granatumL.rind againstB.xylophiluswas 307.08 μmol L-1after 72 h. In addition,it displayed inhibitory activity against acetylcholinesterase,cellulase and amylase of the pine wood nematode,with half maximal inhibitory concentration (IC50) values of 0.60,1.24 and 0.96 mmol L-1,respectively.The treatment with punicalagin 1 resulted in obvious deformation of the surface morphology of the pine wood nematode (Guoet al.2017). However,tannins mainly exhibited a reversible paralytic effect againstM.incognita,and the mobility of the nematodes was restored after removal of the adverse conditions. Tannin might be degraded into tannic acid in the soil,and tannic acid might be a chemical signal of root-knot nematodes that recognizes the plant host to help nematodes gather and locate the best attack area of the roots. The attracting effect of tannin on nematodes in soil might be used to guide the nematodes away from the crop or decrease the ability of the nematodes to search for the host,thereby preventing or delaying the attack by the nematodes on the host (D’Erricoet al.2018). This might be a good management strategy to fully consider the inhibition and attraction of nematodes by natural products. Tannin and cinnamaldehyde also display synergistic effects,with a reasonable combination of multiple natural products possibly achieving good results for the management of nematodes (Ropiaket al.2016).
2.2.Natural nematicidal active compounds of aldehydes
The reported natural products of aldehydes with nematicidal activity are mainly from plants and are fatty and aromatic aldehydes with simple structures,and some of these have exhibited good nematicidal activity (Fig.2). For example,phthalaldehyde,salicylaldehyde,and cinnamic aldehyde displayed EC50values of 11,11,and 12 mg L-1againstM.incognita,respectively. The nematicidal activity of aromatic aldehyde compounds might be closely related to the inhibitory activity of V-ATPase (Caboniet al.2014).For example,(E,E)-2,4-decadienal,(E)-2-decenal,and furfural fromAilanthus altissimahad EC50values of 11.7,20.43,and 21.79 mg L-1againstM.javanica,respectively (Caboniet al.2012a).Trans-2-hexenal is a volatile compound commonly found in with different nematicidal activities againstB.xylophilusduring different life stages. It showed the best nematicidal activity against second-stage juveniles,with an LC50value of 9.87 mg L-1after 48 h. In addition,it had inhibitory effects on egg hatching,nematode growth,respiration rate,movement,reproduction,and egg laying (Chenget al.2017).Trans-2-hexenal affected the metabolism of nutrients and the activity of digestive enzymes in the pine wood nematode,and significantly increases the activity of glutathioneS-transferase,thereby interfering with the normal metabolism and the physiological processes of nematodes (Miaoet al.2012;Zhaoet al.2017).Trans-2-hexenal also showed inhibitory effects againstH.avenaeandM.incognita(Miaoet al.2012). The position and chain length of the double bond might directly affect the nematicidal activity of aldehyde compounds (Seoet al.2010). Compounds 3 and 4 from the methanol extract ofCapparis spinosashowed excellent paralytic activity againstM.incognita,with LC50values of 7.90 and 1.13 mg L-1,respectively (Caboniet al.2012b).
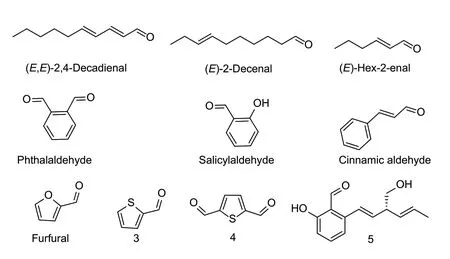
Fig.2 Structures of natural nematicidal active compounds of aldehydes.
The EC50values of furfural againstM.incognitaand eggs were 0.37 and 0.27 mg L-1,respectively (Abdelnabbyet al.2016). In pot experiments,furfural significantly reduced the infestation,egg production,andM.incognitapopulation density. Furfural also caused deformations of the nematode surface (Abdelnabbyet al.2016). When nematodes were treated with 100 mg L-1furfural,the damage caused to the stratum corneum might be due to nucleophilic addition to the stratum corneum amino or thiol residues and the carbonyl function of aldehydes (Caboniet al.2012b). Furfural had an inhibitory effect on the growth of free-living nematodes and tomato plants (Ntalliet al.2018). Biochar extract had no adverse effects on tomato plants (Huanget al.2015).However,in the furfural-biochar composition,when the furfural content was less than 1.0 g kg-1,it enhanced the control effect onM.incognitabut had no adverse effects on tomato plants and free-living nematodes. After treatment,second-stage juveniles caused the epidermis of the nematodes to lose its curl,anus to deform,and stratum corneum to degrade,with the degree of stratum corneum degradation increasing with an increase of furfural content (Abdelnabbyet al.2018). The emergence of furfuralbiochar compositions has allowed furfural to be used to controlM.incognita,which might reduce the adverse effects of certain natural products on non-target organisms. Compound 5 isolated fromMagnaporthe griseahad a nematicidal activity of 66.9% againstC.elegansat 200 mg L-1after 24 h (Yanget al.2020).
2.3.Natural nematicidal active compounds of ketones
The natural products of ketones are extensively found in plants and microorganisms. Several fatty ketone compounds have shown good nematicidal activity and inhibited egg hatching,such as 2-undecanone and 2-nonanone (Huanget al.2010;Ntalliet al.2011). The LC50value of 2-octanone isolated fromPseudomonas putida1A00316 againstM.incognitawas 22.7 mg L-1after 48 h,and 2-octanone showed a repellent effect againstM.incognitaat 1 000 and 10 000 mg L-1(Zhaiet al.2018).This might provide new ideas for the management of nematodes. For example,acetophenone and 2-heptanone released byBacillusB16 acted as powerful attractants appealing to nematodes and caused the nematodes to die on entering their intestines (Niuet al.2010). Compound 6 present in the essential oil of GreekMenthashowed good nematicidal activity with an LC50value of 0.04 mg mL-1(Fig.3). Changes in the positions of the alkenyl,epoxy,and ketone groups affect the nematicidal activity (Kimbariset al.2017). The EC50value of pulegone,which is ubiquitous in peppermint plants,againstM.incognitawas less than 117 mg L-1in 48 h. Stereo configuration,unsaturated double bonds,and changes in the position of the epoxy groups affect the nematicidal activity of these compounds (Ntalliet al.2010a;Caboniet al.2013b). Cytochrome P450 can oxidize pulegone to menthofuran toxic metabolites. The EC50value of compound 7 isolated fromHeterotheca inuloidesplant againstNacobbus aberranswas 21.9 mg L-1at 36 h (Rodríguez-Chávezet al.2019).
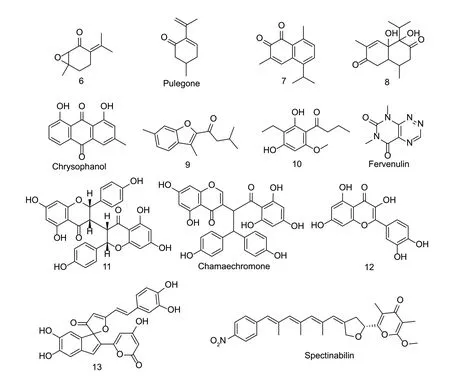
Fig.3 Structures of natural nematicidal active compounds of ketones.
Quinones are electrophilic Michael acceptors that form adducts with nucleophilic biomolecules,and are one of the main sources of reactive oxygen species in cells as redox cycle agents. However,both conditions cause damage to DNA (Daiand Ran-Charvey 2005). The EC50value of compound 8 fromEupatorium adenophorumleaves againstM.incognitawas 151.8 mg L-1(Kunduet al.2016).Chrysophanol isolated fromRheum emodihad an 50% effective dose value of 102.59 mg L-1againstM.incognita.In this structure,the substitution of the 6-position by the hydroxyl group was not conducive to nematicidal activity,and substitution by the methoxy group did not affect nematicidal activity. The nematicidal activity was also decreased when the hydrogen atom on the 3-position methyl group was replaced by a hydroxyl group (Tripathiet al.2014).Compound 9 fromStereumsp.YMF1.04183 showed weak nematicidal activity againstC.elegans(Yanet al.2017).Compound 10 isolated from the underwater culture of the fungusPseudobambusicolathailandicahad an 90% lethal dose value of ≤12.5 mg L-1againstC.elegans(Rupcicet al.2018). The inhibition rate of fervenulin isolated fromActinomycetesagainstM.incognitawas 100% at 250 mg L-1after 96 h;however,its inhibitory effect against eggs was not active (Ruanpanunet al.2011). Compound 11 isolated fromStellera chamaejasmeroots showed good nematicidal activity againstAphelenchoides besseyiandDitylenchus destructorafter 72 h,with LC50values of 2.32 and 0.18 mmol L-1,respectively. The configuration and hydroxyl of the compound are important for its nematicidal activity (Jinet al.2018). Chamaechromone fromS.chamaejasmeshowed strong nematicidal activity againstBursaphelenchus mucronatusandB.xylophilusaftre 72 h,with LC50values of 0.003 and 36.7 μmol L-1,respectively. The difference in the chamaechromone activity for the 2 nematodes might be due to the difference in its metabolism in the 2 nematodes or the difference in its absorption by the 2 nematodes (Cuiet al.2014). The position and relative configuration of the methoxy and hydroxyl groups might be the key to the nematicidal activity of these compounds againstB.xylophilusandB.mucronatus. The LC50value of schaftoside fromArisaema erubescensagainstM.incognitawas 114.66 mg L-1,and the nematicidal activity of its isomer was significantly reduced,which might be caused by the difference in its three-dimensional structure (Duet al.2011).Compound 12 isolated fromSchinus terebinthifoliusleaves had an inhibitory effect on the hatching ofM.incognitaeggs (Abdelet al.2018). The LD50value of compound 13 isolated fromSanghuangporussp.againstC.eleganswas 12.5 mg L-1(Chepkiruiet al.2018). The LC50value of the compound spectinabilin isolated fromStreptomycessp.AN091965 againstB.xylophiluswas 0.84 mg L-1.Spectinabilin effectively inhibited the pine wilt disease ofPinus densiflorain a greenhouse at a dose of 0.9 mg per plant (Liu M Jet al.2019).
2.4.Natural nematicidal active compounds of acids
Recently,the nematicidal activities of several natural acid compounds have been extensively studied,especially those of natural fatty acids (Fig.4),including acetic acid,caproic acid,and butyric acid (Ntalliet al.2010b). Acetic acid and caproic acid caused the paralysis ofM.javanica,with EC50values of 195 and 49 mg L-1,respectively. However,only caproic acid has shown an inhibitory effect onM.javanicaeggs at a concentration greater than 50 mg L-1(Ntalliet al.2020). This might be because the lipophilicity and permeability of acetic acid and caproic acid are different,reflecting the difference in the inhibition of the eggs (Ntalliet al.2016). The lethality and egg hatching inhibition rates of maleic acid againstM.incognitawere 100 and 70.5%,respectively,at 0.37 mmol L-1. Under greenhouse conditions,the combination of maleic acid and copper sulfate enhanced nematicidal activity,and its wettable powder reduced a 51.7% decrease in nematode damage on tomatoes and the density of nematode populations in soil. In a field test,the control effect of the combination of maleic acid and copper sulfate againstM.incognitawas 46.7% (Yeonet al.2019). Maleimide,a derivative of maleic acid,also showed synergistic nematicidal activity with copper sulfate. Its composition might enhance the nematicidal activity by increasingM.incognitasensitivity to oxidative stress (Elohet al.2016). Combining maleic acid and copper sulfate might have the same synergistic effect againstM.incognita,whereas combining organic acids and copper sulfate overcame the impermanence of organic acids in soil (El-Sherifet al.2015). Linoleic acids have been isolated from yellow flowers ofTagetes patulaand showed good nematicidal activity againstHeterodera zeaeat 125 mg L-1,with an inhibition rate of 100%. The nematicidal activity is dependent on the chain length and position of the double bond (Faiziet al.2011). Several aromatic acids have important effects on plants and showed good nematicidal activity,such as salicylic acid and indole acetic acid (Aoudiaet al.2012).
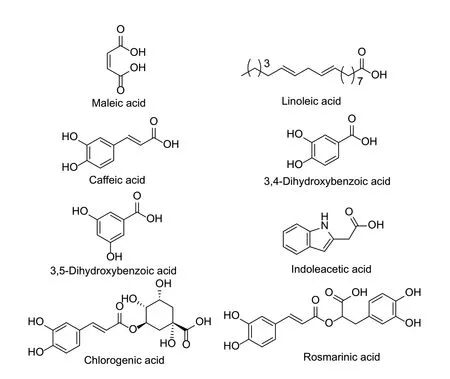
Fig.4 Structures of natural nematicidal active compounds of acids.
The inhibitory rate of compound 3,5-dihydroxybenzoic acid fromBuddlejacrispasp.againstM.incognitawas 96% at a concentration of 0.5% after 24 h (Sultanaet al.2010).3,4-Dihydroxybenzoic acid had a significant inhibitory effect onM.incognitaeggs at low concentrations and caused changes in the egg shape (Nguyenet al.2013). Indole acetic acid fromFusarium oxysporum162 had an LC50value of 104 mg L-1. Indole acetic acid can kill nematodes directly,also induces the defense mechanism of plant resistance. This dual activity might strengthen the management of nematodes (Bogneret al.2017).
Using plant attractants with nematicidal activity to manage nematodes is a good strategy for plant-parasitic nematode management. Caffeic acid and chlorogenic acid fromArtemisia annuahave no nematicidal activity againstM.incognita;however,they have good inhibitory activity againstG.rostochiensisandXiphinema indexat low concentrations (D’Addabboet al.2013). The rosmarinic acid isolated fromZostera marinashowed good nematicidal activity againstB.xylophilusafter 24,48 and 72 h,with LC50values of 1.18,1.05,and 0.95 mg mL-1,respectively (Wanget al.2012). The chain length,the number and position of the double bonds have an important influence on the nematicidal activity of the natural fatty acids. The nematicidal activities of several natural phenolic acids increases as the number of hydroxyl groups increases. There are relatively few studies on the field activity of natural acid compounds against plant-parasitic nematodes,their attracting effects,and the enhancement of plant defenses.
2.5.Natural nematicidal active compounds of esters
Natural nematicidal active compounds of simple structure esters or lactonesThe LC50value of 3-methylbutyl acetate fromF.oxysporumagainstM.incognitawas 198 mg L-1(Fig.5). 3-Methylbutyl acetate inhibited 90% of eggs from hatching and reduced the infectivity ofM.incognitaby 52%. After treatment with 3-methylbutyl acetate,vacuoles were observed in the body ofM.incognitaand the body was linear (Terraaet al.2018). Four volatile compounds have been detected from castor bean cake using the gas chromatography-mass spectrometry method. Among these,γ-decalactone showed good nematicidal activity againstM.incognitawith an LC50value of 7.96 mg L-1(Pedrosoet al.2019). The eggs ofM.incognitahave a glycolipid layer that can resist nematicides to a certain extent. When the embryo continues to develop,the permeability of the eggs changes significantly,which is more likely to be affected by nematicides (Eisenback and Hunt 2009). The EC50values of tulipaline A againstM.incognitaandM.arenriawere 19.3 and 13.6 mg L-1,respectively (Caboniet al.2014).α,β-Unsaturated compounds reacted with SH in amino mercaptan,whereas tulipaline A reacted with SH of cysteine residue (Friedmanet al.1965). V-ATPase is closely related to the horny layer synthesis,osmotic regulation and detoxification of nematodes and is essential in the life cycle of nematodes and can selectively bind to small ligands (Knightand Behm 2012). When the ATP synthase b subunit gene in the mitochondria ofM.incognitawas silenced,the ability of the nematode to infect tomatoes was decreased,and many larvae died (Huanget al.2014). V-ATPase can be used as a potential new target of nematicides,and further clarification of its structure-activity relationship is of great significance for the discovery of nematicides such as V-ATPase inhibitors.
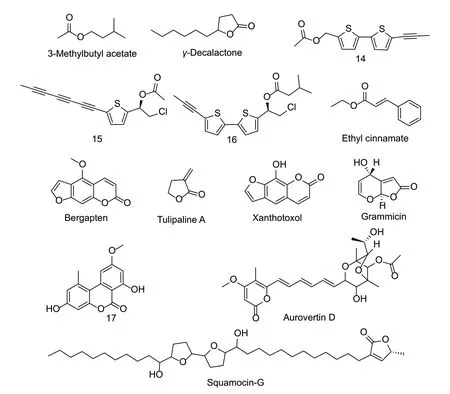
Fig.5 Structures of natural nematicidal active compounds of simple structure esters or lactones.
Eight types of thiophene compounds have been isolated fromArtemisia absinthiumL. Among them,the LC50value of compound 14 againstM.incognitawas 2.69 mg L-1. The lipophilic compound 14 more easily is easier to entered the nematode cell through the less fat-soluble membrane than the hydrophilic compound (Liu T Tet al.2019b). The LC50values of compounds 15 and 16 isolated fromEchinops grijsiiagainstM.incognitawere 2.65 and 2.62 mg L-1,respectively.The nematicidal activity is affected by the amount of acetylene,the chain length at one end of the acyl group and the chlorine atom,with the chlorine atom playing an important role (Liu T Tet al.2019a). Ethyltrans-cinnamate fromKaempferia galangahas shown good nematicidal activity,and when the para-position of the benzene ring was substituted with hydroxyl and methoxy,the nematicidal activity changed significantly,and the hydroxyl substitution was more conducive to nematicidal activity (Konget al.2007;Honget al.2011;Huanget al.2019). The LC50value of bergapten,a coumarin analog isolated fromFicus caricaL.leaves againstB.xylophiluswas 97.08 mg L-1. In addition,the IC50values of bergapten against amylase,cellulase,and acetylcholinesterase ofB.xylophiluswere 372.45,450.40,and 493.11 mg L-1,respectively (Guoet al.2016).Amylase and cellulase are important digestive enzymes of nematodes. Bergapten might weaken the digestive ability of nematodes by inhibiting amylase and cellulase,thereby enhancing its inhibitory effect on nematodes. After bergapten was used to treat the nematodes,the surface of the nematodes became rough and some of the nematodes underwent autolysis (Palomares-Riuset al.2014). The EC50value of xanthotoxol isolated from the aerial part of parsley againstM.incognitawas 68 mg L-1at 24 h. The substitution of the 5-hydroxyl group was important for the nematicidal activity of these compounds (Caboniet al.2015). The EC50values of grammicin fromXylaria grammicaKCTC 13121BP againstM.incognitaand eggs were 15.95 and 5.87 mg L-1,respectively. The isomers of grammicin had weaker inhibitory effects onM.incognita;therefore,the three-dimensional configuration played an important role in its nematicidal activity (Kimet al.2018). The IC50values of compound 17 isolated fromAlternariasp.Samif 01 againstB.xylophilusandC.eleganswere 98.17 mg L-1and 74.62 mg L-1,respectively (Louet al.2016). Aurovertin D fromPochonia chlamydosporiaalso had good inhibitory activity againstM.incognitaat low concentrations and hindered the development ofC.eleganslarvae and reduced the rate of movement. However,aurovertin D had higher inhibitory activity againstC.elegansjuveniles than adults (Wanget al.2015). TheC.elegansjuveniles do not have a mature detoxification system,which might be the factor causing the difference in activity (Lindblomand Dodd 2006).The LC50values of the compound squamocin-G isolated from the seeds ofAnnona squamosaagainstB.xylophilusandM.incognitawere 0.339 and 0.008 mg L-1,respectively (Danget al.2011).
Natural nematicidal active compounds of thiocyanatesThe cyanate ester natural products with nematicidal activity that have been reported are mostly aliphatic and aromatic compounds with simple structures (Fig.6). The EC50values of benzyl isothiocyanate,allyl isothiocyanate,and butyl isothiocyanate fromArmoracia rusticanaagainstM.incognitawere 1.9,6.6,and 12 mg L-1after 24 h (Aissaniet al.2013). The EC50values of the thiocyanate compounds erucin,methyl isothiocyanate,pentyl isothiocyanate,hexyl isothiocyanate,and methyl thiocyanat fromEruca sativaagainstM.incognitawere 3.2,7.9,11.1,11.3 and 18.1 mg L-1,respectively. After treatingM.incognitawith isothiocyanate for 24 h,the nematode became paralyzed,the body was linear,and there were obvious internal vacuoles (Aissaniet al.2015). The same phenomenon appeared after treating nematodes with ATPase inhibitors pyocyanin andα,β,γ,δ-unsaturated aldehydes (Caboniet al.2013a,2014);therefore,the inhibition of nematodes with isothiocyanates might be related to ATPase inhibition.
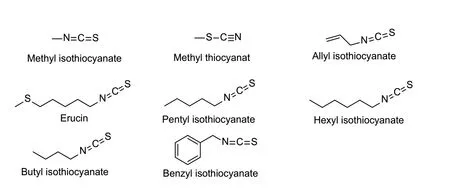
Fig.6 Structures of natural nematicidal active compounds of thiocyanates.
Natural nematicidal active compounds of macrolidesMacrolide compounds have a broad spectrum of biological activities,mainly derived from microbial metabolites such as those of fungi and bacteria (Fig.7). Macrolide compounds,such as abamectin and ivermectin,act on glutamate-gated chloride channel receptors (GluCl) and open ion channels to cause chloride ions to enter cells rapidly,causing hyperpolarization of the cell membranes and ultimately leading to nematode death (Wolstenholme 2012). GluCl is an effective target in nematodes,and the screening of natural compounds based on it might be ideal for discovering new nematicides. Theα,β-dehydrocurvularin from the fungusAspergillus welwitschiaeshowed good inhibitory activity againstMeloidogyne graminicola,with an LC50value of 122.2 mg L-1. Under greenhouse conditions,α,β-dehydrocurvularin decreased the attraction of rice roots toM.graminicolaand inhibited the growth of the nematodes to decrease the root-knot index. Afterα,β-dehydrocurvularin treatment,the cross-sectional volume of giant cells in the rice roots was significantly reduced,possibly because it hindered the development of giant cells,resulting in inadequate food supply for the nematodes,thereby inhibiting their growth (Xianget al.2020). Compound 18 from the thermophilic fungusTalaromyces thermophilushad excellent nematicidal activity againstM.incognita,Bursaphelenches siylopilus,andPanagrellus redivevus,with LC50values of 0.7,0.9,and 0.5 mg L-1,respectively. After the ester groups on the alkyl chain of the compound are replaced by hydroxyl groups,the nematicidal activity decreases (Guoet al.2012). Compound 19 isolated from the fermentation broth ofStreptomyces avermitilisNEAU1069 showed strong nematicidal activity againstC.elegans. The paralytic activity of compound 19 againstC.eleganswas 92.7% at 10 mg L-1(Wanget al.2010). When the polyketide synthases geneaveA3ofStreptomyces avermitiliswas replaced by the polyketide synthases genemilA3of milbemycin,the genetically engineered strainS.avermitilisTM24 was obtained. Then,the macrolide compound 20 was isolated from the fermentation broth ofS.avermitilisTM24,and its LC50value againstB.xylophiluswas 4.30 mg L-1. This is an example of generating new engineered strainsviaspecific gene combinations of different strains,and then purposefully isolating new natural products from the engineered strains (Fenget al.2019). The LD50values of compound 21 fromStreptomyces albogriseolusHA10002 againstM.incognitaandM.javanicawere 7.64 and 7.50 mg L-1,respectively (Zenget al.2013). The LD50values of verrucarin A and roridin A fromMyrothecium verrucariaagainstM.incognitawere 1.8 and 1.5 mg L-1,respectively (Nguyenet al.2018).
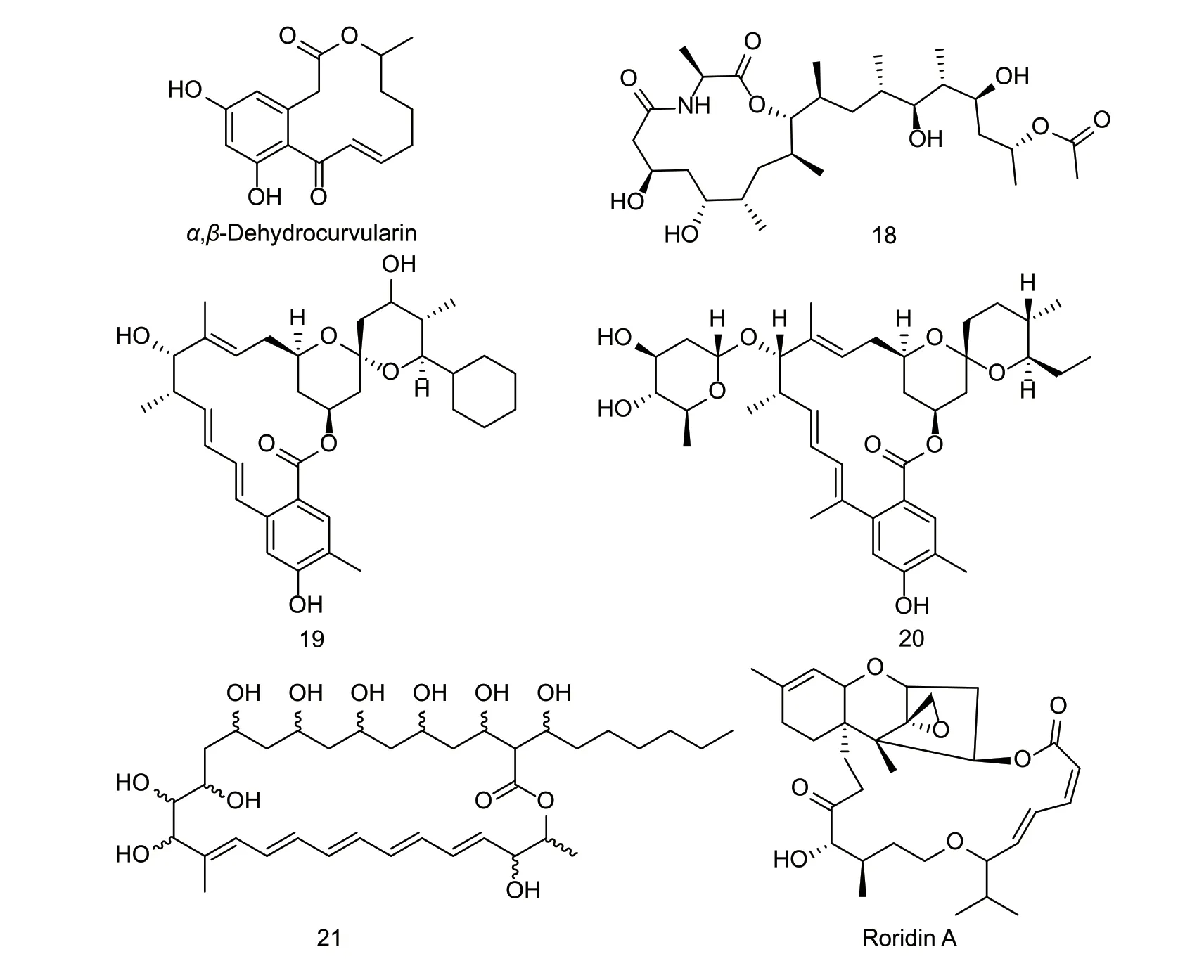
Fig.7 Structures of natural nematicidal active compounds of macrolides.
2.6.Natural nematicidal active compounds of amides
There are relatively few studies regarding natural amide compounds with nematicidal activity (Fig.8). Compound 22 has been isolated from spider parasitic fungusAkanthomyces novoguineensis,however,this compound did not show any nematicidal activity (Helalyet al.2017).The LC50values of compound 23 isolated from bacteriumBacillussp.againstB.xylophilusandD.destructorwere 232.9 and 206.3 mg L-1,respectively (Zenget al.2015).The inhibitory rate of compound 24 fromT.patulaL.yellow flowers againstH.zeaewas 100% at a concentration of 0.125%. Chemically synthesized amide compounds have made great progress in the study of nematicides,whereas natural amide compounds need further attention and research (Banoet al.2019).
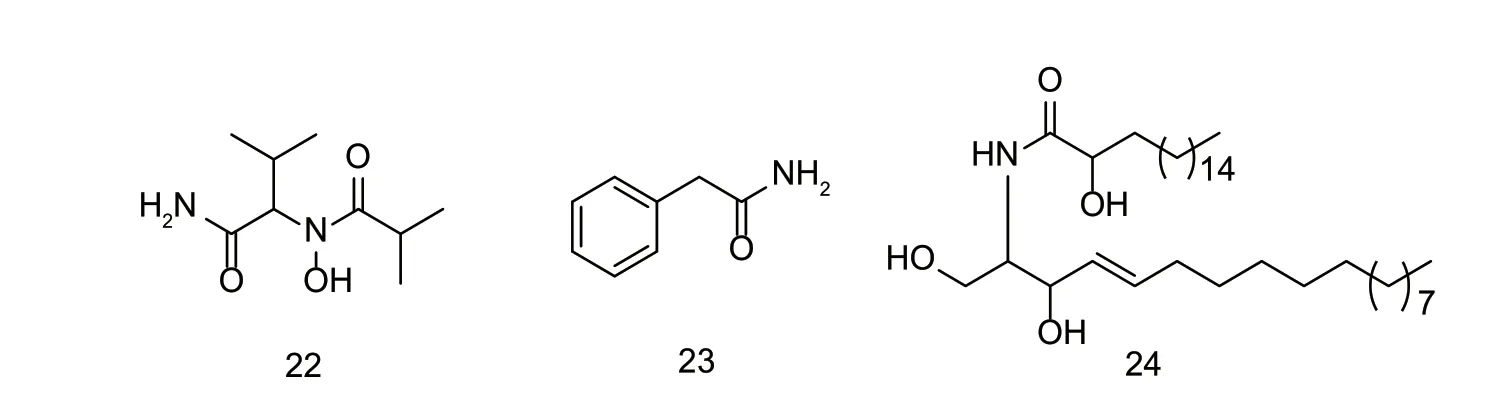
Fig.8 Structures of natural nematicidal active compounds of amides.
2.7.Natural nematicidal active compounds of alkaloids
Recently,alkaloids with nematicidal activity have been found to be ubiquitous in plants (Huanget al.2016),including the family fumariaceae and the speciesStemona parvifloraandCephalotaxus fortune.Betaine from seaweeds is an amino acid that has penetrating and methyl donor functions (Fig.9). The betaine transporter SNF-3 and receptor ACR-23 have been identified inC.elegans. After the transporter SNF-3 is mutated,excessive extracellular betaine might cause excessive contraction and paralysis of the nematode. The mutation of the receptor ACR-23,which encodes the ligandgated cation channel of the cys-loop family,can inhibit the behavioral defects of excessive contraction and paralysis of nematodes. However,when the receptor ACR-23 was activated by betaine,it acted in mechanosensory neurons to maintain the basic movement of the nematode (Pedenet al.2013). The LC50values of pyrrolidine alkaloid 25 fromOrixa japonicaroot bark againstB.xylophilusandM.incognitawere 391.50 and 134.51 mg L-1,respectively (Liu X Cet al.2016). The 4-quinolone alkaloids waltherione E and waltherione A isolated from the methanol extract of the aerial part ofTriumfetta grandidensshowed excellent nematicidal activity againstM.incognita,with LC50values of 0.09 and 0.27 mg L-1,respectively. After 7 days of treatment,the hatching inhibition rates of the eggs were 91.9 and 87.4% at 1.25 mg L-1. The nematicidal activity of these 4-quinolone alkaloids is related to the number of hydroxyl groups on the benzene ring (Janget al.2019). The drupacine isolated fromCephalotaxus fortuneihad good nematicidal activity againstM.incognitaandB.xylophilus,with LD50values of 76.3 and 27.1 mg L-1,respectively.Drupacine can also inhibit the hydrolysis of proteases,which might facilitate the study of this type of alkaloid interaction with nematodes. In anin vivotest,the crude alkaloid extract had no phytotoxicity and significantly decreased the number of eggs and second-stage juveniles on pepper roots. The inhibition rates were 69 and 73% at 0.5 mg mL-1,respectively. The high concentration of crude alkaloids mainly suppressed the nematode control base by killing the eggs and second-stage juveniles (Wenet al.2013). The IC50value of compound 26 from the seeds ofClausena lansiumagainstP.redivevuswas 0.12 mmol L-1(Fanet al.2018).The IC50value ofStemonaalkaloids protostemonine isolated fromS.parvifloraagainstP.redivevuswas 0.10 μmol L-1(Huanget al.2016). The inhibitory rate of protopinium isolated fromFuaria parvifloraroots againstM.incognitaand eggs was 100% at 200 mg L-1. In greenhouse and field tests,protopinium significantly reduced the number of females per gram of root and root knot index at 300 mg L-1. Thecis-andtrans-protopinium showed no significant difference in activities againstM.incognita(Nazet al.2016).Natural alkaloids have great potential for discovering new nematicides,and research on the mechanism of action and safety must be strengthened.
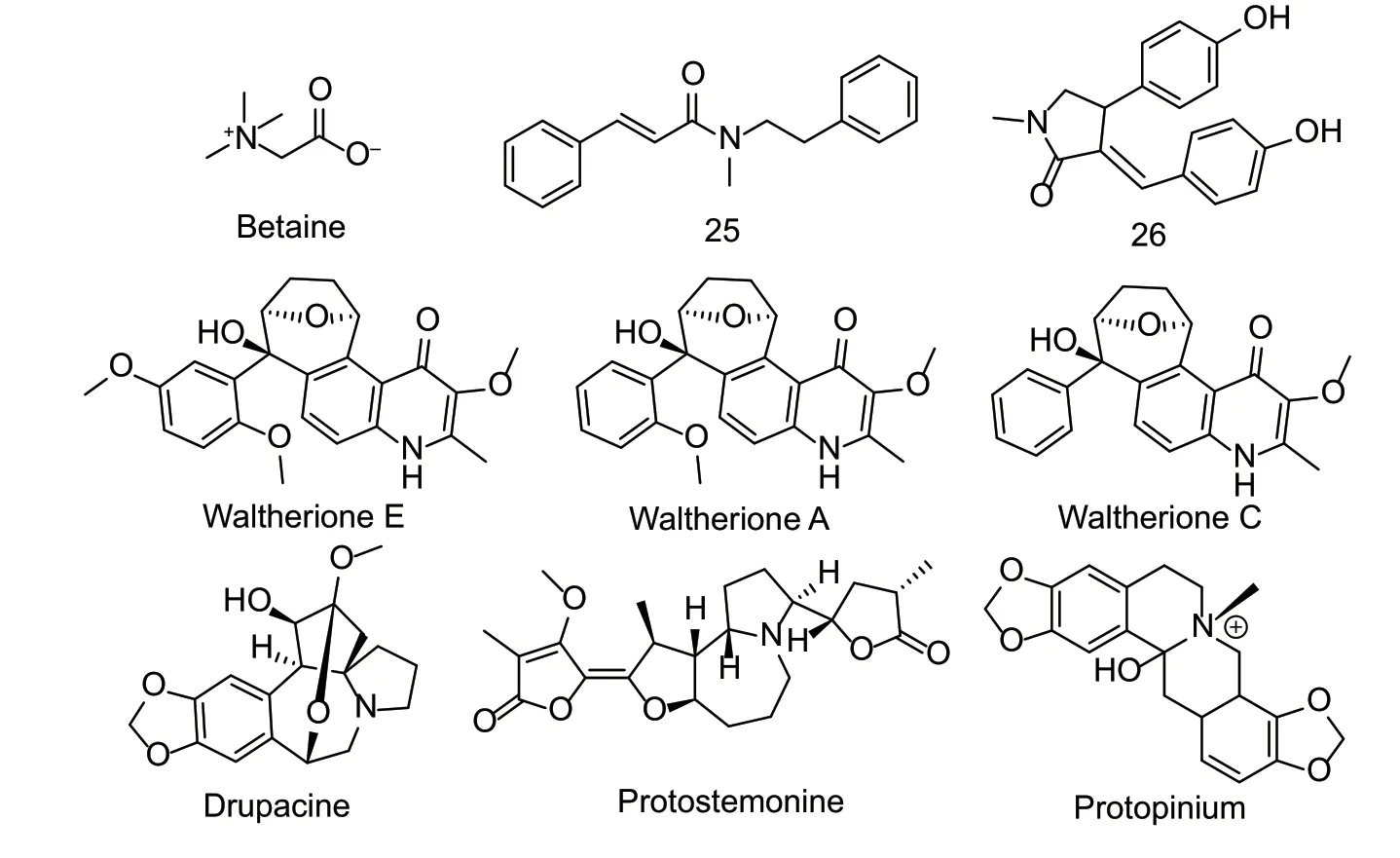
Fig.9 Structures of natural nematicidal active compounds of alkaloids.
2.8.Natural nematicidal active compounds of terpenoids
Terpenoids have a broad-spectrum of biological activities,and there have been many studies undertaken on their nematicidal activity (Fig.10). For example,1,8-cineole,limonene,andβ-pinene have shown good nematicidal activity againstM.incognita,and inhibited egg hatching. Their nematicidal activity is related to the position of the double bond(Ntalliet al.2011). BxACE-1,BxACE-2 and BxACE-3 are the 3 active acetylcholinesterases ofB.xylophilus,and they play different roles in neuronal functions (Kanget al.2011). For example,there are differences in catalytic activities and protein expression levels. Althoughα-pinene has no direct inhibitory effect on the pine wood nematode,(+)-α-pinene has a good inhibitory activity on BxACE-1,BxACE-2,and BxACE-3 ofB.xylophilus,with IC50values of 0.24,0.64,and 0.69 mmol L-1,respectively (Kanget al.2013). The LC50value of compound 27 fromPulsatilla koreanaroot againstM.incognitawas 69.7 mg L-1after 72 h (Liet al.2013). The indole-containing terpenoid gymnoascole acetate isolated from the fungus strainGymnoascus reessiiza-130 had an LC50value of 47.5 mg L-1againstM.incognitaat 24 h. After 7 days of treatment,its inhibition rate of hatching eggs was greater than 90% (Liuet al.2017). Compound 28 isolated fromF.parvifloraroots significantly reduced the number of galls and females per gram of roots in anin vivotest (Nazet al.2013).
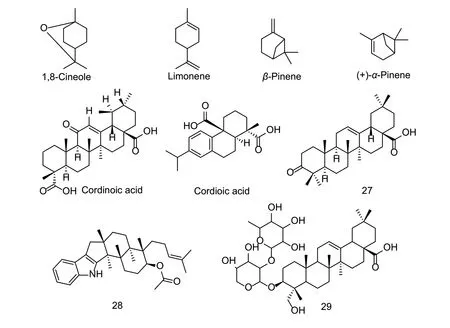
Fig.10 Structures of natural nematicidal active compounds of terpenoids.
2.9.Natural nematicidal active compounds of peptides
Peptide nematicidal active compounds are commonly found in plants and microorganisms,such asPicea sitchensissp.,Fusarium,andBacillus cereus. Most peptide compounds have complex linear or cyclic structures,whereas cyclic peptides combine the head and tail ring backbone and cystine for good stability. The peptide with 2 cysteines forming a disulfide bond in the molecule isolated fromP.sitchensisshowed strong nematicidal activity againstC.elegansat 10 mg L-1after 5 h (Fig.11). When the test concentration was greater than 50 mg L-1,the mortality of nematodes did not increase with the increase in concentration (Liuet al.2011). The linear peptide compound 29 isolated from the culture broth ofXenorhabdus budapestensisSN84 showed good nematicidal activity againstM.incognita,with an LC50value of 27.8 mg L-1(Biet al.2018). The LD90values of compound 30 fromIjuhya vitellinaagainstC.elegansandPratylenchus penetranswere 5 and 100 mg L-1,respectively (Moussaet al.2020). There are relatively few studies the nematicidal activity,mechanism,and toxicology of natural peptide compounds,and more in-depth studies are required in future.
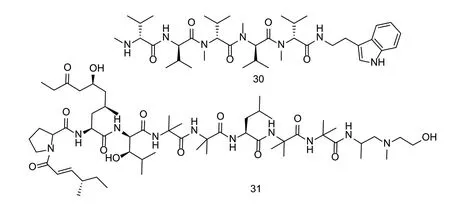
Fig.11 Structures of natural nematicidal active compounds of peptides.
3.Conclusion and outlook
In general,the management of nematodes has changed dramatically. Compared with the direct killing of nematodes with nematicides,comprehensive solutions can manage nematodes better,for example,new models of crop cultivation,soil disinfection before planting,breeding of new resistant varieties and the combined use of different crop rotations and low-toxic nematicides. However,due to the adverse effects of traditional chemical nematicides on the environment and human health,chemical insecticides have become increasingly restricted,with such restrictions continuing in the future. Currently,the discovery of new nematicides that are safe for non-target organisms is still a considerable challenge for nematode management. Using natural products as nematicides can often compensate for the shortcomings of chemical nematicides. For example,through the management of residues and resistance and risks to beneficial organisms. Commonly,low-toxic nematicides are obtained by deriving or intercepting part of the structures with natural products as the lead. Although natural product nematicides have a great potential in the discovery of new nematicides,the determination of their modes and mechanisms of action and targets is still problematic for studying new nematicides,with transcriptomics and metabonomics methods being powerful tools. Focusing on the nematicidal activity of natural products,the analysis of structure-activity relationships,and the study of the mechanisms of action are essential for discovering new and environmentally friendly nematicides.
Acknowledgements
The authors are grateful to the National Key Research and Development Program of China (2018YFD0200100) for supporting the project.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Effects of plant density and nitrogen rate on cotton yield and nitrogen use in cotton stubble retaining fields
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density
- lmproving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele
- Comparative transcriptome analysis of different nitrogen responses in low-nitrogen sensitive and tolerant maize genotypes
- ldentifcation of blast-resistance loci through genome-wide association analysis in foxtail millet (Setaria italica (L.) Beauv.)
