The transcription factor FgNsf1 regulates fungal development,virulence and stress responses in Fusarium graminearum
2021-06-24SHlDongyaRENWeichaoWANGJinZHANGJieJanelfunanyaMBADlANYAMAOXueweiCHENChangjun
SHl Dong-ya,REN Wei-chao,WANG Jin,ZHANG Jie,Jane lfunanya MBADlANYA,MAO Xue-wei,CHEN Chang-jun
1 College of Plant Protection,Nanjing Agricultural University,Nanjing 210095,P.R.China
2 College of Plant Health and Medicine,Qingdao Agricultural University,Qingdao 266109,P.R.China
Abstract Nutrient and stress factor 1 (Nsf1),a transcription factor containing the classical Cys2-His2 (C2H2) zinc finger motif,is expressed under non-fermentable carbon conditions and in response to salt stress in Saccharomyces cerevisiae. However,the role of Nsf1 in filamentous fungi is not well understood. In this study,the orthologue of Nsf1 was investigated in Fusarium graminearum (named FgNsf1),a causal agent of Fusarium head blight (FHB). The functions of FgNsf1 were evaluated by constructing a FgNSF1 deletion mutant,designated as ΔFgNsf1,and its functional complementation mutant ΔFgNsf1-C. Gene deletion experiments showed that the mycelial growth rate,asexual and sexual reproduction of ΔFgNsf1 were significantly reduced,but the pigment production of ΔFgNsf1 was remarkably increased compared with the PH-1 and ΔFgNsf1-C. In addition,the tolerance of ΔFgNsf1 to osmotic pressures,cell wall-damaging agents and oxidative stress increased significantly. Sensitivity tests to different fungicides revealed that ΔFgNsf1 exhibited increased sensitivity to carbendazim (MBC) and tebuconazole,and enhanced tolerance to fludioxonil and iprodione than PH-1 and ΔFgNsf1-C. The virulence of ΔFgNsf1 to wheat coleoptiles and flowering wheat heads were dramatically decreased,which was consistent with the decrease in the yield of deoxynivalenol (DON). All of these defects were restored by target gene complementation. These results indicated that FgNsf1 plays a crucial role in vegetative growth,asexual and sexual reproduction,stress responses,fungicide sensitivity,and full virulence in F.graminearum.
Keywords:Fusarium graminearum,nutrient and stress factor 1,stress responses,virulence
1.lntroduction
Fusarium graminearumis a predominant causal agent of Fusarium head blight (FHB) or scab on staple food crops including wheat,barley and maize in most growing areas (Bai and Shaner 2004;Goswami and Kistler 2004). In addition to severe yield and economic losses,F.graminearumproduces mycotoxins such as deoxynivalenol (DON) and zearalenone (ZEA) in the infected grains,which pose serious threats to human and animal health (Pestka and Smolinski 2005;Stephanieet al.2010). Due to the lack of highly efficient disease-resistant cultivars,fungicides play a prominent role of reliable FHB management (Pirgozlievet al.2003;Bai and Shaner 2004;Figueroaet al.2018). However,with the development of resistance against fungicides,the efficiency of chemical control has been adversely affected (Chen and Zhou 2009). A good knowledge of molecular mechanism of fungal development and pathogensis are great approaches to disease control.
Zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions in order to stabilize the fold (Jantzet al.2004;Michaleket al.2011;Maret 2012;Lee and Michel 2014). To date,more than 40 types of zinc fingers are annotated in UniProtKB,which show structurally diversity and exhibit a wide range of biological functions,including DNA-or RNA-binding,protein-protein interactions,and membrane association (Laityet al.2001). Cys2-His2 (C2H2) zinc finger is one of the most common DNA-binding motifs found in eukaryotic transcription factors (Wolfeet al.2000),which is first identified in transcription factor IIIa (TFIIIa) fromXenopus laeviscontaining nine tandem repeats of the approximately 30 amino acid motif (Brownet al.1985;Milleret al.1985). However,most zinc finger structures identified by genome sequencing have not been confirmed by experiments (Shimberget al.2018).Here,we characterized FgNsf1 which has been reported to be a C2H2zinc fingers protein inF.graminearum(Sonet al.2011).
Nutrient and stress factor 1 (Nsf1/Ypl230w),also named as up in starvation protein 1 (Usv1p),responses to non-fermentable carbon,salt-stress and cell wall biosynthesis (Segalet al.2003;Hlynialuket al.2008) and serves as a negative regulator of sulfur metabolism inS.cerevisiae(Gaillardet al.2009;Bessonovet al.2013).InS.cerevisiae,NSF1bear a homolog,RGM1,which putatively encodes a zinc finger DNA binding transcription factor (Estruch 1991). InMagnaporthe oryzae,GCF6(MGG_03451),the homolog ofFgNSF1,is reported to be involved in development and pathogenicity (Caoet al.2016). Nsf1 has been well documented inS.cerevisiaeandMagnaporthe oryzae,but the specific function of FgNsf1 inF.graminearumis far from well known.
TheFgNSF1gene encodes a 725 amino-acid protein with a typical C2H2domainviahomologous comparison which was conserved among filamentous fungi. The objective of this study was to identify and characterize Nsf1-like protein inF.graminearum,and determine its roles in fungal growth,development and virulence by reverse genetics method.
2.Materials and methods
2.1.Strains and media
Fusarium graminearumstrain PH-1 (NRRL 31084) was used as a parental strain for transformation experiments and wild-type (WT) control. ΔFgNsf1(NSF1knockout mutant ofF.graminearum) and ΔFgNsf1-C(NSF1complementary strain) were generated. For mycelial growth tests,all strains were routinely cultured at 25°C on fresh media (PDA,CM and MM) (Renet al.2018) for 3 days in the dark. To observe the morphology of mycelia,the mycelial plugs (5 mm in diameter) were cultured on cellophane sheets placed on solidified PDA for 24 h. Liquid YEPD (w/v,1% peptone,0.3% yeast extract,and 2% dextrose) was used to prepare the mycelia for the extraction of DNA and RNA. Mung bean liquid (MBL) broth (30 g mung beans boiled in 1 L water for 20 min,and filtered through cheesecloth) (Guet al.2015) was used to produce conidia. For self-crossing assays,perithecia formation was induced on carrot agar (CA) medium (Cavinderet al.2012). Deoxynivalenol (DON) production was assayed with liquid trichothecene biosynthesis induction (TBI) culture (Gardineret al.2009;Zhanget al.2017).
2.2.Gene deletion and complementation by protoplast transformation
To reveal the function of FgNsf1 inF.graminearum,ΔFgNsf1was generated from the parental strainF.graminearumPH-1 using a homologous recombination strategy (Appendix A-a). The gene replacement construct was obtained by a double-joint PCR approach (Yuet al.2004). Protoplast preparation and transformation were performed as the method described previously (Zhenget al.2013;Huet al.2014). The candidate deletion mutants screened from plates with 100 μg mL-1hygromycin B (CalBiochem,La Jolla,CA,USA) were further confirmed by PCR and Southern blot analysis (Cuomoet al.2007). Primers used in this study were listed in Appendix B.
2.3.Construction of GFP fusion cassettes and microscopy
The FgNsf1p-GFP fusion cassette was constructed by amplification of the open-reading frame (without stop codon),and the PCR product was then cloned into the GFP vector by the strategy described before (Renet al.2019). The resulting recombinant plasmids confirmed by sequencing were transformed into the ΔFgNsf1mutant,and the resultant transformants were screened by PCR and GFP fluorescence. To explore subcellular localization,GFP fluorescence and 4´,6-diamidino-2-phenylindole (DAPI) (10 μg mL-1) (Sigma-Aldrich,USA) staining at room temperature in the dark for 10 min were observed by a confocal laser scanning microscope (Leica TCS SP8,Germany).
2.4.Sensitivity of mycelial growth to multiple stresses
To determine the sensitivity of ΔFgNsf1to various stresses,mycelial growth was vertically measured after incubation on PDA plates without or with cell wall-damaging agents 0.05% (w/v) Congo red,osmotic stress agents 1.2 mol L-1NaCl or 1.2 mol L-1KCl,oxidative stress agents 0.1 mmol L-1menadione (MD) or 16 mmol L-1hydrogen peroxide (H2O2) and metal cations 5 mmol L-1ZnCl2(Zn2+) or 0.2 mol L-1MgCl2(Mg2+) (Sonet al.2011),sulfur-containing amino acids 10 mmol L-1cysteine or 10 mmol L-1methionine (Bessonovet al.2013).
To test the sensitivity against different fungicides,the mycelial plug (5 mm in diameter) of each strain was incubated on PDA amended with fludioxonil (0,0.00625,0.0125,0.025,0.05,and 0.1 μg mL-1) or iprodione (0,2,4,8,16,and 32 μg mL-1) or carbendazim (MBC) (0,0.1,0.3,0.5,0.7,0.9,and 1.1 μg mL-1) or tebuconazole (0,0.3,0.5,0.7,0.9,1.1,and 1.3 μg mL-1),all the tests were performed with three replicates in the dark for 3 days at 25°C. The percentage of mycelial growth inhibition and the effective concentration for 50% inhibition of mycelial growth (EC50) were calculated as described previously (Zhenget al.2014). All the experiments were repeated three times independently.
2.5.Asexual and sexual reproduction assays
For conidiation assays,six mycelial plugs (5 mm in diameter) of each strain were taken from the edge of the actively growing colony on the plates,and were transferred into a 50-mL triangular flask containing 30 mL of mung bean liquid (MBL) broth. After 5 days of incubation at 25°C and 175 r min-1in a shaker,the number of conidia in MBL broth in each flask was determined with a hemacytometer.Germination experiments were examined on thin layer of water agar medium as described previously (Zhenget al.2013).
Sexual reproduction was performed on CA plates as previously described (Tanget al.2019). Microscopic examination and photography of perithecia,asci,and ascospores were performed after 2-3 weeks of incubation at 25°C under 12 h light/12 h dark cycle conditions. All the tests were performed three times with three replicates independently.
2.6.Quantitative RT-PCR (qRT-PCR)
RNA samples of wild-type strain PH-1,ΔFgNsf1and ΔFgNsf1-Cwere extracted with total RNA Kit (Tiangen Biotech,Beijing,China) from 2-day-old mycelia grown in liquid YEPD or 3 days old mycelia harvested from DONinduction medium TBI (Gardineret al.2009),respectively. To assay the expression of genes response to osmotic and cell wall stressors in MAPK (mitogen-activated protein kinase) signal pathway,2-day-old mycelia grown in liquid YEPD were treated by 1.2 mol L-1NaCl or 1.2 mol L-1KCl or 0.05% (w/v) Congo red for 4 h,mycelia were harvested for RNA extraction. First-strand cDNA was synthesized with HiScript®II 1st Strand cDNA Synthesis Kit (Vazyme Biotech,Nanjing,China). All quantitative real-time PCR were performed with an ABI 7500 Real-Time Detection System (Applied Biosystems,Foster City,CA,USA). All data were normalized by actin gene expression,and relative abundance of transcripts was calculated by the 2-ΔΔCtmethod (Livak and Schmittgen 2001). All qRT-PCR assays were performed four repeats for each sample,and the experiment was replicated three times independently.
2.7.Virulence assays on wheat coleoptiles and flowering wheat heads
To test the pathogenicity,the infection assays of wild-type strain PH-1,ΔFgNsf1and ΔFgNsf1-Cto coleoptiles and flowering heads of susceptible wheat cultivar Zhenmai 5 were conducted by the previously described method (Wuet al.2005;Liuet al.2016). A total of 10-μL aliquot of spore suspension (3×105mL-1) of each strain harvested from MBL cultures for 5 days was injected to a floret in the central spikelet of single flowering head,or a 2.5-μL aliquot was injected to the tip of coleoptile from the 3-dayold seedlings without the 2-3 mm top,and each strain was inoculated with 20 replicates. After incubation for 7 or 14 days respectively,the brown lesions on diseased coleoptile stems were measured,and the infected spikelets in each inoculated wheat head were recorded. The experiments were repeated three times independently.
2.8.Analysis of deoxynivalenol production in vitro
To determine trichothecene toxin deoxynivalenol (DON) production of the wild-type strain PH-1 and the deletion mutant ΔFgNsf1,fresh conidia were suspended in TBI liquid medium (Gardineret al.2009;Zhanget al.2017) with the terminal concentration 5×104conidia mL-1at 28°C without shaking in the dark. After 7 days of incubation,mycelia were harvested,dried and weighed,and the volume of supernatant was measured. The amount of DON production (μg mL-1filtrate) of each strain was detected by gas chromatography-mass spectrometry (GC-MS) (Zhenget al.2013). Three biological replicates were tested for each strain.
2.9.Statistical analysis
All data were analyzed by the Date Processing System (DPS,version 7.05),the means were analyzed by Fisher’s Least Significant Different (LSD) test. Error bars represent the standard deviation of three independent experiments,and the bars signed with the same letter indicate that the difference is not significant according to an LSD test (P<0.05).
3.Results
3.1.Sequence alignment and phylogenetic analysis of FgNsf1 protein from different organisms
The protein encoded by theFgNSF1gene (FGSG_07928.1) ofF.graminearumcontains 725 amino acids. Sequence alignment and phylogenetic analysis were performed using MEGA7.0 Software. Species names and GenBank accession numbers are as follows:Candida albicans(KHC77091.1),Pyriculariaoryzae(XP_003716477.1),Saccharomyces cerevisiae(NP_015094.1),Neurospora crassa(XP_956438.1),F.verticillioides(XP_018759950.1),F.oxysporum(XP_018238115.1),F.graminearum(XP_011327767.1),Botrytis cinerea(XP_024549613.1),Aspergillus oryzae(XP_001818207.1),Metarhizium anisopliae(KID65101.1). Homologous alignment (http://smart.embl-heidelberg.de) showed that the ZnF_C2H2 domain is similar to other fungi (Fig.1-A). Moreover,phylogenetic analysis showed that the Nsf1 protein ofF.graminearumis evolutionarily conserved (Fig.1-B).
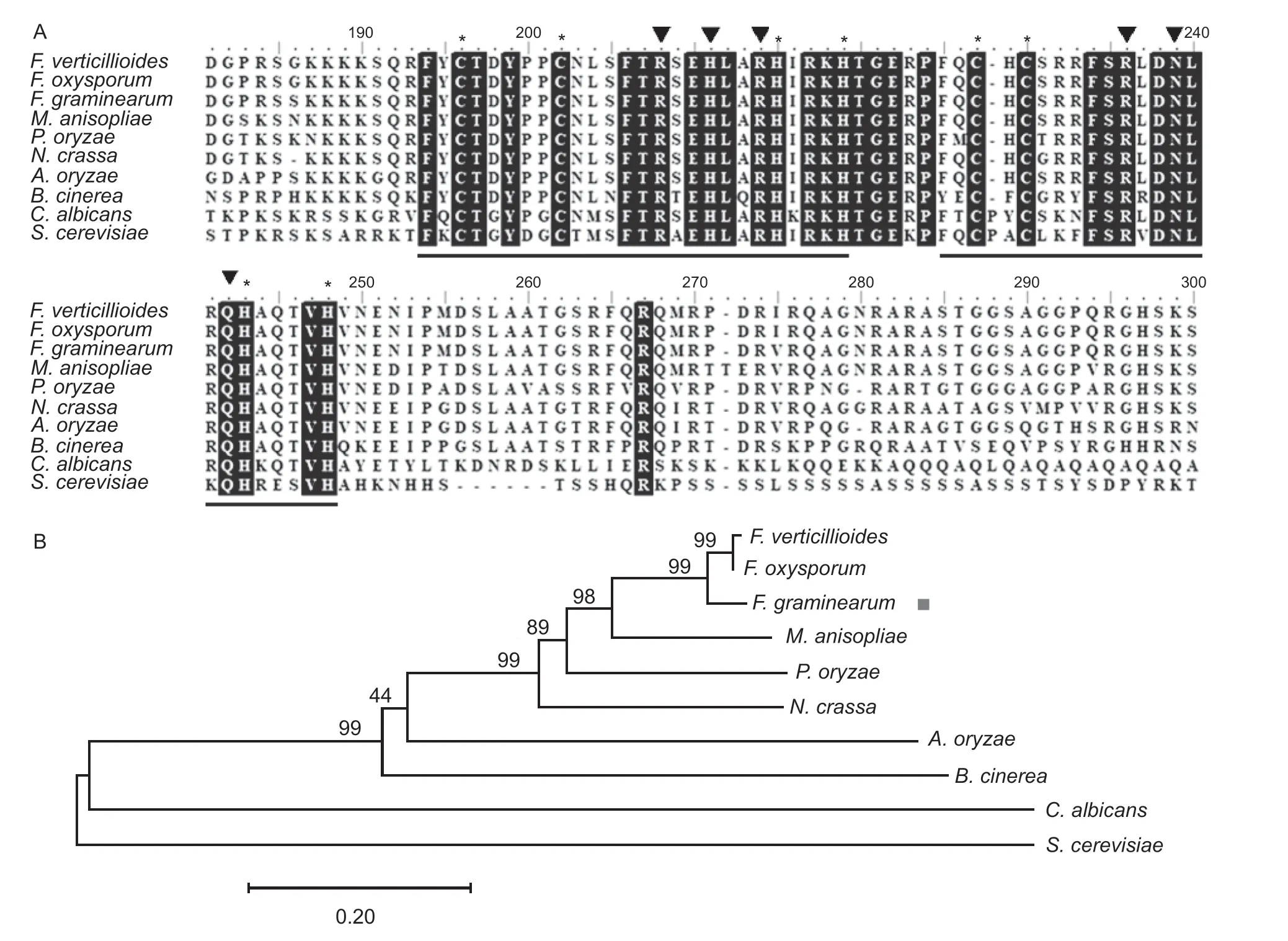
Fig.1 Sequence alignments and the phylogenetic analysis of FgNsf1 and its orthologs. A,sequence alignment of FgNsf1 and its homologs. The two black underlining parts show the classical Cys2-His2 (C2H2) zinc finger motif and amino acids that are identical in all sequences are shaded black. The conserved cysteine and histidine residues of the zinc fingers are marked by asterisks. The black triangles indicate amino acids predicted to make base-specific contacts during DNA binding. B,the phylogenetic tree was constructed with MEGA7 Software based on the amino acid sequence of FgNsf1 proteins from different organisms. Values on the branches of clusters represent the results of bootstrap analysis (10 000 bootstrap replicates) and the target gene of FgNsf1 is marked by a red square.Species names are Fusarium verticillioides,Fusarium oxysporum,Fusarium graminearum,Metarhizium anisopliae,Pyricularia oryzae,Neurospora crassa,Aspergillus oryzae,Botrytis cinerea,Candida albicans,and Saccharomyces cerevisiae.
3.2.FgNSF1 deletion and complementation in F.graminearum
In order to study the biological function of FgNsf1 inF.graminearum,FgNSF1-deleted mutants were constructed by a homologous recombination strategy (Appendix A-a).Three putativeFgNSF1knockout mutants were identified by PCR analysis with three pairs of primers (Appendix B) and further verified by Southern blot hybridization which indicated that the locus of FGSG_07928 in the knockout mutants ΔFgNsf1was replaced by a single-copy insertion of theHPHcassette (Appendix A-b). One of the mutants was randomly selected for further analysis. The resultant complemented strain ΔFgNsf1-Cwas also confirmed by PCR and Southern blot analysis.
3.3.FgNsf1 is involved in hyphal growth and pigmentation in F.graminearum
FgNSF1knockout mutant grew much more slowly than the parental strain PH-1 and its complemented strain ΔFgNsf1-Con PDA,CM,and MM media (Fig.2-A and C;Table 1),while microscopic examination of hyphae grown on cellophane showed no significant difference (Fig.2-B). Furthermore,much more red and yellow pigments were produced by the deletion mutant,especially on PDA medium (Fig.2-A).To further confirm this phenomenon,qPCR assays were performed to determine the expression levels of the four genes (AurJ,AurF,AurO,andAurR2) involved in the biosynthesis of rubrofusarin and aurofusarin (Frandsenet al.2006). The results showed that the four genes were significantly up-regulated in ΔFgNsf1(Fig.2-D) when compared with parental strain. All the results demonstrated that FgNsf1 affects the pigment biosynthesis pathway directly or indirectly.
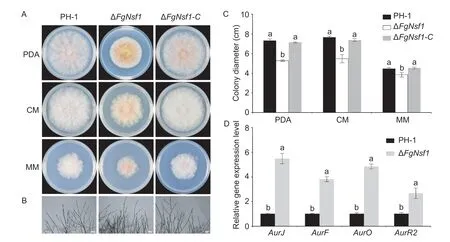
Fig.2 Roles of FgNsf1 in vegetative growth. A,colony morphology of wild-type strain PH-1,ΔFgNsf1 and ΔFgNsf1-C cultured on PDA,CM and MM media at 25°C for 3 days. B,hyphal morphology of each strain with mycelial plugs growing on top of cellophane membranes placed on solidified PDA for 24 h. Bar=100 μm. C,statistical analysis of the colony diameters of each strain. D,relative expression levels of AurJ,AurF,AurO and AurR2 in the wild-type strain PH-1 and the deletion mutant ΔFgNsf1. For each gene,its relative expression in PH-1 was arbitrarily set to 1. Means and standard deviations were calculated from three independent experiments. Values on the bars followed by the same letter are not significantly different at P<0.05.
3.4.FgNsf1 is important for both asexual and sexual reproduction
To determine the effects ofFgNSF1deletion on conidial morphology,conidiation and conidial germination,six mycelial plugs (5 mm in diameter) of each strain were inoculated in MBL medium under conidiation inducing light conditions. After 5 days of shaking at 25°C,microscopic examination of conidia harvested from MBL medium showed that theFgNSF1deletion mutant produced a few abnormal conidia with fragmentation (Fig.3-A and B) which was mainly caused by disarticulation of intercalary and terminal cells of conidia. Subsequent experiments exhibited that the conidiation and the conidial germination rate (8 h on water agar at 25°C) of ΔFgNsf1were remarkably declined (Fig.3-C and D;Table 1). After 12 h of incubation,we found that both the normal and malformed conidia could germinate successfully on the water agar plates,though the germinal tube (12 h) of ΔFgNsf1is much shorter (Appendix C).
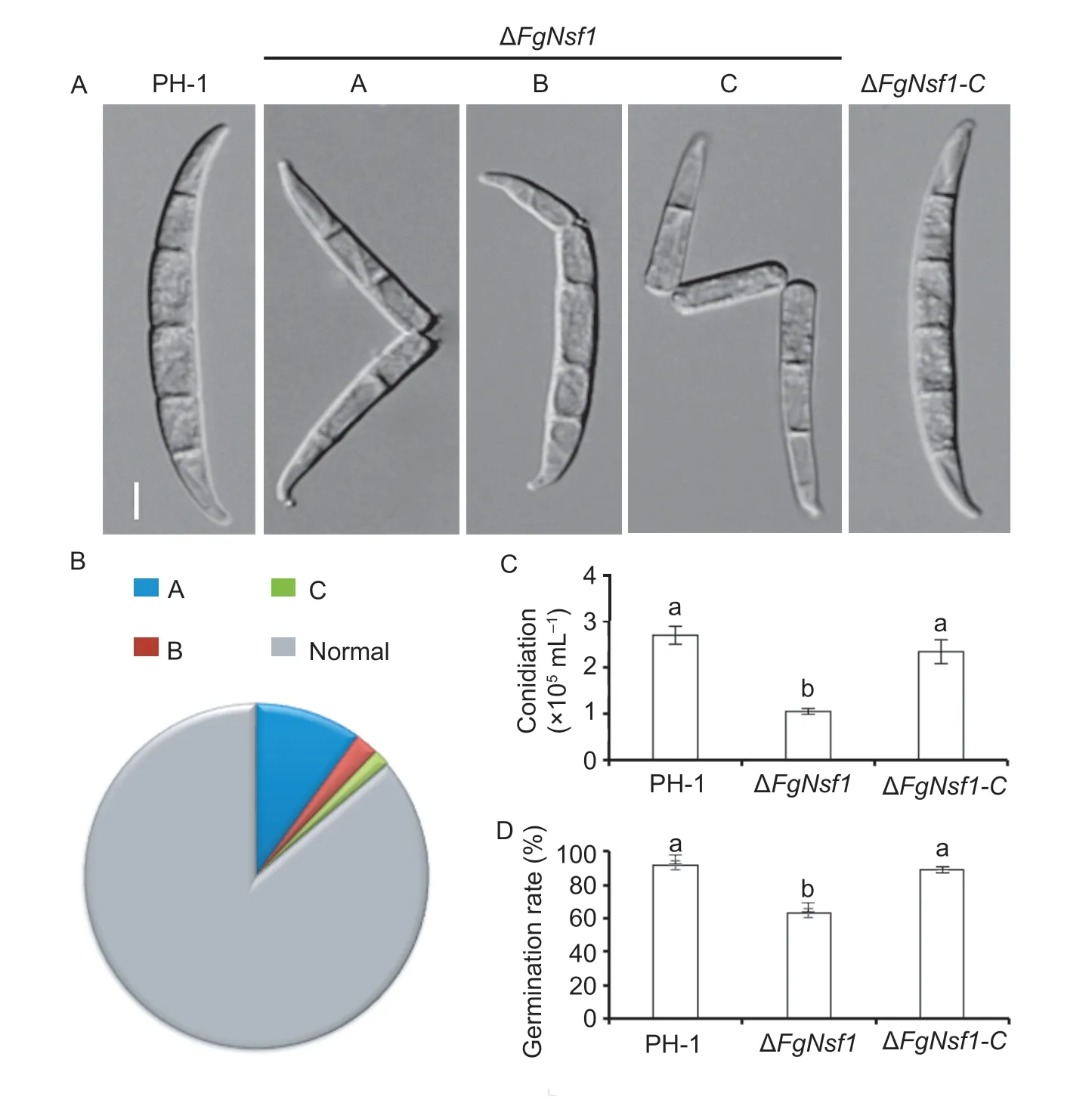
Fig.3 Involvement of FgNsf1 in asexual reproduction in Fusarium graminearum. A,morphology of conidia produced by wild-type strain PH-1,ΔFgNsf1 and ΔFgNsf1-C. Fragmented conidia (A,B,C) were observed in ΔFgNsf1 deletion mutants. Bar=10 μm.B,ratios of different kinds of malformed conidia A (99.8‰),B (22.5‰),C (15.6‰) and normal conidia (862.1‰) in ΔFgNsf1. C,conidiation of PH-1,ΔFgNsf1 and ΔFgNsf1-C. D,germination rate of the conidia produced by PH-1,ΔFgNsf1 and ΔFgNsf1-C on water agar plates at 25°C for 8 h. Means and standard deviations were calculated from three independent experiments. Values on the bars followed by the same letter are not significantly different at P<0.05.
Table 1 Phenotypes of the FgNsf1 deletion mutant (ΔFgNsf1),the parental strain (PH-1) and the complemented strain (ΔFgNsf1-C) in terms of growth,conidiation,deoxynivalenol (DON) production and virulence1)
Sexual reproduction was analyzed on carrot agar plates. After 2 weeks of self-fertilization,the mating plates of PH-1 and ΔFgNsf1-Cproduced abundant fertile perithecia with yellowish cirrhi at the ostiole,while the number of perithecia produced by the ΔFgNsf1mutant was approximately 70% less than PH-1 (Fig.4-A). All the perithecia produced normal ascospores (Fig.4-B),though perithecia on mating plates of ΔFgNsf1mutant exhibited delayed perithecia maturation. These results indicated that FgNsf1 plays critical roles in conidiation,conidial germination and sexual reproduction inF.graminearum.
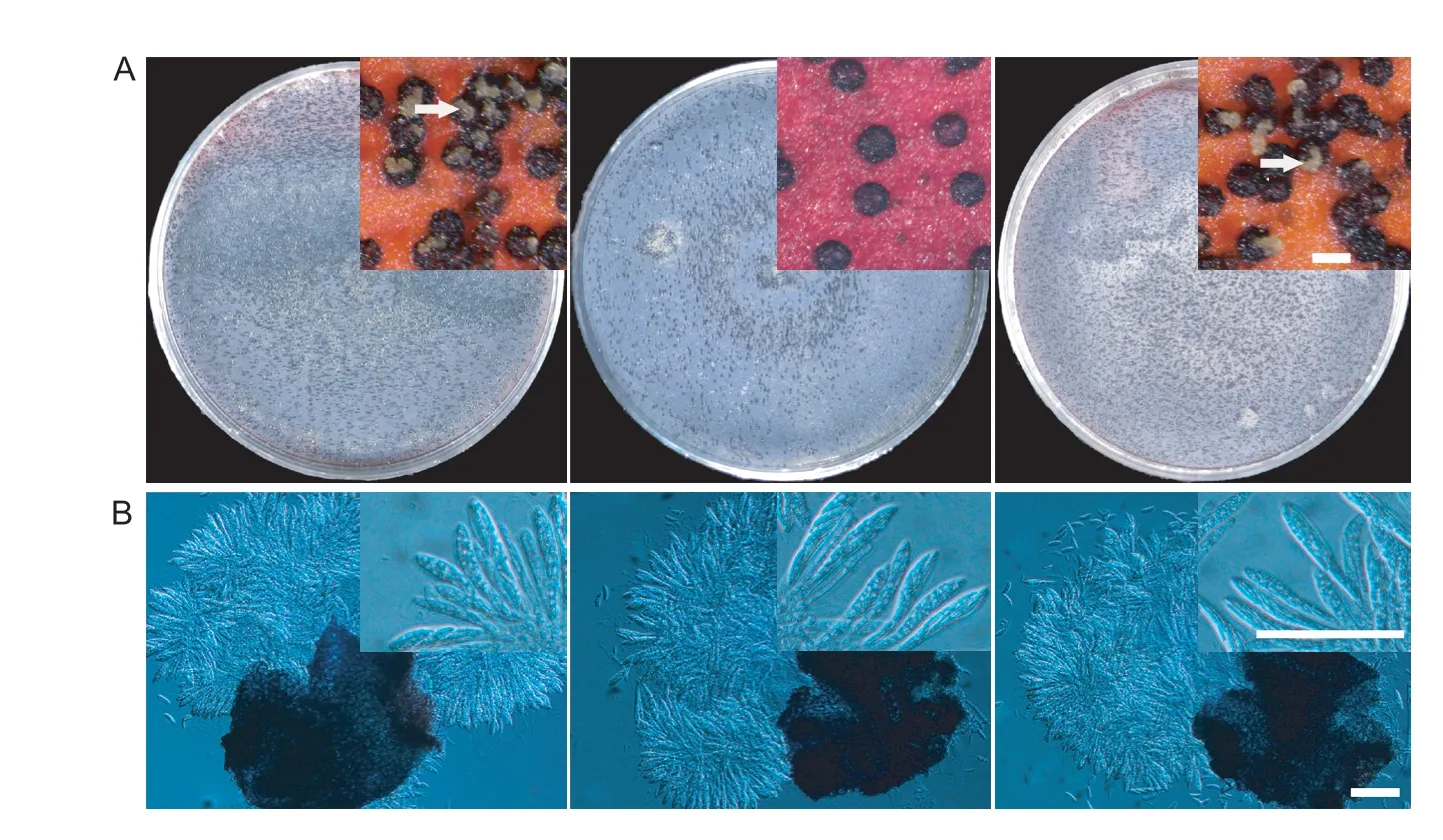
Fig.4 Defects of ΔFgNsf1 mutant in sexual reproduction. A,perithecia produced by wild-type strain PH-1,ΔFgNsf1 and ΔFgNsf1-C were visualized as black structures on carrot agar plates after 2 weeks of inoculation. Cirri (masses of ascospores) formed on the top of perithecia are marked with white arrows. Bar=200 μm. B,fascicles of asci from mature ascetic were observed after squeezing. Scale bar=50 μm.
3.5.Subcellular localization of FgNsf1-GFP in F.graminearum
To obtain some clues on the biological functions from the subcellular localization of FgNsf1,FgNsf1-GFP fluorescence and DAPI (10 μg mL-1) staining were observed using a confocal laser scanning microscope. Similar to the localization pattern of Nsf1 inS.cerevisiae(Hlynialuket al.2008;Bessonovet al.2013),FgNsf1-GFP fusion protein was also present in the nucleus (Fig.5).
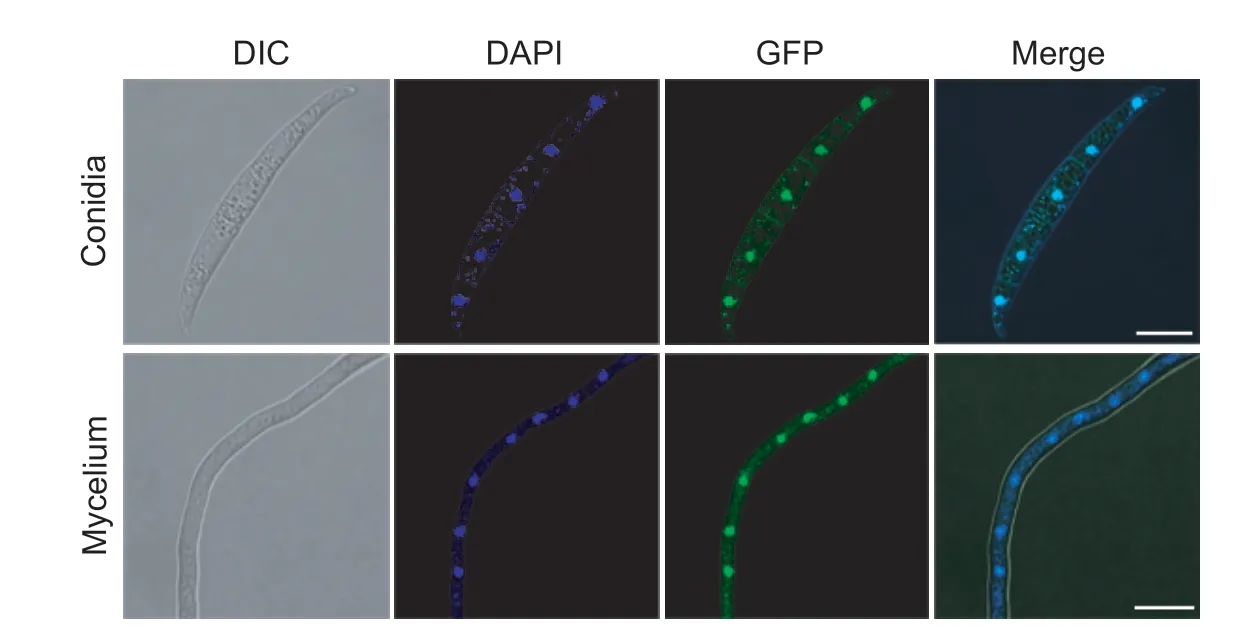
Fig.5 Subcellular location pattern of FgNsf1-GFP fusion protein in conidia and mycelium. Conidia and vegetative hyphae of the FgNsf1-GFP were collected and visualized with a confocal laser scanning microscope (Leica TCS SP8,Germany). In order to observe nuclei,the conidia and hyphae were stained with 10 μg mL-1 DAPI. Bar=10 μm.
3.6.FgNsf1 is involved in osmotic adaptation,metabolism of non-fermentable carbon sources and sulfur in F.graminearum
Nsf1 protein has been reported to be involved in the metabolism of non-fermentable carbon sources,salt stress response inS.cerevisiae(Hlynialuket al.2008;Bessonovet al.2013). To determine whether FgNsf1 has the same functions,the wild-type strain PH-1 was inoculated in YEPD without or with 0.6,0.8,1.0,and 1.2 mol L-1NaCl,YEPethanol (w/v,1%) (YEPE),YEP-glycerol (w/v,1%) (YEPG) and YEP-acetate (w/v,1%) (YEPA). The qRT-PCR results showed that the expression levels ofFgNSF1initially increased and then decreased with the increasing salt concentrations (Fig.6-A). Under non-fermentable carbon sources,the expression levels ofFgNSF1were significantly down-regulated (Fig.6-B) while it is up-regulated inS.cerevisiae(Hlynialuket al.2008).
NSF1was also confirmed to work as a negative regulator in sulfur metabolism in wine yeast (Bessonovet al.2013). To test the effect of FgNsf1 on sulfur metabolism,ΔFgNsf1and the other tested strains were incubated on PDA amended with 10 mmol L-1cysteine or 10 mmol L-1methionine. The result showed that colony growth of ΔFgNsf1was slightly promoted by cysteine or methionine while PH-1 and ΔFgNsf1-Cwere slightly inhibited (Fig.6-C and D),which indicated that FgNsf1 negatively regulates the metabolism of sulfur. All the results implied that the responses ofF.graminearumto salt stress,non-fermentable carbon sources and sulfur are at least partially dependent on FgNsf1.
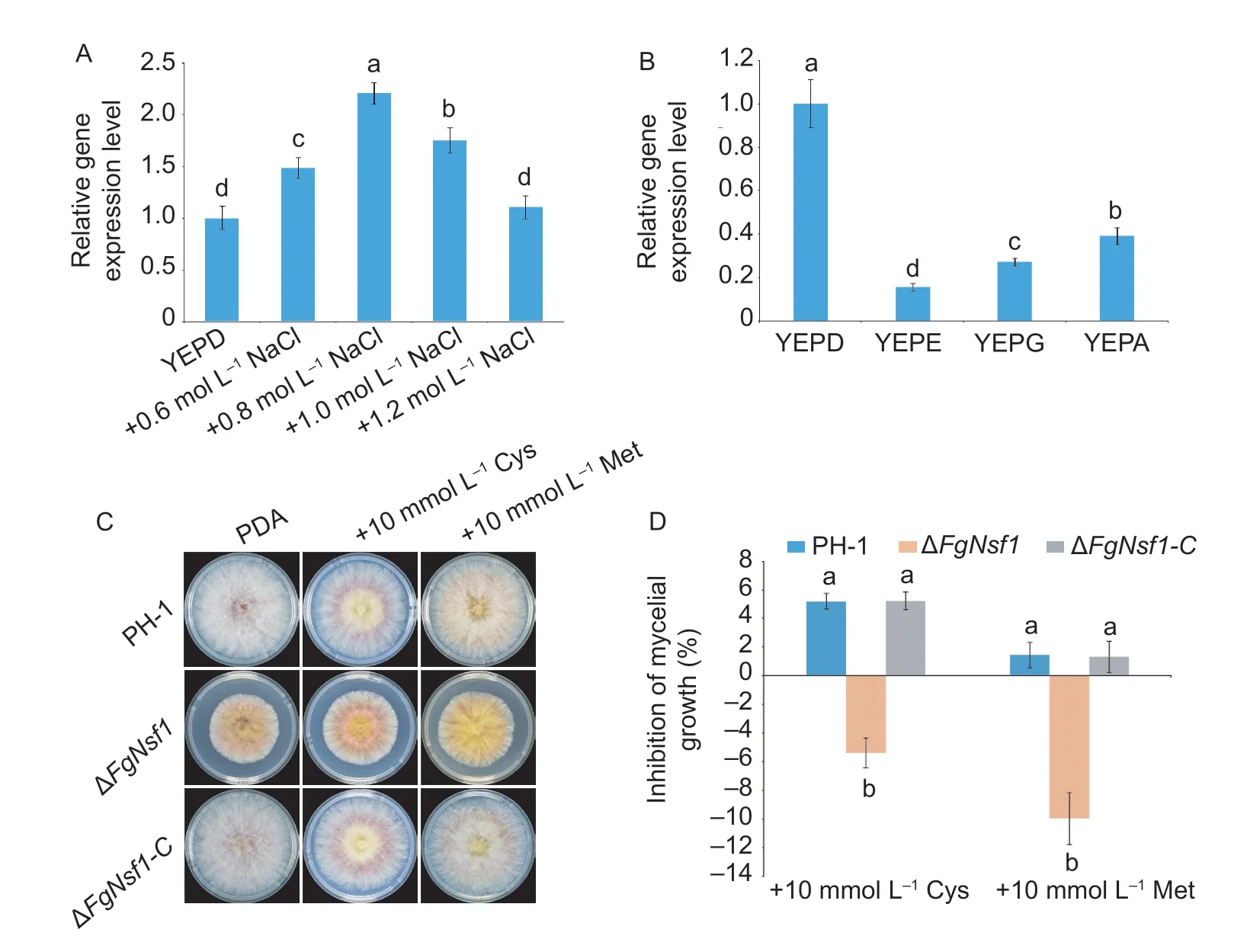
Fig.6 Effects of salt stress and non-fermentable carbon sources on transcription of FgNSF1. A,the wild-type strain PH-1 was cultured in liquid YEPD for 48 h,and then treated without or with NaCl (with the terminal concentration 0.6,0.8,1.0 and 1.2 mol L-1) for 4 h,mycelia were harvested for RNA extraction. B,PH-1 was inoculated in YEPD,YEP-ethanol (YEPE),YEP-glycerol (YEPG) and YEP-acetate (YEPA) for 48 h,mycelia were harvested for RNA extraction. C,effect of FgNsf1 on the sulfur metabolism. PH-1,ΔFgNsf1 and ΔFgNsf1-C were cultured on PDA amended with sulfur containing amino acids 10 mmol L-1 cysteine (Cys) or 10 mmol L-1 methionine (Met). D,inhibition of mycelial growth. Means and standard deviations were calculated from three independent experiments. Values on the bars followed by the same letter are not significantly different at P<0.05.
3.7.Sensitivity of the ΔFgNsf1 mutant to osmotic and cell wall-damaging agents
Nsf1 protein has been reported to be involved in salt stress response and cell wall biosynthesis inS.cerevisiae(Hlynialuket al.2008;Bessonovet al.2013),we were interested in exploring the functions of FgNsf1inF.graminearum.To investigate the sensitivity of ΔFgNsf1to osmotic stress and cell wall-damaging agents,ΔFgNsf1and the other tested strains were incubated on PDA containing 1.2 mol L-1NaCl or 1.2 mol L-1KCl,0.05% (w/v) Congo red. As shown in Fig.7-A and B,ΔFgNsf1exhibited increased resistance to osmotic and cell walldamaging agents. To further confirm this phenomenon,the expression level of genes involved in osmotic stress and cell wall integrity in MAPK signal pathway (Houet al.2002;Zhenget al.2012) were assayed,and the expression levels were slightly up-regulated (Fig.7-C,D and E). All the results indicated that FgNsf1 is involved in the regulation of osmotic stress and cell wall-damaging agents.
3.8.Sensitivity of the ΔFgNsf1 mutant to oxidative stress and metal cations stress
Since FgNsf1 plays an important role in the regulation of osmotic and cell wall damage inF.graminearum,the sensitivity of the ΔFgNsf1mutant to oxidative and metal cations stresses were also investigated. In this study,PH-1,ΔFgNsf1and ΔFgNsf1-Cwere cultured on PDA plates amended with 16 mmol L-1hydrogen peroxide (H2O2) or 0.1 mmol L-1menadione (MD),5 mmol L-1ZnCl2(Zn2+) or 0.2 mol L-1MgCl2(Mg2+). The results showed that ΔFgNsf1was much more tolerant than PH-1and ΔFgNsf1-Cto H2O2,menadione and Zn2+,but exhibited increased sensitivity to Mg2+(Fig.7-A and B). These results suggest that FgNsf1 participates in the adaption ofF.graminearumto oxidation and metal stresses.
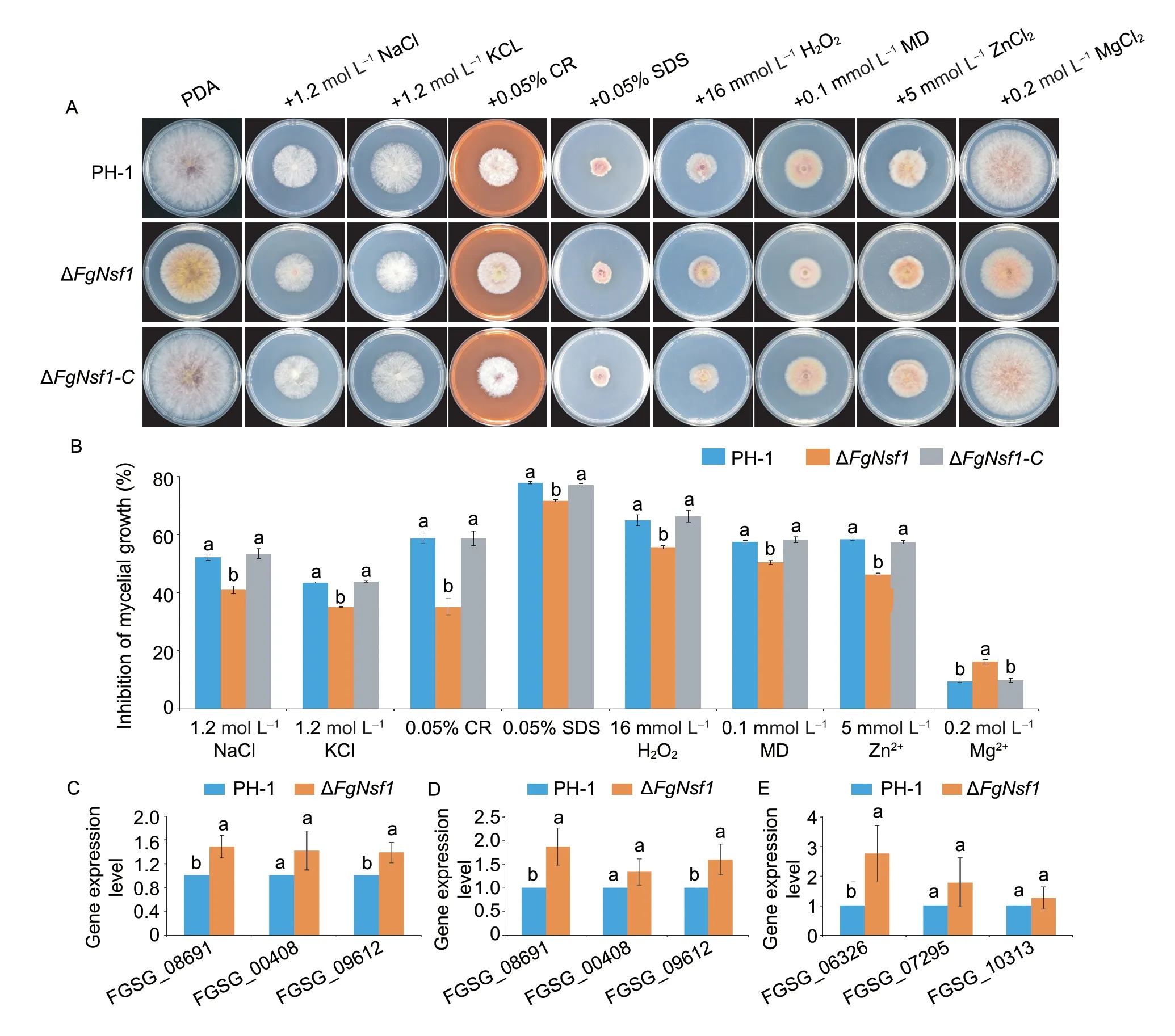
Fig.7 Effects of FgNsf1 on the sensitivity to osmotic stress,cell wall-damaging agents,oxidative stress and metal cations. A,PH-1,ΔFgNsf1 and ΔFgNsf1-C were cultured on PDA medium amended without or with 1.2 mol L-1 NaCl,1.2 mol L-1 KCl,0.05% (w/v) Congo red (CR),16 mmol L-1 H2O2,0.1 mmol L-1 menadione (MD),5 mmol L-1 ZnCl2 (Zn2+),and 0.2 mol L-1 MgCl2 (Mg2+). B,mycelial growth inhibition rates of PH-1,ΔFgNsf1 and ΔFgNsf1-C after incubation at 25°C for 3 days. C,D and E,the expression levels of genes involved in osmotic stress and cell wall integrity in MAPK signal pathway. C and D were treated by NaCl and KCl (terminal concentration is 1.2 mol L-1),respectively;E was treated by CR (w/v,0.05%). FGSG_08691,FGSG_00408,FGSG_09612 were related to osmotic stress,FGSG_06326,FGSG_07295,FGSG_10313 were related to cell wall integrity. Means and standard deviations were calculated from three independent experiments. Values on the bars followed by the same letter are not significantly different at P<0.05.
3.9.Sensitivity of ΔFgNsf1 to different fungicides
The phenylpyrrole fungicide fludioxonil and the dicarboximide fungicide iprodione have been reported to be associated with osmotic pressure in some pathogenic fungi (Kojimaet al.2004),we tested the sensitivity of ΔFgNsf1to the two fungicides and the result showed that ΔFgNsf1exhibited severely increased resistance to fludioxonil and iprodione than wild-type strain PH-1 and the complemented strain ΔFgNsf1-C(Table 2).We were also interested in the susceptibility of ΔFgNsf1to the demethylation inhibitors tebuconazole or benzimidazole carbendazim (MBC),which are the main chemical agents for the management of FHB,the values of EC50showed that ΔFgNsf1exhibited increased sensitivity to MBC and tebuconazole (Table 2). All the results showed that FgNsf1plays an important role in the regulation of the sensitivity to different fungicides.
3.10.FgNsf1 is required for full virulence in F.graminearum
To determine whether the destruction ofFgNSF1impacts the virulence ofF.graminearum,we assessed its pathogenicity to wheat coleoptile and flowering wheat heads with conidial suspensions (Liet al.2016). The stems of the coleoptiles inoculated with PH-1 and ΔFgNsf1-Cturned dark-brown evidently by 7 days post inoculation (dpi),in contrast,the lesion size of the deletion mutant ΔFgNsf1was relatively small (Fig.8-A and B;Table 1). Consistently,the typical symptoms of wheat heads of the parental strain and complemented strain were much more severe than that of deletion mutant at 14 dpi (Fig.8-C),and the percentage of spikelet with symptoms was dramatically lower with ΔFgNsf1than those of the other tested strains (Fig.8-D;Table 1). All these results indicated that the ΔFgNsf1mutant remains a certain ability of infection,but not as aggressive as parental strain or complemented strain.
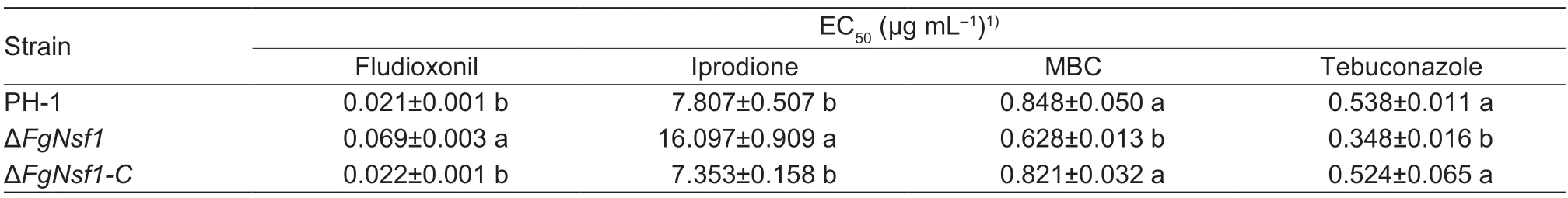
Table 2 Sensitivity of the wild-type strain PH-1,the FgNSF1 deletion mutant ΔFgNsf1 and the complemented strain ΔFgNsf1-C to different fungicides
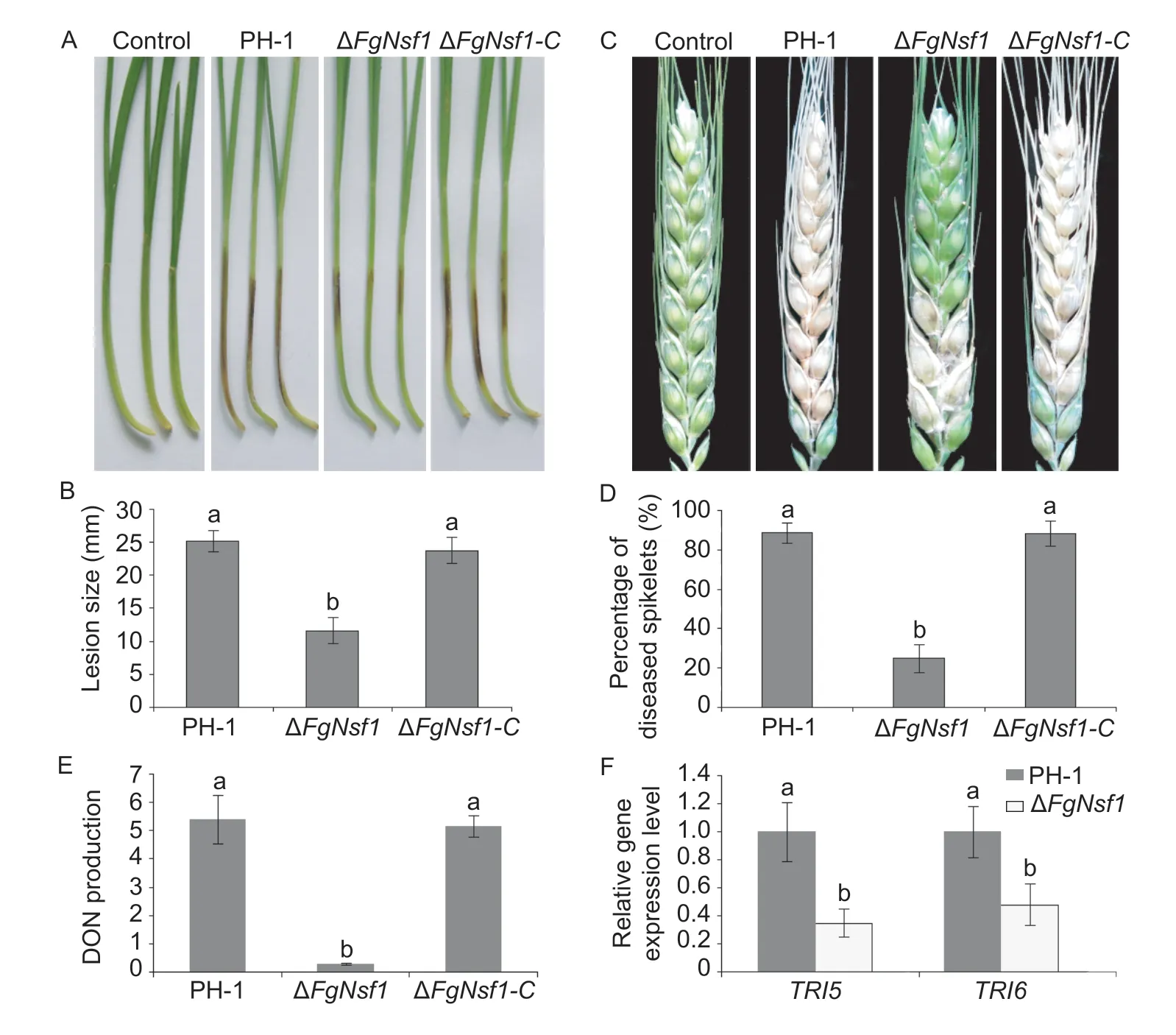
Fig.8 Pathogenicity,deoxynivalenol (DON) production and trichodiene (TRI) synthase genes expression assays in the FgNSF1 deletion mutant. A,wheat coleoptiles were inoculated with equal number of fresh conidia of wild-type strain PH-1,ΔFgNsf1 and ΔFgNsf1-C,brown lesions on the coleoptiles were measured at 7 days post inoculation (dpi). Coleoptiles inoculated with distilled water were taken as the control. B,lesion size of the infested coleoptiles. C,flowering wheat heads were point-inoculated with equal volume of conidial suspension of the tested strains,and maintained in a humid glasshouse. Infected wheat heads were photographed 14 dpi. Distilled water was used as negative control. D,percentage of diseased spikelet on the infected wheat heads. E,DON production (μg g-1 dry mycelia) of PH-1,ΔFgNsf1 and ΔFgNsf1-C were assessed after 7 days in TBI medium containing 5 mmol L-1 arginine. F,the relative expression levels of TRI5 and TRI6 were assessed after 3 days in TBI medium. Means and standard deviations were calculated from three independent experiments. Values on the bars followed by the same letter are not significantly different at P<0.05.
3.11.FgNsf1 affects DON biosynthesis and the expression of TRI5 and TRI6 in F.graminearum
The trichothecene mycotoxins deoxynivalenol (DON) produced byF.graminearumserves as a crucial virulence factor inF.graminearum(Proctoret al.1995;Seonget al.2010). Plant infection assays showed that the deletion ofFgNSF1reduced the virulence ofF.graminearum,so we determined the ability of ΔFgNsf1to produce DON. Compared with the parental strain PH-1,the capacity of the deletion mutant ΔFgNsf1to produce DON was greatly impaired (Fig.8-E;Appendix D). To further confirm this result,the expression levels ofTRI5andTRI6which are crucial trichothecene biosynthesis genes were confirmed by quantitative real-time PCR. The expression levels ofTRI5andTRI6in ΔFgNsf1were substantially decreased in comparison with the wild-type strain PH-1 (Fig.8-F;Appendix D). These results indicated that FgNsf1 plays an important role in the regulation ofTRIgenes expression and the biosynthesis of DON inF.graminearum.
4.Discussion
4.1.Effect of FgNsf1 on development and pathogenesis
Different from rice blast fungus,whose sexual reproduction cannot be achieved under natural field conditions and the rice blast disease is caused by asexual conidia (Talbot 2003;Saunderset al.2010a,b),F.graminearumreproduces asexually and sexually in the field and the disastrous fusarium head blight (FHB) is caused by both asexual and sexual spores (Trailet al.2002;Trail 2009). In particularly,the severity of FHB is often positively correlated with the number of conidia or ascospores (Tenget al.1991),both of them are essential parts of the life cycle inF.graminearum.Due to the knocked out ofFgNSF1,the defective conidial morphology indicates a cell wall defect,which is partly supported by the differential response to Congo red. The yield of conidia or perithecia were affected seriously,especially the pathogenicity of conidia on wheat coleoptiles and flowering heads was significantly decreased (Fig.8-C and D),which may result from malformation of some conidia and retardation of hyphal growth. Consistent with these results,the DON production of ΔFgNsf1was significantly decreasedin vitro. Moreover,the expression levels ofTRI5andTRI6which are the key genes of trichothecene biosynthesis were remarkably decreased in ΔFgNsf1.According to Kazanet al.(2012),most of the gene deletion mutants have reduced toxin synthesis and pathogenicity throughout previous studies,knockout ofFgNSF1may reduce DON biosynthesis and virulence by indirectly affecting secondary metabolism.
4.2.Effect of FgNsf1 on non-fermentable carbon metabolism
FgNsf1 is involved in the regulation of non-fermentable carbon metabolism inF.graminearum. InS.cerevisiae,the metabolism of non-fermentable carbon resources was clearly proved to be partially dependent on intactNSF1,and the transcript ofNSF1was more abundant in nonfermentable carbon sources than that in glucose (Hlynialuket al.2008). However,after taking different non-fermentable carbon sources such as glycerol,ethanol or acetate as sole carbon source,the expression levels ofFgNSF1in PH-1 were significantly down-regulated (Fig.6-B),which was contrary to the situation inS.cerevisiae(Hlynialuket al.2008). This difference may be caused by unique compensation mechanism of metabolism inF.graminearum.
4.3.Effects of FgNsf1 on various stress conditions
FgNsf1also takes an important role in response to various stress conditions. Once inoculated in YEPD and treated with different concentrations of NaCl,the expression levels ofFgNSF1in WT strain were dramatically up-regulated (Fig.6-A). However,different from the directly proportional relationship inS.cerevisiae(Hlynialuket al.2008),the expression levels ofFgNSF1in PH-1 initially increased and then decreased with the increasing salt concentrations (Fig.6-A),this may result from the poor capacity ofF.graminearumin coping with hypertonic pressure. After knocked out ofFgNSF1,the deletion mutant ΔFgNsf1exhibited increased tolerance to osmotic pressures,cell wall-damaging agents,oxidative stress and metal cation Zn2+,but displayed increased sensitivity to Mg2+(Fig.7-A and B). ΔFgNsf1was also slightly promoted by sulfur containing medium (Fig.6-C and D),this may certificate that FgNsf1 act as a negative regulator in sulfur metabolism inF.graminearum.
4.4.Effects of FgNsf1 on different fungicides
We have also found that the lack ofNSF1affects the susceptibility ofF.graminearumto several kinds of fungicides (Table 2),with increased sensitivity to MBC and tebuconazole,and enhanced tolerance to fludioxonil and iprodione than PH-1 and ΔFgNsf1-C. MBC fungicides have been reported to be involved in polymerization of monomeric inF.graminearum(Zhouet al.2016),our research showed that FgNsf1 may play an important role in mitosis. In addition,fludioxonil has been reported to be related to osmotic pressure inBotrytis cinerea,the glycerol contents in mycelia and expression levels ofBcHOG1were significantly increased after treated with fludioxonil (Renet al.2016). Our previous results showed thatFgNSF1deletion mutant was more tolerant to osmotic pressure than wild-type strain PH-1 (Fig.7-A and B),in accordance with this,ΔFgNsf1exhibited increased tolerance to fludioxonil. The deletion ofFgNSF1may cause the increase of glycerol in the mutant ΔFgNsf1,therefore,increasing the tolerance to osmotic pressure and fludioxonil. Although the specific regulation mechanism of FgNsf1 to different fungicides is still hard to be uncover,our work sheds some light on its importance which will be helpful for us to find new potential targets for developing novel fungicides to control FHB in cereal crops.
5.Conclusion
In this study,we elucidated the roles of FgNsf1 by constructing a deletion mutant and its functional complementation mutant. Collectively,our results reveal that the transcription factor FgNsf1 can regulate the vegetative growth,asexual and sexual reproduction,fungicide sensitivity,pathogenicity and DON production ofF.graminearum. Since FgNsf1 is a C2H2zinc finger transcription factor,the next work will mainly focus on identifying the target genes regulated byFgNSF1.
Acknowledgements
This work was supported by the National Key Research &Development Program of China (2016YED0201007,2018YFD0201201 and 2018YFD0201000),the National Natural Science Foundation of China (31672065),the Agricultural Science and Technology Projects of Jiangsu Province,China (BE2018378,BA2018039,PZCZ201715,CX(19)3003,and CX(18)2005),and the Postgraduate Research &Practice Innovation Program of Jiangsu Province,China (KYCX18_0670).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- Comparison of grain yield and quality of different types of japonica rice cultivars in the northern Jiangsu plain,China
- Natural nematicidal active compounds:Recent research progress and outlook
- lmproving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele
- Comparative transcriptome analysis of different nitrogen responses in low-nitrogen sensitive and tolerant maize genotypes
