In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
2021-06-14KOMBOOmarRamadhanWANGXinyuSHENYingLIUJiaweiDONGXianzhenSHAOQiLONGYanpiaoDONGKuoBAKHETShahdFatehElRahmanElkhiderLIBinbin
KOMBO Omar Ramadhan, WANG Xinyu*, SHEN Ying, LIU Jiawei,DONG Xianzhen, SHAO Qi, LONG Yanpiao, DONG Kuo,BAKHET Shahd Fateh El-Rahman Elkhider, LI Binbin*
(1. State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070,China; 2. Biomedical Materials and Engineering Research Center of Hubei Province, Wuhan 430070, China)
Abstract: The 60Fc and 70Fc SF/SA blend scaffolds were prepared to mimic the functions of the native ECM for skin regeneration. Human Umbilical Vein Endothelial Cells (HUVECs) were used to examine the cell cytotoxicity, adhesion, growth factors secretion and the gene expression of associated angiogenic factors. Cell proliferation, adhesion and live-dead analyses showed that HUVECs could better attach, grow, and proliferate on the 70Fc scaffolds compared with 60Fc scaffolds and unmodified controls. Furthermore, the 70Fc scaffolds showed higher levels of specific angiogenic proteins and genes expression as well. This study suggests that the involvement of higher composition of SF (about 70%) than that of SA on the blended scaffolds could be advantageous as it is more suitable to promote angiogenesis, which is potential for vascularization during skin repair.
Key words: vascularization; scaffold; HUVECs; tissue engineering
1 Introduction
The designing of skin-repairing composite scaffolds is critical and has become a very concern aspect in tissue engineering and regenerative medicine. The need for wounds repairing materials has been of significant demand in recent years[1]. A large number of wound dressings are currently used for the treatment of wounds and skin tissues replacement. To achieve proper wound healing and skin regeneration, the scaffolds should provide an ideal environment for cells attachment, migration, proliferation, and differentiation,be highly porous with an interconnected pore network available for cell growth, nutrients and metabolic waste transport, and be biocompatible with skin tissues[2,3].Therefore,in vitroevaluation of the designed scaffolds takes an essential place for the development of the biomaterials on the tissue implants.
The composition and structure of extracellular matrix (ECM) is fundamental in angiogenesis modulating during wound healing and functional reconstruction[2-6]. The ECMs can support cell adhesion, growth,migration and provide an adequate mechanical environment for the cells[7,8]. Special attention has been taken from hydrogels derived from natural polymers due to their excellent compatibility with the natural ECMs[9].Several studies have reported remarkable properties on blended scaffolds composed of SF and SA for the application in tissue engineering[10-12].
Silk fibroin (SF), which is a naturally occurring protein-polymer can support cells attachment, proliferation and differentiation[13]. As a biomaterial, SF has been widely investigated due to its excellent biocompatibility, adjustable biodegradation and immunogenicity[14,15]. Regenerated SF has been verified to support cell adhesion, migration and growth of vascular endothelial cells and smooth muscle cellsin vitro[16]. However, SF films cast from aqueous solution are soluble in water due to the high amount of random coil structures it contains, known as silk I[17]. Therefore, some techniques, such as the introduction of organic solvents[18]or high temperatures[19]can be used to persuade conformational transitions from silk I toβ-sheets (silk II).Though, these techniques turn SF films very brittle in a dry state. Consequently, to enhance its mechanical and physical properties, SF can be blended with other polymers such as Sodium Alginate (SA) which has excellent biocompatibility, biodegradability and high hydrophilicity[10]. SA contains large amounts of free carboxyl and hydroxyl groups, making it easy to form composite materials by combining it with other polymers[20]. Because of its excellent healing properties, including epithelization, vascularization, epidermis characteristics,and hair follicle formation, SA has been widely used as a scaffold in soft tissue engineering[21]. Therefore, the SF/SA blend scaffolds can improve the structure and properties of pure SF, thus provide a proper degradation rate to match the rate of new tissue formation and deliver an extensive network of interconnecting pores and high surface area that would allow cell proliferation,migration, and adhesion, hence mimic the functions of the native ECMs[22,23].
The skin is highly composed of a vascular network. During wound healing, the formation of a functional blood vessel network through angiogenesis is an essential process[1]. Angiogenesis allows the reformation of the normal blood flow, supports cells on the wound with nutrients and oxygen, and controls blood pressure, which is necessary for cell proliferation and viability[24]. Endothelial cells form the inner layer of blood and lymphatic vessel, and they are responsible for controlling the cells passage and other substances to the neighbor tissues. The wound healing process requires an active endothelial migration, proliferation and differentiation to form the new blood vessels from pre-existing ones. HUVECs are one among the most popular models used for endothelial cellsin vitrostudies of the vasculature and angiogenesis[25-27]. HUVECs are famous cell model for studying endothelial cell biology as they are readily available, quite easy to culture, low immunogenicity, highly proliferative, and able to migrate and invade new tissues[28].
To improve the performance of the composite biomaterials, determination of suitable blend composition of scaffolds is crucial[29]. Several studies have explored the characterization of SF/SA blend scaffolds of different compositions to determine the blend scaffolds composition[12,17]. However, further studies for the role of vessel-forming endothelial cells which play a vital role in skin vascularization and angiogenesis are needed.
In our previous study[12], SF/SA scaffolds blended in the ratios 75/25 and 50/50 w/w (named as 75Fc and 50Fc respectively) showed the increase of stability,swelling capacity and porosity, and the decrease in cytotoxicity, cell adhesion and proliferation on the L929 cell model when the SA content increased on blended scaffolds 75Fc towards 50Fc. Based on the study[12], the SF/SA blended scaffolds in the ratios 70/30 and 60/40 were developed as they were suggested to give better results not only in the performance as mentioned above but also in vascularization as they represent in-between composition ratios of scaffolds 75Fc and 50Fc. To explore the vascularization effect on our SF/SA scaffolds,in vitroangiogenic behavior of HUVECs was investigated on these scaffolds[25]. This study aimed to explore the angiogenic behavior of HUVECs on the selected biomimetic composite SF/SA scaffolds based on our previous published study[12]. To study the vascularization effect on SF/SA blended scaffolds, the SF/SA scaffolds fabricated from aqueous solutions of 2 wt% SF and 2 wt%SA at weight ratios of 70/30 and 60/40 were prepared,and the cell cytotoxicity tests, adhesion, growth factors secretion and the gene expression of associated angiogenic factors were investigated on HUVECs model.
2 Experimental
2.1 Materials
Bombyx mori raw silk fibres were purchased from Soho Biotechnology Co. Ltd. (China). SA was obtained from Shanghai Sinopharm Chemical Reagent Co. Ltd.(China). HUVECs were purchased from Chuan Qiu Biotechnology, China (Product code: CQ80252). ECM medium, fetal bovine serum (FBS), Endothelial Cell Growth Supplement (ECGS) and penicillin/ streptomycin were purchased from ScienceCell Research Laboratories (USA). PBS (pH 7.4) was purchased from GE Healthcare Life Sciences (China). Trypsin-EDTA was purchased from Life Technologies Corporation (USA).CCK-8 was purchased from Beijing Labgic Technology Co., Ltd (China). 4′,6-diamidino-2-phenylindole(DAPI) was purchased from Beyotime Institute of Biotechnology (China). Cell live dead kit was purchased from Yeasen Biotech Co., Ltd. (China). Human VEGF ELISA and Human bFGF ELISA kit were purchased from Hangzhou Lianke Biotechnology Co., Ltd. (China). Ethanol absolute, Acetonitrile, Isoamyl acetate,25% glutaraldehyde and other chemicals were purchased from Shanghai Wokai Biotechnology Co., Ltd(China).
2.2 Cell culture
HUVECs were cultured in T25 flasks with complete Endothelial Cell Medium (ECM) supplemented with 5% FBS, 1% Endothelial Cell Growth Supplement(ECGS) and 1% penicillin/streptomycin, and incubated in a 5% CO2humidified incubator at 37 ℃. The culture medium was changed every 2-3 days until the cells reached about 80%-90% confluence. Then the cells were washed by PBS, and 1 mL of trypsin (ETDA)was added before incubated at 37 ℃ for 3 min. Immediately, 4 mL of ECM medium was added and then centrifuged at 1 000 rpm for 5 min. The supernatant was discarded, and the appropriate volume of ECM medium was added in the suspended cells before counted for the scaffold’s evaluation experiments.
2.3 Preparation of scaffolds
The scaffolds were prepared as described by our previous study[12]. Briefly, the SF and SA aqueous solutions, both with concentrations of 2 wt% were mixed in different volumes in a glass beaker. The final weight ratios of SF/SA in mixed solution were 70/30 and 60/40. The EDC, NHS and MES were crosslinked into the solution with a weight ratio of 20%, 10% and 20%against the total weight of SF and SA in the solution,respectively. The mixed solution was stirred gently for 30 minutes at 4 ℃, then poured into a stainless-steel dish for freezing at -40 ℃ for 8 h.
Last week I had a bad day at the office. I know you ve been feeling down lately at work, so I thought I would share my dilemma4 with you to make you realize it s not so bad after all .
2.4 Scanning electron microscopy
The scaffolds 60Fc and 70Fc were first frozen in liquid nitrogen for about 2 min and then were cut laterally with a blade razor. The cross-sectional structures of the scaffolds were then coated with Au, and their morphology was observed under a scanning electron microscope (SEM).
2.5 Cell viability test
The scaffolds were placed in 48-well plates, and the HUVECs at the density of 5×104cells/well were seeded onto the scaffolds and cell culture dishes as control group and incubated at 37 ℃. On days 1, 3, 5 and 7, the cell viability was assessed by CCK8 kit, and the optical density values (ODV) were measured on Thermo LabSystems microplate reader (MK3, USA) at wavelength 450 nm. On 3-days culture, the cells were stained with AM and PI fluorescent dye by following the manufacturer’s protocol and examined by a fluorescence microscope (Olympus, IX71, Japan).
2.6 Cell adhesion on the scaffolds
The HUVECs at the density of 5×104cells/well were seeded onto the scaffolds loaded in 48-well plates and incubated at 37 ℃. After 24 h, the scaffolds were taken out carefully and washed gently with PBS (pH 7.4). 2.5% glutaraldehyde was added to immobilize the cells for 4 h at 4 ℃ then washed them three times with the PBS for 5 min for each wash. The gradient ethanol acetonitrile solution prepared in different ratios was then added (70%, 50%, 30%, 10%, 5% and 0%)for dehydration and finally the samples were taken out and set for drying using the critical drying method for 2 h. The cell adhesion was examined on the scaffold surface by an SEM. On 7-days culture, the scaffolds were submerged with cells for 10 minutes in PBS (pH 7.4) that was then replaced with 4% paraformaldehyde.The fixed samples were stained with 4′, 6-diamidino-2-phenylindole (DAPI), and then were examined by a fluorescence microscope.
2.7 Enzyme-linked immunosorbent assay(ELISA)
The HUVECs were cultured in the samples, and the supernatants were extracted on days 3, 5 and 7. An ELISA was conducted following the manufacturer’s recommended protocol to measure the levels of secretion of proangiogenic cytokines, bFGF and VEGF.
2.8 Total RNA extraction, cDNA synthesis and gene expression analysis by quantitative real-time polymerase chain reaction (qRT- PCR)
Total RNA was extracted from HUVECs seeded on the samples and controlled at 3 and 5-day culture.The procedure was carried out according to the manufacturer’s recommended protocol which includes RNA extraction, Genomic digestion and reverses transcription of cDNA, synthesis of 2 pairs of primers and detection, and detection of expression levels of 2 genes in the samples. The reaction mixture on genomic digestion was prepared on ice according to the composition of 5×gDNA Eraser Buffer (2.0 μL), gDNA Eraser (1.0 μL), Total RNA (7.0 μL) and RNase Free dH2O (Up to 10 μL). On cDNA synthesis, the reverse transcription reaction was prepared by compositions of the reaction liquid of step (1) (10.0 μL), PrimeScript RT Enzyme Mix Ⅰ (1.0 μL), RT Primer Mix (1.0 μL), 5×PrimeScript Buffer 2 (for Real-Time, 4.0 μL) and RNase Free dH2O (4.0 μL). The reverse transcription was carried out immediately after mixing, and the reaction conditions were 15 min at 37 ℃, 5 s at 85 ℃ and 4 ℃ forever. On the q-PCR pre-test detection template and primers for the gene tested, the sequences of the primers used are listed in Table 1. The genes tested were VEGF and bFGF.
The Taq reaction system was composed of 2×Taq Master Mix (10 μL ). Primer-s (0.5 μL), Primer-as (0.5 μL), cDNA (1 μL) and ddH2O (8 μL). Among them, the concentration of Primer-s and Primer-as was 10 μM,and the cDNA template was a 10-fold dilution of the reverse transcription stock solution. The q-PCR detection of relative expression of genes, the primers used in this experiment can be found in Table 1. The qPCR amplification system was composed of 2×SybGreen Mix (5 μL), Primer-s (1 μL), Primer-as (1 μL), cDNA (2 μL), and ddH2O (1 μL). Among them, the Primer-s and Primer-as concentrations were 2.5 μM, and the cDNA template was a 20-fold dilution of the reverse transcription stock. The qPCR amplification was Pre-denaturation 3 min at 95 ℃, Cycle (45 times) 95 ℃, 15 s→60℃ for 30 s and melting curve 55 ℃ → 95 ℃, 0.5 ℃for every 5 s.
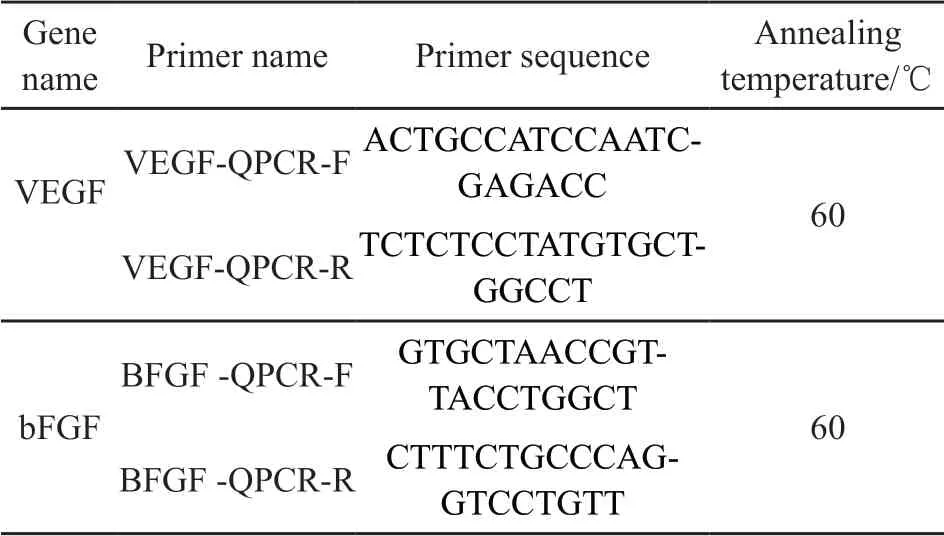
Table 1 Description of primers used in qRT-PCR for gene expression analysis
2.9 Statistical analysis
Results were analyzed with OriginLab Pro 2019 and are shown as mean ± standard deviation.Significant differences between sample groups were determined by analysis of variance (ANOVA) with Fisher LSD where *p< 0.05, **p< 0.01 and ***p<0.001 were considered statistically significant.
3 Results and discussion
3.1 Microstructural analysis
Scaffold pore size is essential for tissue regeneration efficiency[30,31]. The microstructure characteristic of the blend scaffolds was observed by SEM and the resulting images are shown on Fig.1(a). All scaffolds presented a homogeneous pore structure with pore diameters of 92-134 μm. The average pore diameter of 60Fc scaffold was about 122 μm, which showed a slightly significant difference from the average pore diameter of 70Fc scaffold (about 106 μm).
3.2 Cytotoxicity of the scaffolds
The scaffolds needs a good interconnectivity and suitable morphology for cell migration, proliferation,and differentiation[32]. Cytotoxicity is one of the most important indicators for biological evaluationin vitrostudies such as speed, cost reduction and potential for automation. Tests using human cells may be more relevant than somein vivoanimal tests[33].
On 1, 3, 5 and 7 days of culture, CCK-8 assay was carried out[34]to test the cytotoxicity of samples 60Fc, 70Fc and control against HUVECs. The data was read by a microplate reader at the wavelength of 570 nm, and the results are as seen in Fig.1(b). The cells cultured on each scaffold and control proliferated over time. On the first day, there was almost the same number of cells in all samples. The results showed that cell proliferation could increase over time. In the first 3 days, the control showed a fast cell proliferation than the two other samples. However, on day 5, there was a slightly higher optical density on 70Fc scaffold compared to the control, where the 60Fc scaffold showed the lowest value. Furthermore, 70Fc scaffold showed a significantly higher cell proliferation compared to the other samples on 7-day culture.
On 3-day culture, the live/dead assay of HUVECs on the scaffolds was carried out. Fig.1(c) shows photomicrographs of HUVECs stained with AM and PI fluorescent dye after exposure of the cells to extracted media. AM and PI staining were used to confirm the living and dead cells, which are shown on green and red fluorescence, respectively. As the control, both 60Fc and 70Fc scaffolds showed low toxicity. However, compared with 60Fc scaffold, 70Fc scaffold and the control showed the lowest number of apoptotic and dead cells.
Adequate porosity and sufficient mechanical properties of the scaffolds are essential for supporting tissue functioning and integration[35]. From our results, the 70Fc scaffold showed a smaller average pore diameter compared to 60Fc scaffold, hence an increase in specific surface area. It has been reported that an increase in specific surface area increases the ligand density available for cells to bind to[36]. This can explain our findings why the cytotoxicity tests revealed that the 70Fc scaffold could provide a more favorable conditions for cell proliferation (among the samples), with abundant nutrients for cell growth and survivability since they possessed a suitable specific surface area which could increase ligand density for initial cell attachment[37].
3.3 Cell adhesion on scaffolds
In vitroevaluation of biomaterials such as the interaction between cells and the scaffolds should first be considered before being appliedin vivo[38]. The attachment of cells to the ECM is necessary for cell migration. Cell adhesion is critical for the viability and proliferation for many cell types[39]. SEM can be used to monitor cell attachment and the morphology of cells seeded on hydrogels[40].
On 1-day culture, the cell attachment on the scaffolds was assessed via SEM (Fig.2(a)). The results showed that the seeded cells were adhered to both scaffolds and covered the pores after 1-day culture.The 70Fc scaffolds, however, showed better cells attachment compared to 60Fc. On 7-day culture, the cell number and behavior within the scaffolds was monitored by fluorescence microscopy as seen in Fig.2(b).DAPI staining was used to distinguish the HUVECs(grey nuclei) from the scaffolds (in black color). The number of adherent cells (Fig.2(c)) on the surface and within the scaffolds 70Fc was higher compared with 60Fc.
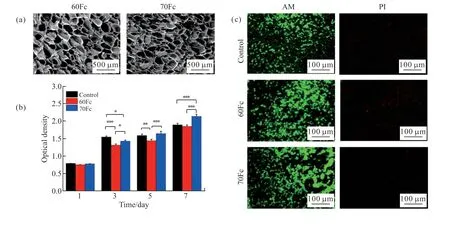
Fig.1 Scaffolds morphology and HUVECs morphology and proliferation in the scaffolds extract media: (a) SEM images of the blend ratios scaffolds at freezing temperature -40 ℃; (b) The cell viability assessed by CCK-8 assay after 1, 3, 7-day exposure with scaffolds extract media (n = 6 per group, *p < 0.05, **p < 0.01, ***p < 0.001); (c) The cells growing in the extracted media of different samples
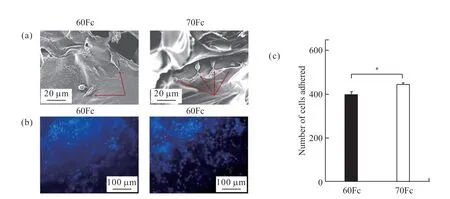
Fig.2 Adhesion and morphology of HUVECs on the scaffolds: (a) SEM images of HUVECs cultured on the surface of scaffolds after 5-day culture; (b) The cells growing on the scaffolds after 7-day culture were stained with DAPI for nuclei; (c) The average number of cells in blue nuclei counted on several images of scaffolds on DAPI (n = 4, *p < 0.05)
Our findings showed a functional cells attachment on the materials due to the biocompatibility of the natural biological scaffolds. The cell adhesion on 70 Fc scaffold was better than that of 60 Fc, because more silk fibroin content in the scaffold could effectively increase the cell adhesion on the material and support more cells attachment which was proved by the previous studies[41-43].
3.4 Measurement of VEGF and bFGF protein secretion
VEGF and bFGF are part of pro-angiogenic growth factors[44]. VEGF is a major angiogenic modulator signal protein produced by cells that stimulates the blood vessels formation[45]. It regulates several developmental processes such as angiogenesis, lymph-angiogenesis and neuronal development[46]. bFGF on the other hand, is a signal protein which is involved in a variety of biological processes including cell growth and tissue repair. It controls the proliferation, differentiation and migration of endothelial cells[27,47]. ELISA assay is a widely used technique, which is a simple one and low-cost analytical tool required for the study and the detection of cytokinesin vitroorin vivo[48].
The concentrations of growth factors, VEGF and bFGF, secreted by HUVECs on the scaffolds were determined quantitatively using ELISA after 3, 5 and 7-days culture. These factors were detectable on both scaffolds, and the results are shown in Fig.3. Although there was no significant difference on the VEGF levels in the first 5 days (Fig.3(a)), the 70Fc scaffold VEGF concentration was slightly higher on 5-day culture compared to the other samples. Against the control on 7-day culture, this value became significantly higher on 70Fc scaffold. On the other hand, the bFGF level on control was noticed to be higher on 3-day culture(Fig.3(b)). Though, from day 5, the 70Fc scaffold was observed to be slightly higher compared to both the control and 60Fc scaffold.
The results on ELISA assay suggest that due to the higher secreted levels of the VEGF and bFGF, the involvement of more silk fibroin in the scaffolds could enhance the angiogenic activity of HUVECs cultured on the sample surface.
3.5 RNA-quality, internal reference quality and q -PCR detection of relative expression of genes
New vessel formation requires an integrated actions of a number of angiogenic growth factors[49].The angiogenic-associated genes VEGF and bFGF are among the major pro-angiogenic growth factors and are known to regulate angiogenesis by promoting endothelial cells proliferation, migration, and vascular formation[50,51].
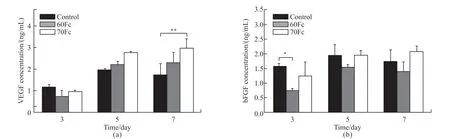
Fig.3 (a) VEGF and (b) bFGF proteins production level of HUVECs cultured on scaffolds surfaces in comparison to the control surfaces at 3,5 and 7 days (Statistical significance was assessed at *p < 0.05, **p < 0.01, ***p <0.001)
To observe the effects of the scaffolds surface structures on the differentiation of HUVECs at the molecular level, the angiogenic genes expressions VEGF and bFGF were examined quantitatively by the q-PCR on 3 and 5-days culture on both sample surfaces, and the results are as shown in Fig.4. Although expression levels of VEGF on the samples were not significantly detected on day 3, they were significantly higher on scaffold 70Fc compared to scaffold 60Fc and the control on day 5 (Fig.4(a)). However, almost no changes were seen on expression levels of bFGF on all samples between day 3 and 5 (Fig.4(b)).
Unlike ELISA results which showed no significant difference in VEGF protein concentration between the control and scaffold 70Fc on day 3, the results of mRNA expression between the two showed a significant difference. Because protein is a downstream product of a gene, these inconsistencies may be the results of delayed variation in the protein level of VEGF and bFGF relative to their mRNA levels with incubation time[27,52]. On the other hand, the results showed poor correlations between mRNA and proteins levels, especially on bFGF levels. Multiple factors could explain this. First, there are many complicated and varied post-transcriptional mechanisms involved in turning mRNA into proteins that are not yet sufficiently well-defined to be able to compute protein concentrations from mRNA. Second, some proteins have a long half-life, while others have to be destroyed immediately for proper function. Another reason is the availability of a significant amount of error and noise in both protein and mRNA experiments that limit our ability to get a clear picture[53,54].
The results were anticipated since SF has been proved to contain the tripeptide Arg-Gly-Asp (RGD)sequences that can support cell adhesion, proliferation,and migration of various cell types including endothelial cells[55,56]. On one study, SF was observed to show no adverse influence on VEGF secretion of endothelial cells[57]. Moreover, because of its excellent biocompatibility, cell adhesion support and biodegradation, SF dressings have been reported to promote angiogenesis by controlling VEGF expressions[58,59].
Skin injury commences a cascade of events,including inflammation, new tissue formation, and tissue remodeling, leading to at least partial repair of the wound[60]. The repair process is initiated immediately after injury by releasing various growth factors and cytokines[60,61]. The inflammatory response is an essential step to the corrected and well-coordinated wound recovery[62]. The secretion of pro-inflammatory cytokines in the early wound plays a vital role in wound repair[63]. However, some wounds(like burn) are associated with a large amount of pro-inflammatory cytokines, which can worsen the tissue damage caused by the initial thermal injury[64]. The excessive expression of pro-inflammatory cytokines within burn wounds has been reported to impair the wound healing process[65]. Several studies have suggested that SF could suppress the excess pro-inflammatory cytokines during inflammation phase of a wound, helping avoid adverse effects in cells and tissues during wound healing[66].
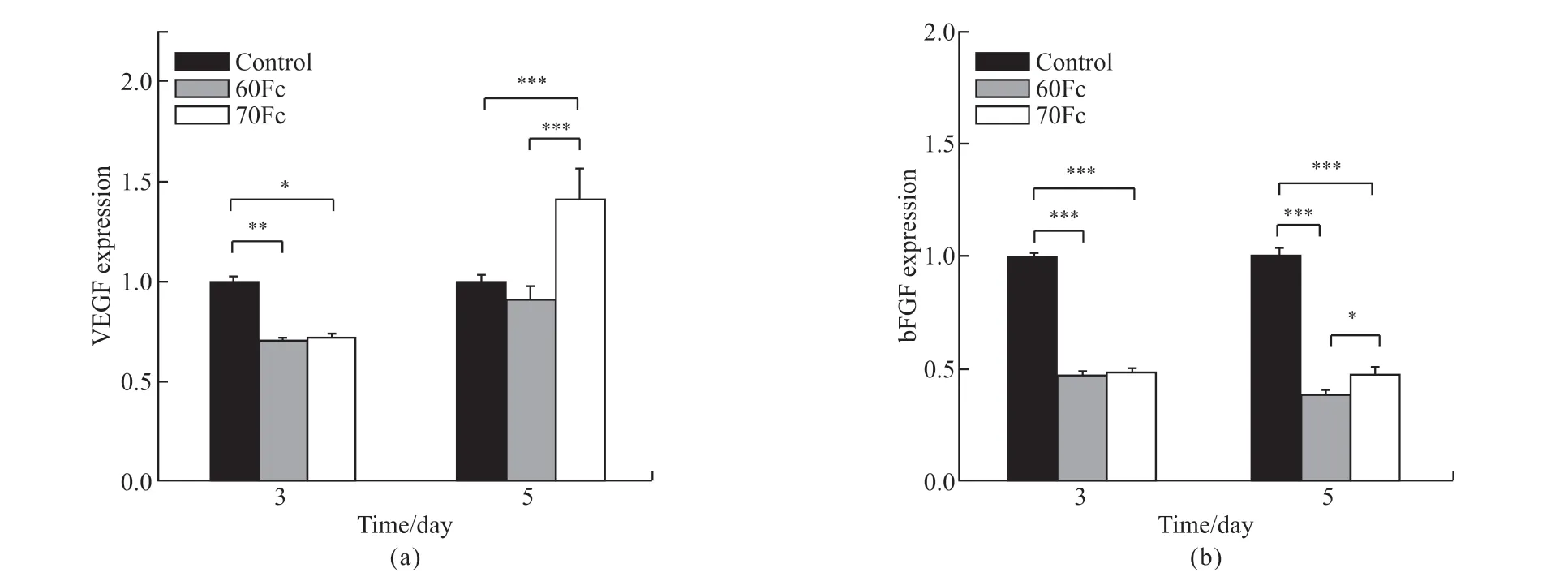
Fig.4 Q-PCR detection of relative expression of genes: (a) VEGF; (b) bFGF on 3 and 5-days culture (Statistical significance was assessed at*p < 0.05, **p < 0.01, ***p < 0.001)
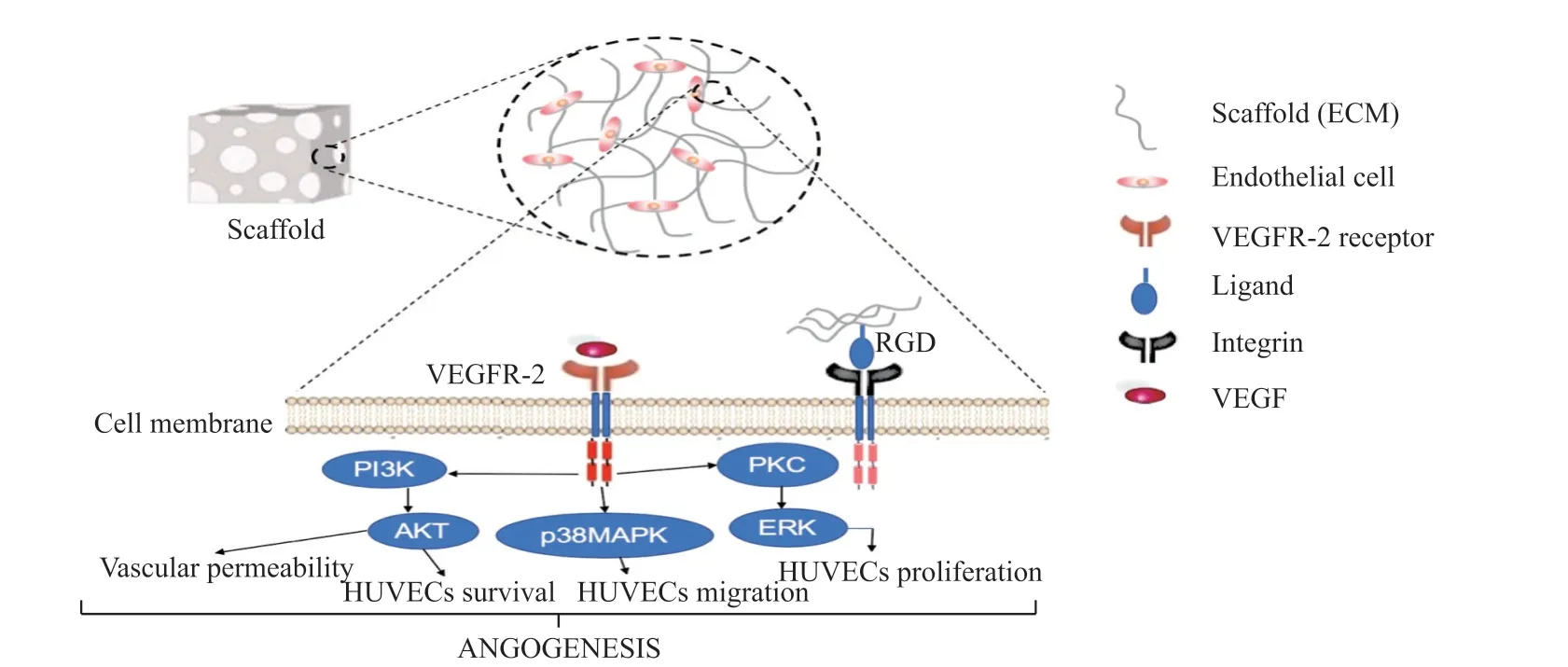
Fig.5 Schematic illustration of the attachment of cells to ECM-based scaffolds. Cells attach to the ECMs molecules (RGD) by formation of ligand-receptor (integrin) complex. VEGF binds to the membrane-bound VEFR2 receptor which mediates activation through the cytoplasmic tyrosine kinase domain conferring signals downstream to PKC, PI3K and p38MAPK signaling, and promote a complex assembly
The regulated pro-inflammatory cytokines and growth factors signaling molecules are secreted from some cells to promote inflammation, which results in several complex cellular signaling during the wound healing process. This cellular signaling plays a significant role in wound healing processes, including AKT signaling and mitogen-activated protein kinase (MAPK)signaling, which occurs in a closely coordinated cascade to heal the injury[67-69]. The availability of more SF on the blend scaffolds resulted to suitable biodegradation and cell adhesion on the materials that could influence the VEGF to bind to the membrane-bound VEFR2 receptor which mediates activation through the cytoplasmic tyrosine kinase domain conferring signals downstream to protein kinase C (PKC), phosphoinositide-3-kinase (PI3K) and p38 mitogen-activated protein kinase (p38MAPK) signaling[70]. The p38 MAPK have an impact on cell migration through increased actin remodeling, PKC results in sequential activation of extracellular signal-regulated kinase (ERK) which influence gene expression and cell proliferation, and activation of PI3K results in sequential activation of Akt which promote cell survival and vascular permeability
[71]. On the other hand, the presence of bFGF on these scaffolds could also stimulate HUVECsin vitroand angiogenesis, and it could further indirectly control the expression of VEGF[72]. Schematic illustration of the cells attached to ECM-based scaffolds is summarized in Fig.5.
4 Conclusions
We were able to examine thein vitroangiogenic behavior of HUVECs on 60Fc and 70Fc scaffolds.Significant enhancement of cell adhesion, proliferation and differentiation of HUVECs on the 70Fc scaffold were observed as compared to 60Fc and the unmodified control. Furthermore, the 70Fc scaffold was noticed to promote the secretion of specific angiogenic proteins and genes expression, VEGF and bFGF. Our findings demonstrate that the composite scaffolds involving a high amount of SF (about 70%), and low amount of SA (about 30%) can interact more efficiently with HUVECs in expediting angiogenesis. Taken all of these under consideration with the characterization of SF/SA composite scaffolds on our previous study, we suggest that the 70Fc scaffold can effectively possess the appropriate biomimetic structure similar to skin that could promote angiogenesis, thus suitable for designing sophisticated, high-performance wound dressings and engineering of skin tissues that require a high degree of vascularization.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
- Fracture Behavior and Processing Deformation of C71500 Cupronickel Alloy during Hot Tensile Deformation
