Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
2021-06-14LINWeiqingLUOWenjunZHANGGuanghuiLIHaifeng
LIN Weiqing, LUO Wenjun, ZHANG Guanghui*, LI Haifeng
(1. School of Civil Engineering and Architecture, Wuhan University of Technology, Wuhan 430070, China; 2. Faculty of Material Science and Chemistry, China University of Geosciences, Wuhan 430074, China; 3. The State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, China )
Abstract: Phlogopite-based geopolymer was first prepared successfully under the activation of lye by compression molding at 50 MPa for 1 minute. The geopolymer was endowed with nonpolar surface via brushing modified liquid at room temperature. Swill-cooked dirty oil, whose main component was fatty acid, was used as nonpolar modifier. The raw materials and geopolymer samples were characterized by XRD, FT-IR and SEM.The compression strength of 7-day specimen run up to 36.8 MPa and its surface static water contact angle could reach 132°. The solubility of phlogopite powder directly affected the compressive strength of geopolymers and the evaluation index of mechanical strength of geopolymer based on the solubility of phlogopite powder was proposed.
Key words: phlogopite; geopolymer; nonpolar modification; solubility; compression strength
1 Introduction
Geopolymer, which was put forward by French scientist Davidovits at 1979[1], has been considered as a substitute for Portland cement due to its environmental friendliness in manufacture process and excellent mechanical properties[2-5]. Current literature reports show that most researchers chose amorphous active materials, such as metakaolin, fly ash,etcto prepare geopolymer materials[6-11]. From kaolin and clay in coal seam to metakaolin and fly ash, they are all the results of structural change from crystalline state to amorphous state in the process of high temperature calcination[12,13]. The analytic results of NMR (including29Si and27Al), FT-IR and XRD in many literatures have proved this transition process[14-17]. Therefore,the raw activity is activated by the alkali. In fact, the first step of alkali activation is that the surface of the raw material particles was dissolved, and then two reactions occurred: one is homogeneous condensationpolymerization between dissolved substances,another is heterogeneous interfacial condensationpolymerization between the dissolved components and surface hydroxyls of non-dissolved particulate cores[18,19]. Since the alkaline solubility of amorphous substances is higher than that of crystalline substances,they exhibit higher activity. However, the solubility of many minerals in nature is not mainly dependent by their amorphous state. Therefore, to broaden the selection range of available materials, it is worthwhile to explore the solubility of other mineral particles under alkaline conditions.
As an alkaline earth metal, magnesium has a higher electronegativity than calcium and is, therefore,a widely researched content in a durable binder.Previous studies show the increasing MgO content contributes to the improved mechanical strength[20,21].As an abundant clay mineral, phlogopite is a viable source of MgO owing to the carbonate-free and naturally abundant characters[22]. It is widespread in 20 provinces in China and contains large amounts of silica and alumina. This makes it possible for alkali activation. However, there are few studies on the application of the phlogopite as geopolymer precursors.Sreenivasan H studied activation potential of the treated and untreated materials through a solubility test in 6 M NaOH, implying the calcined phlogopite has the alkali activation potential, but the properties of geopolymer samples were not studied[22]. In this study, phlogopitebased geopolymer was first prepared by uncalcined phlogopite with sodium hydroxide and sodium silicate as activator. Meanwhile, the relationship between the solubility of raw materials and the compressive strength of geopolymer samples is one of the main contents of this study.

Fig.1 Electrification of surface hydroxyls and research route
Geopolymers have polar surfaces with hydrophilic properties due to the presence of surface hydroxyls,thus water-soluble harmful substances may be enriched on the geopolymer surface, reducing its long-term durability[23,24]. Moreover, the surface hydroxyls have adsorbability to harmful substances by electrostatic attraction, due to the surface charges carried in different pH environments, as shown in Fig.1(a).Nonpolar modification method was adopted, while swill-cooked dirty oil was selected as modifier[25-28].The condition of nonpolar modification corresponds to normal temperature and atmospheric pressure, and the modifying process is surface brushing. The research route was shown in Fig.1(b).
2 Experimental
2.1 Materials and chemicals
Phlogopite was provided by Ling Shou China Technology Mineral Powder Co., Ltd. and its chemical compositions determined using XRF analysis is tabulated in Table 1. The grain size distribution of powdered phlogopite is shown in Fig.2. The activator solution was a combination of sodium hydroxide(NaOH) solution and liquid sodium silicate (Na2SiO3).The NaOH pellet supplied from Tianjin Tianli Chemical Reagent Co., Ltd. has purity of 99%. The Na2SiO3purchased from Hubei Xinrunde Chemical Industry Co., Ltd. contains SiO2/Na2O modulus of 3.1.The modifier (swill-cooked dirty oil) was collected from the university canteen and the deionized water was used throughout the experimentation.

Table 1 XRF analysis of phlogopite (LOI: loss on ignition)/wt%
2.2 Solubility experiment
Mass fraction of sodium hydroxide to phlogopite(MFSP) and curing time were taken into account in the solubility experiment. The whole experiment was conducted at room temperature with three groups of parallel samples in each experimental condition.Different MFSP and same curing time experiments were carried out by the following steps: (i) 2 g phlogopite powder was distributed evenly in four plastic beakers. (ii) Adding 30 mL sodium silicate and sodium hydroxide with the MFSP of 2%, 4%, 6%, 8%,respectively. (iii) After 24 h, 150 mL deionized water was added to wash away the dissolved matter. (iv)Stood still until the clear solid-liquid separation line appeared and poured out the supernatant carefully. (v)Adding 150 mL deionized water again and repeated the fourth step. After that, repeating the fifth step twice. (vi)Filtering, drying and weighing the residual powder, and calculating the solubility of phlogopite using Eq.(1).While the other solubility experiments (same MFSP and different curing time) followed the same steps as the above experiments except for the first water adding time which was 4, 8, 12, 24 and 48 h, respectively:

whereSis solubility of phlogopite,Mpis mass of raw powder,Mp’ is mass of residual powder.

Table 2 Mixing proportions of geopolymer
2.3 Preparation of phlogopite-based geopolymer
Geopolymer specimens were prepared by compression molding method. First, the phlogopite powder and alkali activator were mixed evenly in a fixed proportion. Alkali activator consists of sodium silicate and sodium hydroxide. Therein the sodium silicate was 10 wt% of phlogopite powder and the MFSP was 2%, 4%, 6%, and 8%, respectively. The detailed mixing proportions of geopolymer was shown in Table 2. Then, 6.5 g mixed powder was packed into a steel cylindrical die and pressed at 50 MPa for 1 minute. Finally, 4 group samples (3 samples per group)with 20 mm in diameter and 10 mm in height were prepared and cured in established time for compressive strength test at 20 °C and 45% humidity.
2.4 Nonpolar modification
The 20 mm in diameter and 10 mm in height phlogopite-based geopolymer samples were prepared for nonpolar modification. The nonpolar modifier was synthesized by the following steps: Firstly, 10 mL alcohol solution of 4 wt% swill-cooked dirty oil (1 g swill-cooked dirty oil dissolved in 30 mL anhydrous alcohol) was added into 40 mL distilled water. Then,0.04 g AlCl3as catalyzator was added into it under stirring for 0.5 h at room temperature. Finally, the prepared modifier was evenly brushed on the surface of geopolymer samples, and dried in natural air.
2.5 The characterization of samples
The compressive strength tests were carried out on a universal press (Kehui Test Equipment Co., Ltd,China). Samples with different MFSP and curing time were pressed at a constant displacement rate (1 mm/min), respectively. Scanning electron microscope(SEM) images were taken by a JSM-IT300 instrument.X-ray diffraction (XRD) patterns were carried out on an X-ray diffractometer (X’Pert Pro DY2189, PANalytical,B. V., Netherlands) with CuKα radiation operating at 40 kV and 40 mA. Fourier-transform infrared (FT-IR)spectra measurements were performed on a Nicolet AVATAR 360 instrument using the standard KBr disk method. The zeta potential of material in water was tested by a Zeta-Meter system 4.0 (Ankersmid Co.,Ltd, Netherlands). The surface static water contact angle was measured with the sessile drop method by an OCA20 measuring system (Dataphysics, Filderstadt,Germany) with a maximum error of 0.05°.
3 Results and discussion
3.1 Relationship between solubility and compressive strength
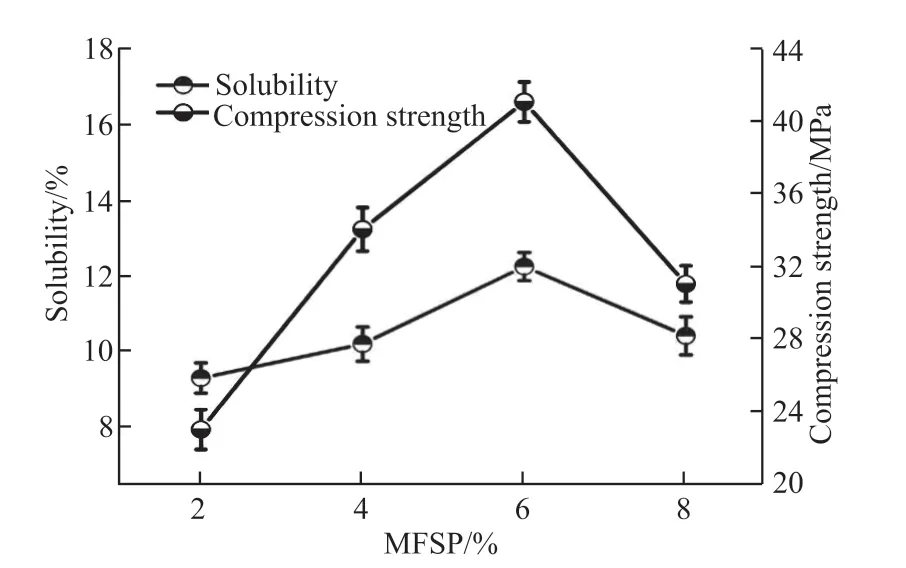
Fig.3 The relationship between solubility and compressive strength
The relationship between the phlogopite’s solubility and the geopolymer’s compressive strength at the same curing time is shown in Fig.3. The experimental data shows the solubility of the raw material has a trend of increasing and then decreasing with the rise of alkali concentration, hitting a peak of 12.2% at 6%. In contrast, the compressive strength of samples has a similar tendency, reaching the highest point of 42 MPa at 6%. It can be found that the geopolymer’s compressive strength is positively correlated with the raw material’s solubility, indicating that the amount of homogeneous polymers formed by dissolved substance directly controls the mechanical strength of the samples. Therefore, the solubility of raw materials could be regarded as a valuable evaluation index of mechanical strength in the preparation of geopolymers.
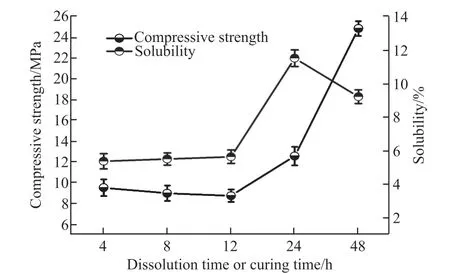
Fig.4 The test results of solubility and compressive strength with the same MFSP (6 wt%) and different time
As shown in Fig.4, the solubility of the raw material and compression strength remain stable at around 6% and 9 MPa, respectively, in the initial state from 4 to 12 h. After that, the solubility and compressive strength present different variation trends:the solubility experiences a dramatic rise and a slight drop between 12 and 48 h, peaking at 12% at 24 h;while the compressive strength maintains a sustained growth, which is most likely due to the fact that new phases (homogeneous polymers) have begun to form between the dissolved substances since 24 h. This phenomenon further confirms that the geopolymer’s compressive strength is directly related to the phlogopite’s solubility, as shown in Fig.3.
These two experimental results both indicate that the uncalcined phlogopite have a certain activity under alkaline conditions, and the solubility of raw materials are related to the variety of alkali concentrations. There is optimum alkali concentration of 6% resulting in the maximum solubility and compressive strength. The establishment of corresponding relationship among alkali concentration, solubility and compressive strength is beneficial for designing higher strength geopolymers in the future.
3.2 Characterization of samples
SEM images of different MFSP (2 wt%, 4 wt%, 6 wt%, 8 wt%) geopolymer samples are shown in Fig.5.The morphology of 2 wt%, 4 wt% and 8 wt% samples mainly present sheet-like. 4 wt% and 8 wt% have more complete forms and closer stacking than 2 wt%, and the edge formation of 4 wt% is better than that of 8 wt%.Compared with them, 6 wt% particles possess a more complete cylindrical boundary at the edge of the flake than 8% and 4%, and stacked more evenly and tightly.The destruction form of 6 wt% is more likely to the fracture of short columns, while the destruction forms of 4% and 8% are similar to slippage of the layer. The above phenomena confirm the trend of compressive strength is that 6 wt% > 4 wt% > 8 wt% > 2 wt%.

Fig.5 SEM images of different MFSP (2 wt%, 4 wt%, 6 wt%, 8 wt%) geopolymer

Fig.6 XRD patterns of phlogopite and its corresponding geopolymer
The XRD patterns of phlogopite and its corresponding geopolymer are presented in Fig.6.Zeolite UTD-1 and phlogopiteogopite-2M1are the major crystalline phases of the phlogopite[29,30].Compared with XRD patterns of phlogopite, there are no new phases formation and the intensity of crystalline diffraction peak at 2 theta angle of 6, 11, 21,27 and 29 shows a marked decrease in the geopolymer.This indicates the dissolution of crystalline phases and the formation of amorphous phases. It is a significant manifestation of geopolymerization. According to previous research[16,31,32], the reduction in the crystalline phases would definitely lead to an increasing of the mechanical strength of geopolymers.
Infrared spectrums of phlogopite, geopolymer and surface nonpolar-modified phlogopite were shown in Fig.7. The absorption bands at about 3 451 and 1 634 cm-1are related to the stretching vibrations of O-H bonds and bending vibrations of H-O-H bonds and they are broader in the spectrum of geopolymer,which indicates the present water molecules during geopolymerization are adsorbed on the surface or encapsulated in the pores of geopolymer. At about 1 430 cm-1, there are stronger absorption bands existed in the geopolymer spectrum, which might be due to the stretching vibration of O-C-O in carbonate groups formed by the reaction of alkali metal hydroxides with atmospheric CO2[33]. The main absorption band at about 1 010 cm-1in all samples indicates the asymmetric stretching vibrations of Si-O-X bonds (X = Si, Al).This absorption band in the geopolymer spectrum is stronger than that in the phlogopite spectrum, which indicates the formation of amorphous aluminosilicate phases and the occurrence of geopolymerization[34,35].In addition, the absorption bands at about 465 cm-1are related to the bending and stretching of O-Si-O and Si-O-Si bonds, which are similar to the Si-O-Al group[36].

Fig.7 FTIR of phlogopite, geopolymer and modified phlogopite
Comparison with the infrared spectrums of phlogopite and geopolymer, the spectrum of surface nonpolar-modified phlogopite shows new obvious bands at about 2 920 and 2 851 cm-1, which corresponds to the tensile vibrations of methyl and methylene. Furthermore, the stretching vibrations of O-H bonds and bending vibrations of H-O-H bonds at about 3 451 and 1 634 cm-1weaken after nonpolar modification. These results indicate the fatty acid molecules in the modifier have successfully combined with the surface of the geopolymer samples via the esterification reaction[13,28].
Waterproof performances of geopolymer before and after surface nonpolar modification are illustrated in Fig.8. The static water contact angle of geopolymer after surface nonpolar modification significantly increases from 33° to 132°, which further demonstrates the success of nonpolar modification of geopolymer surface.

Fig.8 Contact angle of geopolymer before and after modification
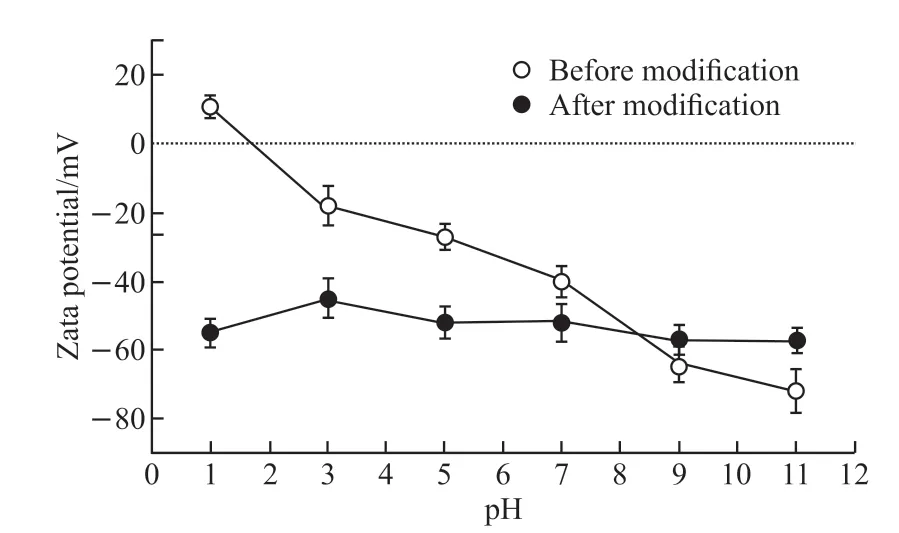
Fig.9 Zeta potential before and after surface nonpolar modification
The change of potential before and after surface nonpolar modification is showed in Fig.9. Compared with the reference powder, the potential of the modified powder has little change in different pH value solutions. This indicates that the surface hydroxyl groups which can occur protonation and deprotonation under different acid and alkali conditions have been exhausted by the modifier. The results are similar to those of FT-IR and contact angle analysis indicating the formation of nonpolar surface.
4 Conclusions
a)The uncalcined phlogopite ores, having a certain reaction activity, can be used as raw material to prepare geopolymers under alkaline conditions,and mechanical properties of the geopolymer samples meet the requirements of the geosynthetics application:compressive strength up to 42 MPa after 28 days. This attempt has expanded the material selection for the preparation of geopolymers.
b) There is a linear positive correlation between the mechanical strength of geopolymers and the solubility of surface materials. This indicates that the strength of the cementing materials is controlled by the amount of homogeneous polymers newly formed by alkali-soluble substances. This can provide the basis for the preparation of geopolymers.
c) The surface nonpolar modification of geopolymers can be achieved by heterogeneous esterification reaction between the geopolymer surface hydroxyl groups and the carboxyl groups of fatty acids, and swill-cooked dirty oil can be selected as a fatty acid raw material. Furthermore, this modifying operation can be carried out only by a simple surface brushing process at normal temperature and atmospheric pressure in the presence of catalyst (Al3+). After nonpolar modification,the static water contact angle of geopolymer samples can reach 132°.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of Nano Silver Modification on the Dielectric Properties of Ag@TiO2/PVDF Composites
- Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
- Effects of Cracks on the Mass Transfer of Polymer Electrolyte Membrane Fuel Cell with High Performance Membrane Electrode Assembly
- Refinement of TiB2 Powders with High-speed Planetary Mill and Its Effect on TiB2 Sinterability
- Fabrication of Ordered Meso-macroporous HPW/TiO2 Catalyst for Efficient Heterogeneous Oxidative Desulfurization
- The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
