Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
2021-06-14ZHANGYingWANGPengshuoSUNGuowenYANGJianmingGAORui
ZHANG Ying, WANG Pengshuo, SUN Guowen , YANG Jianming, GAO Rui
(1.School of Materials Science and Engineering, Shijiazhuang Tiedao University, Shijiazhuang 050043, China; 2.College of Civil Engineering, Yancheng Institute of Technology, Yancheng 224051, China; 3. State Key Laboratory of Mechanical Behavior and System Safety of Traffic Engineering Structures, Shijiazhuang Tiedao University, Shijiazhuang 050043, China; 4. Heibei Key Laboratory of Advanced Materials for Transportation Engineering and Environment, Shijiazhuang Tiedao University, Shijiazhuang 050043, China)
Abstract: A low adsorption clay-resistant polycarboxylate superplasticizer (KN-PC) was synthesized using acrylic acid and isopentenol polyoxyethylene ether as the main reaction materials. The structural characterization and clay-resistant mechanism of the KN-PC were explored using Fourier transform infrared spectroscopy and X-ray diffraction, and the effect of the KN-PC on the performance of composite paste with various montmorillonite (MMT) contents was analyzed. Compared with ordinary polycarboxylate superplasticizer (PC), the KN-PC has a low sensitivity to the MMT. By the action of the MMT, the adsorption dosage of the KN-PC on the MMT is much smaller than that of the PC.
Key words: polycarboxylate superplasticizer; clay-resistant; montmorillonite; performance; synthesis
1 Introduction
Polycarboxylate superplasticizer (PE) has been widely used in structural concrete engineering due to its advantages, such as low dosage requirement, high water reduction rate, small loss of concrete slump, and small impact of dry shrinkage after mixing[1]. However,large-scale infrastructure construction has increased the amount of sand and gravel mining each year, and the quality of sand and gravel resources has become increasingly poor[2-3]. A high clay content in sand and gravel not only reduces strength and durability but also exacerbates the shrinkage of concrete. In particular,when coarse and fine aggregates contain clay impurities(such as MMT, illite, and kaolin), the presence of the impurities significantly reduces the dispersion effect of the PE[4].
Among the clay impurities, MMT has a strong adsorption to PE. The maximum adsorption capacity of MMT to PE containing a polyether side chain about 100 times that of Portland silicate cement[5].Therefore, in the application process, the PE needs to reduce the amount of clay and microporous aggregate that adsorbs on it to improve the PE's water-reducing performance[6,7]. Studies have shown that increasing the amount of PE can reduce the influence of the clay on the PE. However, this procedure not only reduces the strength and durability of the hardened concrete but also increases the cost[8].
Based on the design of the molecular structure of polycarboxylate acid and the diversity of monomers, molecular design has introduced clayresistant monomers, and these monomers can improve dispersibility to a certain extent. A series of patents by Hirata[9,10]shows that the specific relative molecular weight distribution of the carboxylic acid polymer and a certain proportion of hydrophilic functional groups on the main chain of the polymer are the key factors affecting the monomers' application performance.
Leiet al[11]synthesized a series of PE containing light alkyl side-chain functionalities. The results shown that a PE containing light alkyl groups on the side chain could significantly reduce the sensitivity to clay. Chenet al[12]used modified polyether as raw material to synthesize a new type of PE with hydroxy as the terminal group, and improved the dispersion performance of PE in concrete with clay. Sunet al[13]synthesized an amino-modified polycarboxylate acid PE using a self-made quaternary ammonium salt of maleic acid, acrylic acid, and TPEG. This modified PE was less affected by MMT. The adsorption capacity of the amino-modified PE on the clay was less than that of the traditional PC. Xinget al[14]copolymerized acrylic acid, itaconic acid, and dimethylaminoethyl methacrylate to synthesize a clay-resistant PE containing tertiary amine groups. It was found that the PE had difficulty being adsorbed on MMT, and the PE was sensitive to temperature during synthesis by modern analysis instrument.
Therefore, we introduced some small functional monomers into the macromonomers of TPEG and synthesized KN-PC under a redox initiation system.The reaction temperature, the acid-ether ratio, and the amounts of initiator, chain transfer agent, sodium methyl vinyl sulfonate, acrylamide, and phosphate ester clay-resistant monomer are fully considered in the synthesis process to obtain the optimum dosage of the KN-PC. To test the anti-adsorption effect and mechanism of the synthesized KN-PC, an MMT with strong adsorption capabilities is selected for the macro and micro experiments.
2 Experimental
2.1 Experimental materials
The synthetic materials used include acrylic acid, TPEG (mW=2 400 g/mol), ammonium persulfate,acrylamide, sodium hydroxide (NaOH), and sodium methyl vinyl sulfonate (all 99% purity, purchased from Heibei Chemical Reagent Co., Ltd, Shijiazhuang,China). The phosphate ester clay-resistant monomer was synthesized in the laboratory.
Other materials include the following: reference cement P.I.42.5 (C), a type of cement specially used to examine the performances of concrete admixture,was produced by China Resources Cement Group. The MMT, with a particle size smaller than a 200-mesh sieve, was purchased from Aladdin Co., Ltd, Shanghai,China. The PC with a solid content of 49.6% and water-reducing ratio of 28.5% was produced by Hebei Qinghua Building Materials Co., Ltd. Experimental measurements by X-ray fluorescence revealed the chemical composition of C and MMT, as shown in Table 1.
2.2 Experimental methods
Instruments used in the experiment include the following: a constant temperature water bath, a fournecked flask, a thermometer, an HL-2 constant current pump, a JJ-1 precision electric agitator, a high-speed centrifuge, and a pH meter.
Fourier transform infrared spectra (FTIR) were collected by a VECTOR-22 FTIR spectrometer;the wavelength range was 4 000 to 400 cm-1using potassium bromide as the diluent and binder. XRD powder analysis was recorded by a D/max2200/PC X-ray diffractometer in steps of 0.02° using Cu Kα radiation, and the scanning range was 0° to 50°.
A TOC analyzer was used to test the adsorption properties of the samples with the added KN-PC and PC. The adsorption amountQ(mg/g) was calculated as follows:
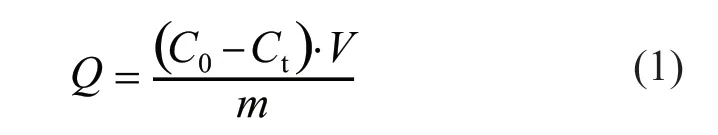
whereC0is the initial concentration of the sample solution, g/L;Ctis the residual concentration after adsorption, g/L;Vis the total volume of the solution,mL; andmis the mass of the MMT or C, g.

Table 1 Chemical compositions of C and MMT/%
2.3 Synthesis process of KN-PC
TPEG (320 g) and deionized water (200 g) were mixed in a four-neck round-bottom flask. The fourneck round-bottom flask was placed in a constant temperature water bath with a stirrer and continuously stirred; the reaction temperature increased under reflux conditions. In order to observe the temperature change in the reaction process, the thermometer were inserted into the four-necked flask. After stirring, ammonium persulfate was added and stirred for 5 minutes until completely dissolved. A certain amount of acrylic acid and phosphate ester clay-resistant monomer was weighed and put into a beaker with deionized water,then stirred to form Solution A; a certain amount of acrylamide, and sodium methyl vinyl sulfonate was weighed and put into a beaker with deionized water,then stirred to form Solution B. Solutions A and B were simultaneously added to the four-neck round-bottom flask at the same rate. After this addition, the reaction was terminated with a small amount of ascorbic acid solution. After the reaction of heat preservation was 1 to 2 h, the product was neutralized to pH 7 with an NaOH solution. The KN-PC was obtained and named KN-PC. The schematic diagram of the synthesis reaction is shown in Fig.1.
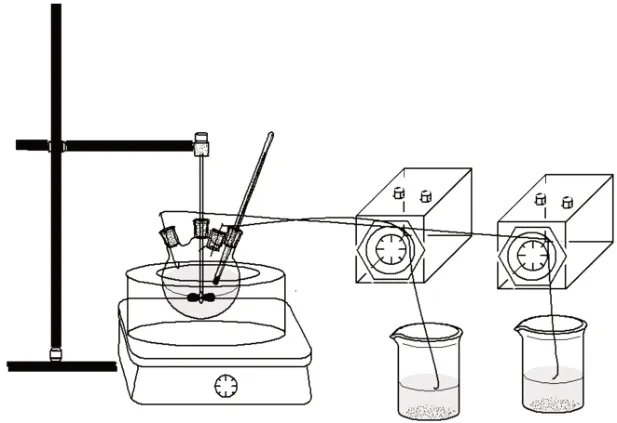
Fig.1 Schematic diagram of the KN-PC synthesis reaction
2.4 Preparation of samples
2.4.1 Total organic carbon
Deionized water was respectively added to the KN-PC and PC to prepare solutions with mass concentrations of 1, 2, 3 and 4 g/L. The concentration of KN-PC and PC in solution was obtained from the TOC results. The C (1 g) and MMT (1 g) were mixed into the superplasticizer solution (50 mL) with the various mass concentrations. After being added to the beaker, they were oscillated in a constant temperature water bath for 15 minutes; then they were held still for 5 minutes, and finally the upper suspension was separated using high-speed centrifuge and diluted with deionized water, which reached the test range of the TOC.
2.4.2 XRD and FTIR
The MMT (5 g) and C (5 g) were respectively added to different deionized water (200 g) to prepare the solution, which was stirred with a precision electric stirrer. The pH of the solution was adjusted to 12 with NaOH, and then the PC (4 g) or KN-PC (4 g) was added and stirred continuously for 5 minutes. Finally,the solution was filtered, and the solid obtained was tested with XRD and FTIR analysis.
Table 2 Factors and levels of L16(45) experiment
3 Factors affecting the synthesized KN-PC
This section reports on how the orthogonal test method is used to investigate the influence of the acidether ratio, the reaction temperature, and the amounts of the initiator, the acrylamide, and the chain transfer agent on the synthesis of KN-PC. The sodium methyl vinyl sulfonate and ammonium persulfate are added as the chain transfer agent and initiator in the experiment,respectively. The amount of chain transfer agent and of initiator is the percentage of the total mass of the reaction material. The changes in the fluidity of the composite paste with a certain amount of MMT added to the synthesized KN-PC are tested under different conditions. The factors of significant influence and how different levels of the same factor affect the fluidity of the composite paste are obtained, and the reasons for this influence are analyzed.
The fluidity of the composite pastes containing the KN-PC and PC were examined according to the standard method GB/T 8077-2012[15]given in the National Standards of China. The fluidity of the composite paste with the MMT and KN-PC content of 1% and 0.18% of the mass of the C is used to measure the effect of KN-PC, and the water-to-cement ratio is 0.29.
3.1 Orthogonal experimental design and results
Combined with the influencing factors being tested, the orthogonal test design scheme of L16(45) is adopted. Table 2 shows the specific factors and level.Table 3 shows the experimental results and the range analysis.
The results of the range analysis in Table 3 show the following:
a) The values ofRof the orthogonal test are arranged from large to small: C > D > B > A > E. That is, during the synthesis reaction, the order of influence on the KN-PC is as follows: acid-ether ratio > reaction temperature > chain transfer agent dosage > initiator dosage > acrylamide dosage.
b) The orthogonal experimental results show that typical conditions of KN-PC include a reaction temperature of 40 ℃, acid-ether ratio of 4, acrylamide dosage of 0.7 mol, and dosages of chain transfer agent and initiator of 1.5% and 0.5%, respectively.
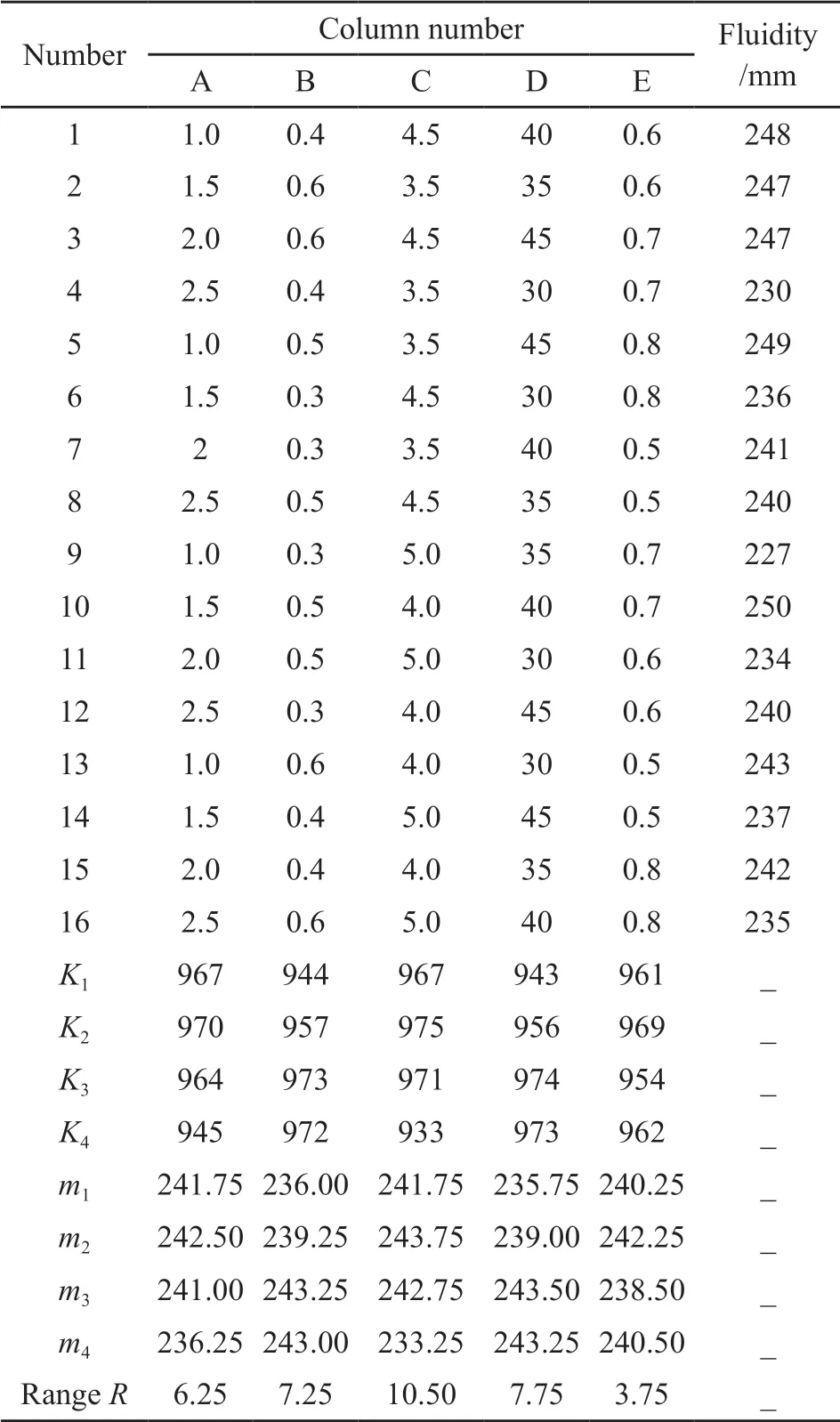
Table 3 L16(45) experiments and analysis
3.2 Single factor optimization experiment of KN-PC
To obtain the optimized synthesis conditions,based on the orthogonal test results, the effect of each single factor on the performance of the KN-PC is investigated.
With an increase of reaction temperature, the fluidity of the composite paste first increases from 37 to 43 ℃, and then decreases from 43 to 47 ℃, and the highest flow value is 253 mm. This result indicates that at 43 ℃, the optimized clay-resistant can be obtained in the synthesized KN-PC. In the following experiment,the temperature is set at 43 ℃.
3.2.1 Effect of acid-ether ratio
To investigate the effect of the acid-ether ratio on the KN-PC, the amounts of chain transfer agent and initiator was set as 0.5% and 1.5%, respectively.
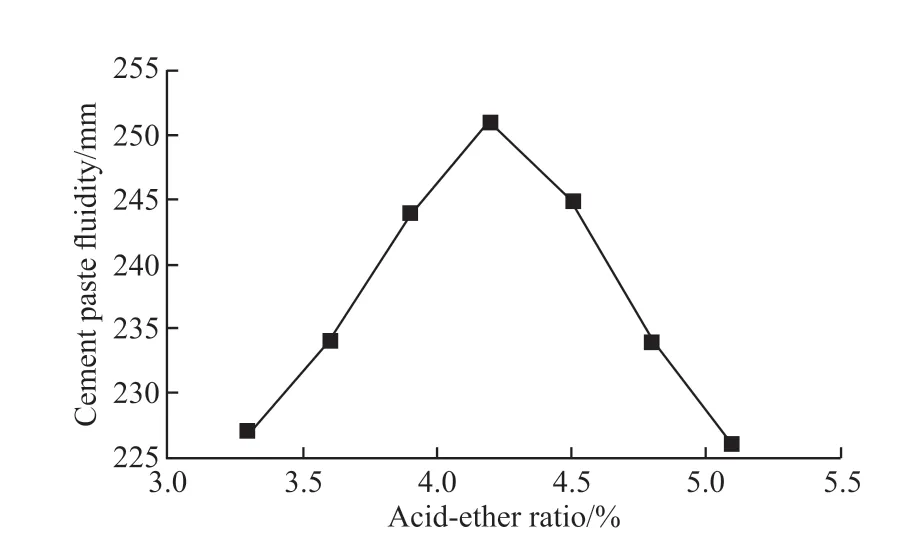
Fig.2 Effect of acid-ether ratio on the dispersibility of KN-PC
Fig.2 shows that with an increase of the acidether ratio, the fluidity of the composite paste first increases and then decreases, and the highest flow value is 251 mm, with an acid-ether ratio of 4.2. This result indicates that this acid-ether ratio, at which the composite paste flow value reaches a maximum, gives the optimized clay-resistant in the synthesized KN-PC.
The acid-ether ratio mainly affects the number of carboxyl groups in the KN-PC[16]. When the acidether ratio is less than 4.2, with an increase in the acid-ether ratio, the number of carboxyl groups in the KN-PC increases, along with the number of carboxyl groups between the macromonomers. Furthermore, a higher acid-ether ratio leads to a higher density of the polyether side chain, whose degrees of freedom may also increase. Also, with the increase in the acid-ether ratio, the number of encapsulated carboxyl groups should increase, leading to an improved degree of polymerization. All of these effects will increase the dispersion ability of the composite paste. Conversely,when the acid-ether ratio is greater than 4.2, some of the carboxyl groups cannot participate in the reaction,and the excess carboxyl groups exist in the form of groups together with the density of the side chain of polyether. These factors lead to a lower degree of polymerization, which leads to a decrease in the waterreducing effect of KN-PC.
3.2.2 Effect of amount of chain transfer agent
To investigate the effect of the amount of chain transfer agent on the KN-PC, the following reaction conditions are selected: an acid-ether ratio of 4.2, and an amount of initiator of 1.5%.
The experimental results shows that with an increase in the chain transfer agent dosage, the fluidity of the composite paste first increases at 0.36% to 0.45%, and then decreases at 0.45% to 0.54%, and the highest flow value is 253 mm, with the chain transfer agent dosage of 0.45%. This result indicates that this chain transfer agent dosage, at which the composite paste flow value reaches a maximum, gives the optimized clay-resistant in the synthesized KN-PC.
The amount of chain transfer agent mainly affects the length of the polymer molecular chain of the KNPC. When the amount of chain transfer agent is less than 0.45%, an increase in the amount of chain transfer agent decreases the length of the polymer chain. In the course of polymerization, the number of free radicals transferring to the chain transfer agent is small, and cannot prevent the extension of the molecular chains of the polymers, which makes the molecular weight of the polymers larger. When the amount of chain transfer agent is greater than 0.45%, the number of free radicals transferred to the chain transfer agent during the polymerization process is small, and the molecular chain of the polymer cannot be prevented from becoming elongated, so that the molecular weight is too large. This large weight leads to a decrease in the water-reducing effect of the KN-PC[17].
3.2.3 Effect of amount of initiator
To investigate the effect of the amount of initiator on the KN-PC, the following reaction conditions are selected: an acid-ether ratio of 4.2, and an amount of chain transfer agent of 0.45%.
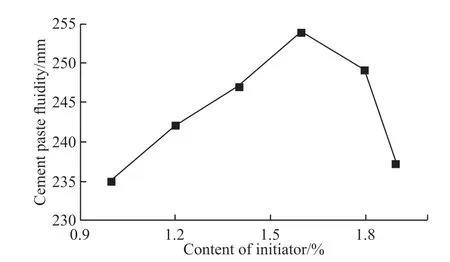
Fig.3 Effect of initiator on the dispersibility of KN-PC
Fig.3 shows that with an increase in the amount of initiator, the fluidity of the composite paste first increases and then decreases, and the highest flow value is 254 mm, with the initiator dosage of 1.6%. This result indicates that this amount of initiator, at which the composite paste flow value reaches a maximum,gives the optimized clay-resistant in the synthesized KN-PC.
The amount of initiator mainly affects the number of free radicals produced in the KN-PC[18]. When the amount of initiator is less than 1.6%, the molecular weight of the polymer is too large because the number of free radicals produced is insufficient, and the surface activity of the polymer decreases as the number of free radicals increases. All of these factors decrease the dispersion ability of the C. Conversely, when the initiator dosage is greater than 1.6%, the number of free radicals generated is too large and the molecular weight of the polymer is too small, and the phenomenon of flocculation occurs easily with the composite paste,leading to a decrease in the water-reducing effect of the KN-PC.
3.2.4 Effect of amount of phosphate ester clay
resistant monomer
To investigate the effect of the amount of phosphate ester clay-resistant monomer on the KN-PC,the following reaction conditions are selected: an acidether ratio of 4.2, and amounts of chain transfer agent and initiator of 0.45% and 1.6%, respectively.
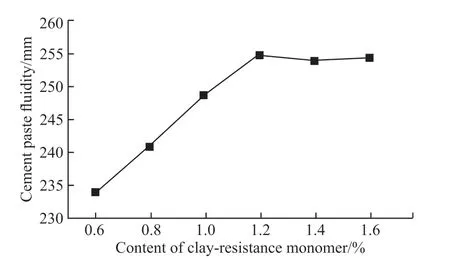
Fig.4 Effect of amount of phosphate ester clay-resistant monomer on the dispersibility of KN-PC
Fig.4 shows that with an increase in the amount of phosphate ester clay-resistant monomer, the fluidity of the composite paste first increases and then decreases slowly, and the highest flow value is 255 mm, with a number of monomers of 1.2 mol. This result indicates that this number of monomers, at which the composite paste flow value reaches a maximum, gives the optimized clay-resistant in the synthesized KN-PC.
When the number of monomers is less than 1.2 mol, the number of phosphate groups increases along with the number of phosphate ester clay-resistant monomers. It can not only reduce the adsorption and retarding effect of MMT on KN-PC[19], but also can crosslink the polymer and monomer in the alkaline environment of cement hydration. All of these factors increase the dispersion ability of the C. When the number of monomers is greater than 1.2 mol, the crosslinking density of the polymer decreases because the phosphates cannot cause the crosslinking reaction between the polymers and the monomers, and the steric hindrance decreases, leading to a decrease in the waterreducing effect of KN-PC.
3.2.5 Effect of dripping time of Solution B
To investigate the effect of the dripping time of Solution B on the KN-PC, the following reaction conditions are selected: an acid-ether ratio of 4.2,dosages of chain transfer agent and initiator of 0.45%and 1.6%, respectively, a dripping time of Solution A of 2 h, and amounts of phosphate ester clay-resistant monomer of 1.2 mol.
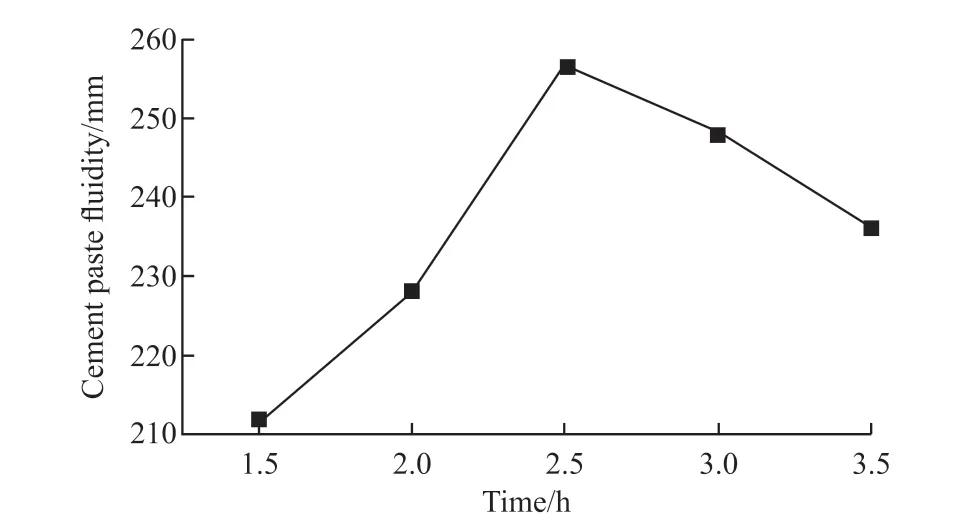
Fig.5 Effect of dripping time of Solution B on the KN-PC
Fig.5 shows that with an increase in the dripping time of Solution B, the fluidity of the composite paste first increases and then decreases, and the highest flow value is 257 mm, with a dripping time of 2.5 h. This result indicates that this dripping time, at which the composite paste flow value reaches a maximum, gives the optimized clay-resistant in the synthesized KN-PC.
The dripping time of Solution B mainly affects the molecular weight of the KN-PC. When the dripping time of Solution B is less than that of Solution A (2 h),after Solution B is completely added, the amount of reactant in the reaction is reduced because the added Solution A cannot fully act under the chain transfer agent or the reducing agent, which reduces the waterreducing effect of the synthesized KN-PC. In contrast,when the dripping time of Solution B is longer than that of Solution A, the concentration of the chain transfer agent added per unit time increases, and the molecular weight of the polymer decreases as the dripping time of Solution B increases. Furthermore, when the dripping time of Solution B is greater than 2.5 h, the molecular weight of the KN-PC continues to decrease, which is not conducive to the adsorption of the C on the surface of the C and MMT, causing the deterioration of the clay-resistant of the KN-PC.
3.2.6 Analysis of single factor results of KN-PC
Based on the above analysis, the optimum conditions of synthesis are as follows: a reaction temperature of 43 ℃; an acid-ether ratio of 4.2;amounts of sodium methyl vinyl sulfonate and initiator of 0.45% and 1.6%, respectively; amounts of acrylamide and phosphate ester clay-resistant monomer of 0.7 mol and 1 mol, respectively; and dripping times of Solution A and Solution B of 2 and 2.5 h,respectively.
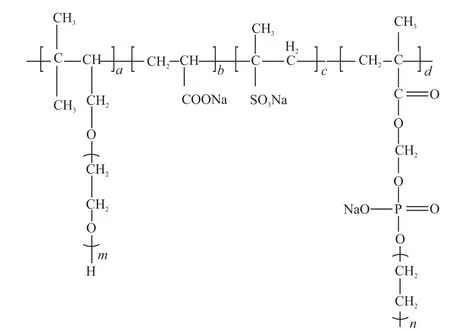
Fig.6 Structural diagram of the KN-PC
Based on the above analysis, the synthesized KN-PC, gives the optimized clay-resistant and fluidity of the composite paste. The KN-PC can be obtained by introducing carboxyl (-COOH), sulfonic (-SO3H),phosphate (-PO3H2), hydroxyl (-OH), carbonyl(-COOR), polyoxyethylene ether (-CH2CH2O-)n,ether (C-O-C), and other functional groups from each monomer. Fig.6 show the general formula of the molecular structure diagram of the KN-PC.
4 Results and discussion
To compare the effect of the KN-PC and the PC on the fluidity of composite paste with the same MMT dosage, a series of macro and micro expriments are conducted.
4.1 Dispersion analysis
The fluidity of the composite pastes containing the KN-PC and PC were examined according to the standard method GB/T 8077-2012[15]of China. The composite paste consists of C and MMT replacing certain mass of the C. The C is replaced by the MMT according to the internal mixing method, and the amount of KN-PC or PC is 0.18% of the mass of C.
Fig.7 shows that the fluidity of the composite paste with different contents of MMT and with the PC and KN-PC decreasing by 49.6% and 18.7%,respectively. The effect of the KN-PC on the fluidity of the composite paste is smaller than that of the PC with the same amount of MMT. The main reasons are as follows: First, the MMT has strong water absorption and expansion abilities, and its interlayer adsorption force is small[9]. As it comes into contact with water,it absorbs water and swells, along with decreasing the free water content, all of which increases the viscosity of the composite paste, and thus decreases the fluidity of the composite paste. Second, the MMT and C have a competitive adsorption relationship in regards to the KN-PC[20]. The MMT has a higher capacity of adsorption to the KN-PC than the C does[21].
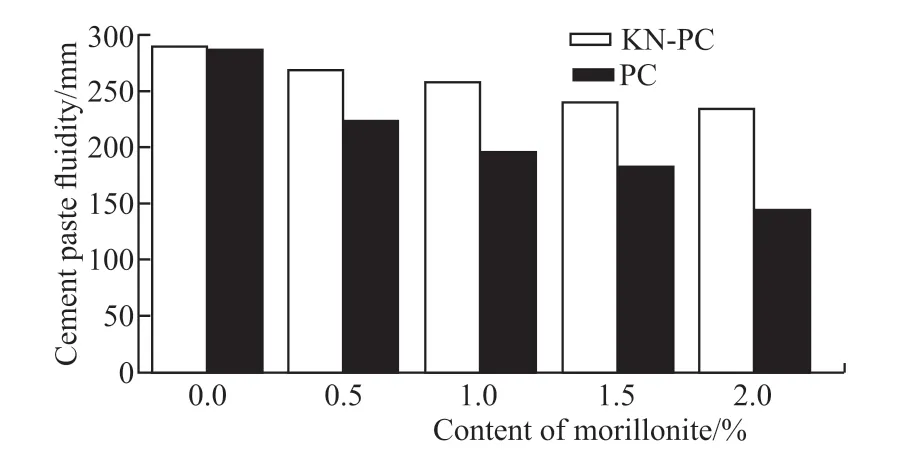
Fig.7 Fluidity of composite pastes on different content MMT by the KN-PC and PC
4.2 Adsorption analysis
To compare the difference in the adsorption ability of the MMT and C on the KN-PC and PC,different concentrations of KN-PC and PC solutions are prepared with deionized water, and samples of MMT are treated with KN-PC and PC solutions and tested by a TOC analyzer.
Fig.8 shows the adsorption amount results. The adsorption of the KN-PC and PC on the surface of C and MMT first increases and then remains constant as the KN-PC and PC concentration increases. The main reasons are as follows: When the KN-PC and PC concentration are very small, the surfaces of the MMT and C have a large reaction space, and the KNPC and PC is easily adsorbed by the MMT and C. With the increase of the KN-PC and PC concentration, the reaction space on the surface of the MMT particles becomes more occupied and might even reach saturation. It becomes difficult for the KN-PC and PC to adsorb on the MMT and C, so the adsorption dosage of the KN-PC and PC on the MMT and C remains basically unchanged.
Fig.8(a) shows that when the concentrations of the KN-PC and PC remains the same, the amount of KN-PC adsorbed by the C is greater than that of the PC. The reasons are as follows: First, both the KN-PC and PC are anionic surfactants. The C has a positive charge on the surface of its particles in the initial stage of hydration, and the C adsorbs the the KN-PC and PC. Second, the KN-PC introduces an anionic phosphate group and a sulfonic acid group with strong electronegativity, so that the adsorption ability of the C to the KN-PC is greater than that to the PC.
Fig.8(b) shows that the adsorption dosage of MMT to the KN-PC is less than that to the PC, when the concentrations of the KN-PC and PC are the same.The reasons are as follows: First, the phosphate group is negatively charged, which will form a competitive adsorption relationship to MMT with the KN-PC; the structures of the P-O tetrahedron and Si-O tetrahedron are similar. Compared with PC, the KN-PC has a low sensitivity to the MMT. Second, the MMT can adsorb the phosphate group by a van der Waals force, which increases the negative charge of the MMT and reduces its adsorption on the KN-PC.
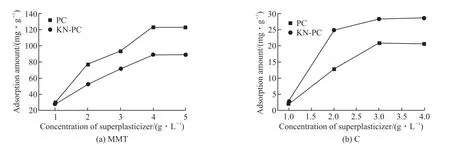
Fig.8 Adsorption amount of the MMT and C to KN-PC and PC with different concentration
4.3 FT-IR analysis
Fig.9 shows the FT-IR of MMT and C after the KN-PC and PC treatments, respectively. The figures show the absorption peaks of the MMT: a stretching vibration absorption peak of -OH at 3 441 cm-1, a strong band of a Si-O stretching vibration absorption peak at 1 028 cm-1, and the bending vibration absorption peaks of Al-O and Si-O at 400 to 530 cm-1. The FT-IR of MMT and C after the KN-PC treatment are almost the same. The peak height of the infrared spectrum of MMT after H2O treatment at 798 cm-1is larger than that of KN-PC and PC treatment, and when the absorption peak is at 2 990 cm-1, the peak height is less than PC and greater than KN-PC. The PC characteristic absorption peaks appear in the FT-IR of MMT after the PC treatment: the vibration absorption peaks of -CH-,-CH2- and -CH3at 2 980-3 000 cm-1.
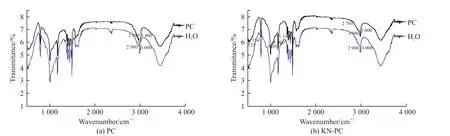
Fig.9 FT-IR spectra of MMT treated by the PE
The KN-PC characteristic absorption peaks appear in the FT-IR of MMT after the KN-PC treatment: the vibration absorption peaks of -SO3H at 532, 554 and 1 190 cm-1,the vibration absorption peaks of -PO43-at 1 050,1 069 cm-1, and the C-O-C stretching vibration absorption peak at 2 760 cm-1.The absorption peak at 2 990 cm-1, the peak height of MMT after H2O treatment is greater than KN-PC. It can be concluded that the carboxylic acid, phosphate and sulfonic acid groups of KN-PC entered the interlayer of the MMT.
Since the test sample is treated with KN-PC and PC, the surface of the MMT is rinsed with deionized water, so that no PC and KN-PC remains on the surface of the sample. It can be concluded that the KN-PC and PC entered the interlayer of the MMT.
4.4 XRD analysis
The interlayer spacing of the MMT in both groups is measured by XRD. Fig.10 shows that the surface diffraction peak 2θ of the MMT is 5.761. Using the Bragg equation, 2dsinθ=nλ, the interlayer spacingdcan be calculated; the interlayer spacing of MMT is 1.49 nm. The surface diffraction peaks 2θ of the MMT treated by PC and KN-PC are 4.826 and 5.732,respectively. The interlayer spacings with KN-PC and PC are 1.78 and 1.50 nm, respectively.
The surface diffraction peaks of the MMT treated with the KN-PC and PC are shifted to a lower angle than the surface diffraction peak of the dried MMT,and the interlayer spacing of the MMT treated with PC increases by 0.29 nm. The MMT belongs to the type 2:1 of interlayer structure silicate minerals[22]. Fig.11,Fig.12 and Fig.13 show MMT’s interlayer structural characteristics. The interlayer -OH bond and H2O result in hydrogen bonding, and the oxygen atom on the side chain EO group of the KN-PC or PC, and the interlayer of H2O also produce hydrogen bonding, making PC and MMT difficult to separate[23]. Thus, the MMT adsorbs the KN-PC and PC.
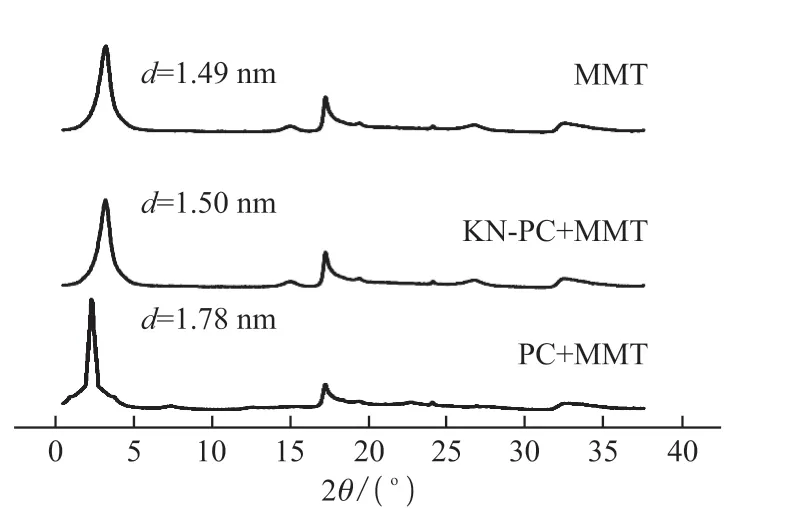
Fig.10 XRD results
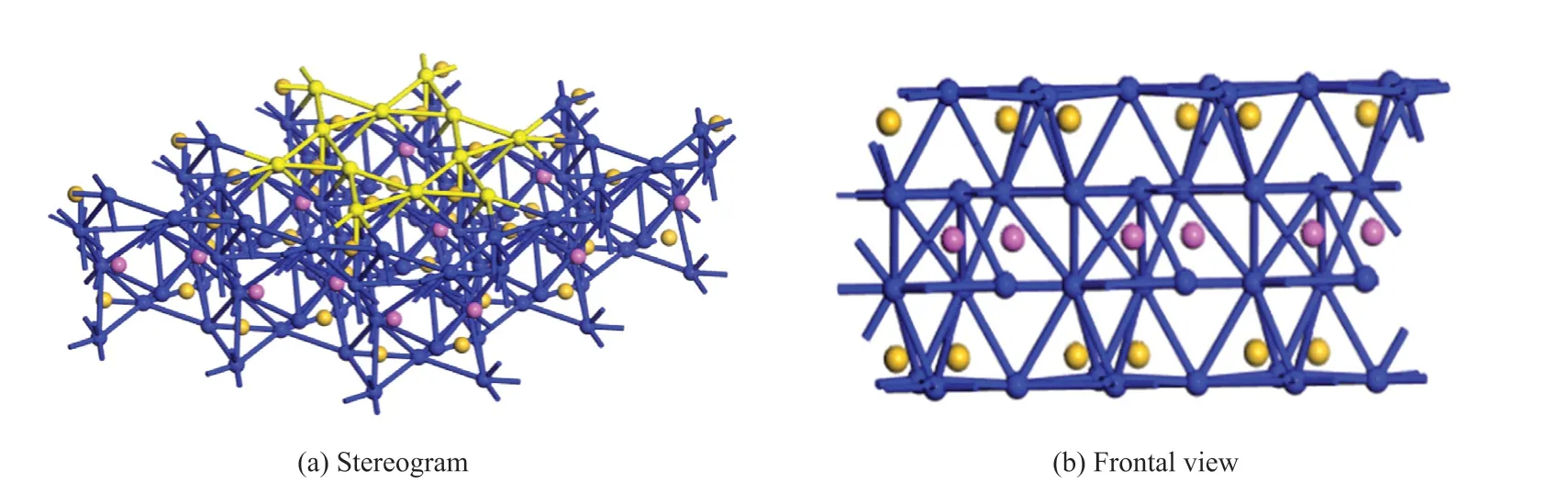
Fig.11 Crystal structure diagram of MMT
Compared with the interlayer spacing of the dried MMT, the interlayer spacing of MMT treated with KN-PC increased by 0.01 nm. The carboxylic acid, phosphate and sulfonic acid groups of KN-PC entered the interlayer of the MMT. The reason is that the KN-PC polyether side chain belongs to the segment structure, and the chain structure makes the molecular chain activity relatively free, which reduces the interresistance effect of the KN-PC, so that the KN-PC has difficulty existing in the MMT. The side chain formed by the introduction of acrylamide and sodium methyl vinyl sulfonate in the KN-PC is short, and the short side chain cannot enter the MMT interlayer, which is consistent with the research results of Planket al[24,25].
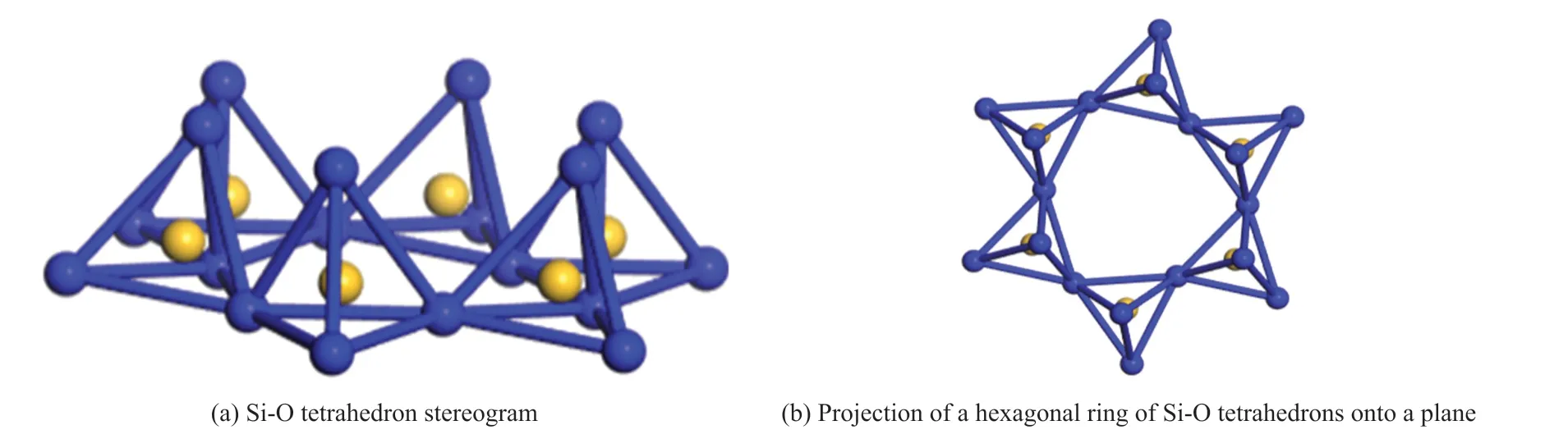
Fig.12 Si-O tetrahedron
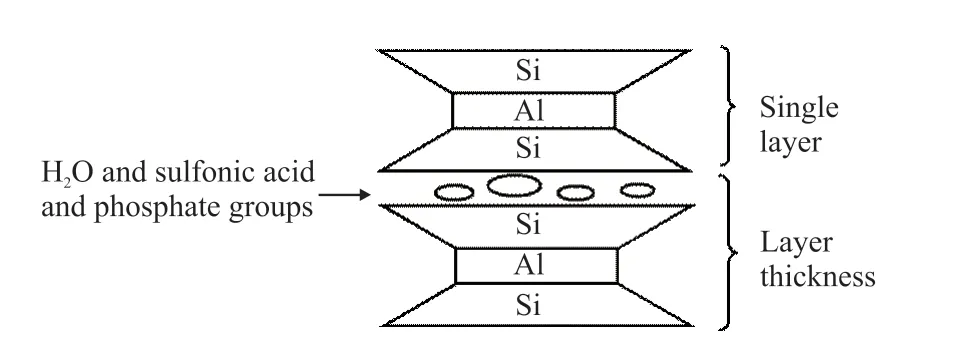
Fig.13 Model diagram of crystal interlayer of MMT(2:1)
4.5 Low adsorption and clay-resistant mechanism of the synthesized KN-PC
In the initial stage of hydration, the surfaces of the C particles are positively charged, and the surface of the MMT particles are negatively charged. The results of TOC test show that MMT and C will adsorb the mixed PC, and XRD test shows that PC can be intercalated on MMT, which increases the layer spacing of MMT. There will be three different forms of PC adsorbed by MMT: full adsorption, semi adsorption and non adsorption (Fig.14). With the adsorption of MMT on PC, the amount of PC is reduced, so that the amount of PC adsorbed by MMT is reduced.
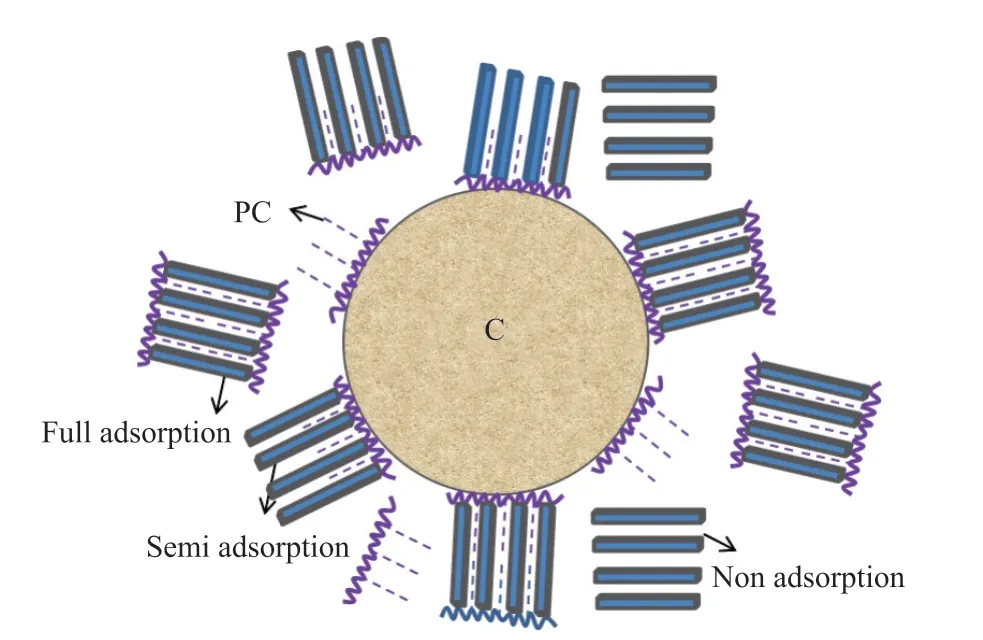
Fig.14 Schematic diagram of mechanism of the PC and MMT interaction
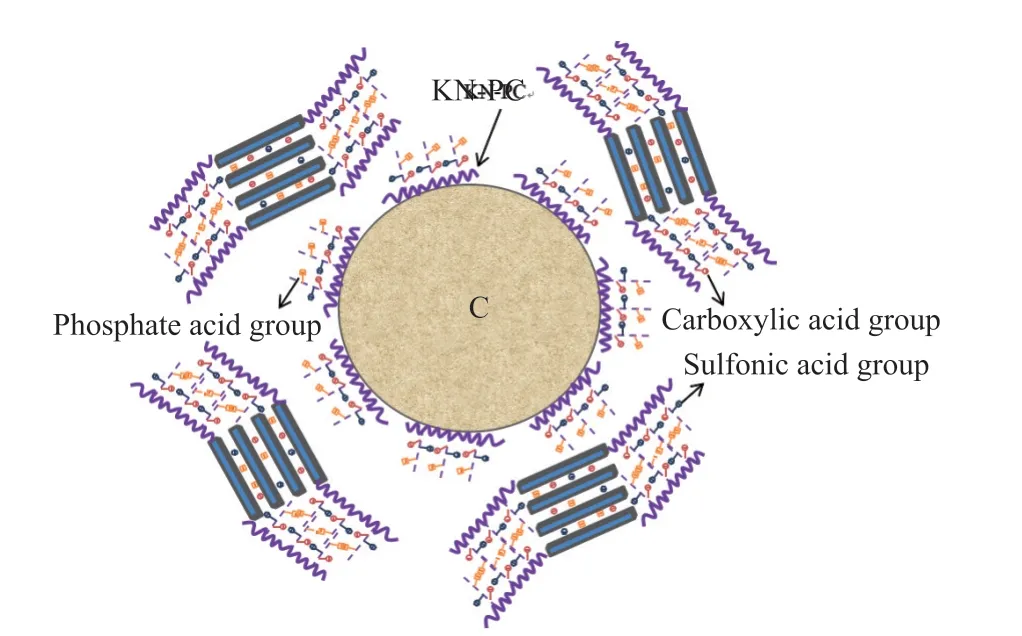
Fig.15 Schematic diagram of mechanism of the KN-PC and MMT interaction
Fig.16 Schematic diagram of the mechanism of the KN-PC
The TOC test shows that the C has the capacity to adsorb KN-PC. The crosslinking reaction of the phosphate ester on the main chain of KN-PC enhances the steric hindrance and decomposes the molecule formed into a strongly electronegative carboxylic acid and phosphate. The carboxylic acid group has great dispersibility, water reduction ability, and sustained release ability. The carboxylic acid, phosphate and sulfonic acid groups can also complex with metal ions and exchange ions with the MMT, thereby reducing the adsorption of the high-valence metal ions in the composite paste on the KN-PC. Enhancing the electronegativity of the KN-PC increases the adsorption capacity of the C to the KN-PC. The more the KNPC is adsorbed by the C, the greater the increase in the electrostatic repulsion between the C particles (Fig.15).
Fig.16 shows that when the surface tension of the water and cement pores is reduced, the water can more easily enter the cement particles. The water contained in the pores is more easily removed, so that the flocculation structure between the C particles is destroyed, and the fluidity of the composite paste is improved.
5 Conclusions
a) Based on the principle of free radical polymerization, acrylic acid and isopentenol polyoxyethylene ether are the main reaction materials.The KN-PC with the optimized performance can be obtained under the following conditions: a reaction temperature of 43 ℃; an acid-ether ratio of 4.2;amounts of sodium methyl vinyl sulfonate and initiator of 0.45% and 1.6%, respectively; amounts of acrylamide and phosphate ester clay-resistant monomer of 0.7 and 1.2 mol, respectively; and dripping times of Solution A and Solution B of 2 and 2.5 h, respectively.
b) The fluidity of the composite paste and the test of adsorption amount show that the adsorption capacity of the MMT to the PC is higher than that to the KN-PC.With an increase in clay content, the fluidity reduction rate of the KN-PC is much lower than that of the PC.
c) The FT-IR and XRD tests show that the side chains of the KN-PC are short, and the short side chains cannot enter the interlayer of the MMT. The increase of electrostatic repulsion and the decrease of steric hindrance of the polyether side chains making the increase of interlayer spacing of the MMT smaller.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
- Fracture Behavior and Processing Deformation of C71500 Cupronickel Alloy during Hot Tensile Deformation
