Effects of Different Electrolytes on Stress Corrosion Properties of 2A12 Aluminum Alloy
2021-06-14JIANGGenBAIZhenhaiLUOBinghuiWANGShuaiHEChuan
JIANG Gen, BAI Zhenhai,2*, LUO Binghui,2, WANG Shuai, HE Chuan
(1.School of Materials Science and Engineering, Central South University, Changsha 410083, China; 2. Key Laboratory for Nonferrous Materials(MOE), Changsha 410083, China)
Abstract: The stress corrosion cracking (SCC) behaviors of 2A12 aluminum alloy after annealing treatment were studied by slow strain rate testing (SSRT), electrochemical polarization measurement, scanning electron microscope (SEM), energy dispersive spectrometer (EDS) and transmission electron microscopy(TEM). Various concentrations of NaCl, H2SO4 and HCl aqueous solution were prepared to act as the corrosive solution. The experimental results show that regarding the SCC, 2A12 alloy performs best in NaCl solution but worst in HCl solution and intermediately between the above mentioned two cases in H2SO4 solution. For the SSRT carried out in room temperature, there is a higher decrease in elongation without large strength loss for the alloy immersed in NaCl solution. With the test conducted in H2SO4 solution, there is a higher strength loss and a relatively less loss of elongation compared to the one immersed in NaCl solution. With the test conducted in HCl solution, there is a relativel level loss of strength and elongation compared to either result carried out in NaCl solution or H2SO4 solution.
Key words: 2A12 aluminum alloy; stress corrosion cracking; corrosive electrolyte; SSRT
1 Introduction
2xxx Al-Cu-Mg alloys are applied in aerospace and automotive industries due to their excellent combination of high strength, low density, excellent toughness, and good machinability[1]. However, the application of Al-Cu-Mg alloys is limited by their stress corrosion cracking (SCC), occurring in the ocean or atmosphere[2,3]. So, it is significant to investigate the machanism of the SCC in Al-Cu-Mg alloys, aiming to reduce their SCC susceptibility. Many factors, including environmental media, heat treatment, internal stress and applied stressetc, can affect the SCC susceptibility of Al alloys by changing their electrochemical and mechanical behavior during the service[4]. In the past few decades, a lot of research was carried out to solve the stress corrosion cracking problem in high-strength Al alloys. These solutions mainly focus on optimizing the composition or the heat treatment process of the alloy, introducting surface treatment, and eliminating residual stress[5-9]. While these works were carried out in neutral NaCl solutions with different pH values,and few were carried out in acidic solution without the presence of Cl-.
In the research, annealed 2A12 aluminum alloy was used as the research object to rule out the internal stress in the alloy, which may have a great influence on the SCC of the alloy. Slow strain rate testing (SSRT),electrochemical polarization measurement, scanning electron microscope (SEM) equipped with energy dispersive spectrometer (EDS) and transmission electron microscopy (TEM) were used to investigate the microstructure, mechanical properties and corrosion behaviors of the alloy. In the research, the SCC properties of 2A12 alloy were studied according to the tests carried out in three kinds of solutions (NaCl,H2SO4, HCl). The effects of Cl-and H+on the SCC properties of the alloy were analyzed.
2 Experimental
Homogenized commercial AA 2A12 Al ingot(chemical composition (wt%): 4.19Cu-1.48Mg-0.53Mn-0.16Fe-0.08Si-0.01Ti) was hot rolled (450 ℃,75 % reduction, from 10 to 2.5 mm), and then annealed at 380oC for 2 h to eliminate the internal stress.
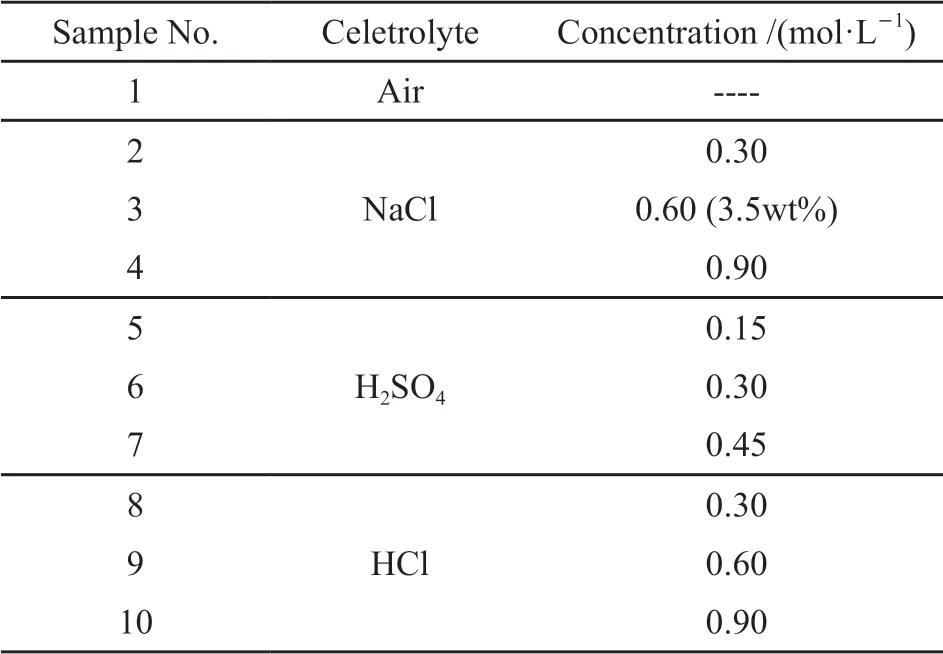
Table 1 Corrosion solutions in the experiment
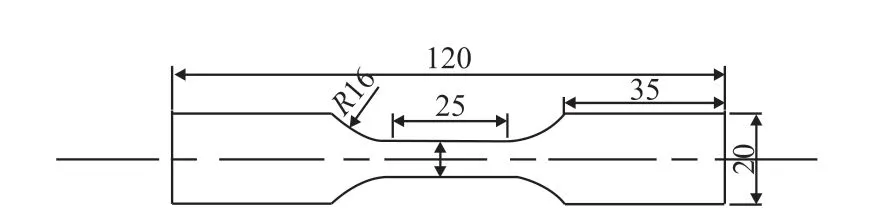
Fig.1 Schematic diagram of the SSRT tensile specimen
The SSRT was carried out in different solutions to evaluate the SCC behavior of the alloy (Table 1).Specimens for the SSRT were machined in accordance with GB/T 228.1- 2010 and GB/T 15970.7 - 2000,whose thickness is 2 mm (Fig.1). A RWS50-CRIMS machine was used to conduct SSRT with a slow strain rate of 6.67×10-6s-1at room temperature. A 150 N force was loaded to eliminate mechanical errors before starting the test. SCC susceptibility index (ISSRT) is the result of mathematical treatment of various mechanical properties obtained by SSRT experiments, which can reflect the SCC susceptibility compared with the single mechanical property index[10]. In the research, theISSRTwas calculated by Eq.(1) according to HB 7235-95. The larger value ofISSRTrepresents poorer SCC resistance:
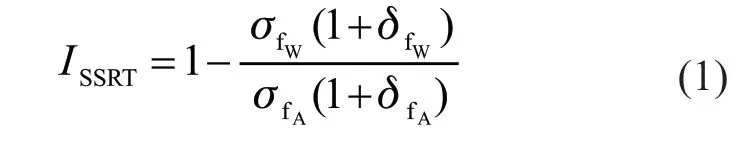
whereσfWis the fracture strength of specimen conducted in corrosive media (MPa),σfAis the fracture strength of specimen conducted in inert medium (dry air) (MPa),δfWis the elongation at break of specimen conducted in corrosive media (%), andδfAis the elongation at break of specimen conducted in dry air (%).
Siron 200 scanning electron microscope was used to observe the fracture morphology and corrosion morphology. For transmission electron microscope(TEM) study, the sample was mechanically thinned down to nearly 80 μm slice on SiC papers. Discs with 3 mm diameter were punched from the slice and thinned by a twinjet electrolytic polishing unit (MTP-1) with an applied potential of 20 V in 30 vol% nitric acid + 70 vol% methanol solution at -25--30 ℃.Then the microstructural features were characterized by TEM. Electrochemical polarization tests were carried out by AUTOLAB M204 electrochemical workstation at 25 ℃. A three-electrode structure was used to inverstigate the eletrochemical behaviors of the alloy: an area of 1 cm2made from the alloy was the work electrode, a platinum electrode was the counter electrode and an SCE (saturated calomel electrode)was the reference electrode. The experiments were conducted in different solutions mentioned at Table 1. The applied scanning voltage was from -1.0 V to-0.4 V and the scan rate was 0.002 V/s. The corrosion potential (Ecorr) and corrosion current density (icorr),were calculated by NOVA software.
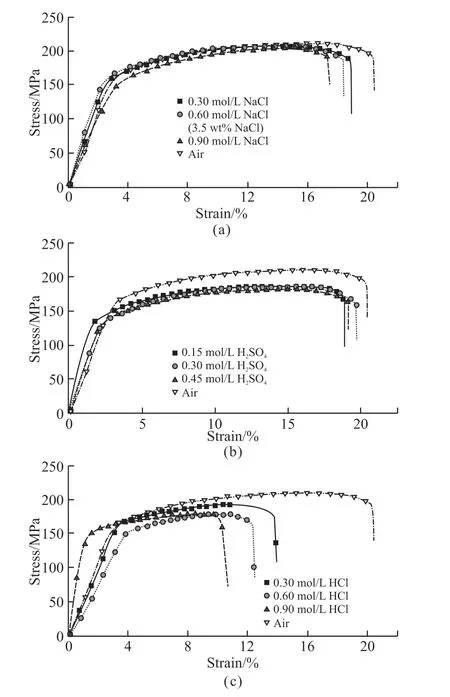
Fig.2 Stress-strain curves of 2A12 aluminum alloy tested in various corrosion solutions: (a)NaCl, (b) H2SO4, (c)HCl
3 Results
3.1 Slow strain rate testing
Fig.2 shows the stress-strain curves of 2A12 aluminum alloy subjected to air or various corrosive solutions with different concentrations. Table 2 gives the SCC susceptibility, tensile properties andISSRT.Fig.2 and Table 2 show various SCC susceptibility of 2A12 aluminum alloy in different corrosion medium.For the solutions that have the same concentration but different kinds of solute, the SSRT susceptibility of the alloy was as follows: HCl>NaCl>H2SO4; for the certain electrolyte with different concentrations, the SSRT susceptibility of the alloy increases with the increase in concentration. The strength and elongation of 2A12 aluminum alloy is 194 MPa and 20.5% respectively,when the test was carried out in the air. When the samples were tested in NaCl solutions with different concentrations, the average strength of the alloy is 187.3 MPa, and the average elongation is 18.2%. It suggested that NaCl solution causes little effect on the strength of the alloy, but has obvious influence on the elongation. There is about 11.2% loss in the elongation of the sample tested in NaCl solution compared with that in the air. When the samples were tested in H2SO4solutions with different concentrations, the average strength and average elongation is 164 MPa and 19.24%. Compared to the test carried out in the air,there is about 6.1% lose in the elongation and 15.5%strength loss.
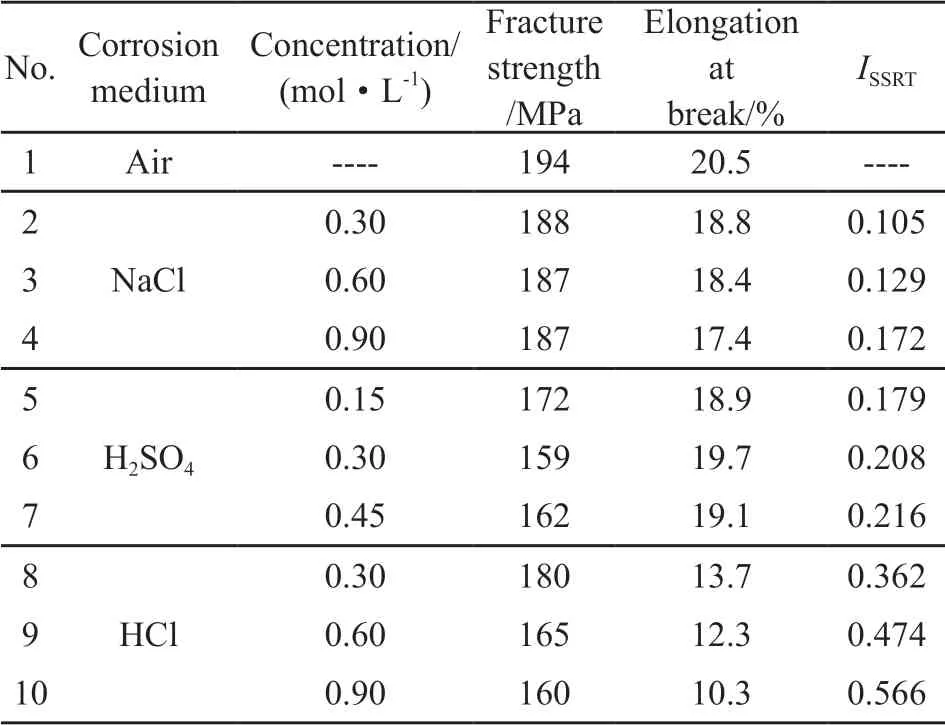
Table 2 Stress corrosion cracking index of 2A12 aluminum alloy
3.2 Fractography
Fig.3 shows the SEM images pertaining to the fractography of the specimens after the SSRT. Fig.3(a)shows the SSRT fracture of the alloy tested in the air.It reveals that the intergranular fracture dominates the main morphology of the alloy. For the alloy tested in NaCl solution, the fracture in the middle area (transient fracture zone) (Fig.3(b)) is similar to that in the air,while a cleavage plane dominates the fracture in the perpheral area (SCC zone, marked by white line).Both of the alloy that were tested in H2SO4solution(Fig.3(c)) and HCl solution (Fig.3(d)) feature similar fracture morphology after the SSRT. The cleavage planes are dominant in the peripheral area (the SCC zone), while fracture in the middle area (transient fracture zone) is characterized by ductile transgranular type. The number of dimples in the fracture surface of the alloy tested in H2SO4solution is much larger than that in HCl solution.
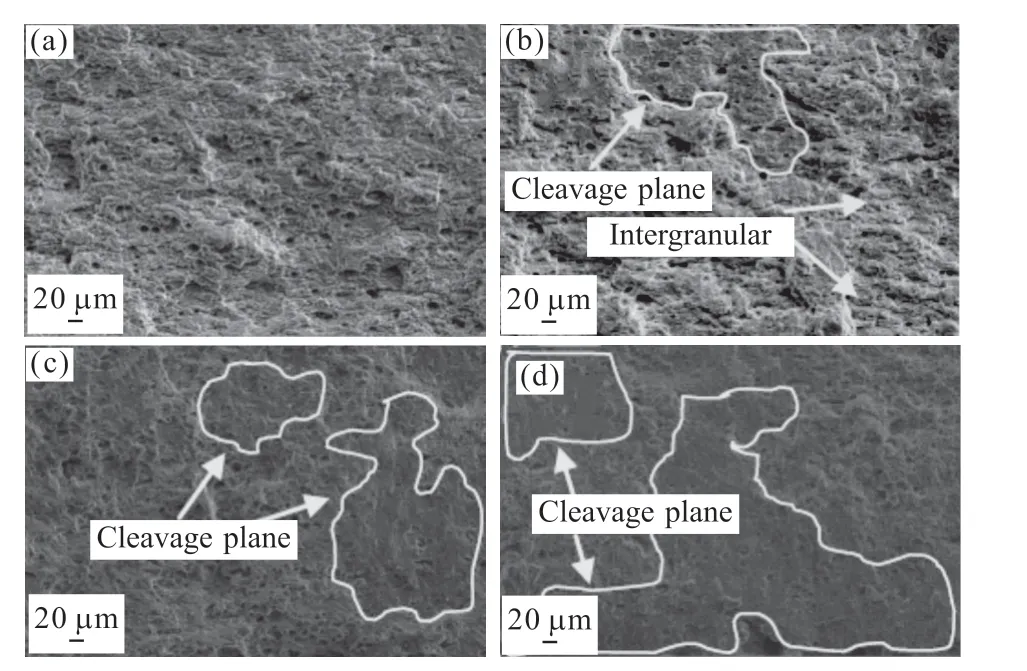
Fig.3 SEM images regarding the fracography of 2A12 aluminum alloy after SSRT in various corrosion solutions: (a)Air,(b)3.5 wt% NaCl (0.6 mol/L), (c) 0.3 mol/L H2SO4, (d)0.6 mol/L HCl
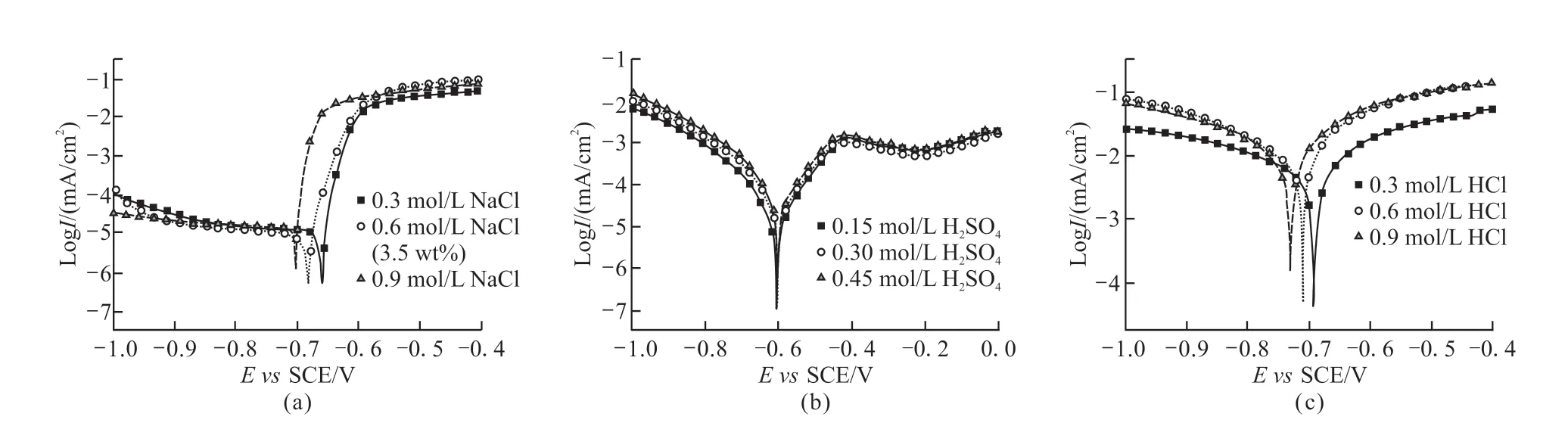
Fig.4 Polarization curves of the 2A12 aluminum alloy in various solutions:(a)NaCl, (b) H2SO4, (c)HCl
3.3 Potentiodynamic polarization
Fig.4(a-c) show the polarization curves of 2A12 aluminum alloy in various electrolytes. The electrochemical data, including the corrosion potential(Ecorr) and corrosion current density (icorr) are given in Table 3. The samples tested in H2SO4solutions show three typical changing steps at the anodically polarized curves: active-passive-transpassive (Fig.4(b)). TheEcorrare similar, but theicorrincreases with the increasing concentration. The samples tested in NaCl and HCl solutions show typical activated metal dissolution at the anodic polarized curves (Fig.4). Even though the polarization curves regarding these two cases show similar trend at the anodically polarized part, theicorrobtained from the case of HCl solutions is double to triple than that in NaCl solutions. Besides, the relative magnitude regarding theEcorrof the alloy tested with different electrolytes is H2SO4>NaCl>HCl. In addition, with the increase in Cl-concentration, theEcorrdecreases and theicorrincreases.
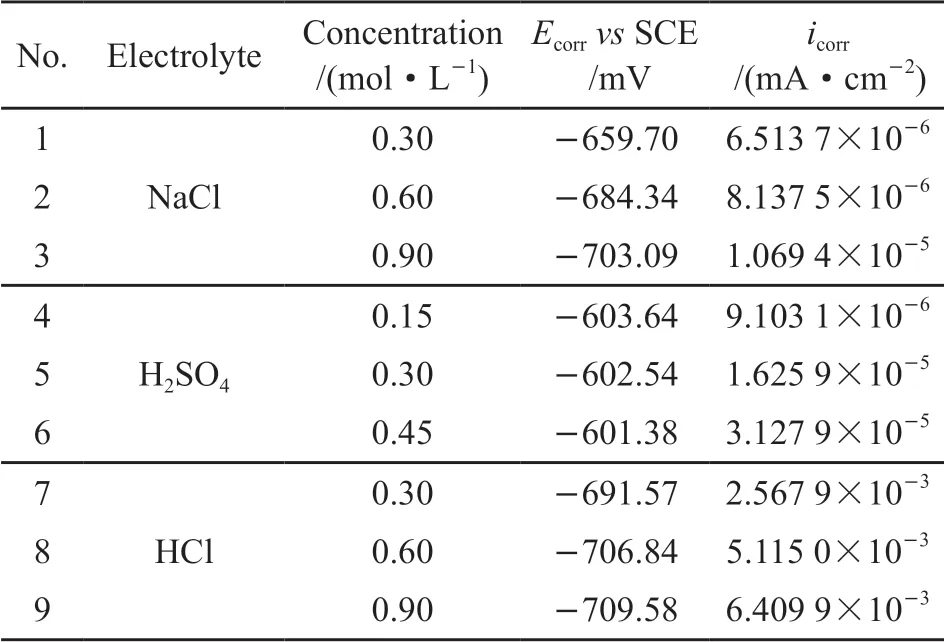
Table 3 Electrochemical data of the 2A12 aluminum alloy in various corrosion solutions
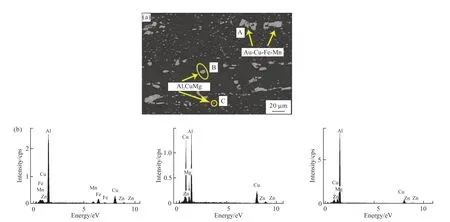
Fig.5 SEM images and EDS results of the annealed 2A12 aluminum alloy: (a)microstructure of the alloy; (b) EDS results corrspnding to (a)
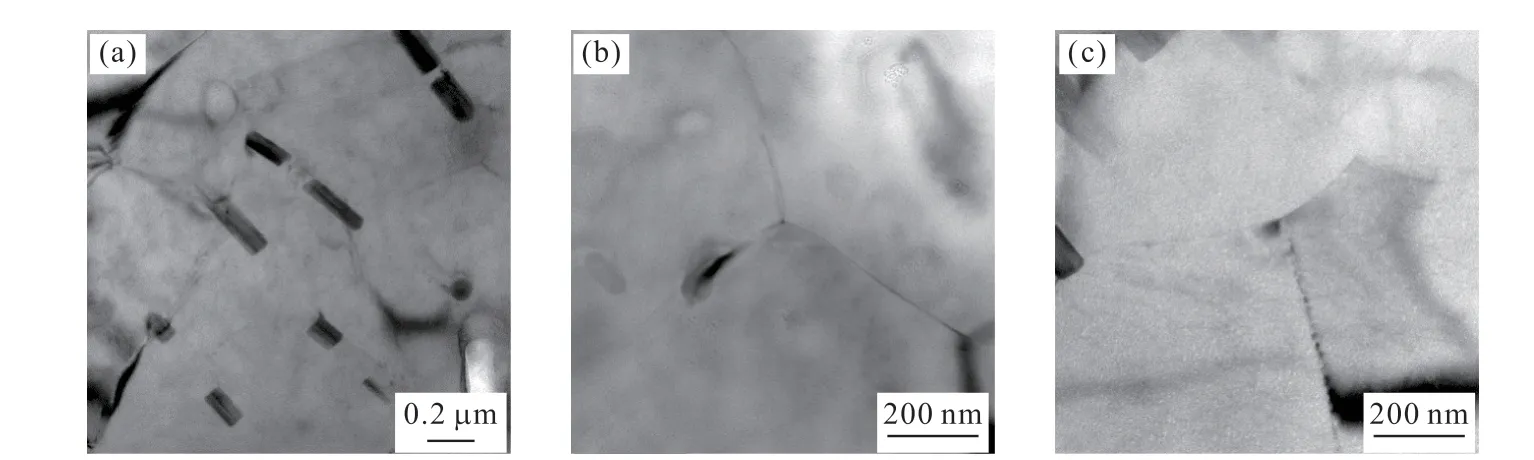
Fig.6 TEM images of the annealed 2A12 aluminum alloy
3.4 Morphology of microstructure
Fig.5 shows the SEM images and EDS results of the microstructure of 2A12 aluminum alloy. There is a large amount of large undissolved Fe(Mn)-rich particles(Al-Cu-Fe-Mn) and S phase (Al2CuMg) in the annealed 2A12 aluminum alloy (Fig.5(a)). Fig.6 shows the TEM micrograph of 2A12 aluminum alloy after annealing.Fig.6(a) indicates that few short rod-like phase (T phase) distributes within the grains. This phase is known as “dispersoid” typical of the microstructure of the alloy after a homogenization heat treatment[11].However, few precipitates can be observed along most grain boundaries, and only few and fine precipitates can be observed in few grain boundaries.
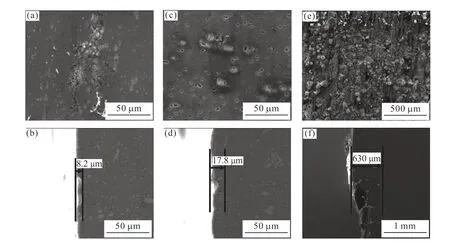
Fig.7 SEM images of 2A12 aluminum alloy immersed in various corrosion solutions: (a) 0.6 mol/L (3.5 wt%) NaCl, (c) 0.3 mol/L H2SO4,(e) 0.6 mol/L HCl; and the correponding cross section: (b) 0.6 mol/L (3.5 wt%) NaCl, (d) 0.3 mol/L H2SO4, (f) 0.6 mol/L HCl
3.5 Immersion corrosion test
Fig.7 shows the SEM images of the surface and cross section of 2A12 aluminum alloy immersed in various corrosive solutions for 4 h. Fig.7(a) indicates that a local corrosion dominates the sample immersed in 0.6 mol/L NaCl solution. As for the sample immersed in 0.6mol/L H2SO4solution for 4 h, it shows that the S phase dissolves partly and it is stripped out from the matrix eventually, and the Al matrix around the Al-Cu-Fe-Mn phase dissolves slightly, as shown in Fig.7(c).As for the alloy immersed in 0.6 mol/L HCl solution for 4 h, (Fig.7(e)), severe general corrosion is found on the surface, and there is much corrosion product on the surface. In addition, the corrosion depth can represent the corrosion resistance of the alloy, so the cross section of the samples was observed, as shown in Fig.7(b), 7(d) and 7(f). The lowest corrosion depth of 8.2 μm is the sample immersed in NaCl solution. The sample immersed in H2SO4solution shows a corrosion depth of 17.8 μm, a value that between the other two kinds of solutions. The corrosion depth of 630 μm for the sample immersed in HCl solution is the highest.
4 Discussion
4.1 Potentiodynamic polarization
In the case of NaCl electrolyte, the polarization curves of the alloy exhibits typical characteristics of aluminum alloys. The corrosion mechanism of aluminum alloy can be described by the following equations[12,13]:
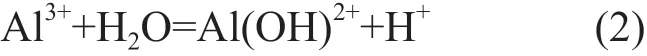
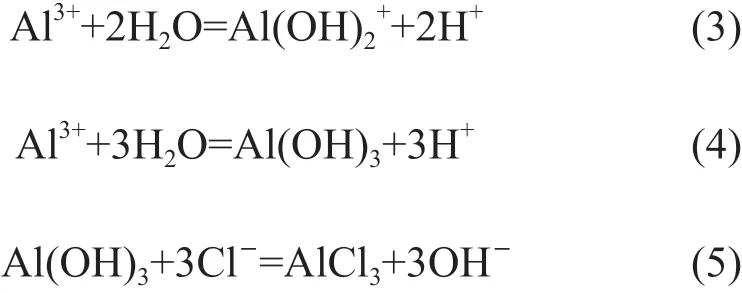
In neutral solution, the concentration of OH-ion is approximately 10-7mol/L so that Al can continuously dissolve concomitant with positive potential shift.Then the Al(OH)3will precipitate when the product of interfacial OH-and Al3+ion concentration surpasses the solubility product of Al(OH)3(Eq.(2)-(4)). But chloride ions can always replace the oxygen consisted in the oxide film to form metal chlorine (Eq.(5)), which results in the initiation of pitting corrosion[13,14]. It is believed that Cl-concentration has a great influence on the corrosion rate of aluminum alloys[12]. So, theicorrincreases with the increasing Cl-concentration, while theEcorrdeceases, as shown in Fig.4 and Table 3. As for the samples immersed in H2SO4solution, the oxide film is more soluble in acid solution; on the other hand, the high concentration of H+makes the Mg-rich S phase become more active[12,14]. All of the factors result in the highericorrthan that in NaCl solution as seen in Fig.4.The dissolution of aluminum in H2SO4solution can be expressed by the following equations[15]:
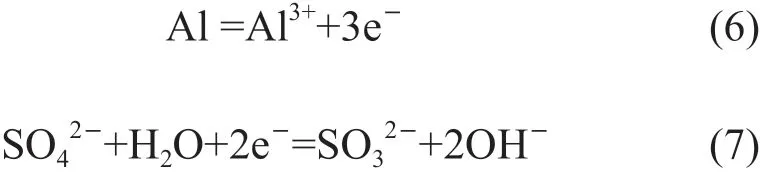
For the alloy immersed in HCl solution, the high concentration of H+and Cl-ion can destroy the oxide film on the alloy and prevent it from forming new passive film. This is different from the sample immersed in NaCl solution, which can reduces the corrosion rate by the Al(OH)3corrosion products on the alloy surface. Then the Cl-can participate into the metal dissolution reaction (Eq.(8)), because there is no oxide film or Al(OH)3on the alloy surface. So, theicorrin HCl solution is higher than the case of in NaCl solution. The more negativeEcorrcan be explained by the more active anodic reactions due to the presence of high concentration of H+and Cl-. Higher solute concentration can lead to more rapid reaction listed above, which results in the highericorror self-corrosion rate. Aluminum dissolution in HCl solution can be expressed by the following equations[12,13]:
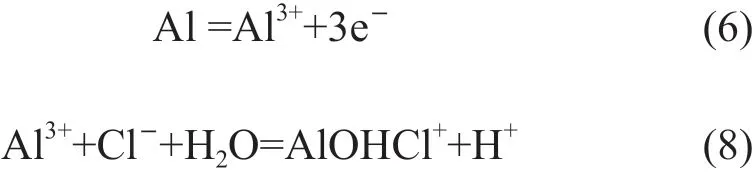
4.2 Stress corrosion cracking
Al-Cu-Fe-Mn and S phase, two major phases exist in 2A12 aluminum alloy after homogenization heat treatment, are the main cause that induce the corrosion process of the alloy (Fig.5). The electrode potential of S phase is -1 061 mV (vsSCE) in neutral NaCl solution, which is lower than that of the Al matrix, so S phase should suffer corrosion attack preferentially[16-18].What’s more, with the preferential dissolution of Al and Mg in the S phase, it transforms into a Cu-rich cathode and thus promotes the dissolution of Al matrix[19]. Al-Cu-Fe-Mn phase acts as a cathode and promotes the dissolution of Al matrix in NaCl solution[18,20]. Fig.7(a)shows the local corrosion and slight dissolution of the S phase, and the Al matrix around the Al-Cu-Fe-Mn phase is not corroded. Considering that theicorris the lowest for the sample immersed in NaCl solution,the corrosion depth of the sample in NaCl solution is only 8.2 μm (Fig.7(b)). A serious dealloying process occurs in the S phase because of the presence of a large amount of H+. Then the S phase dissolves and separates from the Al matrix, leaving the corrosion pitsin situ.Meanwhile, the Al matrix around the Al-Cu-Fe-Mn phase also dissolves to some extent. Thus, the corrosion depth of the sample immersed in H2SO4solution is 17.8 μm (Fig.7(d)). However, for the sample immersed in HCl solution, the corrosion depth of 630 μm (Fig.7(f))can be attributed to the high concentration of Cl-and H+, which results in the most negativeEcorrand the highesticorr(3 orders of magnitude higher than the other two) (Table 3). There are many corrosion pits with different sizes occurring in the alloy during a SSRT,where the formation of the initial cracks initiates. Then,with the slowly increasing stress, the initial cracks start to aggregate and propagate, forming the fracture crack finally[21].
The SCC mechanisms can be explained by the combination effect of anodic dissolution and hydrogen embrittlement (HE)[22]. For the overaged alloys, the grain boundary precipitate (GBPs) with large size can act as hydrogen-trapping site to nucleate hydrogen bubbles, reducing hydrogen concentration to a critical value and retarding HE[7]. However, there are only few precipitates along the grain boundary for the annealed 2A12 aluminum alloy as shown in Fig.6. So, it cannot effectively prevent the occurrence of HE which can reduce the strength of the alloy.
Similar fracture morphology is observed in the samples tested in the air and NaCl solution as shown in Fig.3 (a) and (b). Cl-in NaCl solution can only destroy the passive film, resulting in a coupling between the fresh metal and passive film[23,24]. This kind of cell can accelerate the corrosion of the small anode (the fresh metal), so it can promote the crack of the sample.However, this kind of cell cannot change the strength of the sample, as proposed in many articles[7,23-25], and it also cannot change the fracture mode at the fast fracture area of the sample tested in NaCl solution.So the main SCC mechanism of 2A12 alloy is anodic dissolution in NaCl solution. For the alloy immersed in H2SO4solution containing a large amount of H in the environment, once the initial crack initiate from the corrosion pit, H atoms can diffuse along the crack to the crack tip. However, they can hardly be trapped by those GBPs, because there are few GBPs along the grain boundary in the annealed 2A12 alloy (Fig.6)[7,25],which will result in HE. Therefore, the strength of the alloy decrease to some extent, and the fracture consists of many small cleavage facets and transgranular fracture. The existence of plenty of Cl-and H+in HCl solution change both of the mechanical properties and the fracture mode. Therefore the elongation at break and the strength of the alloy decrease sharply(Fig.1(c)). The fracture, as shown in Fig.3(c), consists of large cleavage facets and transgranular fractures, and the number of dimples is lower than that of the alloy tested in H2SO4solution.
5 Conclusions
a) The stress corrosion resistance of 2A12 aluminum alloys is the best in NaCl aqueous solution but the worst in HCl aqueous solution and intermediate between these two cases in H2SO4aqueous solution.For the same corrosion electrolyte, the stress corrosion resistance of 2A12 aluminum alloys decreases with the increase in the concentration of corrosive solution.
b) Compared to the experimental results of the tensile test in room temperature, for the alloy immersed in NaCl solution, there is higher decrease in the elongation without large strength loss, which may be caused by the presence of Cl-. With the alloy immersed in H2SO4solution, there is higher strength loss and relatively less loss of elongation because of the presence of high H+concentrations. With the alloy immersed in HCl solution, there is relatively level loss of strength and elongation due to the existence of plenty of Cl-and H+.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
- Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
