Bioprocess-inspired Fabrication of Lead Iodide Coexisting with Crystalline Nanosheet and Amorphous Nanorod for Perovskite Solar Cells
2021-06-14CHIWenhaoZHUYuexuFUZhengyiXIEJingjing
CHI Wenhao, ZHU Yuexu, FU Zhengyi, XIE Jingjing
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070,China)
Abstract: A recombinant protein ChiSifiCa, which was originally designed for regulation of calcium carbonate, was utilized to direct the mineralization of PbI2. By the regulation of ChiSifiCa protein, PbI2 nanoparticles composed of crystalline nanoflakes and amorphous nanorods were fabricated under environmental benign conditions. Synthetic PbI2 was successfully applied for preparation of perovskite precursors to fabricate solar cells. This regulation of ChiSifiCa on PbI2 improves the power conversion efficiency of corresponding perovskite solar cells to 16%. The present study may open a new avenue in the design and synthesis of materials with novel structures and functions.
Key words: bioprocess-inspired fabrication; biomineralization; ChiSifiCa protein; lead iodide;perovskite solar cells
1 Introduction
Inspired by exquisite structures and unique functions of biomaterials, bio-inspired materials or bioinspired functions have been designed and fabricated[1-4]. In contrast to anthropogenic technologies wherein harsh conditions are commonly requisite,natural structure-forming processes can be realized under environmentally benign condition. Hence, natural structure-forming processes of biomaterials are also worth learning to develop new synthesis technology.This research field can be developed and referred to as“bioprocess-inspired fabrication”[5-8].
Biological processes normally contain a series of biochemical reactions. As a typical biological process,biomineralization involves the functional presentation of various proteins and other organic molecules[9,10].These organic matrices especially proteins can act as templates or inducers in the formation of biomineralsin vivo. In studying and mimicking the role of proteins in biomineralization, scientists have succeeded in directly utilizing single or multiple natural proteins to synthesize various materialsin vitro[11,12]. Moreover,in order to improve the mineralization efficiency,recombinant proteins that contain different functional segments to establish an artificial process have been designed for synthesis of novel materials. Duet alreported the formation of supramolecular assembly of amelogenin nanospheres, which effectively controlled the oriented growth of apatite crystalsin vitro[13]. Inoueet alused two kinds of genetically modified cageshaped proteins to synthesize a thermo-stable carbon nanotube-TiO2nanocomposite, which was able to be applied in dye-sensitized solar cells.[14]However,normally a certain recombinant protein can only regulate the synthesis of specific materials according to the purpose of protein construction, which limits their application in material synthesis. Recently, inspired by the species and distribution of organic matrix in nacre, we constructed a multifunctional protein ChiSifiCa containing domains of chitin binding(Chi),silk fibroin(Sifi) and calcium ion binding(Ca) to direct the growth of vaterite on the surface of chitin[15,16]. We conceive whether this recombinant protein designed according to specific functions can be used to regulate the synthesis of other inorganic materials.
Lead iodide(PbI2) have unique electrical and optical properties, which lead to wide potential application in chemical sensors, photovoltaic devices,supercapacitor and perovskite solar cells[17-19]. The common structure of lead iodide nanoparticles is nanosheet[20,21]. Also, a few studies have reported the synthesis of lead iodide nanorod. Kaviyarasuet alutilized hydrothermal method to synthesize PbI2nanorods.[17]Barnakovet alfabricated PbI2nanorods in toluene by addition of NaNO3[22]. Wanget alproduced surface-modified PbI2nanorods by adding polyethylene glycol as surfactant[23]. Nevertheless, heat treatment or introduction of chemical additives that can be toxic or hard to remove are essential in these synthetic approaches, limiting the application in the perspective of energy saving and environmental protection.
In the present study, we selected the recombinant protein ChiSifiCa, which was originally designed for directing the growth of calcium carbonate, to regulate the mineralization of PbI2in aqueous solution at room temperature. The microstructure of obtained PbI2nano powders are composed of crystalline flakes and amorphous nano rods, which has not been reported before. Furthermore, the potential application of prepared PbI2on CH3NH3PbI3perovskite solar cells was explored.
2 Experimental
2.1 Preparation of ChiSifiCa protein
The preparation of ChiSifiCa protein was realized by the culture and proliferation of modified Escherichia coli[24]. Expression and purification process of ChiSifiCa Protein followed previous procedures.
[15]Concentration of the protein was measured by Bradford Protein Assay Kit (Sangon Biotech). Purified ChiSifiCa Protein solution was stored at 4 ℃ to delay the degradation and maintain the purity before use.
2.2 Mineralization of PbI2 assisted by ChiSifiCa protein
0.1 M Pb(NO3)2(Sinopharm, China) were prepared respectively. 66.4 mg KI (Sinopharm, China)was added into 18 mL purified ChiSifiCa solution with continuous stirring. Then the pH of the solution was adjusted to 7.5. The mineralization was initiated by adding Pb(NO3)2solution drop by drop with continuous stirring at room temperature. The initial concentrations of KI and Pb(NO3)2are 20 and 10 mM, respectively,in the mixed solution before the reaction. After the precipitation was collected by centrifugation at 1 000 g for 5 min and washed with deionized water for three times. Finally the sample was freeze-dried for 12 h.
2.3 Characterization of mineralization products
The phase composition and crystallinity were examined by X-ray diffraction (XRD) using a Bruker D8 Advance with Cu Kα radiation (40 kV, 40 mA)in a 2θrange from 10°-70°. Surface morphology information was revealed by field emission scanning electron microscopy (FESEM, Hitachi S-4800) at an accelerating voltage of 5 kV. High resolution transmission electron microscopy (HRTEM)examination was carried out with a Talos F200S at 200 kV. The Fourier transform infrared (FTIR) spectra was obtained on a Bruker Vertex 80 V FT-IR spectrometer.Thermogravimetric (TG) analysis was performed in a Netzsch STA449F3 at a heating rate of 10 ℃·min-1from 40 to 900 ℃.
2.4 Fabrication and measurement of perovskite solar cell
Perovskite precursor preparation: The MAPbI3perovskite precursor solution was prepared by dissolving 158 mg MAI and 461 mg PbI2powder into 800 uL DMF/DMSO (4:1 by volume) mixed solvents.The perovskite precursor solutions were filtrated after oscillation for 1 hour.
Device fabrication: The FTO/glass substrates were etched using a femtosecond laser machine and washed by DI water and ethyl alcohol under ultrasonic cleaning for 20 min, respectively. Then the compact SnO2films were prepared by spin coating 50 mL of 0.075 m SnCl4·5H2O isopropanol solution onto the UV-ozone processed FTO/glass substrates at 3 000rpm for 30 s, followed by annealing at 180 ℃ for 1 h.The perovskite absorber was deposited on UV-ozone processed SnO2/FTO/glass substrates by spinning a 25 mL perovskite precursor solution at 5 000 rpm for 30 s with an accelerated speed of 1 000 rpm before dropping 100 mL antisolvent (ethyl acetate) during the last 10 s. The films were then annealed at 100 ℃for 15 min. After cooling down to room temperature,a 25 μL Spiro-OMeTAD solution which was abtained by dissolving 73 mg Spiro-OMeTAD into 1 mL chlorobenzene followed by the addition of 18 μL Li-TFSI (predissolved as a 520 mg/mL stock solution in acetonitrile) and 30 μL 4-tert-butylpyridine was spun on the corresponding mixed perovskite films at 3 000 rpm for 30 s. Finally, a 70 nm-thick layer of gold was deposited on the top of spiro-OMeTAD layer by a thermal evaporator as the back electrode to complete the whole devices. TheJ-Vcurves of the PSCs were measured using a solar simulator (Oriel 94023A, 300 W) and a Keithley 2400 source meter. The intensity(100 mW/cm2) was calibrated using a standard Si solar cell (Oriel, VLSI standards). All devices were tested under AM 1.5G sun light (100 mW/cm2) using metal masks of 0.16 cm2for the characterization.
3 Results and discussion
3.1 PbI2 mineralization regulated by Chi-SifiCa protein
Before used in mineralization reaction, the purity of ChiSifiCa(CSCa) was confirmed. SDS-PAGE analysis shows that the observed molecular weight of the ChiSifiCa matched well with the theoretical one,which means over 95% homogeneity of ChiSifiCa is achieved (Fig.1)[15]. PbI2mineralization in the presence of ChiSifiCa were explored and compared with that in the absence of ChiSifiCa.
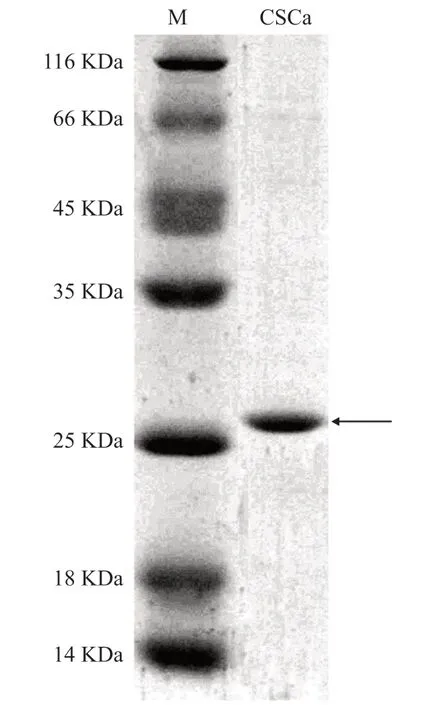
Fig.1 SDS-PAGE analysis of purified ChiSifiCa protein
X-ray diffraction was performed to investigate the composition and crystalline phase of samples with different ChiSifiCa concentration (Fig.2). The XRD patterns show that the crystallographic phase of all the mineralized products is lead iodide(JCPDS No. 07-0235). With the concentration of ChiCaSifi increasing from 0(control group) to 2% (w/w), the intensity of the diffraction peaks becomes weaker,which indicates reduction of crystallinity. Broadening of diffraction peaks reveals the decrease of PbI2crystal size, which demonstrates the influence of ChiSifiCa on the mineralization of PbI2. Meanwhile,morphology changes of products were analyzed by FESEM. In control group, only flake-like PbI2can be observed, with particle size exceeding 2 μm (Fig.3(a)).The addition of ChiSifiCa leads to the size reduction of flake-like PbI2and the appearance of novel PbI2nanorods. A large amount of stacked nanoflakes and a few nanorods was observed in the sample from 0.5%concentration of ChiSifiCa(Fig.3(b)). With the increase of ChiSifiCa concentration, the relative content of nanorods in products rises obviously(Fig.3(c)). When the concentration reaches 2%, nanorods occupy the main morphology in microstructure with a small amount of nanoflakes(Fig.3(d)). The average diameter of the nanorods is about 50 nm(inset in Fig.3(d)).Therefore, ChiSifiCa can play a marked role on the variation of PbI2crystallinity and microstructure.
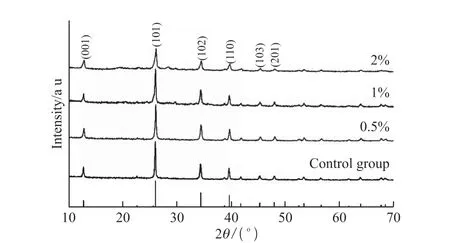
Fig.2 XRD patterns of PbI2 minaralized with different ChiSifiCa concentration from 0 to 2%
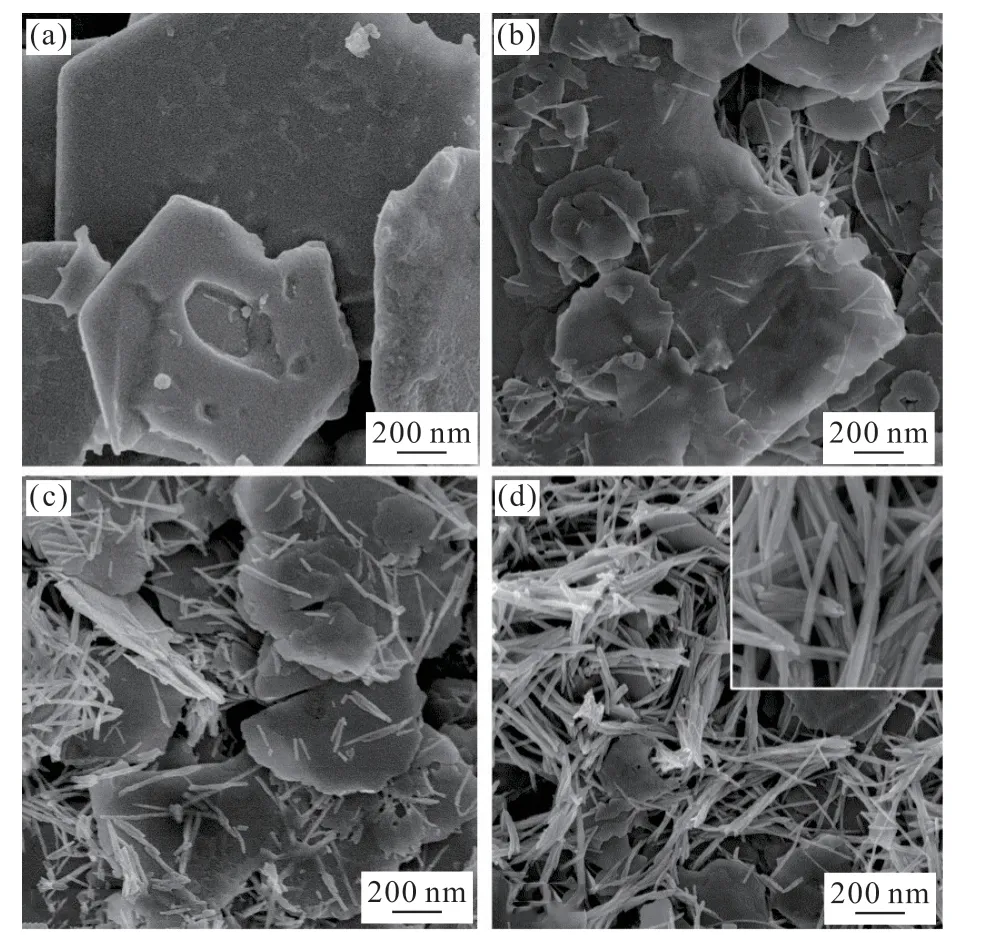
Fig.3 SEM images of PbI2 minaralized with different ChiSifiCa concentration: (a) 0; (b) 0.5%; (c) 1%; (d) 2%
Fine structure of ChiSifiCa(2%(w/w)) regulated PbI2was observed more clearly by TEM(Fig.4(a),4(b)).HRTEM image shows the good crystallinity of flakes in samples(Fig.4(c)). The crystal lattice, with an interplanar spacing of 0.396 nm, is parallel to the(100) plane of PbI2. However, no distinct attice fringes emerge in single nanorod (Fig.4(d)). The related selected area electron diffraction (SAED) pattern further indicates amorphous state of PbI2nanorods(inset in Fig.4(d)). Especially, a coating layer with about 5 nm thickness around the nanorod was observed, which is likely to be organic matter.
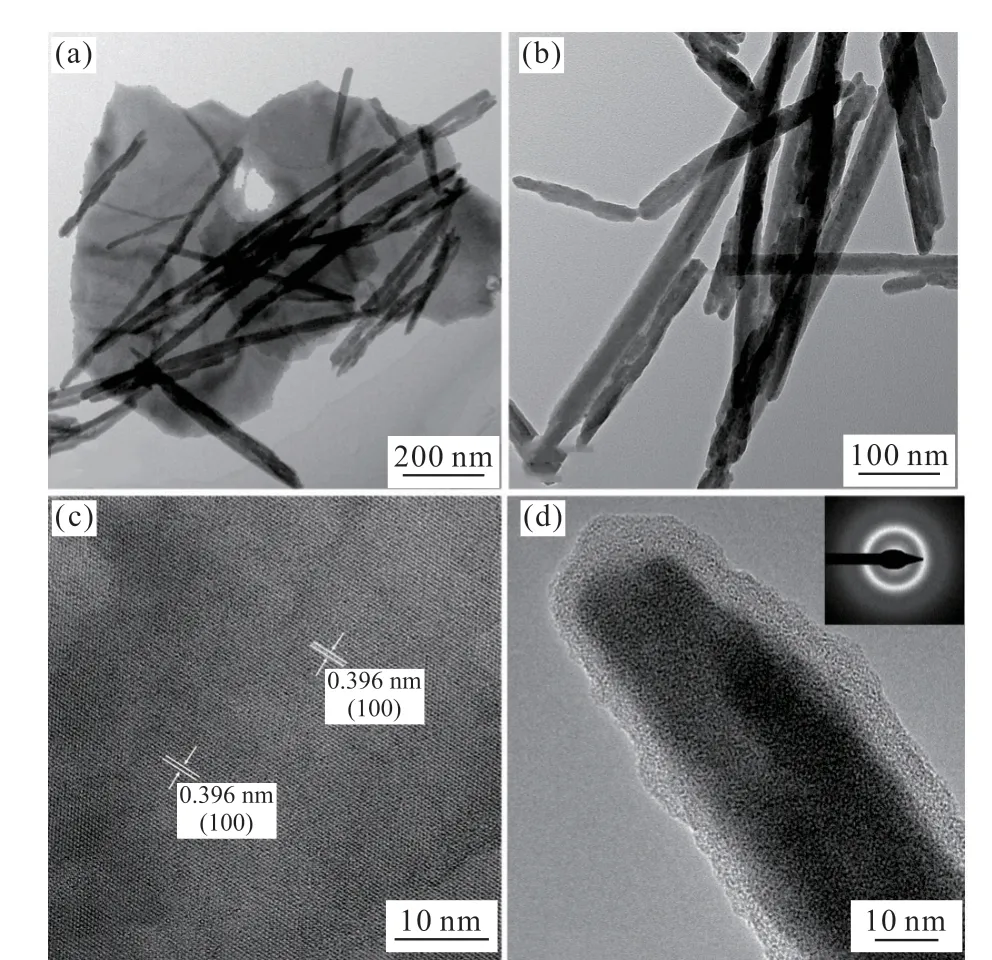
Fig.4 (a,b) TEM images of ChiSifiCa regulated PbI2; (c) HRTEM of nanoflake; (d) TEM image of single nanorod (Inset: the SAED pattern)
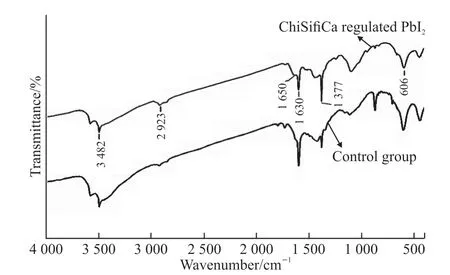
Fig.5 FTIR spectra of PbI2 samples with or without the regulation of ChiSifiCa
Chemical composition of PbI2samples was analyzed by FTIR spectroscopy(Fig.5). The absorption band centered at 3 482 cm-1, and weak peaks at 1 630 and 606 cm-1are attributable to the stretching and bending vibrations of O-H from absorbed water in samples, respectively[25,26]. Peaks near 1 377 and 2 923 cm-1agree with the stretching vibrations of Pb-I bonds. Furthermore, the band associated with protein is observed, corresponding to amide I vibrational modes at 1 650 cm-1, which confirms the existence of protein[27]. To estimate the organic content, we applied a thermogravimetric analysis procedure to ChiSifiCa regulated PbI2(Fig.6). Weight loss below 200 ℃ is probably due to loss of water, and 6.06% mass loss above 200 ℃ is induced by decomposition of organic matrices[28]. The decomposition of PbI2occurs at 500-750 ℃. Therefore, it demonstrated that ChiCaSifi protein participated in the mineralization of PbI2and promoted the change of crystallinity and the formation of amorphous PbI2nanorods. The result is probably because chain-like ChiCaSifi proteins bound to PbI2which impeded its crystallization and induced its oriented growth in one dimension.
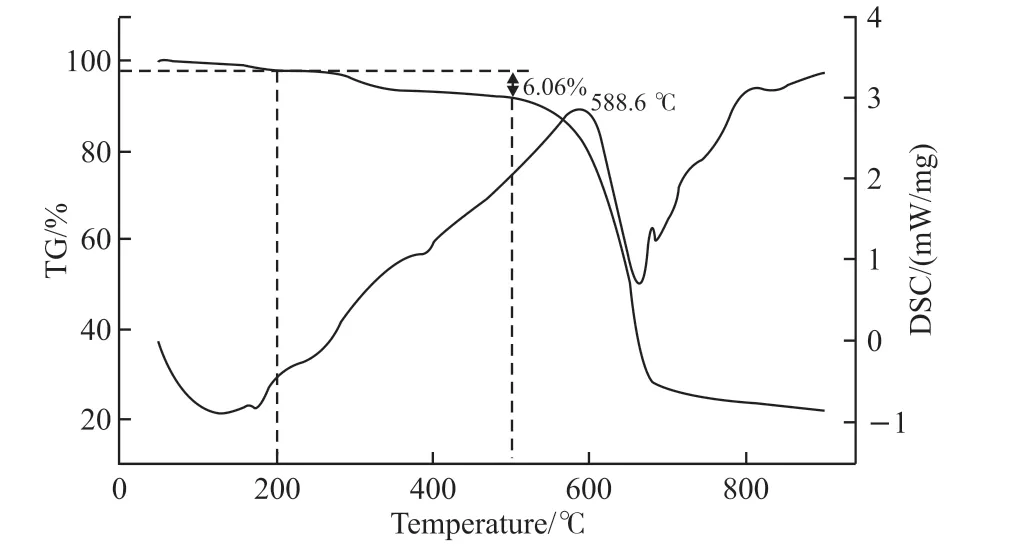
Fig.6 Thermogravimetric analysis of ChiSifiCa regulated PbI2
3.2 Measurement of CH3NH3PbI3 perovskite film and solar cell
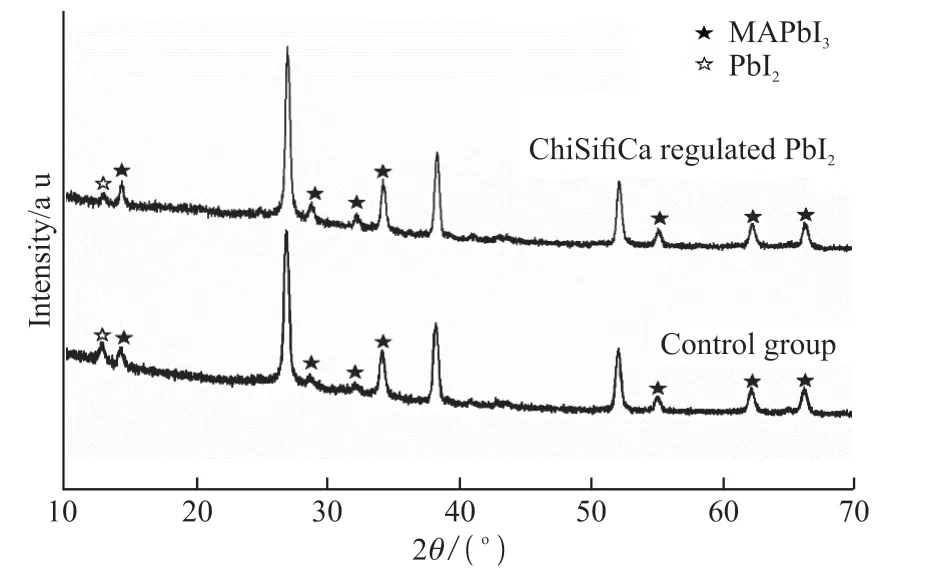
Fig.7 XRD patterns of CH3NH3PbI3 films prepared from fabricated PbI2
To explore the application of ChiSifiCa regulated PbI2on perovskite solar cell(PSC), the product was employed to synthesize CH3NH3PbI3film of perovskite solar cells. PbI2fabricated without the regulation of ChiSifiCa is as the control group. In the process of dissolving PbI2in DMF/DMSO mixed solvents,ChiSifiCa regulated PbI2shows a much better solubility than that of control group, which is significant for the fabrication of CH3NH3PbI3[29,30].
XRD pattern of CH3NH3PbI3film synthesized from ChiSifiCa regulated PbI2shows higher peak intensity and narrower peak width of MAPbI3, and lower peak intensity of residual PbI2compared with control group, indicating better crystallinity and purity(Fig.7). From cross-sectional SEM images,the average thickness of the CH3NH3PbI3film is estimated to be 650 nm in control group(Fig.8(a)).As for the CH3NH3PbI3films prepared from PbI2regulated by ChiSifiCa, the thickness reaches nearly 700 nm(Fig.8(b)). Moreover, plenty of pores and flakelike PbI2impurities can be observed on the surface of the film in control group(Fig.8(c)). The particle size of CH3NH3PbI3is relatively uniform and the film surface is relatively flat in CH3NH3PbI3film synthesized from ChiSifiCa regulated PbI2, which is beneficial to the improvement of solar cell performance(Fig.8(d)).
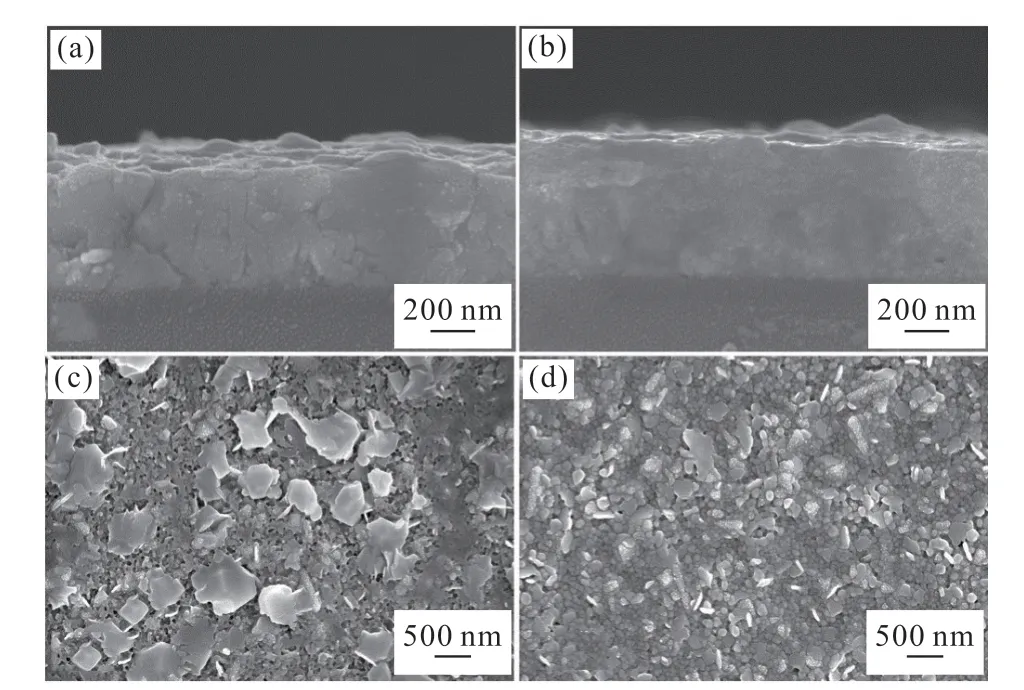
Fig.8 Cross-sectional SEM images of CH3NH3PbI3 films prepared from PbI2 in (a)control group; (b) ChiSifiCa regulated samples; Plane-view (insets) SEM images of CH3NH3PbI3 films prepared from PbI2 in (c)control group; (d) ChiSifiCa regulated samples
We applied these different PbI2based perovskite precursors to fabricate solar cells. The planar PSC with a structure of FTO/SnO2/perovskite/Spiro-OMeTAD/Au is shown in Fig.9(a). Fig.9(b) indicates the championJ-Vcurves of different PbI2based PSCs.The control device exhibits a low power conversion efficiency(PCE) of 9.72%, with aVocof 1.028 V, aJscof 16.35 mA/cm2, and a fill factor (FF) of 0.58. This lower current density can be ascribed to the poor solubility of control PbI2powder, which induces a much thinner absorber films with poor crystallinity. As for ChiSifiCa regulated PbI2, a higher concentration of perovskite precursor solution was achieved, resulting in suitable thickness of perovskite films, as well as improved crystallinity. Therefore, the corresponding PSC shows dramatically increase ofJscto 21.99 mA/cm2, and the FF also increases to 0.71, thus leading to a champion PCE exceeding 16%. The corresponding steady state output current densities under maximum power point tracking are also shown in Fig.9(c). The control device exhibits a fast descent of current at the beginning of light exposure, while the target device shows a smoother output current even under light illumination of 100 s, indicating the better stability of target device.By comparison, target device shows superior power conversion efficiency exceeding 16% with better stability than control group. The improvement of performance is probably because that amorphous PbI2nanorods have higher reactivity than PbI2nanosheets as raw materials for the synthesis of CH3NH3PbI3, which in turn leads to the improvement of film quality and final performance.
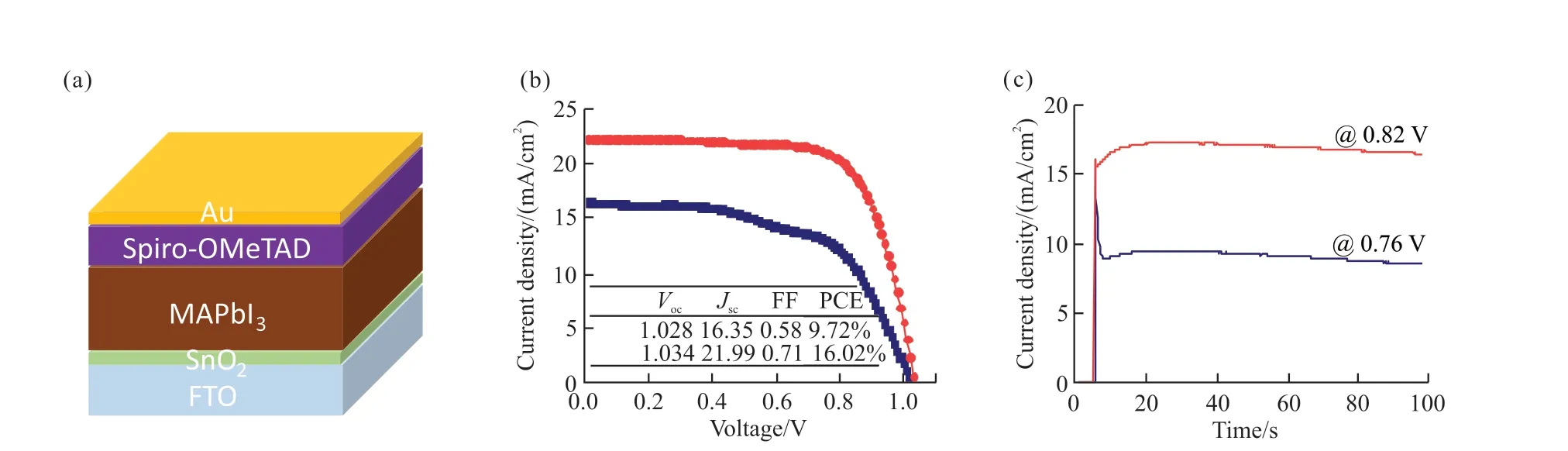
Fig.9 Photovoltaic performance of different PbI2 based PSCs: (a) Schematic of the device architecture; (b) J-V curves and (c) steady state output current density under maximum power point tracking
4 Conclusions
PbI2nanoparticles composed of crystalline nanoflakes and amorphous nanorods were fabricated by the regulation of ChiSifiCa protein. The protein can play a marked role on the variation of PbI2crystallinity and microstructure by participating in the mineralization. The diameter of obtained amorphous PbI2nanorods is about 50 nm. The concentration of ChiSifiCa can obviously affect the relative content of nanorods in products. Furthermore, we applied obtained PbI2as based material for preparation of perovskite precursors to fabricate solar cells successfully. The results of performance comparison indicate that target device shows superior power conversion efficiency exceeding 16% with better stability than control group,which demonstrates the significance of regulation of ChiSifiCa protein on PbI2. This study provides broader insight into biomineralization by recombinant proteins and also opens a potential path for fabrication of materials with new structures and functions.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
- Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
