Stabilization of low-valence transition metal towards advanced catalytic effects on the hydrogen storage performance of magnesium hydride
2021-05-21JinZhngShuiYnGunglinXiXiojieZhouXinzhengLuLinpingYuXuebinYuPingPeng
Jin Zhng, Shui Yn, Gunglin Xi, Xiojie Zhou, Xinzheng Lu, Linping Yu,Xuebin Yu, Ping Peng
a Hunan Provincial Key Laboratory of Intelligent Manufacturing Technology for High-performance Mechanical Equipment, Changsha University of Science and Technology, Changsha 410114, China
b Department of Materials Science, Fudan University, Shanghai 200433, China
cSchool of Chemistry and Biological Engineering, Changsha University of Science and Technology, Changsha 410114, China
d College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 30 December 2019; received in revised form 7 February 2020; accepted 16 February 2020
Available online 8 November 2020
Abstract
Keywords: Magnesium hydride; Hydrogen storage; Dehydrogenation; Catalysts; First-principles calculations; Solid-solution.
1. Introduction
The increasing environmental pollution resulting from the use of conventional fossil fuels has stimulated the extensive exploration of renewable and sustainable clean energy.Hydrogen has been widely considered as one of the most promising energy carriers due to its abundance, high energy density,environmental friendliness, and renewability [1,2]. Nevertheless, the safe, efficient and compact storage of hydrogen is still one of the most difficul challenges for the advancement of the proposed hydrogen economy [3,4]. Owing to its high volumetric (110g L-1) and gravimetric (7.6wt.%) hydrogen storage densities, low cost, and abundance, magnesium hydride (MgH2) has received great attention as a potential hydrogen storage material [5-8]. Its sluggish kinetics and stable thermodynamics, however, are severely hindering the practical application of MgH2as an on-board hydrogen material[9-12].
The addition of catalysts is an effective strategy to alleviate the kinetic and thermodynamic barriers for the reversible hydrogen storage of MgH2. Among them, 3d-transition metal oxides (such as TiO2, V2O5, Nb2O5, Fe2O3, CoO2, etc.) have attracted intense intention due to their superior catalytic effects [13-21]. Owing to the high reductivity of Mg/MgH2,these transition metal oxides (TMOs), however, are easily reduced and transformed into low-valence transition metal oxides or hydrides, which have been experimentally recognized as the most effective catalysts to promote the hydrogenation and dehydrogenation of MgH2[22-25]. This mainly results from the strong interaction between the s-states of H and dstates of low-valence transition metals, owing to the electron pairing effect, which could weaken the Mg-H bonds of MgH2and hence facilitate its reversible hydrogen storage performance [26-29]. Unfortunately, it should be noted that, along with the reduction of TMOs, MgO with a dense rock-salt structure will be produced, which is hydrogen-impermeable and inactive in terms of improving the hydrogen storage performance of MgH2[30-34]. The formation of MgO could not only hinder the physical interaction between the catalysts and Mg/MgH2, but also impedes the transportation of hydrogen to some extent. In addition, the oxidation of MgH2/Mg to form MgO leads to the consumption of active Mg/MgH2,and hence, the overall hydrogen storage capacity will be decreased during the cycling process.
To address these issues, we theoretically and experimentally evaluated the direct doping of low-valence transition metals (TMs) into MgO to achieve advanced catalytic effects towards improving the hydrogen storage performance of MgH2. The doping of low-valence TMs into MgO could not only stabilize the low-valence state of TMs, which facilitates their catalytic effects towards improving the hydrogen storage performance of MgH2and hence turns catalytically inactive MgO into active catalysts, but also avoids the consumption of MgH2/Mg during the synthetic and/or cycling processes.First-principles studies demonstrate that, after doping lowvalence TMs into MgO, the interaction between Mg(TM)O could effectively not only weaken the Mg-H bonds of MgH2,leading to decreased hydrogen desorption energy, but also reduce the activation energy for the hydrogen storage process,leading to enhanced hydrogen storage kinetics, and prevent the sintering and particle growth of MgH2, leading to enhanced cycling stability. These results were further verifie by experimental observations. Therefore, this strategy is expected to provide important guidance for developing advanced MgH2-based hydrogen storage materials by screening and designing suitable catalysts towards practical application.
2. Computational and experimental details
2.1. Computational details
Herein, MgH2monomer was firs constructed to simulate the MgH2hydride (Figure S1a in the Electronic Supplementary Information). Based on the MgO crystal with a rock-salt structure (Figure S1b), a MgO(100) (2×2) surface supercell model with 3-layer slabs was further constructed to simulate the MgO oxide with the most stable cleavage plane [35,36].The bottom layer of the MgO slabs was fi ed at the bulk position, while the top two layers of slabs could be relaxed freely. Then, the TM (TM=Ti, V, Nb, Fe, Co, Ni) doped MgO(100) surface models were constructed to simulate the Mg(TM)O oxides by substituting one Mg atom in the second layer of the pristine MgO slabs with one TM atom.In order to screen for the magnesium-based oxides with superior catalytic effects towards MgH2and understand their catalytic mechanisms on the hydrogen desorption properties of MgH2, adsorption models of MgH2monomer on the pristine MgO(100)and Mg(TM)O(100) surfaces were further constructed (Figure S1c and d). The vacuum spaces of all surface models with and without adsorbates were set at 20 °A to avoid mutual interference between the adjacent slabs.
The structural relaxation and energy calculations for all models were performed by first-principle calculations based on the density functional theory (DFT) through the Cambridge Serial Total Energy Package (CASTEP) [37,38]. The generalized gradient approximation (GGA) parameterized by the Perdew-Burke-Ernzerhof (PBE) model was adopted as the exchange-correction functional [39]. The plane wave cut-off energy of 340eV was employed for all models. For all surface models with or without MgH2monomer adsorption, the k sampling was set by a 4×4×1 k-point mesh in Brillouin zone [40]. To evaluate the dehydrogenation kinetics of MgH2,the transition states of H2desorption from MgH2monomer in different systems was calculated by using the linear synchronous transit (LST)/quadratic synchronous transit (QST)methods [41]. In the variable-cell relaxation [40], the convergence criteria for energy, force, stress, and displacement were 1.0×10-5eV/atom, 0.03eV/°A, 0.05GPa, and 0.001 °A,respectively.
2.2. Experimental details
MgH2(98 wt%) with a particle size of 200 mesh was purchased from Alfa Aesar. MgO (97 wt%) with a particle size of 22 mesh and Nb2O5(99 wt%) with a particle size of 100 mesh for use as an additive were purchased from the Shanghai Taitan Technology Company, China. Ball milling was carried out on a QM-3C high-speed vibrating ball mill,in which several stainless-steel balls 10mm in diameter were used as the milling medium. The Nb-doped Mg(Nb)O oxide was synthesized by ball milling the reactant mixture of MgH2and Nb2O5(6:1 molar ratio)for 24h.Then,the MgH2-5 wt%Mg(Nb)O composite was further ball milled for 2h to study its microstructures and dehydrogenation properties. For comparison, the pristine MgH2, MgH2-5 wt% MgO, and MgH2-5 wt% Nb2O5powders were also ball-milled under the same conditions. The milling speed was 1000rpm, and the ball-topowder weight ratio was 30:1. To avoid the effects of excessive temperature on the powders, the ball-milling machine was stopped to cool for 15min in every 1h. Moreover, the milling vial was also cooled for 1h in the glove box after ball milling, and the hardened-steel vial was fille with argon gas during ball-milling.
X-ray diffraction (XRD) was performed on a Rigaku D/Max 2500 diffractometer with Cu Kɑ radiation source using a step size of 0.02° The morphology of the samples was revealed using scanning electron microscopy (SEM)performed on a TESCAN MIRA3 LMU electron microscope and transmission electron microscopy (TEM) conducted on a JEOL 2011 F. Energy dispersive spectroscopy (EDS) mapping analysis was performed on a 150 mm2X-MaxN electron microscope operating at 20kV. Fourier transform infrared spectroscopy (FTIR) measurements were performed on a Nicolet IS 50 instrument. X-ray photoelectron spectroscopy(XPS) was performed on a VG Scientifi instrument with an Al Kɑ radiation source using a pass energy of 100eV.High resolution spectra were recorded at the normal emission take-off angle with a pass energy of 30eV.
The hydrogen storage performance of the as-prepared samples was firs investigated via differential scanning calorimetry (DSC) measurements (HP204, Netzsch) at various heating rates with an argon fl w of 50mL min-1. Thermogravimetric analysis (TG; Netzsch STA 449 F3) was performed in a glove box fille with argon using a heating rate of 10oC min-1. The hydrogen desorption and adsorption kinetics measurements as well as the cycling performance testing were conducted on a Sieverts apparatus (GRC, Advanced Materials Corp., USA) at various temperatures.
3. Results and discussion
3.1. Theoretical predictions of the catalytic effects of MgO and Mg(TM)O on the hydrogen desorption performance of MgH2
The catalytic effect of Mg(TM)O on the hydrogen storage performance of MgH2was firs evaluated by investigating the bonding structure of the relaxed MgH2monomer adsorbed on different MgO or Mg(TM)O surfaces (Fig. 1 and Figure S1 in the Electronic Supplementary Information (ESI)). As shown in Fig. 1a, the two Mg-H bonds of the pristine MgH2monomer have an equal length of 1.712 °A. Under the support of MgO, the corresponding Mg-H bonds are stretched with only a slight increase of bond length by 0.064 °A and 0.053 °A (Fig. 1b), respectively. It is interesting to note that,upon doping of Ti, V, and Nb atoms into MgO, the lengths of the Mg-H bonds were significantl increased relative to the MgO-modifie MgH2system (Fig. 1c-e) and more importantly, a certain degree of distortion could be clearly observed for the relevant MgH2monomers. Among them, the maximum Mg-H bond length was demonstrated to be 1.912 °A in the MgH2/Mg(Nb)O system. In comparison, only minor changes in the Mg-H bond lengths and configuratio were achieved for MgH2when interacting with MgO doped with Fe, Co, and Ni (Fig. 1f-h). This result indicates that doping Ti, V, and Nb into MgO effectively weakens the Mg-H bonds and induces destabilization effects on MgH2. To verify this,the hydrogen desorption energy(ΔEdes)from MgH2under the support of Mg(TM)O, which is an important thermodynamic parameter used to determine the hydrogen desorption temperature [42], was subsequently calculated. It was demonstrated that the ΔEdesvalue of MgH2decreases with increasing Mg-H bond length (dMg-H), which confirm the inverse relationship between ΔEdesand dMg-H(Fig. 1i). The ΔEdesvalue of pristine MgH2monomer was calculated to be 1.360eV, while the corresponding value for the MgO-modifie MgH2system was slightly decreased to be 1.018eV, which agrees well with the experimental observation that the addition of MgO into MgH2leads to only a limited enhancement of its hydrogen storage performance [43]. In strong contrast, it is interesting to notice that, with the doping of TM atoms (especially Ti, V, and Nb) into MgO, the ΔEdesvalues of these MgH2/Mg(TM)O systems were significantl decreased. In particular, due to the large average dMg-Hof 1.884 °A, an ΔEdesas low as 0.24eV could be observed for the Mg(Nb)O-modifie MgH2system(Fig. 1i), which provides a direct evidence of the superior catalytic role of Mg(Nb)O towards the hydrogen storage performance of MgH2.
Subsequently, the activation energy was further calculated to explore the effects of Mg(TM)O oxides on the hydrogen desorption kinetics of MgH2. In this work, the relaxed MgH2/MgO and MgH2/Mg(TM)O models were set as the initial states (IS) of dehydrogenation reactions and the relaxed Mg/MgO and Mg/Mg(TM)O models with H2adsorption in vacuum region were set as the fina states (FS) of dehydrogenation reactions. Subsequently, the transition states(TS)between IS and FS were calculated using LST/QST tools and the activation energies of dehydrogenation were determined [41,44]. As shown in Fig. 2a, all the Mg(TM)O oxides were found to boost the dehydrogenation of MgH2to different degrees, with decreased dehydrogenation activation energies relative to the MgH2/MgO system. In particular, the dehydrogenation activation energy for the MgO-modifie MgH2system was calculated to be 436.50kJ mol-1. Upon doping TMs into MgO, the calculated activation energies were decreased to 424.91kJ mol-1, 360.49kJ mol-1, 330.06kJ mol-1,312.19kJ mol-1, 257.80kJ mol-1, and 246.62kJ mol-1for the MgH2/Mg(Ni)O, MgH2/Mg(Fe)O, MgH2/Mg(Co)O,MgH2/Mg(V)O, MgH2/Mg(Ti)O, and MgH2/Mg(Nb)O systems, respectively. Apparently, MgO doped with Nb exhibited the most outstanding catalytic effect towards enhancing the hydrogen desorption kinetics of MgH2due to its having the minimum dehydrogenation activation energy.
It is well known that the occurrence of sintering and aggregation during the reversible hydrogen storage process significantl degrades the cycling performance of MgH2.Hence, the binding energies (Ebin) between MgH2monomer and the surfaces of Mg(TM)O [45,46], as well as the adsorption heights, were further calculated to evaluate the confine ment effects of Mg(TM)O upon cycling de/re-hydrogenation(Fig. 2b). It was verifie that the larger adsorption height of MgH2monomer on the surfaces of various oxides corresponds to a smaller Ebin, indicating the inverse relationship between adsorption height and Ebin. Specificall , the adsorption height of 2.026 °A and an Ebinof 1.132eV were calculated for the MgH2/MgO system. On doping Fe, Co,and Ni into MgO, the adsorption height increased, and the Ebinvalue decreased relative to the MgH2/MgO system,indicating the weak interaction between MgH2monomer and MgO oxides doped with Fe, Co, V, and Ni. In comparison, a large increase in the binding energies was observed between MgH2and the MgO oxides doped with Ti and Nb, suggesting their strong confinemen effects on MgH2monomer. Notably,the maximum value of Ebin, i.e., 1.441eV, was obtained in the MgH2/Mg(Nb)O system, and hence, the Mg(Nb)O oxide exhibited the most pronounced confinemen effect on MgH2particles, which could limit the growth and agglomeration of MgH2particles to some extent and facilitate their cycling stability. Therefore, based on the above results, it could be concluded that the doping of TM into MgO could effectively not only tailor both the desorption energy and the activation energy of MgH2, but also enhance the confinemen effect on MgH2and, among them, the doping of Nb into MgO was verifie to be most effective. Inspired by this, the practical hydrogen storage performance of the MgH2/Mg(Nb)O system was further investigated experimentally.
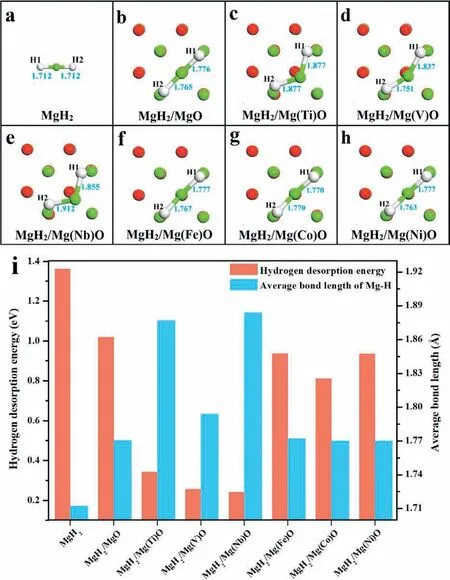
Fig. 1. (a) MgH2 monomer and (b)-(h) its stable adsorption configuration on MgO and Mg(TM)O surfaces; (i) calculated hydrogen desorption energies and average bond lengths between Mg and H of MgH2 monomer within the various systems.
3.2. Experimental verification of the catalytic effects of Mg(NB)O on the hydrogen desorption performance of MgH2
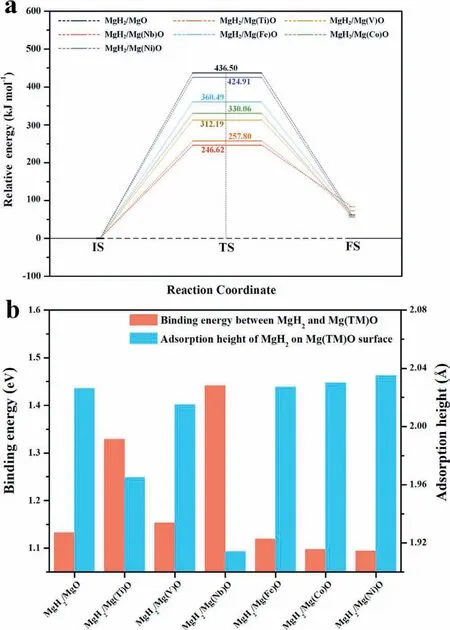
Fig. 2. (a) Calculated activation energies for the hydrogen desorption from MgH2/MgO and MgH2/Mg(TM)O systems, (b) calculated binding energy between MgH2 monomer and various Mg(TM)O surfaces, and measured adsorption height of MgH2 monomer on various Mg(TM)O surfaces. (For interpretation of the references to colour in this figur legend, the reader is referred to the web version of this article.)
Its X-ray diffraction(XRD)pattern demonstrates that MgO has a typical rock salt type structure (Fig. 3a), which is similar to that of NbO, indicating that the formation of a solid solution would be possible between MgO and NbO due to their closely matching lattice constants and oxidation states [47,48]. Hence, a solid-state reaction between MgH2and Nb2O5, which could lead to the simultaneous reduction of Nb2O5with the formation of MgO, was adopted to synthesize the Mg(Nb)O compound. After the milling process,the characteristic diffraction peaks of both MgH2and Nb2O5disappeared, indicating the complete reaction between them,and all the diffraction peaks could be indexed to the standard pattern of MgO, indicating the formation of a single phase with a similar crystal structure to MgO. Specificall , it should be noted that the diffraction peaks in the range from 30° to 55° shifted to lower angles in comparison with pure MgO, demonstrating the enlarged lattice parameters, which is mainly attributed to the larger size of the Nb atom compared with Mg and indirectly confirm the formation of solid solution Mg(Nb)O. The FTIR spectra (Fig. 3b) further validated the absence of the bond vibration of Nb-O indexed to Nb2O5and the appearance of broad absorption peaks within the range of ~550-820cm-1in the as-synthesized Mg(Nb)O compound, which agrees well with the results reported in the literature [49]. In addition, the XPS survey spectrum verifie the high-purity of the as-synthesized Mg(Nb)O sample (Figure S2) and the characteristic peaks of Nb 3d5/2observed at 203.4eV and 206.6eV (Fig. 3c) provide further evidence of the successful doping of Nb into the MgO lattice.
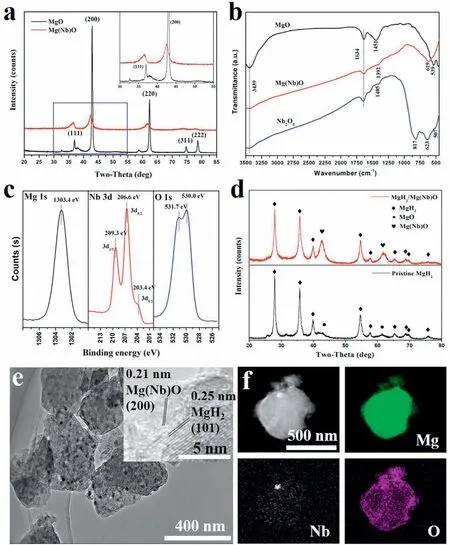
Fig. 3. (a) XRD patterns of MgO and Mg(Nb)O samples, with enlargement in the inset. (b) FTIR spectra of MgO, Nb2O5, and Mg(Nb)O samples. (c)High-resolution XPS spectra of Mg 1s, Nb 3d, and O 1s for the Mg(Nb)O sample. (d) XRD patterns of the pristine MgH2 and MgH2/Mg(Nb)O samples. (e)TEM image (inset: HRTEM image) and (f) the corresponding elemental mapping of the MgH2/Mg(Nb)O composite.
The scanning electron microscope (SEM) images show that the as-synthesized Mg(Nb)O oxides have small irregular particles with uniform distributions of Mg, Nb, and O (Figure S3). Compared with ball-milled MgH2, the characteristic XRD peaks of MgH2and Mg(Nb)O in the MgH2/Mg(Nb)O composite were evidently lower and broader, which verifie that the relatively refine MgH2grains in the MgH2/Mg(Nb)O sample originated from the milling aid effects of Mg(Nb)O oxide on MgH2(Fig. 3d). After the addition of Mg(Nb)O, the MgH2particles were obviously refine and had uniform sizes of 0.2-1μm (Fig. S4a), while a wide range of uneven particle sizes and severe agglomeration could be observed in the milled MgH2(Fig. S4b). Furthermore, the transmission electron microscopy (TEM) image (Fig. 3e) and the related elemental mapping(Fig.3f)validated the uniform distribution of Mg(Nb)O on the surface of MgH2particles. High-resolution TEM (HRTEM) image (Fig. 4e) confirm the simultaneous presence of MgH2and Mg(Nb)O with intimate contact.It verifie the role of Mg(Nb)O oxide on confinin MgH2particles should be attributed to the strong interactions between them as evidenced by the theoretical calculation (Fig. 2b), which could prevent their growth and agglomeration and facilitate their uniform distribution.
Differential scanning calorimeter (DSC) spectra demonstrates an onset and peak dehydrogenation temperatures of 410 and 442, respectively, for pristine MgH2and only limited reduction of dehydrogenation temperature was observed for the MgH2/MgO composite (Fig. 4a). With the addition of Nb2O5,the peak temperature for dehydrogenation from MgH2could be decreased to 371 °C, 34oC lower than that of the MgH2/MgO composite. A total dehydrogenation capacity of around 7wt.% was observed for the MgH2/Nb2O5composite(Fig. 4b), slightly lower than that of MgH2/MgO composite,which mainly results from the oxidation of MgH2during the milling process. In strong contrast, the onset and peak dehydrogenation temperature of MgH2/Mg(Nb)O composite was significantl decreased to only 280 and 321 °C, respectively.This result directly demonstrates the superior catalytic role of Mg(Nb)O in comparison with both MgO and Nb2O5, which could be attributed to the weakened Mg-H bonds induced by Mg(Nb)O and hence the lower hydrogen desorption energy as evidenced by the theoretical calculation results. Thermogravimetric (TG) analysis demonstrated that there was a weight loss of 7.2wt.% for the MgH2/Mg(Nb)O composite owing to the release of hydrogen (Fig. 4b), coinciding with its theoretical dehydrogenation capacity, which confirm that no reaction took place between MgH2and Mg(Nb)O during the milling process.
The isothermal dehydrogenation kinetics of the MgH2/Mg(Nb)O composite was further investigated in comparison with the MgH2/MgO composite. As shown in Fig. 4c, only a hydrogen capacity of less than 1wt.% could be released from the MgH2/MgO composite at 280oC within 120min. In strong contrast, under the identical conditions, the hydrogen desorbed from MgH2/Mg(Nb)O composite could reach 5wt.%. Moreover, on increasing the temperature to 340°C, a hydrogen capacity of around 7.2wt.%, approaching the theoretical hydrogen density of the MgH2/Mg(Nb)O composite, could be achieved in less than 50min. To quantitatively investigate the improved hydrogen desorption kinetics of the MgH2/Mg(Nb)O composite compared with the MgH2/MgO composite, the apparent activation energy (Ea) for the dehydrogenation of the MgH2/Mg(Nb)O composite was calculated by the Kissinger method [50]. Based on the differential scanning calorimetry (DSC) curves with various heating rates(Figs. 4d), which exhibited reduction of the peak temperature with decreasing heating rates, the Eafor the MgH2catalyzed with Mg(Nb)O was determined to be 84.1kJ mol-1,which is much lower than that of the MgH2/MgO system(121.1kJ mol-1). This directly demonstrates that doping Nb into MgO could effectively improve the catalytic effect of MgO towards enhancing the hydrogen desorption kinetics of MgH2, which agrees well with the results of the theoretical calculations.
The cycling performance of the MgH2/Mg(Nb)O composite was subsequently evaluated using the isothermal dehydrogenation and hydrogenation method. It should be noted that,according to their hydrogen storage performance, the dehydrogenation of the MgH2/Mg(Nb)O composite was conducted at 340oC for 60min with an initial pressure below 0.02 atm and the reversible hydrogenation process at 300oC under 50 atm within 60min. In comparison, the hydrogen desorption of the MgH2/MgO system was performed at 420oC with an initial pressure below 0.02 atm and the reversible absorption at 350oC under 50 atm. As shown in Fig. 4f, clear capacity degradation was observed for the MgO-catalyzed MgH2with only a capacity of 4.9wt.% after 5 cycles, corresponding to a capacity retention of 68%. In strong contrast, the hydrogen desorption and adsorption curves of the MgH2/Mg(Nb)O composite remained almost unchanged, and the dehydrogenation capacity could reach 6.97wt.%,corresponding to a capacity retention of 96.8%. This result provides direct evidence of the enhanced confinemen provided by Mg(Nb)O over MgO towards limiting the particle growth and aggregation of MgH2during repeated hydrogen storage processes, leading to enhanced cycling stability, which is in good agreement with the superior confinemen effect of the Mg(Nb)O on MgH2obtained from the theoretical results (Fig. 2b).
3.3. Catalytic mechanism analysis of the activity of Mg(Nb)O in the hydrogen desorption performance of MgH2
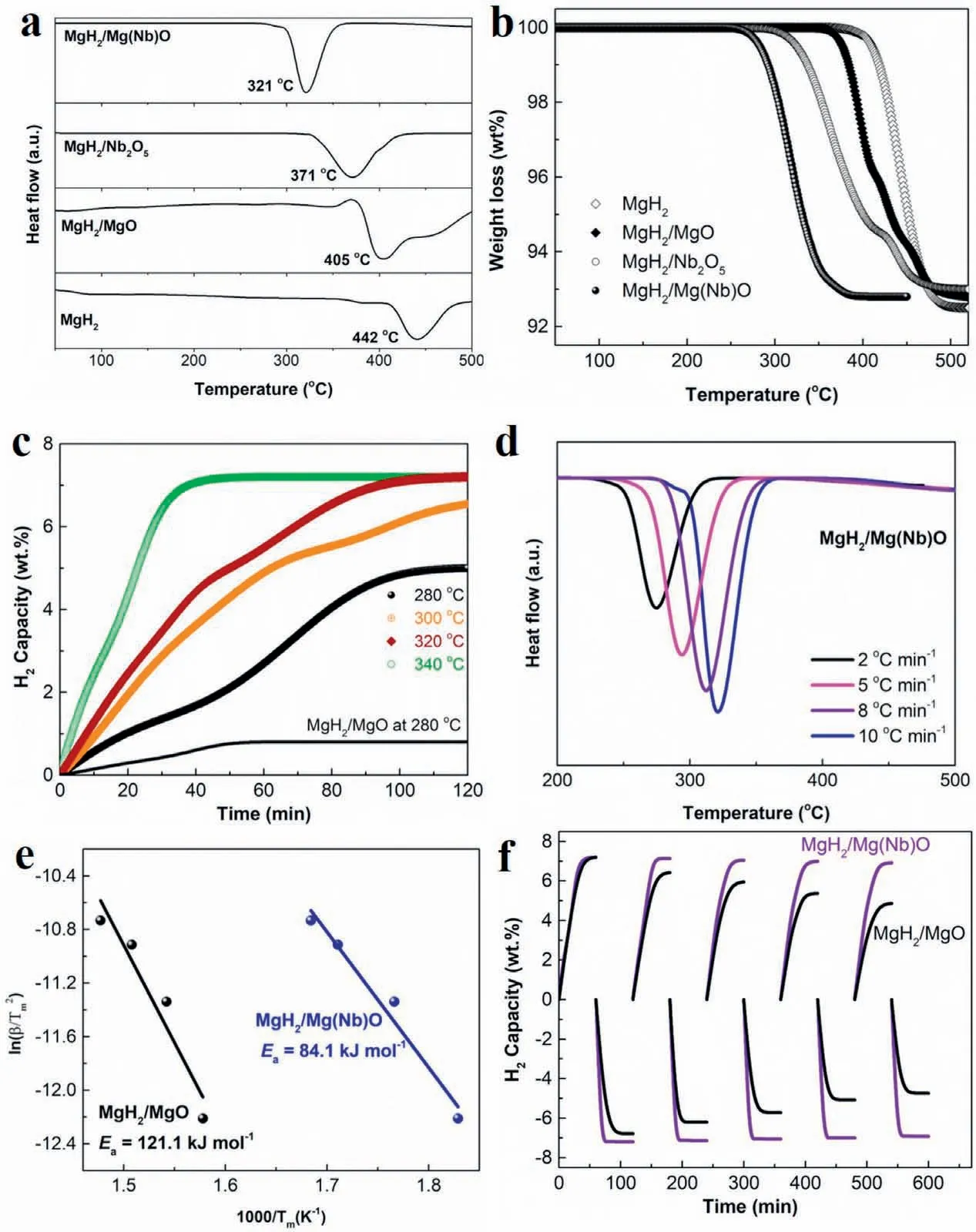
Fig. 4. (a) Differential scanning calorimetry (DSC) and (b) TG analysis curves of the MgH2/Mg(Nb)O composite in comparison with MgH2, MgH2/MgO,and MgH2/Nb2O5. (c) Isothermal hydrogen desorption curves of MgH2/Mg(Nb)O composite at various temperatures, with the MgH2/MgO composite included for comparison. (d) DSC curves of MgH2/Mg(Nb)O composite at various heating rates, and (e) estimation of the apparent activation energy (Ea) for the dehydrogenation of MgH2/Mg(Nb)O composite using the Kissinger method. (f) Reversible hydrogen desorption and adsorption of the MgH2/Mg(Nb)O composite, with the MgH2/MgO composite included for comparison.
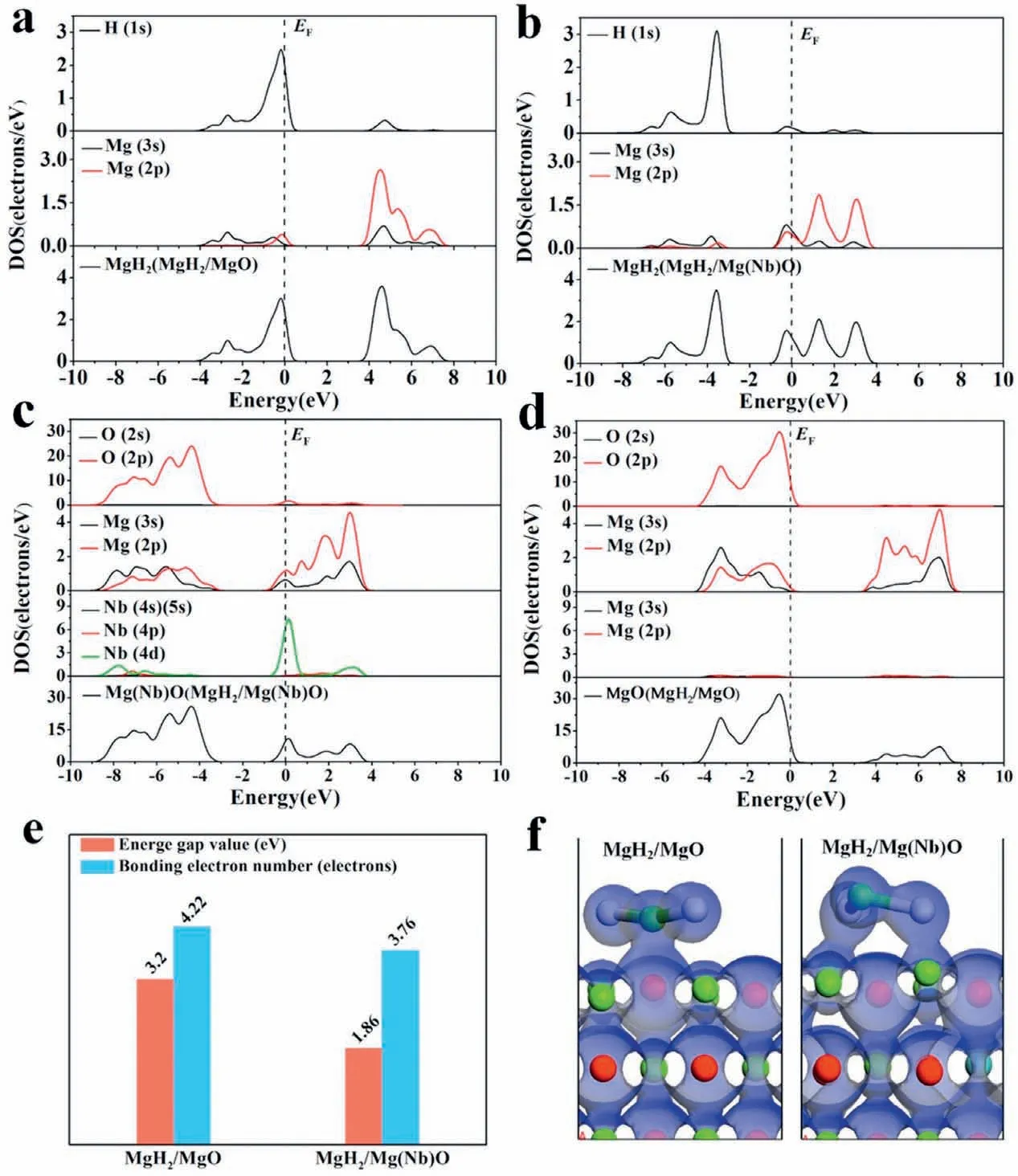
Fig. 5. (a) DOS of MgH2 in the MgH2/MgO and (b) MgH2/Mg(Nb)O models. (c) DOS of Mg(Nb)O in the MgH2/Mg(Nb)O model. (d) DOS of MgO in the MgH2/MgO model. (e) The calculated energy gap values and bonding electron numbers of the MgH2/MgO and MgH2/Mg(Nb)O models. (f) Charge density plots (with blue representing the valence electron cloud) in the MgH2/MgO system and the MgH2/Mg(Nb)O system, respectively. (For interpretation of the references to colour in this figur legend, the reader is referred to the web version of this article.)
To unravel the catalytic mechanisms of Mg(Nb)O towards the hydrogen desorption performance of MgH2, the total and partial density of states (DOS) of MgH2monomer and oxide supports (i.e., MgO and Mg(Nb)O) in MgH2/MgO and MgH2/Mg(Nb)O systems, which could reveal the nature of bonding interaction of various atoms for understanding the structural stability of MgH2in detail, were calculated as plotted in Figs. 5a-d, respectively. It should be noted that the Fermi level (EF) was set at zero and used as a reference in this work. As shown in Fig. 5a and b, the coexistence of ionic and covalent bonding interactions in MgH2leads to high strength of the Mg-H bonds, resulting in the requirement of high temperature for its dehydrogenation. In the presence of MgO, the DOS of MgH2is mainly distributed within the range of -5-8eV, while, with the doping of Nb into MgO oxide (Fig. 5b), the DOS of MgH2shifts to the lower energy range of -8-5eV, demonstrating that Mg(Nb)O could effectively weaken the stability of MgH2. This mainly results from the contribution of Nb 4d orbitals (Fig. 5c), leading to a lower energy level of Mg(Nb)O compared with the MgH2/MgO system (Fig. 5d), which results in the remarkable orbital hybridization between Mg(Nb)O and MgH2, the narrowed energy gap, and the decreased bonding electron number of MgH2monomer (Fig. 5e) [51]. This result was further supported by the increased overlapping of electron clouds between MgH2monomer and Mg(Nb)O oxide in comparison with MgO(Fig.5f).The Mulliken charges of MgH2monomer(Table S1) verifie that the total charge (-0.67 e) of MgH2monomer in the MgH2/Mg(Nb)O system was threefold higher than that of MgH2monomer(-0.19 e)in the MgH2/MgO system [52]. Hence, it is concluded that the doping of Nb into MgO effectively promotes the charge transfer from MgO to MgH2monomer, leading to significantl weakened stability of the MgH2, which could effectively enhance the interaction between them and thus enhance the hydrogen storage performance.
4. Conclusions
In this work, we have theoretically and experimentally demonstrated that the doping of low-valence of TMs into MgO could effectively not only weaken the Mg-H bonds of MgH2, resulting in a decreased hydrogen desorption energy,but also reduce the activation energy of the hydrogen storage process and prevent the sintering and particle growth of MgH2, resulting in enhanced cycling stability. Among all the investigated TMs, Nb was verifie to be the most effective in tailoring the hydrogen storage performance of MgH2. Owing to the superior catalytic effect of Mg(Nb)O, the apparent activation energy for the dehydrogenation of MgH2was reduced to 84.1kJ mol-1, and the reversible capacity could be maintained at around 7wt.% after 5 cycles, with a capacity retention of 96%. Theoretical calculations demonstrated that the doping of Nb into MgO could effectively increase the charge transfer induced by the remarkable orbital hybridization between Mg(Nb)O and MgH2, and hence weaken the stability of MgH2, resulting in enhanced hydrogen storage performance. This novel strategy provides new insights into the design of effective catalysts for improving the hydrogen storage performance of metal hydrides.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2018YFB1502101), National Science Fund for Distinguished Young Scholars (51625102), National Natural Science Foundation of China (Nos. 51874049,51401036; 51901045), the Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-07-E00028), the Science and Technology Commission of Shanghai Municipality (17XD1400700), the Changsha Science and Technology Program Project (No. kq1907092), the Science Research Project of Hunan Province Offic of Education (No.20A024), and the Programs for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jma.2020.02.029.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- Investigating TiO2-HA-PCL hybrid coating as an efficien corrosion resistant barrier of ZM21 Mg alloy✩
- Effect of yttrium modificatio on the corrosion behavior of AZ63 magnesium alloy in sodium chloride solution
- Effect of lithium content on the mechanical and corrosion behaviors of HCP binary Mg-Li alloys
- Poly caprolactone/titanium dioxide nanofibe coating on AM50 alloy for biomedical application
- New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys
