New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys
2021-05-21ShngjuLioBoxingYuXulingZhngXiopngLuPngZhouChunynZhngXioBoChnToZhngFuhuiWng
Shngju Lio, Boxing Yu, Xuling Zhng, Xiopng Lu, Png Zhou, , Chunyn Zhng,XioBo Chn, To Zhng, , Fuhui Wng
a Corrosion and Protection Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 62 Wencui Rd, Shenyang 110016, China
b School of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China
c Shenyang National Laboratory for Materials Science, Northeastern University, 3-11 Wenhua Rd, Shenyang 110819, China
d Shanghai Spaceflight Precision Machinery Research Institute, No. 76 Guide Road, Songjiang, Shanghai 201600, China
e School of Engineering, RMIT University, Carlton, VIC 3053, Australia
Received 27 August 2019; received in revised form 24 December 2019; accepted 30 December 2019
Available online 20 August 2020
Abstract
Keywords: Phosphate conversion coating; Design principles; Magnesium alloy; TA/pH; Corrosion resistance.
1. Introduction
1.1. Cracking and thin thickness: two major obstacles to improve the corrosion resistance of conversion coating on magnesium alloy
Poor corrosion resistance under various service conditions obstacles the wide range of applications for magnesium (Mg)alloys [1-3]. Conversion coatings are appealing due to their high protective effectiveness, low-cost and ease in operative procedure [4-6]. Chromate conversion coating (CCC) is the most effective conversion coating due to its unique selfhealing capability[7-9].However,a strict restriction has been imposed on the chrome-pickle treatment due to the carcinogenicity of Cr(VI) [10-12].
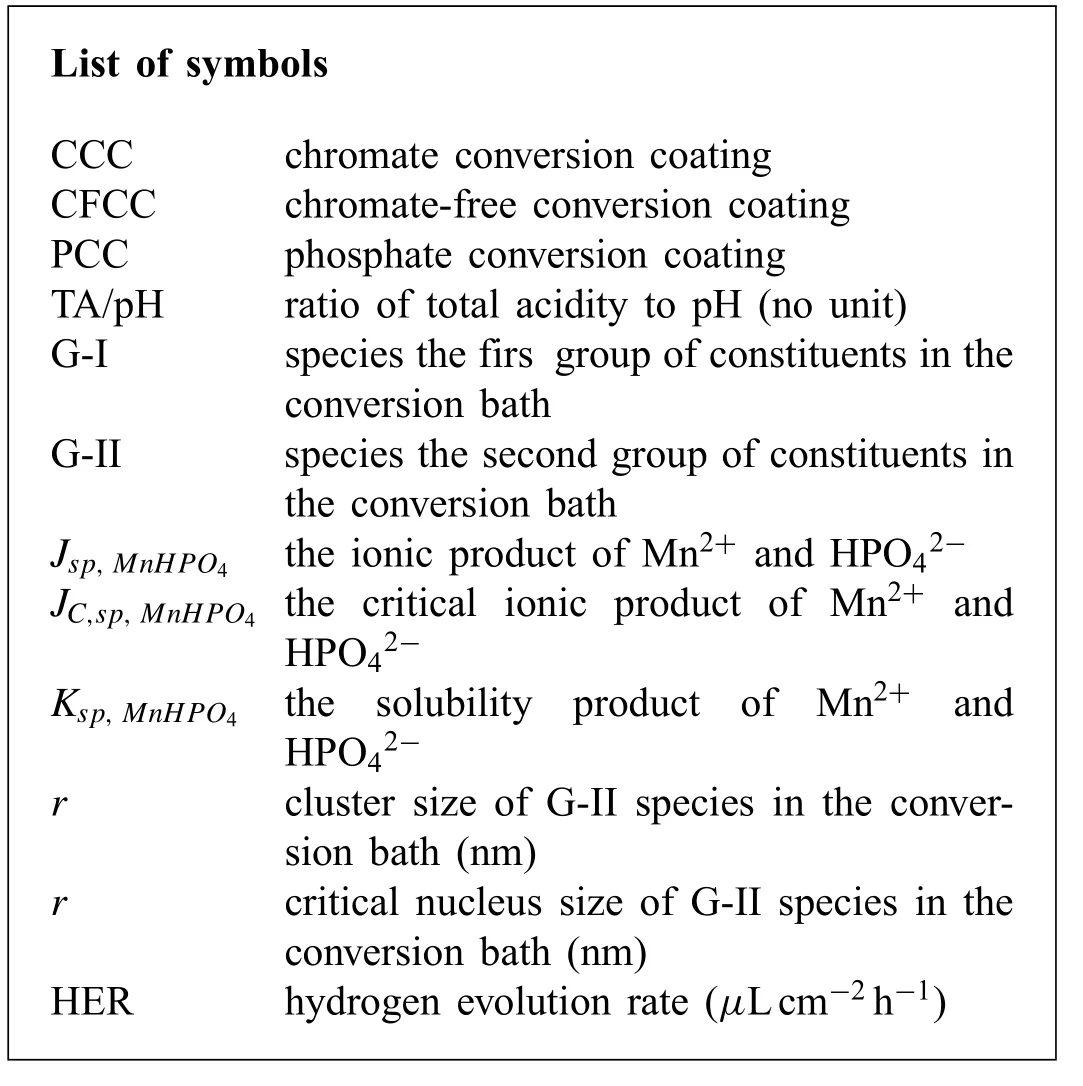
List of symbols CCC chromate conversion coating CFCC chromate-free conversion coating PCC phosphate conversion coating TA/pH ratio of total acidity to pH (no unit)G-I species the firs group of constituents in the conversion bath G-II species the second group of constituents in the conversion bath Jsp,MnHPO4 the ionic product of Mn2+ and HPO42-JC,sp,MnHPO4 the critical ionic product of Mn2+ and HPO42-Ksp,MnHPO4 the solubility product of Mn2+ and HPO42-r cluster size of G-II species in the conversion bath (nm)r critical nucleus size of G-II species in the conversion bath (nm)HER hydrogen evolution rate (μLcm-2 h-1)?
During the past decades, research into alternatives of CCCs led to the development of phosphate [13-19], stannate [20,21], phosphate-permanganate [22-24], hydrotalcite[25,26],rare earth[27-29],vanadium[30],zirconium[31],titanate [32] and phytic acid conversion coatings [33,34]. However, the corrosion resistance of these chromate free conversion coatings (CFCCs) is still significantl inferior than that of CCCs. The formation mechanism of CFCCs can be summarized as follows: (a) Mg substrate reacts with the solutes in the bath, and generate a corrosion-product-like hydrated oxide/hydroxide/salt coating; (b) due to dehydration effect,the shrinkage of conversion coating leads to the tensile stress during the drying process, which becomes worse with the increase of coating thickness;(c)consequently,the conversion coating releases the stress through cracking. Unlike the excellent self-repairing property of CCCs, the ubiquitous cracks within CFCCs act as passways for Cl-penetration, leading to a serious localized corrosion on Mg substrate. Although stannate conversion coating has small semispherical particle morphology and shows crack-free feature [20,21], the formation of a compact stannate conversion coating inhibits the further reaction between the Mg substrate and the bath, which decreases the reaction rate and results in a thinner coating(around 1μm). Usually, only a thicker coating with consistent compactness could provide reliable protectiveness for Mg substrate.So an ideal design of CFCC should exhibit a feature of both crack-free and high thickness.
By far, the unsatisfie corrosion performance of CFCCs might be attributed to the lack of appropriate design rationale of the treatment baths. Conventional designs are based on two con~icted processes: (a) the uninterrupted reaction of Mg with solutes to form a corrosion-product-like protective film and (b) the growth of the protective film Once a protective coating is formed upon Mg surface, it will isolate Mg substrate from any further reaction, resulting in a protective but thin conversion coating. A case in point is the stannate conversion coating. When a conversion coating grows continuously in a bath, channels exist to allow aggressive ions to approach underlying Mg substrate, indicating poor protective performance of the coating. Though the coating could grow without being interrupted by the existed protective layer, its large size particle will not facilitate the release of tensile stress inside the coating during the dehydration, giving rise to the emergence of physical defects, e.g. cracks. In other words, it remains a challenge to design chemical conversion baths to produce coatings with a combined feature of high protectiveness, thickness and crack-free.
1.2. Designing idea for the bath towards crack-free conversion coatings
In this work, we present a new idea for fabricating crackfree CFCC on Mg alloys. Based on their roles in coating formation, the selected solutes in bath design were categorized into two groups. The firs group (noted as G-I) reacts with Mg substrate and generates an inner layer consisting of corrosion products and cracks. The second group (G-II)displays a supersaturated state, thus forming nuclei and crystallizes into a compact outer layer. G-I solutes react with Mg and increase pH at the interface of metal and electrolyte. The pH increase breaks the ionic equilibrium of G-II species and leads to rapid the crystallization of G-II species into conversion coating. Such a design will lead to a CFCC microstructure with two discrete layers. Due to the high nucleation rate of G-II species, grain size of the ingredients in out layer is significantl refined which facilitates the formation of abundant grain boundaries. Such a high density of grain boundary will mitigate the tensile stress that is accumulated during the dehydration process. As a result, a crack-free and compact structure is achieved.Moreover,due to the supersaturated state of G-II species, their crystallization process is irrelevant to the reactions of G-I species,and thus accelerates the formation of a thick coating.In summary,the essential difference in designing rationale between our and conventional baths lies in the decoupling coating growth (outer layer, G-II species)from the dissolution of Mg substrate(inner layer,G-I species).Both growth rate and grain size will be dominantly affected by the degree of ionic supersaturation in bath. Through such a way, it is feasible to produce crack-free and thick CFCC upon Mg alloys for effective corrosion protection.
In this article,the fabrication of phosphate conversion coating (PCC) was selected as an example to validate the proposed designing rationale. H2PO4-is a G-I species in the bath, and its reactions can be expressed as follow:
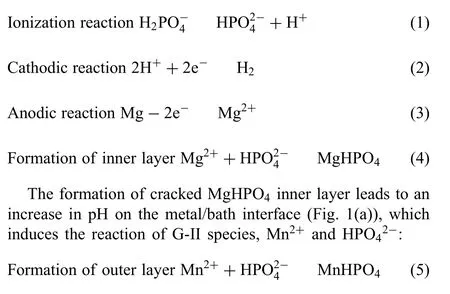
Fig. 1. Schematic illustration of the formation mechanisms of the crack-free PCC coated Mg alloy AZ91D specimens in the bath: (a) cracking inner layer,(b) nucleation of MnHPO4, (c) growth and coalescence of nuclei and (d) crack-free outer layer.
MnHPO4nuclei accumulates on Mg substrate (Fig. 1(b)),grows and coalesces (Fig. 1(c)). The growth rate of MnHPO4grains in the supersaturated solution can maintain at a high level, which is seldomly in~uenced by the decrease in the reaction rate of reactions (2) and (3). Thus, a MnHPO4outer layer with high thickness could be generated on the surface of Mg alloy. Moreover, the refine MnHPO4grains could avoid the stress concentration in the process of formation and dehydration. As a consequence, a crack-free PCC with high thickness was fabricated on the surface of Mg alloy (Fig. 1(d)).
Although some researchers have attempted to establish similar designing ideas towards the preparation of CFCCs,the protective performance of those coatings is not satisfie[15-19]. This might be attributed to the improper designing for the [Mn+], [HPO42-] and pH. We recently proposed a new concept, i.e., the ratio of total acidity to pH (TA/pH),for the firs time, to determine the design of conversion coating baths [35]. Results demonstrated that the microstructure and corrosion resistance were well improved with the decrease of TA/pH. However, some questions remain unanswered, such as physical significanc and valid boundary of TA/pH in improving the quality of PCC[35].In this work,the significanc of TA/pH was interpreted in terms of the ionic product ([Mn+]×[HPO42-]), and the composition of the conversion bath was further adjusted by adding complex agent(EDTA4-), oxidizing agent (NO3-) and catalysts (NH4+),which in turn changes the TA/pH value simultaneously. The intrinsic property of TA/pH in designing conversion bath is proposed.
2. Experimental details
2.1. Materials and preparation of conversion coating
Commercial ASTM AZ91D Mg alloy ingots(Nanjing Yunhai Special Metals Co. Ltd., China) were used in this work.Its chemical composition (8.70 Al, 0.64Zn, 0.23 Mn, 0.014 Si, 0.002 Fe, 0.002 Cu and balance Mg, all in wt%) was analyzed using inductive coupled plasma-atomic emission spectrometry (ICP-AES, X series II, Thermofishe Scientific US).The as-received ingots were machined into a dimension of 20mm×20mm×5mm and mechanically ground with silicon carbide papers up to 2000 grit. The specimens were dried in compressed cold air prior to the fabrication of PCC.
The benchmark phosphate conversion baths were composed of 0.2M Mn2+and 0.3M H2PO4-(Sinopharm Chemical Reagent Co.Ltd.,China).Different quantities of EDTA4-,NO3-and NH4+were added into the benchmark bath as required, aiming to adjust TA/pH value and accelerate nucleation, respectively. Chemicals were dissolved into deionized water at room temperature with magnetic stirring. 0.1M NaOH was used to adjust pH of the phosphate conversion solutions through a pH meter (PHSJ-4A, SPSIC, China). TA was determined by measuring the consumption volume of 0.1M NaOH during the titration of conversion bath using phenolphthalein as indicator as reported previously [36]. The specimens were treated for 10min at 60°C, followed by rinsing with running deionized water, dried with a cold air ~o w and aged in air for 12h prior to subsequent characterizations.
2.2. Microstructure characterization
Surface and cross-sectional morphology of PCCs was examined using field-emissio scanning electron microscopy(SEM, Philips XL-30FEG, the Netherlands) equipped with energy dispersive spectrometry (EDS). Samples for crosssectional imaging were prepared as follows. Firstly,a selected PCC coated Mg alloy AZ91D specimen was placed vertically into a silicon mould and fi ed using glue, followed by pouring a mixture of epoxy and hardener (with a mass ratio of 5:1) into the mould; the embedded specimen was left on a~at surface at room temperature over 24h for curing. Secondly, the embedded specimens were automatically ground using 2000-grit silicon carbide papers at a metallographic grinding machine with a rotating speed less than 100rpm. To avoid mechanical fracture of conversion coating evoked by shear stress, coating/metal interface was maintained parallel to the tangent direction of grinding disc during the grinding process. Finally, the ground specimen was polished using alumina slurry (2.5μm) suspension solution.
2.3. Corrosion tests
Hydrogen evolution tests were performed to evaluate the corrosion performance of bare and PCC coated specimen with 9 cm2contact area to 3.5 wt% NaCl solution. Hydrogen evolution rate (HER) was measured by a home-made automatic setup. This setup couples the reversely placed funnels to the multi-channel digital scales, and automatically measures the weight of water being drained away due to the corrosion of samples beneath the funnels. At least fi e parallel samples were tested, ensuring the reproducibility of the measurement.The hygrogen volume was obtained by converting the weight of water into volume. The detection limit of hygrogen emission is 0.03mL for this setup.Salt spray tests based on ASTM B117-03 standards were carried out to evaluate the corrosion resistance of PCC coatings up to 48h. The macroscopic morphology of the substrate and coatings was obtained using a digital camera. Potentiodynamic polarization curves of bare and coated samples were recorded using a potentiostat (Zennium, Zahner-elektrik GmbH & CoKG, Germany) in 3.5 wt%NaCl solution at room temperature. A typical three-electrode cell was used, consisting of a platinum foil as counter electrode, a saturated calomel electrode (SCE) as the reference electrode and the coated sample as working electrode. The samples were polarized towards anodic and cathodic direction after 10min stabilization at open circuit potential (OCP) at a scan rate of 0.33mV s-1. Each measurement was repeated at least fi e times at 30±1°C for reproducibility.
2.4. Nucleus size measurement
For determination of the size distribution of MnHPO4clusters, 10mL conversion bath containing 0.2mol/L Mn2+and 0.3mol/L H2PO4-was placed in a quartz cell. Then, pH of a TA/pH of conversion bath was adjusted using 0.1M NaOH within the range of 11.5-12.3. The size distribution of MnHPO4clusters was determined using a Zetasizer Nano S90 laser particle size analyzer in order to explore the relationship between the critical nucleus size and TA/pH.
3. Results and discussion
3.1. Roles of TA/pH in PCC growth
In our previous work, we demonstrated that morphology of the outer layer (MnHPO4) of PCC was strongly in~uenced by the value of TA/pH of coating baths. Decreasing TA/pH leads to increasing nucleation density and reduction in particle size, which are beneficia to the formation of a uniform and compact MnHPO4out layer upon Mg alloys [35]. In this work, the roles of TA/pH in regulating the growth of PCCs are explored in details by borrowing two key concepts from metallurgy, i.e., constitutional supersaturation degree and critical size of nucleus.
3.1.1. Broadening the window of TA/pH values without compromising the stability of supersaturated conversion bath: effect of complexing agents
In theory, TA/pH could be reduced by means of either decreasing TA or increasing pH. But the strong buffering effect of conversion bath requires a significan change of composition to achieve a significan change of pH.The direct increase in pH by adding NaOH leads to the forward shift of reaction(1) and the increase of HPO42-concentration. Subsequently,reaction (5) also shifts to the forward direction, evoking the precipitation of MnHPO4. As a result, the conversion bath fails before the conversion treatment. Thus, it is very diffi cult to decrease TA/pH simply via the addition of NaOH. In other words, TA/pH is under the restriction of the equilibrium of reaction (5) in the bath and can’t be simply decreased by adjusting pH.
However, the addition of complexing agent, such as EDTA4-, will avoid the breach of chemical equilibrium by a complexation reaction in the bath:

Due to the complexation reaction, the concentration of free Mn2+in bath is significantl decreased, indicating the great decrease in ionic product of MnHPO4, Jsp,MnHPO4=[Mn2+]×[HPO42-]. As a consequence, TA/pH could be easily decreased without compromising the stability of conversion bath, as is shown in Table 1.
The surface morphology of PCC coated Mg alloy prepared in the bath containing various EDTA4-is shown in Fig. 2.With the addition of various EDTA4-(0-0.06M), the density of MnHPO4particles increases with the decrease of particle size, resulting in the increasing coverage of outer layer(Fig. 2(a)-(d)). With the further addition of EDTA4-, both the density and size of MnHPO4particles decreases obviously(Fig. 2(e)-(h)). The cross-sectional morphology of PCCs in Fig. 3 demonstrates a similar trend: the quality of MnHPO4layer formed on Mg alloy was firstl improved gradually,then deteriorated and formed sparsely distributed but large MnHPO4particles.
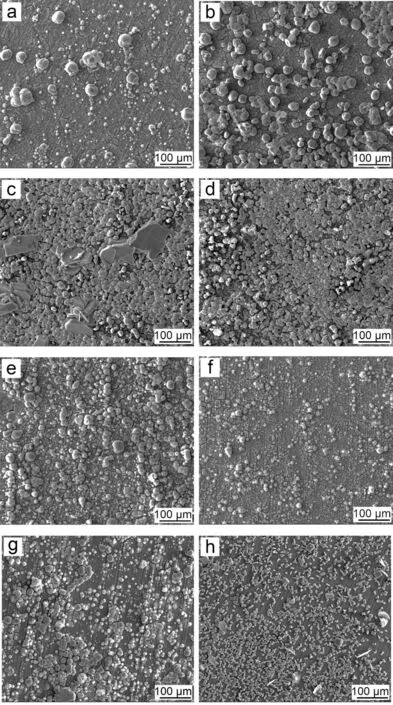
Fig. 2. Surface morphology of the PCCs fabricated from the benchmark baths with the different concentration of EDTA4-: (a) 0molL-1, (b) 0.02molL-1,(c) 0.04molL-1, (d) 0.06molL-1, (e) 0.08molL-1, (f) 0.11molL-1, (g) 0.16molL-1 and (h) 0.21molL-1.
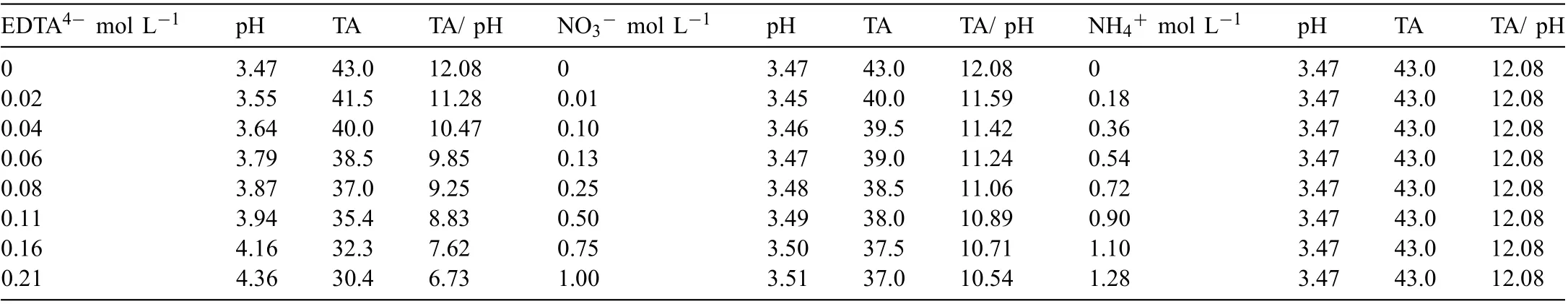
Table 1 Calculated and measured value for pH, total acidity (TA) and their ratio (TA/pH).
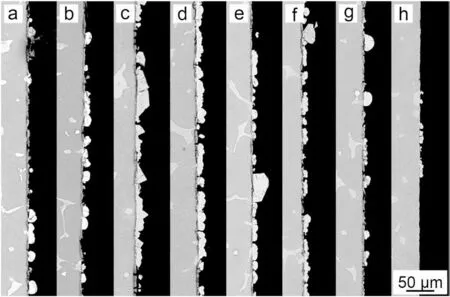
Fig. 3. Cross-sectional morphology of the PCCs fabricated from the benchmark baths the different concentration of EDTA4-: (a) 0molL-1,(b) 0.02molL-1, (c) 0.04molL-1, (d) 0.06molL-1, (e) 0.08molL-1, (f)0.11molL-1, (g) 0.16molL-1 and (h) 0.21molL-1.
The hydrogen evolution rate (HER) of the PCCs prepared in the bath containing various EDTA4-is listed in Table 2. With the increase of EDTA4-concentration, HER decreases from 42.27±4.80 μL cm-2h-1(benchmark bath)to 17.12±0.25 μL cm-2h-1(0.06M of EDTA4-), and then increases to 42.92±3.04 μL cm-2h-1(0.21M of EDTA4-).The macroscopic morphology of PCCs after 48h salt spray test is shown in Fig. 4. It can be seen that PCC fabricated in the benchmark bath was severely corroded and many corrosion pits were observed on the surface (Fig. 4(a)). After the addition of EDTA4-, the density of corrosion pits decreases and then increases (Fig. 4(b)-(h)). It could be found that the results of spray salt tests are in good agreement with the morphology observations and HER test.
3.1.2. Supersaturation of bath: constitutional supersaturation degree during the formation of outer layer
With the addition of EDTA4-, the reaction equilibrium in solution can be expressed as in Eqs. (1), and (6) to (8):
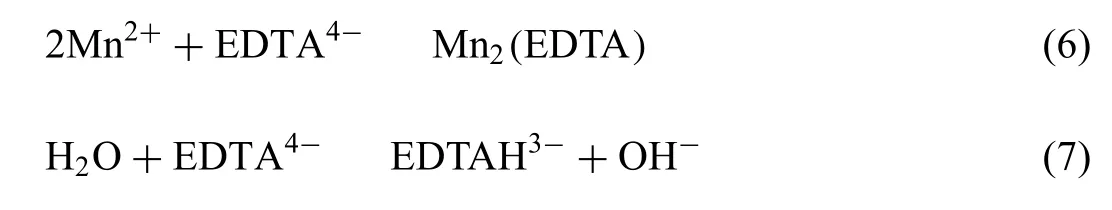

The equilibrium constant of the aforementioned reactions is 6.34×10-8, 6.31×10-13, 1.02×10-2and 7.59×10-8,respectively [37]. According to these constants, TA/pH,Jsp,MnHPO4, the concentration of H+, HPO42-and free Mn2+in the conversion baths were calculated and illustrated in Fig. 5. With the addition of complexing EDTA4-in to baths,TA/pH decreases from 12.8(0.0M)to 6.7(0.21M)(Fig.5(a)),which also leads to the decreasing concentration of free Mn2+ions and increasing concentration of free HPO42-ions(Fig. 5(b)). Meanwhile, Jsp,MnHPO4increased gradually to a peak value of 2.28×10-5(at TA=9.25) and then decreased to 1.34×10-5(at TA=7.62), as shown in Fig. 5(c). Considering the formation process at a constant temperature (60 °C),the denotation of Jsp,MnHPO4is somehow similar with constitutional supersaturation degree during the solidificatio of metals[38].The increase in Jsp,MnHPO4by its nature is to increase the supersaturation degree of G-II species in the bath, suggesting a stronger driving force for the nucleation of MnHPO4[38]. This explains the phenomenon we proposed previously that a dense and refine PCC can be obtained by decreasing TA/pH [35]. As a summary, the promotion of nucleation could be achieved by adding either complexing agent, e.g.EDTA4-or alkaline compound, e.g. NaOH, which in turn increases the solubility product (Ksp) of G-II species. The increase in solubility product increases the supersaturation degree of G-II species,thus promoting the formation of compact PCC.
3.1.3. Critical supersaturation degree and critical size of nucleus: crucial factors for the formation of PCCs with a compact structure
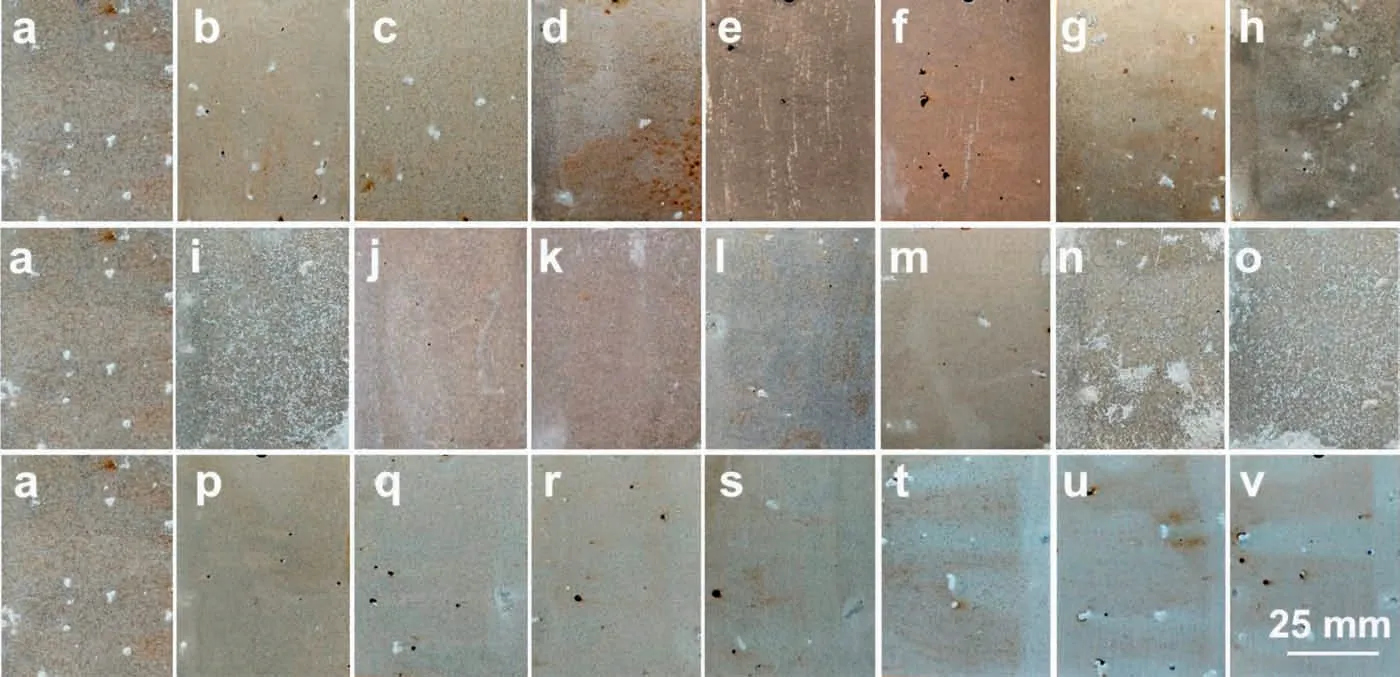
Fig. 4. Macroscopic morphology of the PCCs after salt spray test for 48h, which fabricated from the benchmark baths with the different concentration of EDTA4- (b)-(h), NO3- (i)-(o) and NH4+ (p)-(v), respectively: (a) 0molL-1, (b) 0.02molL-1, (c) 0.04molL-1, (d) 0.06molL-1, (e) 0.08molL-1, (f)0.11molL-1, (g) 0.16molL-1, (h) 0.21molL-1, (i) 0.01molL-1, (j) 0.10molL-1, (k) 0.13mol L-1, (l) 0.25molL-1, (m) 0.50molL-1, (n) 0.75molL-1, (o)1.00molL-1, (p) 0.18molL-1, (q) 0.36molL-1, (r) 0.54molL-1, (s) 0.72molL-1, (t) 0.90molL-1, (u) 1.10molL-1 and (v) 1.28molL-1.
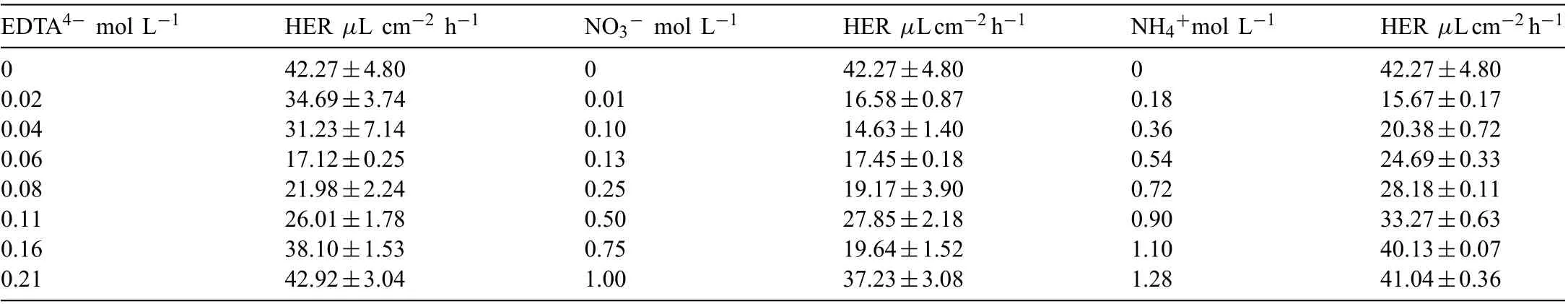
Table 2 HER of the PCCs coated Mg alloy AZ91D specimens fabricated from the benchmark bath with the addition of different compositions.
From a thermodynamic perspective, the nucleation of MnHPO4crystalline particles as main constituents of outer layer upon Mg alloys is triggered when the ionic product of free ions in a coating bath exceeds the solubility product (Jsp,MnHPO4>Ksp,MnHPO4) of a compound consisting of those ions. However, in terms of kinetics, the nucleation is accelerated only when the ionic product exceeds the critical ionic product (Jsp,MnHPO4>JC,sp,MnHPO4) [39,40]. The introduction of those parameters shed lights on the mechanistic effects of TA/pH on formation of PCCs and consequent corrosion protection upon Mg alloys (Fig. 6). During the formation of PCCs, the reaction of G-I species, i.e., dissolution of metallic Mg into Mg2+and hydroxide ions, significantl increases pH at the interface, which in turn promotes Reaction (1). During the formation of the outer layer (Eq. (5)),low TA/pH values boosts Jsp,MnHPO4climbing up and readily exceeding JC,sp,MnHPO4, which results in rapid nucleation of MnHPO4on the surface of Mg alloy. Such a high nucleation rate of MnHPO4grains renders the formation of a compact MnHPO4outer layer with a crack-free and well refine structure [35]. In contrast, high TA/pH values indicate low Jsp,MnHPO4, far below its JC,sp,MnHPO4. As a consequence, a low number density of MnHPO4nuclei is formed and the spacious room will facilitate growth of MnHPO4particles into big size on Mg substrate but incomplete surface coverage(Fig. 2(a)), as such, poor protection to underlying Mg alloy parts against corrosion takes place.
Under the circumstances of Ksp,MnHPO4<Jsp,MnHPO4<JC,sp,MnHPO4, though a large quantity of MnHPO4clusters smaller than their critical nucleus size (r <r ), precipitation of MnHPO4particles could not occur upon Mg alloy until r >r , i.e., Jsp,MnHPO4>JC,sp,MnHPO4. This is analogous to the critical nucleus size during the solidificatio of metal melts.
The measurement of r will be of critical significanc to offer guidance for the design of premium PCC baths.By adding NaOH into the benchmark conversion bath (i.e.,0.2mol L-1Mn2+and 0.3mol L-1HPO42-), the ratio of TA/pH and the cluster size of MnHPO4were altered simultaneously. The cluster size was measured using a laser particle size analyzer (Fig. 7). It is not surprising that the addition of NaOH gradually changed the conversion bath from transparent to turbid, indicating the homogenous precipitates of solutes from bath (Fig. 7(a)). When 11.85 <TA/pH <12.30 (A to D in Fig. 7(a)), the cluster size of MnHPO4was distributed within the range of [30nm, 100nm] (Fig. 7(b)).With the further decrease in TA/pH, i.e., 11.85 <TA/pH<11.72, r was increased to the range of [100nm, 300nm].The cluster size as a function of TA/pH was plotted and presented in Fig. 7(c). A quasi-sigmoidal relationship was observed: the decrease of TA/pH leads to a rapid increase of cluster size from less than 50nm to ca. 250nm, then stabilizes at this value. Meanwhile, the ionic product, Jsp,MnHPO4,increases gradually and stabilizes at 1×10-3when TA/pH decreases to ca. 11.8. The further decrease of TA/pH results in the turbidity of the conversion bath, making the calculation of Jsp,MnHPO4meaningless. Note that when TA/pH was in the range from 11.8 to 12.25, the decrease of TA/pH results to a rapid increase of Jsp,MnHPO4, but r was kept at ca. 25nm with a negligible increase. This indicates that within this range, the decrease of TA/pH results in an increase of cluster density in the bath. The critical nucleus size(r ) should be in the range from 100nm to 250nm, corresponding to a critical supersaturation degree, JC,sp,MnHPO4, of 1.0×10-3.
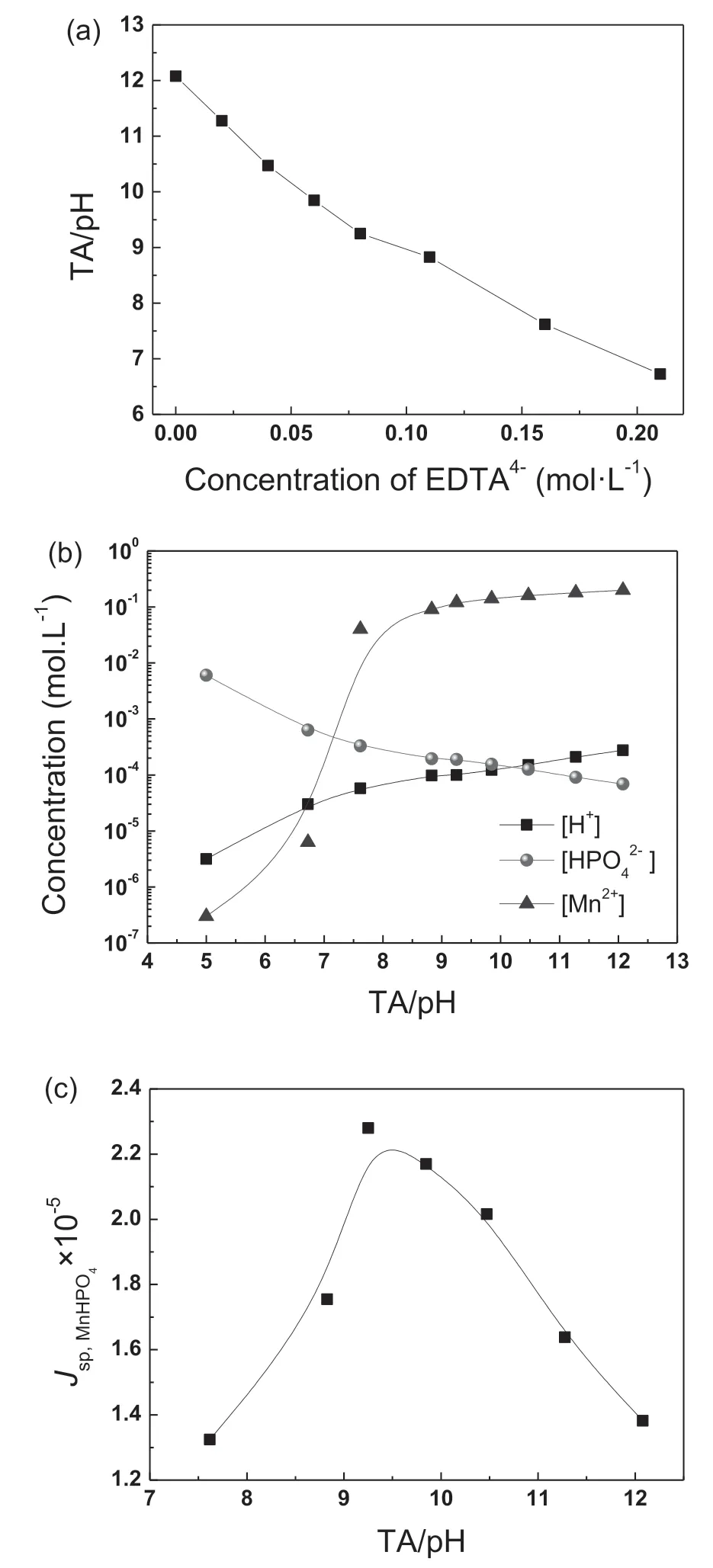
Fig. 5. (a) TA/pH of the baths as a function of concentration of EDTA4-,(b) effect of TA/pH on the concentration of H+, Mn2+ and HPO42-, (c)relationship between the solubility product of MnHPO4 and TA/pH.
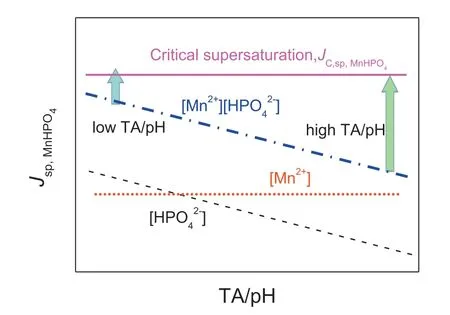
Fig. 6. Schematic illustration of the solubility product,Jsp,MnHPO4, and critical supersaturation solubility product of MnHPO4,JC,sp,MnHPO4, as a function of concentration of TA/pH.
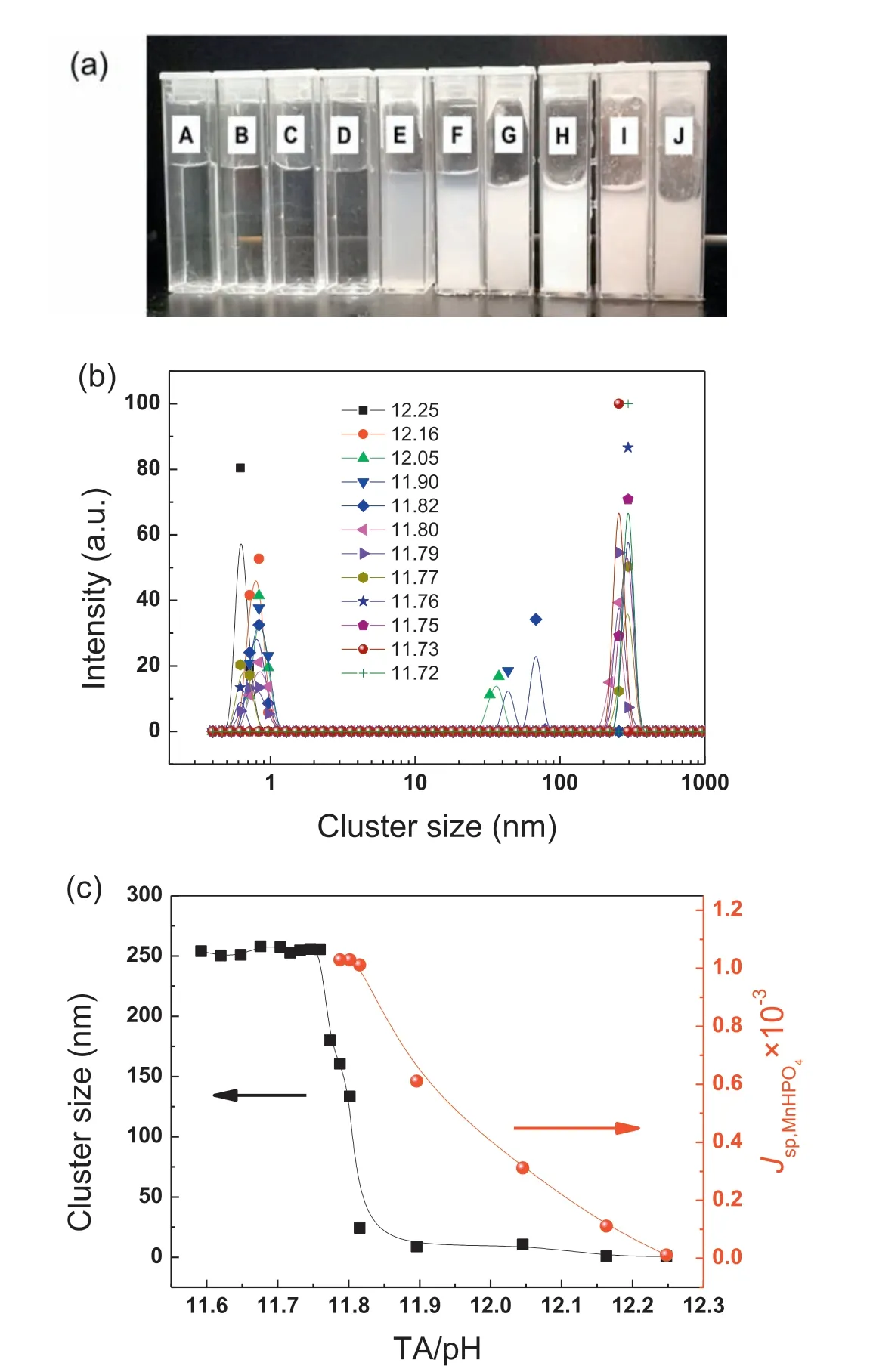
Fig. 7. (a) Macroscopic morphology and (b) size distribution of MnHPO4 cluster of the benchmark bath with the different TA/pH, (c) cluster size and Jsp,MnHPO4of the bath as a function of TA/pH.
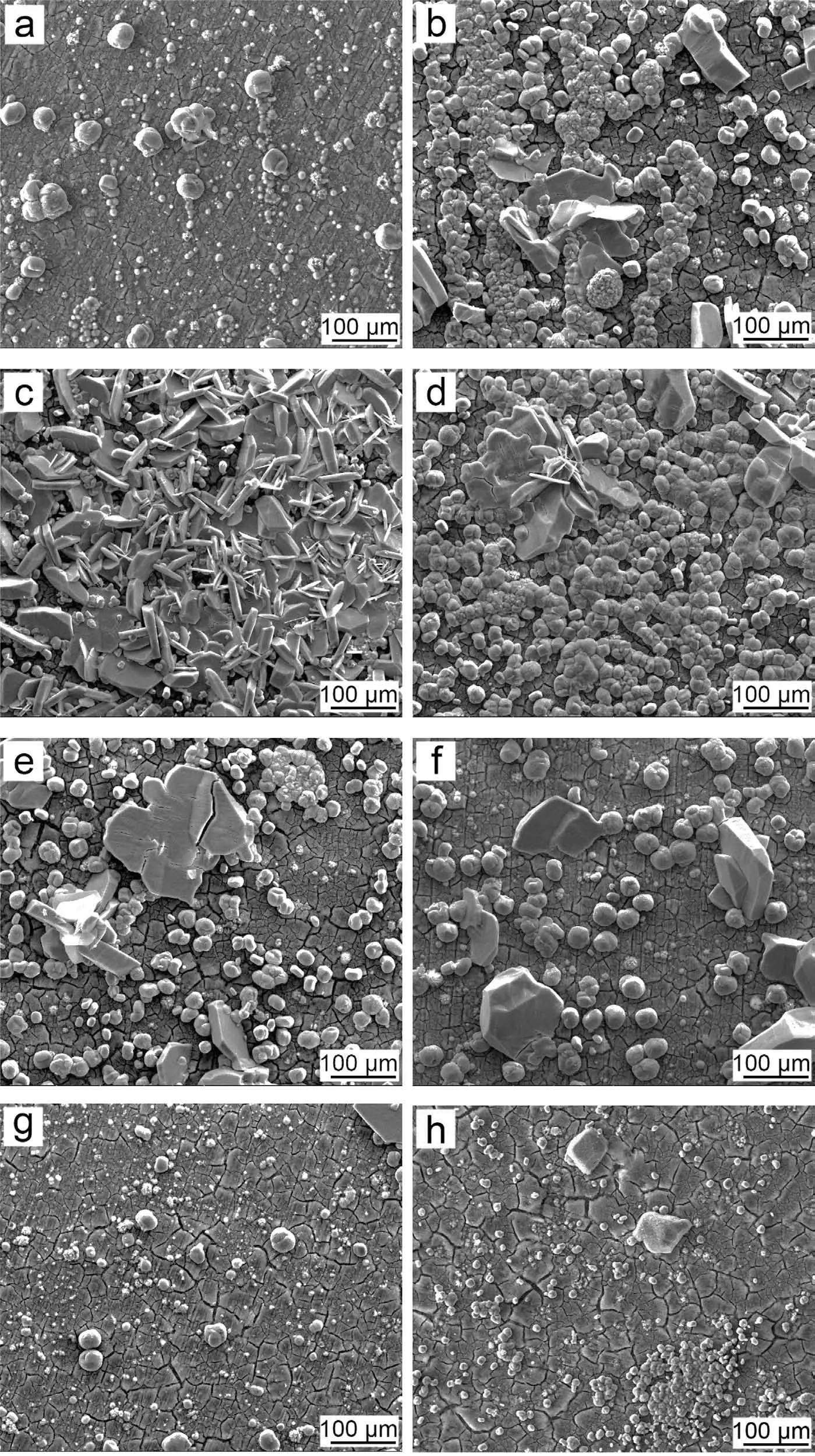
Fig. 8. Surface morphology of the PCCs fabricated from the benchmark baths with the different concentration of NO3-: (a) 0molL-1, (b) 0.01molL-1, (c)0.10molL-1, (d) 0.13molL-1, (e) 0.25molL-1, (f) 0.50molL-1, (g) 0.75molL-1 and (h) 1.00molL-1.
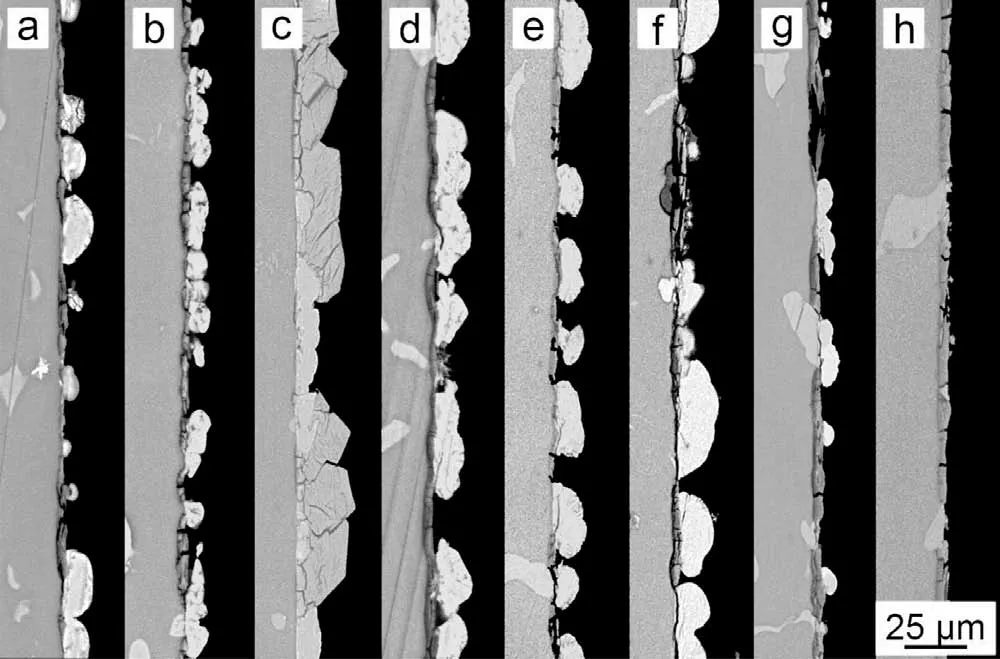
Fig. 9. Cross-sectional morphology of the PCCs fabricated from the benchmark baths with the different concentration of NO3-: (a) 0molL-1,(b) 0.01molL-1, (c) 0.10molL-1, (d) 0.13molL-1, (e) 0.25molL-1, (f)0.50molL-1, (g) 0.75molL-1 and (h) 1.00molL-1.
The above results demonstrate that in order to obtain a PCC with improved quality, Jsp,MnHPO4in the bath should be increased as much as possible,to the maximum of 1.0×10-3.Only by this way can we obtain enough MnHPO4nucleus and control their growth, avoiding the cracks and large size particle on the substrate. This is the fundamental principle for the designing of conversion bath.
3.2. Formation kinetics of PCCs
The decrease of TA/pH via increasing pH in the conversion bath, will decrease the reaction rate of (2) and (3). While the addition of EDTA4-will not compromise the stability of conversion bath, the complexing effect will decrease the concentration of free Mn2+, which is also detrimental to reaction (5). Previous work has demonstrated that the decrease of TA/pH was always accompanied by the decrease of PCC thickness [35]. So the adjustment of TA/pH alone is not a comprehensive solution to optimize the quality of PCC.
3.2.1. Electrochemical reaction rate: effect of oxidizing agent
The decrease of TA/pH will lead to the loss of H+at the interface, which was detrimental to the interfacial reaction rate.The addition of strong oxidizing agent, such as NO3-, will compensate the interfacial reaction rate. Besides, the TA/pH will be decreased as well (Table 1). Figs. 8 and 9 present the morphology evolution with the addition of various NO3-into the bench mark bath. With the addition of NO3-up to 0.1M,the substrate was covered by a dense and compact PCC layer(Figs. 8(c) and 9(c)). The further increase of NO3-leads to the adverse effect: the cracked inner MgHPO4layer from GI species was unable to be completely covered by MnHPO4((d)-(f) in Figs. 8 and 9). Especially for the case that NO3-exceeds 0.75M, only a small quantity of large MnHPO4particles was observed on top of the cracked MgHPO4inner layer, suggesting the deposition of MnHPO4was seriously inhibited ((g)-(h) in Figs. 8 and 9). The HER test shows that when NO3-was 0.1M, the PCC exhibits the lowest HER at 14.63±1.40 μL cm-2h-1. The further increase of NO3-deteriorates the corrosion resistance. A similar tendency was also observed from the salt spray test for 48h ((i)-(o) in Fig. 4).
These results demonstrate that the addition of NO3-has two completing effects on the growth of PCC: on the one hand it will accelerate the interfacial reaction rate by promoting the redox potential; on the other hand, it will result in the inhibition of interfacial reaction by passivating the Mg surface. When the former effect dominates, the conversion bath will benefi from the increasing addition of NO3-. When the latter dominates,the further addition of NO3-will slow down the interfacial reaction and large MnHPO4particles will form.
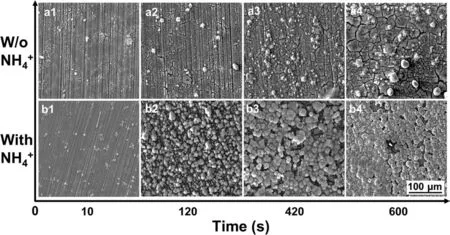
Fig. 10. Morphological evolution of the PCCs fabricated on Mg alloy AZ91D after immersion in the baths: (a1-a4) with and (b1-b4) without NH4+ for 10s,120s, 420s and 600s, respectively.
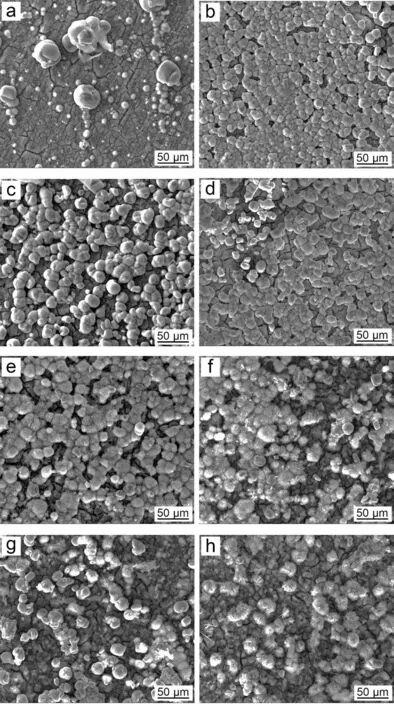
Fig. 11. Surface morphology of the PCCs fabricated from the benchmark baths with the different concentration of NO3-: (a) 0molL-1, (b) 0.18molL-1, (c)0.36molL-1, (d) 0.54molL-1, (e) 0.72molL-1, (f) 0.90molL-1, (g) 1.10molL-1 and (h) 1.28molL-1.
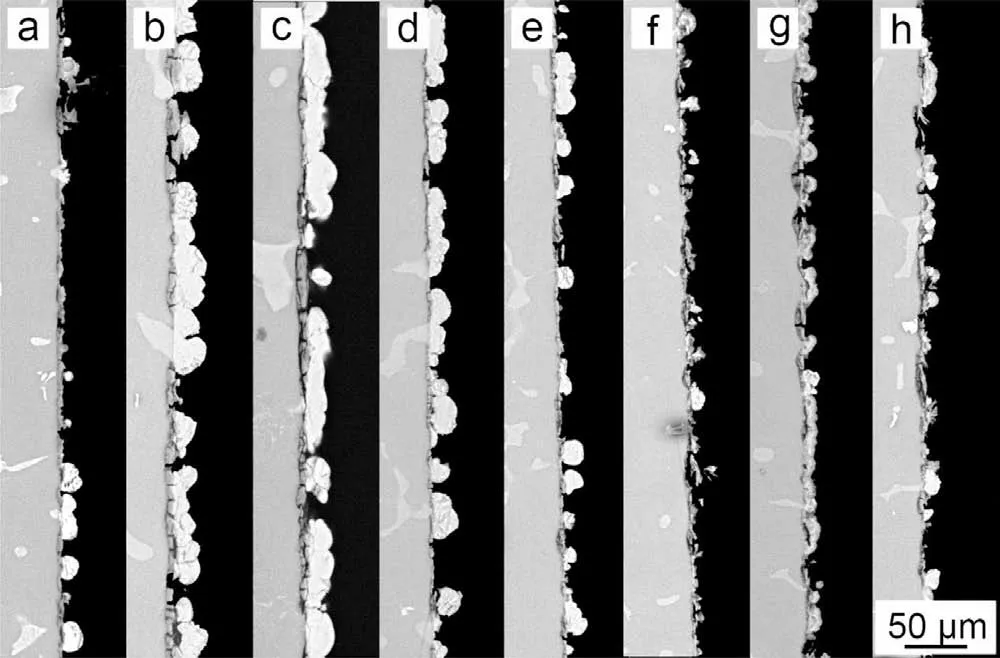
Fig. 12. Cross-sectional morphology of the PCCs fabricated from the benchmark baths with the different concentration of NO3-: (a) 0molL-1,(b) 0.18molL-1, (c) 0.36molL-1, (d) 0.54molL-1, (e) 0.72molL-1, (f)0.90molL-1, (g) 1.10molL-1 and (h) 1.28molL-1.
3.2.2. Precipitation reaction rate: effect of catalyst
During the growth of PCC, the diffusion effect results in the fact that the concentration of free Mn2+at the interface is smaller than that in the bulk solution. This concentration gradient is detrimental to the formation of PCC, since it will slow down the reaction rate of Eq. (5). The addition of catalyst is an effective way to improve the reaction rate via the following reactions:
Fig. 10 demonstrates the remarkable difference by adding NH4+as catalyst. The quantity of MnHPO4and its size were both significantl improved by adding NH4+with various time duration. The morphology of PCC by adding various catalysts were presented in Figs.11 and 12.The PCC exhibits the best quality when 0.18M NH4+was added to the bath((b)in Figs. 11 and 12). The further increase of NH4+addition leads to the deterioration of PCC, since uncomplete coverage of MnHPO4was found in (c)-(h) of Figs. 11 and 12. The cross-section observation reveals that a coarser metal/coating interface was presented, due to the excessive addition of catalyst (>0.9M) ((f)-(h) in Fig. 12). This was due to the significan decrease of interfacial pH via reaction (11), which in turn results in the increase of TA/pH. The increase of TA/pH finall inhibits the nucleation of MnHPO4, leading to the deterioration of PCC. Besides, the decrease of pH also accelerates the electrochemical reaction of Mg substrate, resulting in the severely corroded Mg/coating interface. Similar to the double role played by NO3-, the addition of catalyst is also two-edged: a proper addition of NH4+will accelerate the nucleation of MnHPO4by catalyzing reaction (13), but an excessive addition of NH4+will lead to the increase of TA/pH,thus inhibiting the nucleation of MnHPO4.
3.3. Design principles of PCC baths
Based on the above result and discussion, the designing idea for crack-free PCC is proposed:
(1) the conversion bath should be consisted of two types of constituents:G-I species(H2PO4-)and G-II species(Mn2+and HPO42-).G-I species directly reacts with Mg substrate and forms a cracked inner MgHPO4layer. The reaction will increase the pH at the interface, promoting the nucleation of supersaturated G-II species and forming an outer MnHPO4layer.
(2) TA/pH could be tuned by adding complexing agent(EDTA4-). The decrease of TA/pH promotes the supersaturation degree of G-II species. The increase of Jsp,MnHPO4is critical to obtain a crack-free and refine PCC coating. In this work, the upper limit of tuning Jsp,MnHPO4is the critical ionic product of G-II species,i.e., JC,sp,MnHPO4.0×10-3.
(3) The adverse effect of decreasing TA/pH can be balanced by adding oxidizing agent (NO3-) and catalyst (NH4+).The addition of NO3-and NH4+are both doubled edged,an optimal addition should be determined.
Following the guidance of the above design principles, the benchmark conversion bath (0.2M Mn2++0.3M H2PO4-)was further optimized by adding 0.06M EDTA4-, 0.1M NO3-and 0.18M NH4+. Jsp,MnHPO4was finall adjusted by adding NaOH, to the value of approximately 1.0×10-3. The TA/pH of the optimized conversion bath was 11.80.
The SEM images of the PCC prepared using the benchmark bath and the optimized bath are both shown in Figs. 13 and 14. The PCC prepared in the benchmark bath demonstrates a sparsely distributed but large MnHPO4particles on top of the cracked MgHPO4layer ((a) and (b) in Fig. 13). The average thickness of PCC was ca. 3.0μm. The PCC prepared in the optimized bath exhibits an excellent morphology with a completely coverage of the compact and refine MnHPO4layer. The average thickness was measured to be approximately 10.0μm. The PCC prepared using the optimized bath demonstrates a significan improvement of corrosion resistance by decreasing the HER from 42.27±4.80 μL cm-2h-1to 9.46±1.47 μL cm-2h-1, a factor of 4.5 times more than the PCC using the benchmark bath and 17.7 times more than the Mg substrate. The potentiodynamic polarization experiment indicates that the PCC using optimized bath inhibits both the anodic and the cathodic reaction (Fig. 15).The 120-hour salt spray test also demonstrates that the PCC significantl improves the corrosion resistance (Fig. 16). The above tests have demonstrated a significan improvement of corrosion resistance of PCC by optimizing the conversion using the new designing principle.
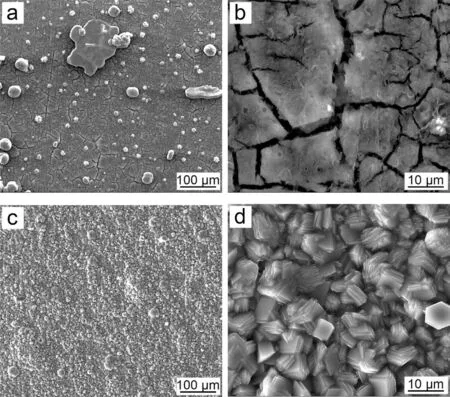
Fig. 13. Surface morphology of the PCCs fabricated from the (a) benchmark and (c) optimized baths. (b) and (d) large magnificatio for (a) and (b),respectively.
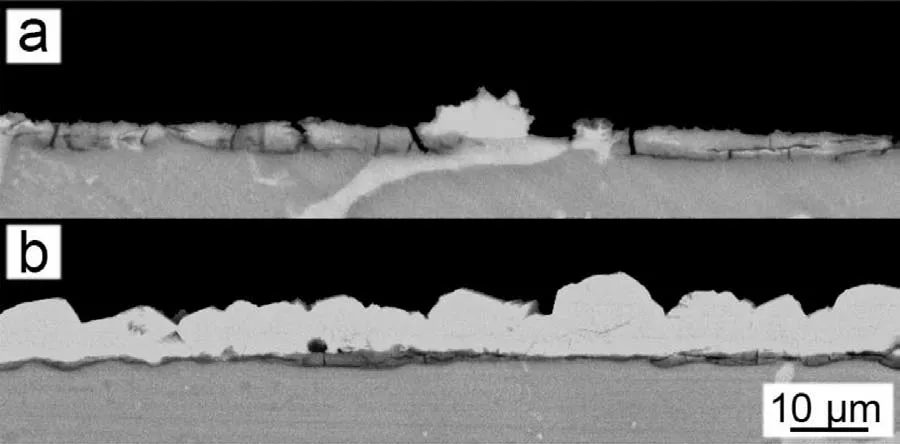
Fig. 14. Cross-sectional morphology of the PCCs fabricated from the (a)benchmark and (b) optimized baths.
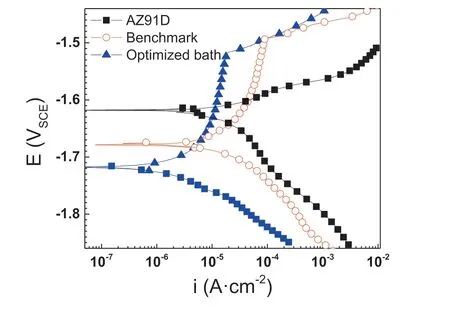
Fig. 15. Polarization curves of the bare alloy and alloys coated with different PCC in 3.5 wt% NaCl solution.
Note that in actual production environment, the precise control of the composition of treatment bath is impractical for the commercialized non-stop automated assembly line. The conventional strategy to maintain the quality of conversion bath, simply by maintaining the concentration of constituents,or adjusting the pH, is not effective, as was justifie previously [35]. The use of TA/pH as an indicator to optimize the treatment bath, offers an effective route to retain the productivity, since both TA and pH are parameters that could be acquired on-site. TA/pH is much more sensitive than pH alone.The insufficien or overdose of additional chemicals will lead to a significan deviation of TA/pH from the predetermined optimal range. However, a knowledge base that clarifie the relationship between TA/pH and different constituents is inevitable.
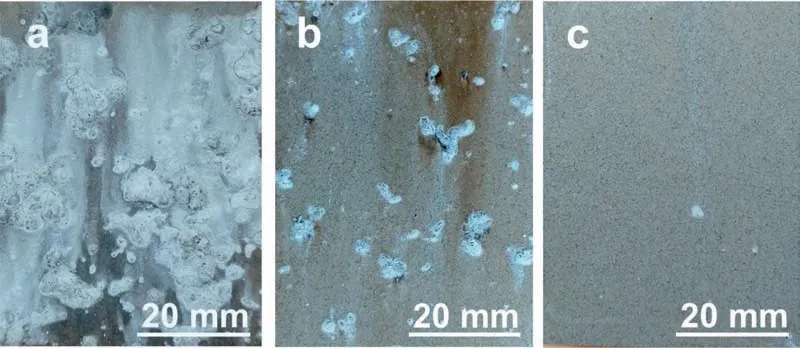
Fig. 16. Macroscopic morphology of the (a) bare and PCCs fabricated from the (b) benchmark and (c) optimized baths after salt spray test for 120h.
Further, this work takes the phosphate conversion coating as an example, not only because the phosphate conversion coating is one of the best alternatives for chromate conversion coating, also this family of coating was formed in a buffered conversion bath that could not be optimized by adjusting the pH alone. The core of the strategies proposed in this work is to clarifying the role of each constituent in the treatment bath during the growth of coating, and to further optimize the bath using TA/pH as an indicator. Theoretically, this strategy is applicable to all chromate-free conversion coatings. However, one may raise the stannate conversion coating as an exception, in which the treatment bath exhibits a pH range of 12-13. Admittedly the measurement of total acidity is not applicable, but the growth of the stannate conversion coating is not essentially deviating from conventional conversion coating. It is known that the thinness of stannate conversion coating is the major defect that underperforms their protectiveness [21]. This lack of thickness in stannate conversion coatings is a perfect cautionary example that could be well justifie by the strategies proposed: the lack of G-II species in the design of treatment bath could never form a protective coating with enough thickness.
4. Conclusions
The preparation of crack-free PCC should comply with the following principles:
(1) The conversion bath should contain two categories of species: G-I species and G-II species. G-I species reacts directly with Mg substrate, forming a cracked inner layer. The pH will be increased due to the reaction of G-I species, promoting the nucleation of supersaturated G-II species. The rapid nucleation of G-II species will form a compact and refine PCC. Due to the high density of nucleation, the PCC will be rich with grain boundaries, which will facilitate the release of local stress and mitigating the formation of cracks.
(2) The ionic product (Jsp) and the critical ionic product(JC,sp) of G-II species are crucial to the designing of conversion bath. The closer Jspto JC,sp, the bigger nucleation density and the smaller grain size one can obtain on Mg substrate, and the better quality PCC one can acquire. The nature of tuning TA/pH is to increase Jspto the level of JC,sp, thus promoting the cluster size of G-II species to its critical nucleus size. This is very similar to the concept of constitutional supersaturation and critical nucleus size.
(3) The adjustment of TA/pH should be accompanied by the addition of complexing agent; the acceleration of reaction rate should be achieved by adding oxidizing agent and catalysts.
(4) The conversion bath was optimized by adding 0.06M EDTA4-, 0.1M NO3-and 0.18M NH4+into the benchmark bath, the TA/pH value was further adjusted using NaOH, corresponding to a value of 11.80 and a Jspvalue of approximately 1.0×10-3. The PCC prepared using the optimized conversion bath shows significan improvement of thickness and corrosion resistance.
Declaration of Competing Interest
The authors declare that they have no known competing financia interests or personal relationships that could have appeared to in~uence the work reported in this paper.
Acknowledgments
The authors are grateful to the financia support from the National Natural Science Foundation of China (51531007 and 51771050), China Postdoctoral Science Foundation(2019M651128), the National Program for Young Top-notch Professionals, the Fundamental Research Funds for the Central Universities (N170205002).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- Investigating TiO2-HA-PCL hybrid coating as an efficien corrosion resistant barrier of ZM21 Mg alloy✩
- Effect of yttrium modificatio on the corrosion behavior of AZ63 magnesium alloy in sodium chloride solution
- Effect of lithium content on the mechanical and corrosion behaviors of HCP binary Mg-Li alloys
- Poly caprolactone/titanium dioxide nanofibe coating on AM50 alloy for biomedical application
- Pore characterization of PM Mg-0.6Ca alloy and its degradation behavior under physiological conditions
