Investigating TiO2-HA-PCL hybrid coating as an efficien corrosion resistant barrier of ZM21 Mg alloy✩
2021-05-21NvdeepSinghUmBtrKmlKumrAnilMhptro
Nvdeep Singh, Um Btr, Kml Kumr, Anil Mhptro
aDepartment of Metallurgical and Materials Engineering, Punjab Engineering College, Chandigarh 160012, India
bDepartment of Mechanical Engineering, Punjab Engineering College, Chandigarh 160012, India
cDepartment of Biomedical Engineering, Wichita State University, Wichita, KS 67260, United States
Received 12 April 2020; received in revised form 25 June 2020; accepted 1 August 2020
Available online 1 October 2020
Abstract
Keywords: Corrosion resistance; TiO2-HA-PCL; Contact angle; Adhesion strength.✩Statement of novelty:Mg and its alloys are not developed fully as orthopedic implant element due to high degradation rate in physiological media.A TiO2-HA-PCL hybrid coating is produced on ZM21 Mg alloy that reduced the Ecorr from -1.444V (Ag/AgCl) for machined ZM21 Mg sample to -0.407V (Ag/AgCl). The microporous layer on top facilitates the passage of H2 gas, as a result no delamination is reported for the period of 28 days of immersion in SBF. Electrostatic interaction of PCL with Mg of substrate and its hydrogen bonding with HA of TiO2-HA layer provide good adhesion strength well sustained during 28 days of immersion test. A compact corrosion product developed over TiO2-HA-PCL coating indicating its better bioactivity, faster bone growth tendency and corrosion resistance.
1. Introduction
Worldwide research on orthopedic devices using degradable biomaterials such as magnesium (Mg) alloys has been accelerated in recent past. The intent is to reduce the need of second surgery for implant removal after complete healing of tissues and the severe stress shielding of permanent implants [1]. Due to limitations of permanent implant materials,it is necessary to fin alternative degradable implant materials.Mg can be a promising and fascinating alternative biomaterial because of its Young’s modulus(45GPa)similar to that of human bone (40-57 Gpa), light weight (density=1.74g/cm3)and biodegradability in a physiological environment. Mg participates in essential metabolic processes like mitochondrial activity, nucleic acid synthesis and other cellular functions in human body [2]. The excessive amount of Mg is excreted from body as waste product [3]. Recently, different compositions of Mg alloys and coatings have been explored to improve degradation resistance by researchers but the rapid degradation of Mg alloys in physiological environment is still a challenge for the orthopedic application [4-6]. The strategic idea in this research fiel is to delay initial rapid corrosion and control of its degradation. The key point is retardation of corrosion process and not its complete stoppage [7].
Many recent studies have focused on the application of coatings on Mg surface and have found that the nonbiodegradable coatings pose two most serious problems: (i)its remains in the human body may lead to undesirable effects like inflammatio etc.,(ii)localized corrosion due to the presence of cracks, discontinuities in the coating [6]. This raises considerable interest in the biodegradable and bioactive coatings to combat the above problems.
In terms of the bioactivity, Hydroxyapatite (HA) coating has been recommended in past due to its comparable chemical composition and crystallographic structure to the mineral component of natural bone [8]. But poor adhesion strength,brittleness and fragile nature of HA coatings demands its use in combination with other materials for load bearing application. So far, HA has been used as reinforcement in bioinert ceramics like TiO2or ZrO2matrix to produce composite coatings that resulted in chemical stability compared to apatite structure [9]. HA reinforced TiO2coating has displayed good bioactivity and faster bone growth as well as corrosion resistance. Moreover, TiO2-HA coating has higher bonding strength than HA coating [10]. Cell culture studies have reported improved biocompatibility and cell adhesion on HATiO2coated alloy [11]. While HA-TiO2coating on Mg alloy is porous and has low adhesion strength, this promotes penetration of electrolyte leading to decreased protection and compromised mechanical properties [12-14]. Biodegradable and biocompatible polymers such as polyacrylic acid(PAA),polycaprolactone (PCL), polylactic acid (PLA) and poly(lactic-co glycolic) acid (PLGA) degrade slowly, by water uptake and hydrolysis, releasing biocompatible by-products that are either used in various metabolic processes or excreted from the body [15]. While these polymers have been efficien to control the degradation rate and to functionalize the surface of Mg implants [16,17], the dissolution of the polymer and its hydrolysis leads to local pH acidificatio that further accelerates corrosion of Mg [18]. Among the mentioned polymers,PCL is inexpensive and is a fully degradable [19]. PCL is a U.S. Food and Drug Administration approved implantable material. PCL coatings have remarkable toughness and good biocompatibility [20,21]. PCL has an intrinsic hydrophobic chemical nature, and its poor surface wetting and interaction with biological fluid avoid cell adhesion and proliferation. It is a semi-crystalline aliphatic polymer that displayed slower degradation rate and higher fracture energy than most biocompatible polymers [22-25].
Based on these characteristics, PCL has been investigated for various biomedical applications [26]. Wong et al. has reported that PCL coating on AZ 91 Mg alloy resulted in good biocompatibility but it showed poor bioactivity and poor adhesion strength [27]. From the literature, it is obvious that a singular coating has not been able to fulfil requirements for the development of a bio-implant comprehensively. So far,various composite and hybrid coating strategies have been adopted by researchers. For example, Bakhsheshi-rad et al.applied hybrid coating comprising Fluorine doped HA-PCL on Mg-2Zn-3Ce alloy thereby resulting in the shift of Ecorrfrom -1.60 to -1.25V [28]. Chen et al. prepared PDATiO2hybrid coating on pure Mg and the Ecorrimproved from-1.58V for pure Mg to -1.28V [29]. Abdalla-hey et al. fabricated nHAp-PCL composite coating on AM50 Mg alloy and Ecorrimproved from -1.340 to -1.21V for coated substrate[30]. The corrosion potential (Ecorr) for various other hybrid coatings reported for Mg alloy has Ecorrranging from -1.728 to -1.057V [31]. However, no study has been carried out on TiO2-HA-PCL hybrid coatings as biodegradable and bioactive coatings on Mg-base substrate. In addition, the relationship between physicochemical properties of TiO2-HA-PCL hybrid coating and Mg substrate corrosion are still unclear.Thus the research is still required to defin an appropriate hybrid coating that is biodegradable, bioactive and suits the application requirement.
In the present study, the effect of hybrid coating comprising TiO2-HA (inorganic part) and PCL (organic part) on its coating properties, electrochemical corrosion and degradation of Mg-base substrate were examined.
Wire electric discharge machining(WEDM)was employed for machining of Mg-base substrates.WEDM is a non-contact machining suitable for conductive metallic materials irrespective of their hardness that provides high dimensional accuracy and complex geometries required for the manufacturing of bio-implants. Manufacturing processes directly affect the surface and sub-surface properties of the implants and hence affect the corrosion, bio-functionality and fatigue life [32,33].
The hybrid coating was fabricated on ZM21 alloy using a facile wet chemical method in order to obtain uniformly distributed interconnected pores in PCL layers which would be beneficia in two ways. The proposed hybrid coating aims to slow down the degradation of Mg substrate rather complete stoppage as done by polymer coatings. Additionally, the chances of localized degradation would be reduced, often encountered in the inorganic coatings. For this purpose, TiO2and HA were selected as promising inorganic components which are bioactive and PCL was chosen as organic component which is biodegradable. Dip coating of hybrid coating on ZM21 were characterized by a number of techniques.The corrosion behavior of ZM21 substrate and coated samples was measured by Potential Polarization curves, EIS and immersion testing in simulated body fluid The results of this research are optimistic with the promising use of developed hybrid coating in the fiel of orthopedic devices production.
2. Experimental
2.1. Materials and substrate preparation
A 7mm thick extruded plate of ZM21 Mg alloy with a nominal composition of Mg-2.0Zn-1.0Mn (wt%) was ma-chined into 20×20×4mm samples using wire electric discharge machining WEDM (ELPULS 15 Electronica, India) at given parameters (Table 1). The machined samples were polished down to 1200 grit on SiC paper wet with ethanol and then ultrasonically cleaned. As-polished substrates were used to deposit the coatings.

Table 1 WEDM parameters for machining of ZM21 Mg alloy.
2.2. Preparation of coatings
2.2.1. TiO2–HA coating
The TiO2sol was prepared by dropwise adding titanium(IV) n-butoxide (TiO2, Alfa-Aesar, USA) to a mixture of ethanol (EtOH) and acetic acid (HAc) using the molar ratio of TiO2:EthOH:HAc=9:1:0.1 while continuous stirring at 600rpm at 45°C for 1 h. The acetic acid acts as a chelating agent and retards hydrolysis of titanium alkoxide [34].Nanocrystalline pure hydroxyapatite powder was synthesized by methodology given by Batra et al. [35]. The nanopowder synthesized by sol-gel route was mixed with TiO2Sol(1:1w/v) and stirred continuously for 24 h to obtain homogenous TiO2-HA sol as shown in Fig. 1. In the present work,TiO2-HA composite coating was deposited onto the polished ZM21 Mg alloy substrate by dip-coating at withdrawal speed of 5mm/min with 1min immersion time. The dip coating cycle was repeated for fi e times and each cycle was followed by drying at 60°C for 1 h. The coated samples were calcined at 350°C for 3 h according to experimentally determined conditions in order to transform amorphous TiO2to anatase phase and retain the HA phase as such. The anatase phase is more stable and biocompatible than rutile phase [36].
2.2.2. TiO2–HA–PCL coating
The TiO2-HA-PCL hybrid coating was deposited onto the TiO2-HA coated samples by dip-coating. A chloroform solution of PCL prepared with 5% (w/v) prepared by dissolving PCL pellets (Mn= 80,000, Sigma-Aldrich) in Trichloromethane (TCL) while continuous stirring at 500rpm for 3 h. The dip coating is performed at a withdrawal speed of 10mm/min with immersion time of 1min. The dip coating cycle was repeated for fi e cycles and each cycle was followed by drying at 30°C for 1 h. The coated samples were heat treated at 50°C for 12 h for uniform densificatio of polymeric coating. An interconnected microporous layer of PCL layer was fabricated onto the TiO2-HA-PCL coated sample from the previous step by using non-solvent induced phase separation (NIPS) technique [37-39]. The schematic representation of coating process is shown in Fig. 2.
2.3. Surface morphology and adhesion strength
A JSM-IT100 scanning electron microscope (SEM) with energy dispersive spectrometer (EDS) was employed to characterize the morphology and elemental composition of asmachined surface,as-polished substrate,TiO2-HA coated surface, and TiO2-HA-PCL coated surface. The cross-section of coated samples was examined using SEM to measure the thickness of coatings. The surfaces after electrochemical corrosion and degradation studies were also observed using SEM-EDS. Fourier transform infrared spectroscopy (FT-IR,Bruker OPTIK) was used in the spectral domain of 400-4000 cm-1at a resolution of 2 cm-1.X-ray diffraction(XRD)was performed by Cu-Kα radiation over diffraction angles of 10° to 99° to detect the phases and crystalline structures of coatings using X’PERT Pro-Panalytical XRD. The crystallite sizes of TiO2, HA powders were also calculated by XRD using Scherrer equation D=kλ/βcosθ where, k=0.94,λ=1.54178 °A, β is full width at half maximum (FWHM)and θ is Bragg’s law diffraction angle [40]. Contact angles(CA) measurements were carried out with simulated body flui (SBF) by using Drop Shape Analyzer (DSA25S KRÚSS GmbH, Germany to determine hydrophobicity and surface properties of substrate and coatings.In the present work,10μl SBF drop was used. The adhesion strength of TiO2-HA and TiO2-HA-PCL coatings applied on as-polished substrates was measured using scratch tester (TR-104, Ducom, USA). The scratch test was performed at a scratch speed of 10mm/min over a scratch length of 3mm according to ASTM standard C 1624-05. Three samples were tested for each type of coating and an average value of load has been reported. Cross-cut tape adhesion test was performed on PCL coated, TiO2-HA coated and TiO2-HA-PCL hybrid coated ZM21 Mg alloy according to ASTM standard D3359-17.
2.4. Electrochemical corrosion study and immersion test
All the electrochemical measurements were carried out using an electrochemical workstation (Autolab PGSTAT-302N,Metrohm, Switzerland) with three electrode configuration Ag/AgCl (1M KCL) and graphite rod served as reference electrode and counter electrode respectively, the samples (surface area of 1 cm2) was the working electrode and the electrolyte was SBF at 37°C. The SBF was prepared according to Kokubo et al. and it was buffered at pH 7.4 with trishydroxymethyl aminomethane and 0.1M HCl [41]. The ion concentrations in the SBF solution was 142.0 Na+, 5.0 K+,1.5 Mg2+, 2.5 Ca2+, 147.8 Cl-, 4.2 HCO3-, 1.0 H2PO4-,and 0.5 SO42-mM, being close to those in human blood plasma [42]. Potentiodynamic polarization (PDP) were performed at a 1 mV s-1scan rate using potential window-900mV to +1400mV at step potential of 1mV. Electrochemical Impedance Spectroscopy(EIS)was performed using same electrochemical workstation (Autolab instrument) Nova version 1.10.Nyquist plots were obtained for as-machined,aspolished, TiO2-HA coated, TiO2-HA-PCL coated samples at pre-determined OCP values of-1.473V,-1.447V,-1.112V,-0.261V respectively (vs Ag/AgCl) in the frequency range of 0.01Hz to 100kH and 10mV of amplitude.
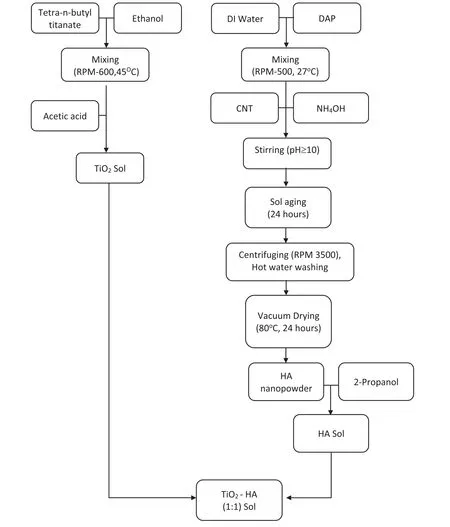
Fig. 1. Flowchart for the preparation TiO2-HA composite sol.
In order to further investigate the degradation behavior, the immersion test was performed on the as-machined,as-polished, TiO2-HA coated, TiO2-HA-PCL coated samples according to ASTM G31-72. For the present work,the samples were immersed in SBF at 37± 1°C for 7d,14d, 21d and 28d and the volume to exposure area ratio was kept same as 0.20ml/mm2. The corroded samples were cleaned with chromic acid and subsequently rinsed with deionized water and dried in vacuum oven. The weight loss was measured. The corroded surfaces were examined using SEM-EDS.
2.5. Hydrogen evolution test
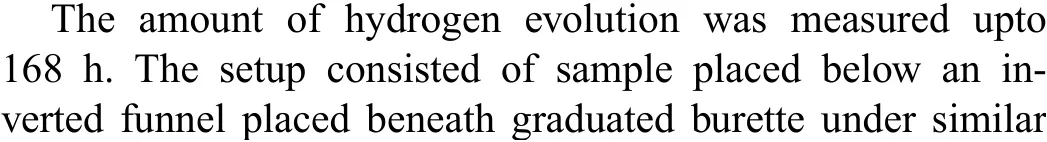
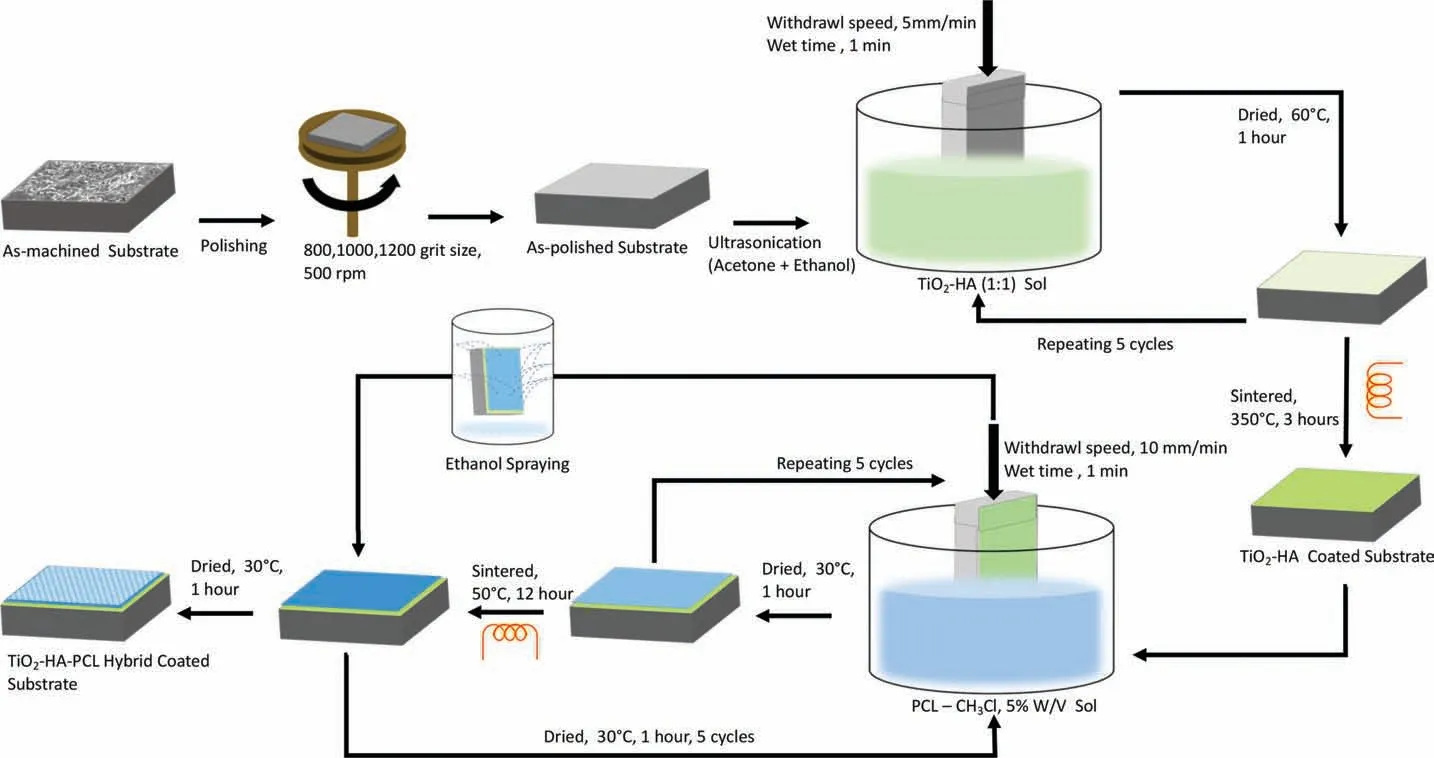
Fig. 2. Schematic representation of creating TiO2-HA and TiO2-HA-PCL hybrid coatings on ZM21 Mg substrate.
3. Results and discussion
3.1. Surface analysis
The surface roughness of the as-machined and as-polished ZM21 samples was measured by surface roughness tester.The surface finis of as-machined sample is described by the maximum height of the profil (Rt= 23.180 μm), arithmetic average roughness (Ra= 3.934 μm) and the root mean square roughness (Rq= 5.012 μm). In comparison, as-polished sample has Rt=2.661μm, Ra= 0.228 μm and Rq= 0.340 μm.This indicated the level of improvement in the surface finis achieved by polishing after machining. The SEM micrograph of as-machined sample is shown in Fig. 3(a).The machined surface consists of overlapped craters (of diameter 70-100 μm) formed due to bombardment of ions generated by repetitive sparking and collapsing of vapors bubble causing melting and evaporation of material. Quick heating and cooling effect in WEDM causing re-solidificatio of melted material resulting into micro cracks and pores on machined surface. SEM micrograph of as-polished sample is shown in Fig. 4(a). The craters and perturbances have been rubbed off completely. The EDS spectrum were obtained for asmachined and as-polished samples. In as-machined sample,the corresponding amount of Mg, Zn, Mn and O was found to be 75.80,0.00,1.96 and 22.24(inat%,as given in Fig 3(b)and in as-polished sample, it was found to be 98.69, 1.17,0.14 and 0.00 (in at% as shown in Fig. 4(b). The presence of appreciable amount of O in as-machined sample inferred the formation of oxides of Mg and Mn on the surface. The EDS results (Fig. 4(b)) also revealed that the oxides formed during WEDM were removed on polishing.
The SEM micrograph of TiO2-HA composite coating is shown in Fig. 5(a.i). It was found that coating was uniform but it consisted of a small number of micro-cracks and pits. The internal stresses and small amount of shrinkage during heat treatment of coating could possibly result into micro-cracks and pits, respectively [45,46]. The micro-cracks and the pits can develop potential sites for the penetration of electrolyte and subsequently for activating corrosion process.Fig. 5(b.i) shows the surface morphology of TiO2-HA-PCL hybrid coating. Unlike TiO2-HA coating, the TiO2-HA-PCL coating was more homogeneous having micro-pores of 2 to 5μm size and free of micro-cracks and pits. EDS spectra were obtained from TiO2-HA as well as TiO2-HA-PCL coatings. In TiO2-HA coating, the corresponding amounts of Mg, Ti, Ca, P and O were found to be 5.17, 5.53, 15.78,12.09 and 61.42 (in at%, as given in Fig. 5 (a.ii). The ratios Ca/P, (Ca+Mg) /P and Mg/Ca were calculated from the EDS results, these were found to be 1.30, 1.73 and 0.32 respectively. The stoichiometric ratio Ca/P in hydroxyapatite is 1.67 [47]. In the present work, the Ca/P ratio was lesser than 1.67 implying that the HA is calcium deficient Moreover,the ratio (Ca+Mg)/P was more than 1.67 suggesting that Mg could have leached out from the Mg alloy substrate through the micro-cracks and pits in the TiO2-HA coating.
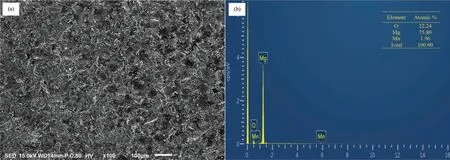
Fig. 3. SEM and EDX analysis of as-machined ZM21 substrate.
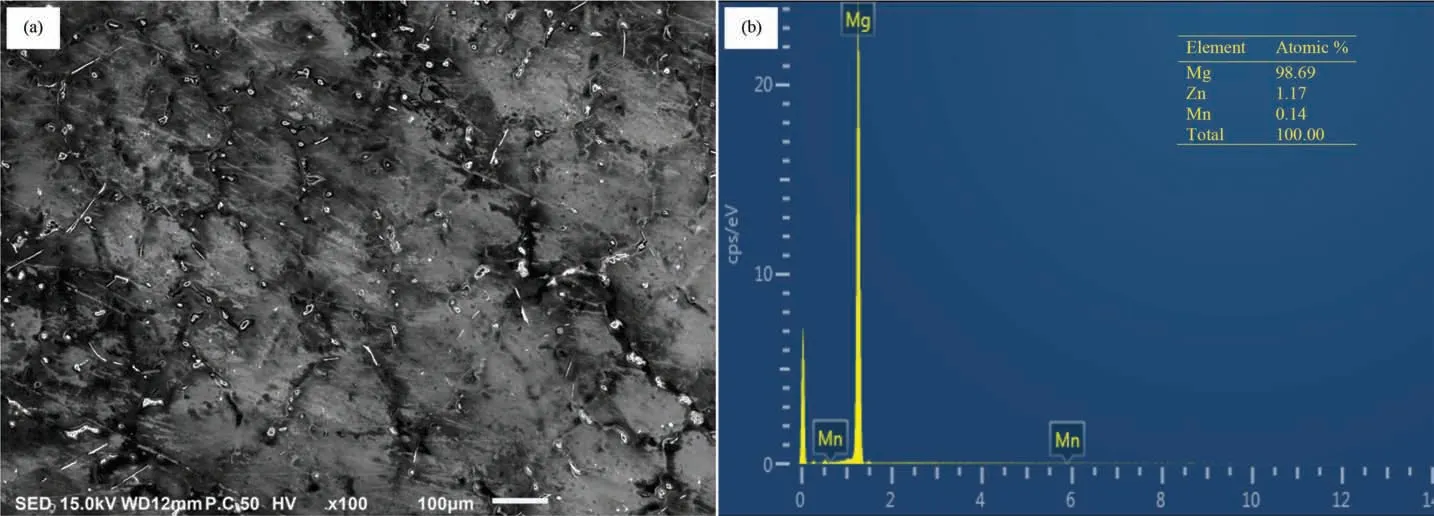
Fig. 4. SEM and EDX analysis of as-polished ZM21 substrate.
In TiO2-HA-PCL coating, the corresponding amounts of Mg, Ti, Ca, P and O were found to be 8.83, 0.00, 10.97, 9.14 and 69.28 (in at%) as shown in Fig. 5(b.ii). In EDS spectra of TiO2-HA-PCL coating titanium was absent. It suggested that Ti from the TiO2-HA layer did not interact with the PCL layer. However, presence of Mg, Ca, and P in EDS spectra of TiO2-HA-PCL coating indicated that there was an interaction of PCL with HA from the TiO2-HA layer as well as Mg from the substrate. The ratios Ca/P, (Ca+Mg) /P and Mg/Ca from the EDS spectra of TiO2-HA-PCL coating were 1.20, 2.16 and 0.80, respectively. In comparison with TiO2-HA coating, the lower Ca/P ratio and higher (Ca+Mg)/P and Mg/Ca ratios in TiO2-HA-PCL coatings seemed to suggest that more amount of Mg leached out from the substrate during the formation of PCL layers which interacted with HA of TiO2-HA layers and PCL of outer layer. Since the amounts of Mg as well as O in TiO2-HA-PCL coatings are higher than in TiO2-HA coatings, that suggests formation of MgO due the interaction of Mg with O of PCL. The SEM micrographs of the cross sections of TiO2-HA coating and TiO2-HA-PCL coating are shown in Fig. 6. It was revealed that the thickness of TiO2-HA coating was 14-20μm(Fig. 6a) whereas, TiO2-HA-PCL hybrid coating comprised 14-20μm of TiO2-HA inner layer and 49-52μm of PCL outer layer (Fig. 6b).
The formation of various bonds in TiO2-HA coating and TiO2-HA-PCL coating was confirme by using FTIR spectroscopy (Fig. 7). The peaks observed in the FTIR spectra of TiO2-HA coating at 3443 cm-1was of hydroxyl group stretching frequency. The bending vibrations at 1631 cm-1was due to H2O and Ti-OH.The PO43-groups of HA crystal exhibits peaks at 1038-1093 cm-1due to asymmetric stretching (ν3and ν4) and the peaks at 564 cm-1due to bending mode [35] . The peak obtained at 1384 cm-1was due to Ti-O bonds which also overlaps with band of CO32-having formed due to reaction of OH-with CO2from air [48].The band at 615 cm-1was due to TiO2. On comparing with FTIR of pure TiO2and pure HA, it was found that the FTIR spectra of TiO2-HA coating has peaks of TiO2and HA only(Fig. 7).
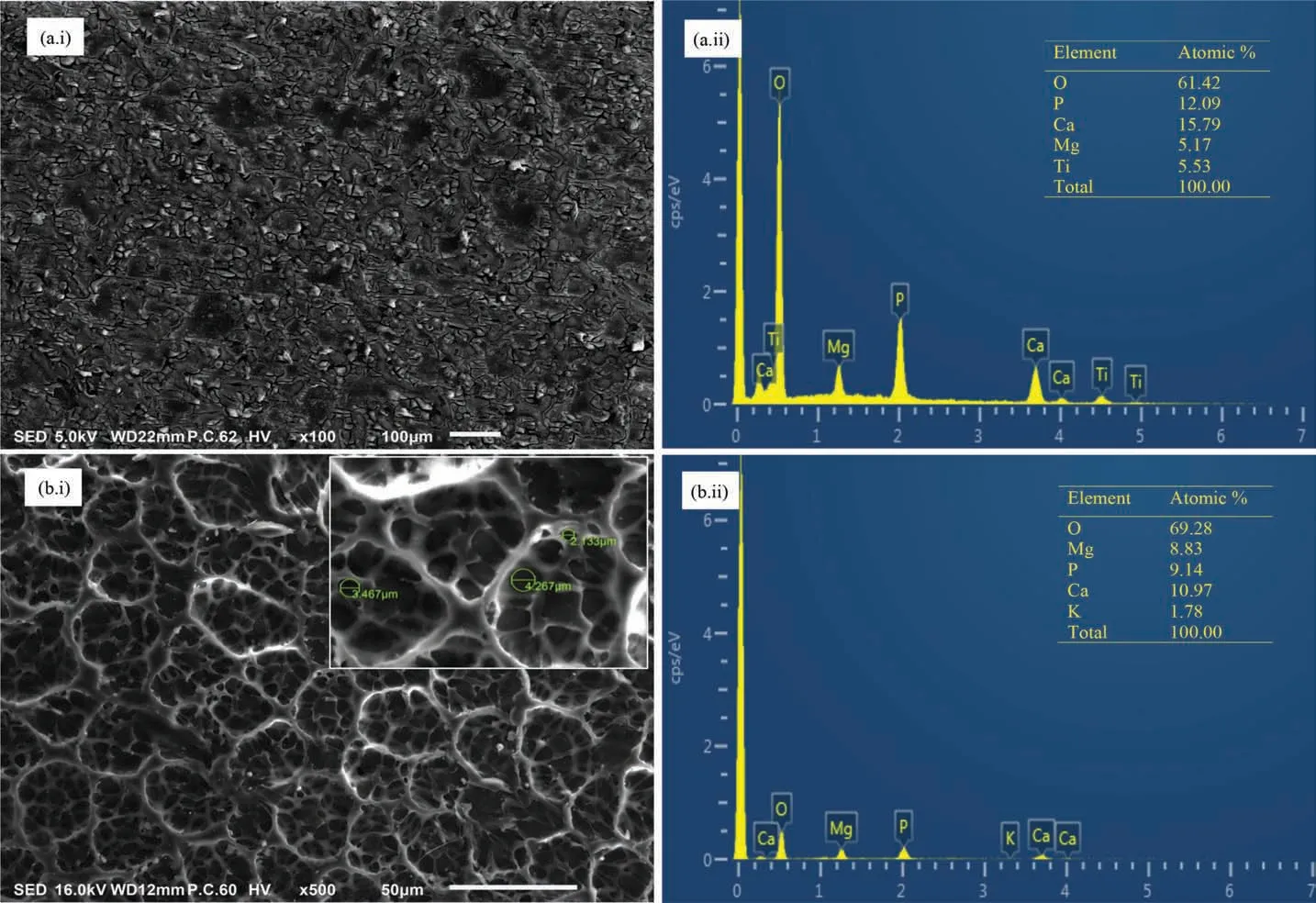
Fig. 5. SEM and EDX analysis of (a) TiO2-HA coating and (b) TiO2-HA-PCL hybrid coating.
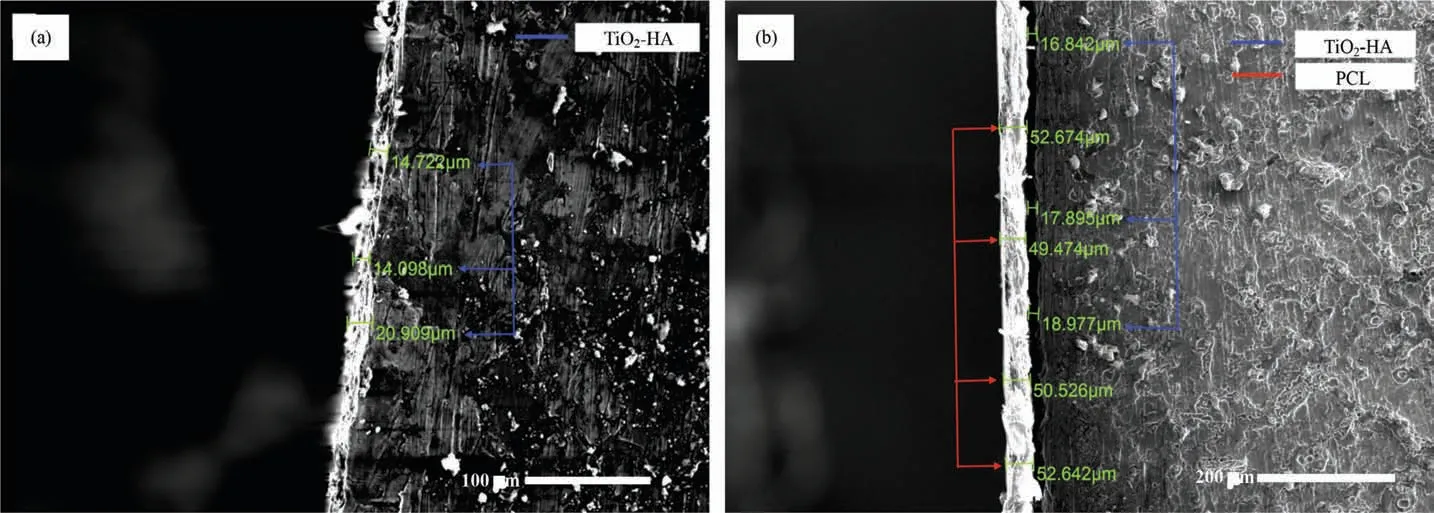
Fig. 6. SEM micrographs from the cross sections of (a) TiO2-HA coating and (b) TiO2-HA-PCL hybrid coating.
In the TiO2-HA-PCL hybrid coating, the Peaks observed at 2944 cm-1and 2864 cm-1attributes asymmetric and symmetric stretching of CH2.The absorption peak at 1708 cm-1was due to the stretching vibration of carbonyl group of PCL.The peak observed at 1470 cm-1and 1376 cm-1were related to the methylene C-H bending and C-H in plane scissoring mode respectively [49] . The peaks at 1169 cm-1and 1242 cm-1were attributed to C-O-C symmetric and asymmetric stretching of PCL amorphous phase. The characteristic peaks corresponding to CH2bending vibrations were observed at 1045 cm-1, 961 cm-1and 731 cm-1were ascribed to O-C vibrations. The peak at 671 cm-1was due to TiO2.When compared with FTIR spectra of pure PCL,it was found that the peak at 1726 cm-1in PCL has been shifted to 1708 cm-1in the TiO2-HA-PCL hybrid coating {i.e. Δν (C=O)=18 cm-1}. The measure of this shift signifie the occurrence of hydrogen bonding between HA and PCL as shown in Fig. 7 [50-52].
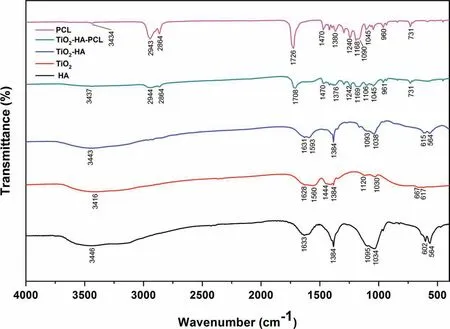
Fig. 7. FT-IR spectra of HA, TiO2, TiO2-HA, TiO2-HA-PCL hybrid coating and pure PCL.
As shown in Fig. 8, In TiO2-HA spectra characteristic peaks of TiO2appearing at 2θ of 25.38ο, 37.97ο, 48.06ο,53.25ο,55.10ο,62.77ο,68.88ο,70.17οand 75.29οcorresponds to (011), (004), (020), (015), (121), (024), (116), (220) and(125)crystal planes(JCPDS card no.96-900-9087).HA peaks appearing at 2θ of 25.38ο, 31.99ο, 33.05ο, 48.06ο, 49.54ο,53.25οcorresponds to (002), (211),(112),(132), (213) and(004) crystal planes (JCPDS card no. 96-900-2217). MgO peaks(JCPDS card no.96-901-3222)detected at 2θ of 37.97°,62.77°, 75.29° corresponds to (111), (022), (113) crystal planes. The presence of TiO2, HA and MgO phases suggested that the coating has interaction with Mg ions from substrate. In the XRD pattern of TiO2-HA-PCL hybrid film the presence of orthorhombic PCL was observed at values of 2θ=21.2ο, 22οand 23.5οcorresponding to planes (110),(111), (200) respectively. These peaks attributed the intermolecular interaction between polymer side chains due to the hydrogen bonding [53]. TiO2peaks are observed at 2θ of 25.26ο, 38.00ο, 47.98ο, 53.93ο, 54.97ο, 62.59ο, and 75.03οcorresponds to (011), (004), (020), (015), (121), (024) and(125) crystal planes. HA peaks seen in the hybrid fil at 2θ of 25.26ο, 31.87ο, 47.98ο, 53.93οcorrespond to (002), (211),(132) and (004) crystal planes. MgO peaks in hybrid coating is detected at 2θ of 35.98°,43.43°,62.59°,75.03°corresponds to (111), (002), (022), (113) crystal planes. This implied that the coating has interacted with the Mg from the substrate.
The wettability of SBF with TiO2-HA coating and TiO2-HA-PCL coatings on the ZM21 alloy surface was evaluated by means of contact angle (CA) measurement as shown in Fig. 9. The CA value of as-machined (56°) and aspolished (53.9°) surfaces are comparable. The TiO2-HA coating demonstrate a higher CA value (60.7°) as compared aspolished substrate. However, the TiO2-HA-PCL hybrid coating has still higher CA value of 82.5°.
3.2. Scratch testing
Fig. 10 shows the scratch morphology and adhesion between the as-polished ZM21 Mg alloy substrate with PCL(Fig. 10a), TiO2-HA coating (Fig. 10b) and TiO2-HA-PCL hybrid coating (Fig. 10c). Fig 10d presents the load vs sliding distance graphs for TiO2-HA and TiO2-HA-PCL hybrid coatings. It was found that the TiO2-HA-PCL hybrid coating was able to bear a critical load of 5.936N which is nearly 1.4 times higher for TiO2-HA which could bear only 4.159N. The critical load borne by the outer most layer in hybrid coating comprising PCL was 3.729N. According to the literature, adhesion strength values between Mg alloy and various coatings have been reported as 1.8N for PCL[52], 1.35N for TiO2-PCL [54], 2.2N HA-PCL [55]. For the sake of comparison, PCL layer was directly deposited on ZM21 Mg substrate and it displayed an adhesion strength of 2.195N (Fig. 10a). After 28 days of immersion in SBF, the adhesion strength decreased by 28.38%, 27.69% and 15.11%,respectively, for PCL coating (Fig. 10e), TiO2-HA coating(Fig. 10f), TiO2-HA-PCL hybrid coating (Fig. 10g). The results from the ASTM tape adhesion test on the as deposited coatings displayed adhesion grades of 2B, 3B and 4B respectively for PCL coating (Fig. 11a), TiO2-HA coating(Fig. 11b), TiO2-HA-PCL hybrid coating (Fig. 11c) which decreased to 1B,2B and 3B,respectively,after 28 days of immersion in SBF.Obviously,the TiO2-HA-PCL hybrid coating displayed significantl high adhesion strength with Mg alloy as compared with other similar coatings reported in literature so far. The close examination of the results of FTIR and scratch resistance revealed that interfacial interaction happening between the organic and inorganic matrices of TiO2-HAPCL hybrid coating.
Each HA unit cell in the TiO2-HA layer consists of two OH groups as strong hydrogen bonding sites, which could result into good interfacial bonding between OH groups of HA and =O sites of PCL as shown schematic diagram in Fig. 12(i) [56]. Besides, the electrostatic interaction between Mg2+and=O sites of PCL in the manner shown in Fig.12(ii)[54]. This is supported by the EDS results that show the presence of Mg in the TiO2-HA layer.Both the hydrogen bonding provided by OH groups of TiO2-HA layer and Vander Walls attraction due to Mg gives combined effect to provide strong interfacial bonding between TiO2-HA and PCL layers in hybrid coating.
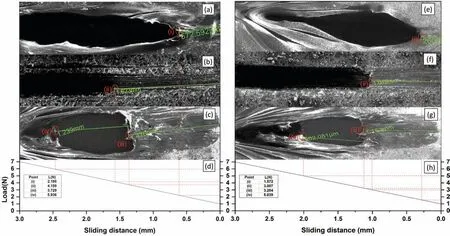
Fig. 10. Scratch morphology of (a) PCL, (b) TiO2-HA coating, (c) TiO2-HA-PCL hybrid coating, (d) load vs sliding distance graph before immersion and(e) PCL, (f) TiO2-HA coating, (g) TiO2-HA-PCL hybrid coating and (h) Load vs sliding distance graph after immersion in SBF for 28 days.
Besides, our observation of the EDS results on the higher amount of Mg present in TiO2-HA-PCL coating than in TiO2-HA coating seems to suggest that the PCL in the former makes direct contact with the Mg substrate through the pores or cracks in the intermediate TiO2-HA layer as shown in Fig. 12 (iii). In order to understand the phenomena, PCL was coated directly on as-polished substrate. PCL has poor oxygen weight ratio in its molecular structure, thus interfacial bonding is mainly dominated by electrostatic interaction by means of Vander walls forces instead of hydrogen bonding.Vander walls interaction are generally weaker than hydrogen bonding. However, few hydrogen bonding sites are available but they are very less in number. Such kind of weak electrostatic interaction and poor hydrogen bonding of PCL with Mg resulted into adhesion strength of 2.195N as compared to adhesion strength of 3.729N of PCL layer over TiO2-HA coating in hybrid coating.
3.3. Electrochemical measurements
Fig. 13a shows the potentiodynamic polarization (PDP)curves of ZM21 samples obtained after different types of processes as-machined, as-polished, as-TiO2-HA coated and as-TiO2-HA-PCL hybrid coated samples. According to the Table 2, the corrosion potential (Ecorr) of as-machined, aspolished, TiO2-HA coated samples were more negative than for as-TiO2-HA-PCL hybrid coated sample. The polishing of as-machined samples shifted the Ecorrto the positive direction (-1.444V to -1.395V), TiO2-HA coating shifted it even further (-1.017V). TiO2-HA-PCL hybrid coating displayed additional jump in Ecorrto -0.407V. This positive shift implies that TiO2-HA composite coating and TiO2-HA-PCL hybrid coating retarded the corrosion of as-polished sample in SBF. A breakdown down potential is observed at 0.56±0.01V in anodic branch of the PDP curve of TiO2-HAPCL coated sample, which seemed to suggest that it had tendency of self-healing. Cui et al. reported that occurrence of a breakdown potential in anodic branch of polarization curve suggested the self-healing capability of the coating[57].The current density (Icorr) exhibited by the TiO2-HA-PCL hybrid coating (7.31×10-8A/cm2) was significantl lower when compared with TiO2-HA coating (4.03×10-4A/cm2)and which were further lower than observed in as-machined(2.2×10-3A/cm2) and as-polished (1.2×10-3A/cm2) samples. This suggested that hydrophobic TiO2-HA-PCL coating effectively protected Mg alloy substrate in the SBF. The presence of a thin and compact TiO2-HA layer was able to restrict the penetration of SBF solution and therefore resulted into lower Icorrthan as-machined and as-polished ZM21 samples. The Nyquist plots of as-machined, as-polished and TiO2-HA coated samples (Fig. 13b) comprised of two capacitive loops at high and medium frequency due to charge transfer resistance between substrate and corrosive media,diffusion of ions through corrosion product and coating respectively and one inductive loop at low frequency region due to adsorption processes [58]. The Nyquist plot for TiO2-HAPCL hybrid coated sample comprised of a capacitive loop followed by a diffusional tail which signify porous nature of coating. The impedance modulus increases at nearly 45° in low and medium frequency regions suggesting that the corrosion product formed in-between pores is highly resistant and stable [59].
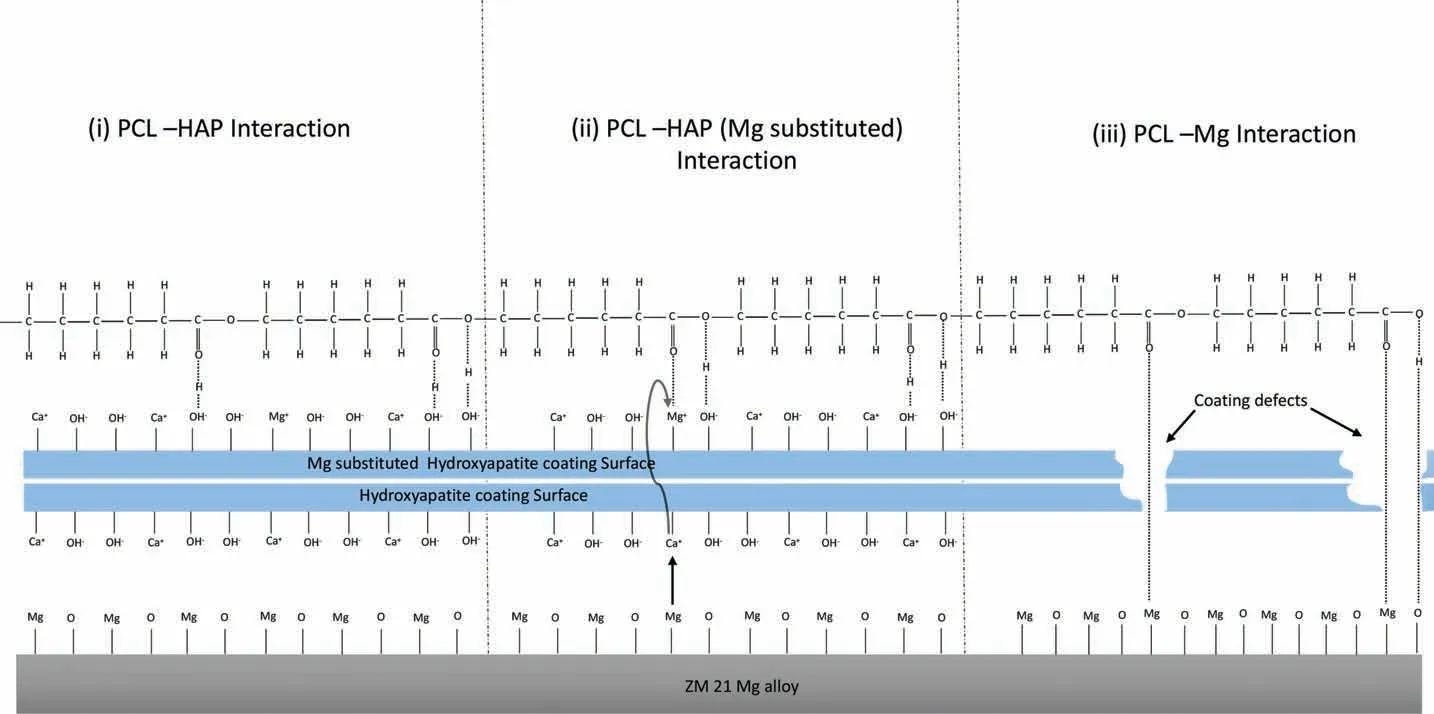
Fig. 12. Schematic illustration of electrostatic interaction between (i) PCL coating with HA, (ii) PCL coating with Mg substituted HA, (iii) PCL coating with ZM21 Mg alloy.
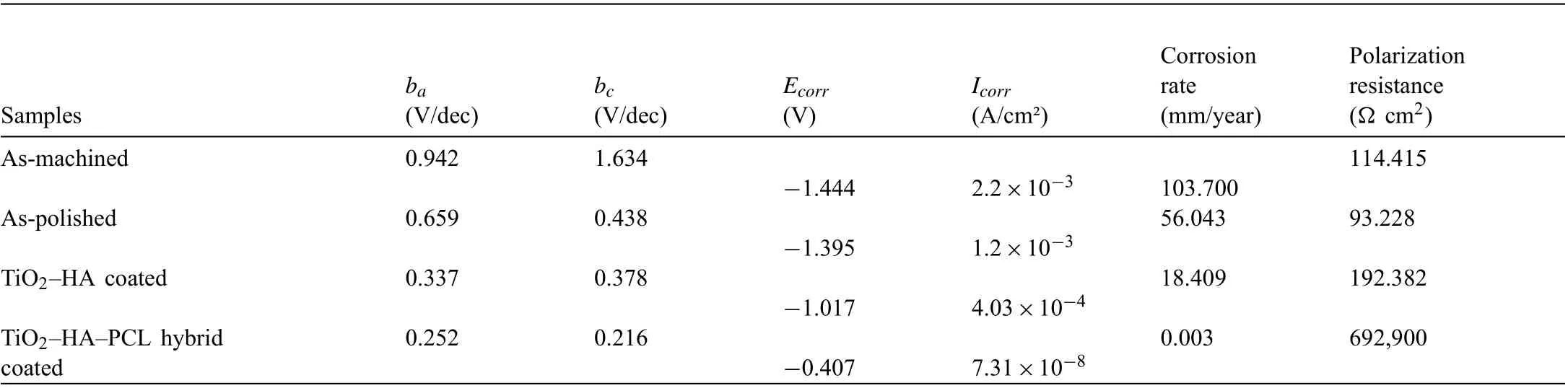
Table 2 Electrochemical parameters of the polarization curves in SBF.
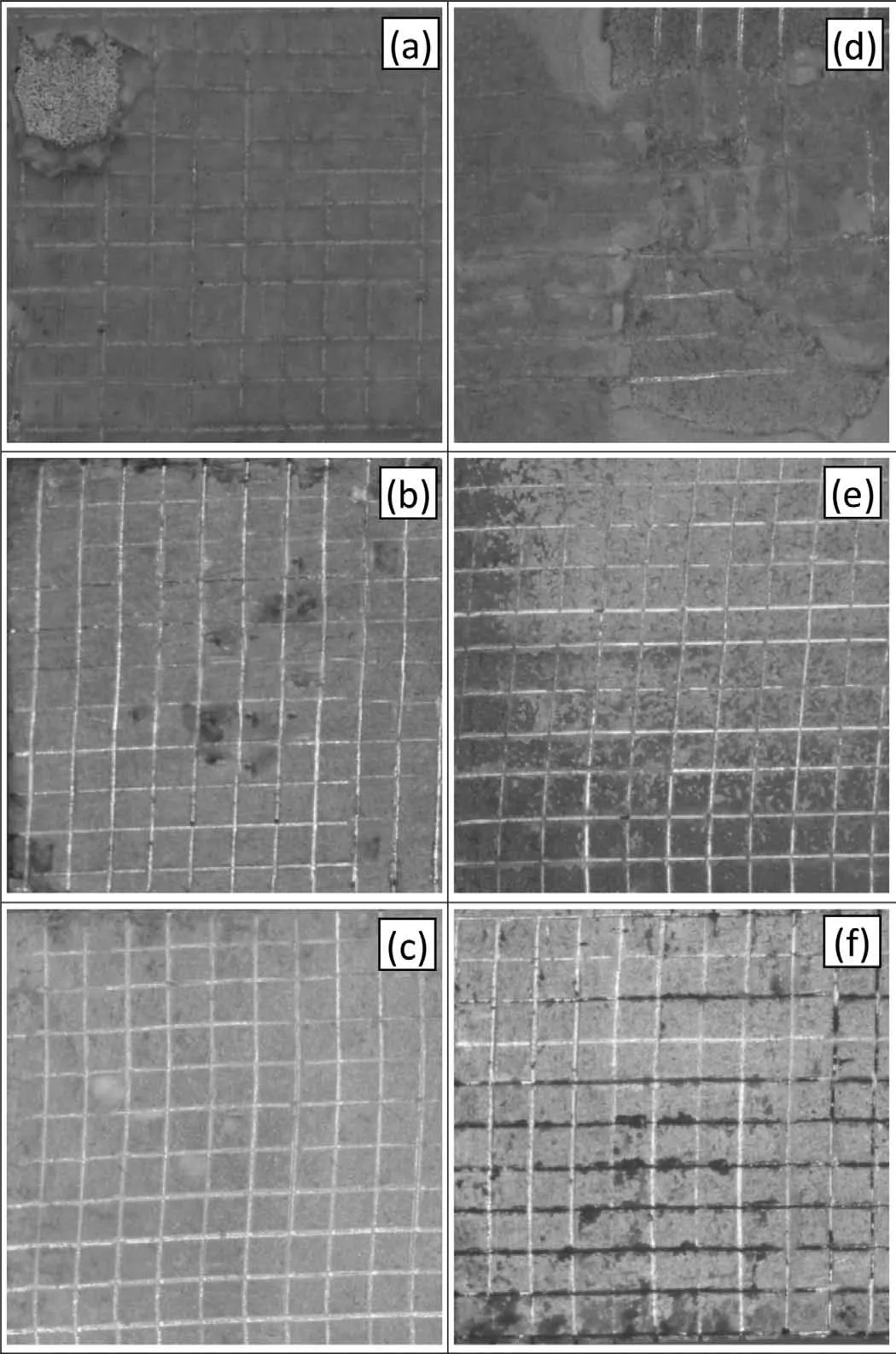
Fig. 11. Tape test results of (a) PCL, (b) TiO2-HA coating, (c) TiO2-HAPCL hybrid coating before immersion and (d) PCL (e) TiO2-HA coating (f)TiO2-HA-PCL hybrid coating after immersion in SBF for 28 days.
The charge transfer resistance (Rct) is a measure of diameter of high frequency capacitive loop [58] . The Rctof TiO2-HA-PCL was nearly 104times of the value obtained for TiO2-HA coating. In comparison the Rctvalue for asmachined and as-polished ZM21 alloy were much smaller.The as-polished substrate presented low Rctdue to the formation of loose and porous protecting fil such as Mg(OH)2that could not effectively protect the substrate against the infiltra tion of corrosive medium.The visibly larger Rctof TiO2-HAPCL hybrid coated sample was due to its hydrophobic characteristics that reduced contact area with the corrosive medium.
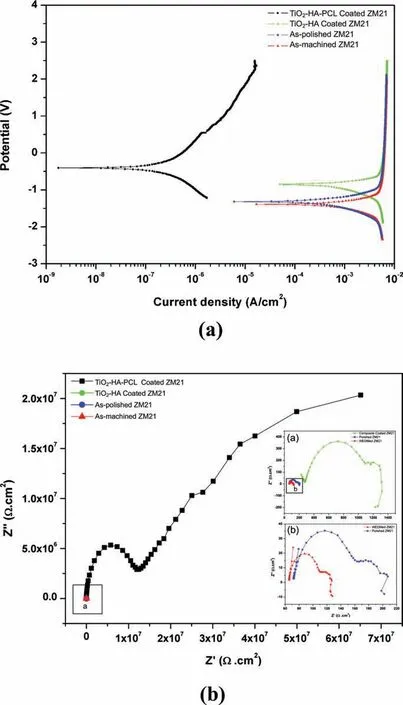
Fig. 13. Electrochemical measurments of as-machined, as-polished, TiO2-HA and TiO2-HA-PCL hybrid coated ZM21Mg substrate: (a) PDP curves and (b) Nyquist Plots.

Fig.14. Morphology comparison of the specimens;(a)as-machined,(b)as-polished,(c)TiO2-HA coated,(d)TiO2-HA-PCL hybrid coated ZM21 Mg samples after immersion in SBF for 28 days.
3.4. Immersion testing
Fig. 14 shows the surfaces of samples immersed in SBF for 28 days and subsequently cleaned with chromic acid. The corroded surface of as-machined exhibited largest sizes of pits and cracks among all four types of samples. The size of pits and cracks was smaller in as-polished samples than in asmachined samples. The sizes were further lower in TiO2-HA coated sample. In contrast the TiO2-HA-PCL hybrid coated sample surface did not show pitting or crack formation.
Hydrogen evolution test was used to study the corrosion behavior of the samples as a function of time. Higher amount of H2gas evolution during implantation results in increase in pH which leads to hemolysis of cells [60]. Fig. 15(a) shows the H2evolution rate (HER) for the as-machined, as-polished,TiO2-HA coated and TiO2-HA-PCL hybrid coated samples during immersion in SBF for 7 days. Due to presence of cracks on the surface of as-machined surface and low electrochemical potential (-1.444V), bubbles appeared as soon as the sample was immersed in SBF and the H2evolution rate after 24 h was 3.3ml/cm2/day and the corresponding pH of SBF was 7.85. During this period the degradation rate became faster and more pits appeared on the surface.After 24 h,H2evolution rate was found to be comparable in as-polished and TiO2-HA coated sample. As the corrosion proceeded,the apatite formed on the surfaces and H2evolution was suppressed.The apatite growth was easily noticeable in TiO2-HA coated sample than as-polished and as-machined samples. At the end of 144 h, apatite growth became so effective that the H2evolution rate in TiO2-HA coating and hybrid coating sample became comparable. After 172 h, H2evolution increased for TiO2-HA coated sample. It might be due to the delamination of coating layer/ corrosion product caused by evolved hydrogen. In contrast to as-machined, as-polished and TiO2-HA coated sample, TiO2-HA-PCL hybrid coated sample was more protected and restricted the attack of aggressive ions as no bubble appeared for firs 12 h and later some bubbles were observed at isolated places. The hydrogen evolution rate was very low and sample appeared unaffected as compared to as-machined, as-polished and TiO2-HA coated samples throughout the immersion period. Even after 168h,the HER was 0.16ml/cm2/day, the pH of SBF was 7.46 and TiO2-HA-PCL hybrid coating was intact without any sign of cracks or delamination. Fig. 15(b) shows the variation of pH of the SBF during 7 days of immersion at regular interval of 24 h for as-machined, as-polished, TiO2-HA, and TiO2-HAPCL hybrid coated samples. Interestingly, the change in pH was lower for coated samples than for un-coated samples.It was significantl lower for TiO2-HA-PCL coated sample than all other samples.
Fig. 15(c) displayed the weight loss percentage for asmachined, as-polished, TiO2-HA, and TiO2-HA-PCL coated samples after 28 days of immersion in SBF. The weight loss trend indicated that polishing, TiO2-HA coating and TiO2-HA-PCL coating has synergetic effect on corrosion protection during immersion time. The coating effectiveness can be assessed in the terms of weight loss (%) ratio for asmachined, as-polished, TiO2-HA, and TiO2-HA-PCL coated samples being 1: 1/2.5: 1/7: 1/9.
Fig. 16 shows the surface morphology of samples immersed in SBF for 28 days. In the as-machined sample, the corrosion product was porous and uneven. There was severe degradation of as-machined sample. As-polished sample has relatively denser and evenly distributed corrosion product on the surface. The density and evenness of the corrosion product was improved for TiO2-HA coated sample. The corrosion product on TiO2-HA-PCL coated sample was the most compact and evenly spread among all the samples. SEM-EDS in Fig. 17 shows the morphology and elemental analysis of corrosion product deposits. The corrosion product deposit on as-machined, as-polished and TiO2-HA coated samples had numerous cracks and pits but the deposit on TiO2-HA-PCL coated sample was defect free and evenly spread. According to EDS analysis, the ratios Ca/P, (Ca+Mg)/P and Mg/Ca of deposits are compared in Table 3 for the as-machined, aspolished, TiO2-HA coated and TiO2-HA-PCL coated samples. The atomic percentage of Ca, P and Mg elements is shown in Fig. 18.
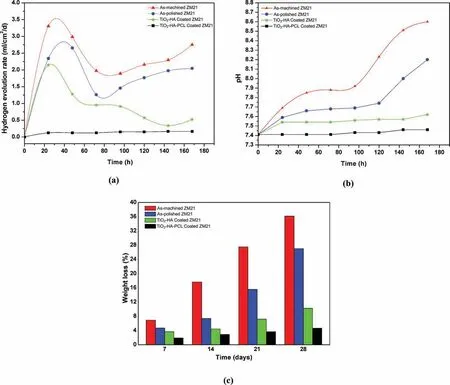
Fig. 15. Hydrogen evolution rate (a), pH change (b) and weight loss percentage (c) for as-machined, as-Polished, TiO2-HA coated and TiO2-HA-PCL hybrid coated ZM21 Mg samples after immersion in SBF for 28 days.

Fig. 16. Morphology comparison of the corrosion product deposited over; (a) as-machined, (b) as-polished, (c) TiO2-HA coated and (d) TiO2-HA-PCL hybrid coated ZM21 Mg samples after immersion in SBF for 28 days.
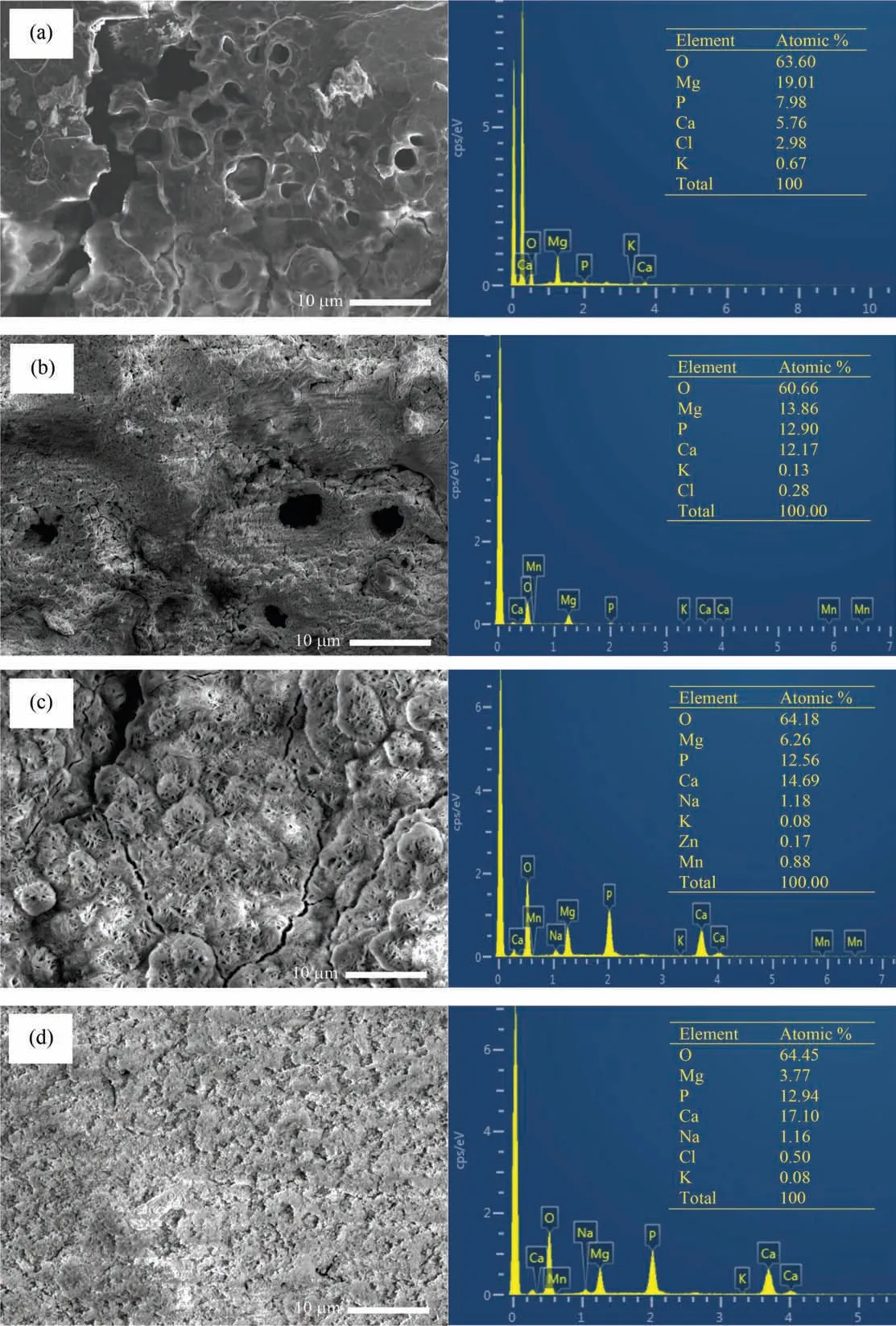
Fig. 17. SEM morphology and EDS spectra of (a) as-machined, (b) as-polished, (c) TiO2-HA coated and (d) TiO2-HA-PCL hybrid coated ZM21Mg samples after immersion in SBF for 28 days.
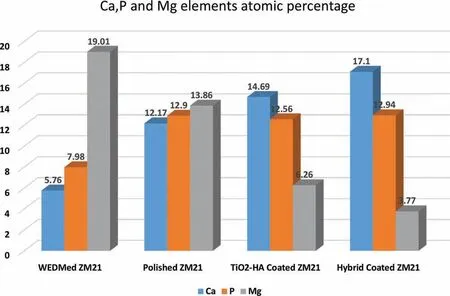
Fig. 18. Atomic percentage of Ca, P and Mg elements of as-machined, as-polished, TiO2-HA and TiO2-HA-PCL coated ZM21Mg substrate immersed in SBF for 28 days.
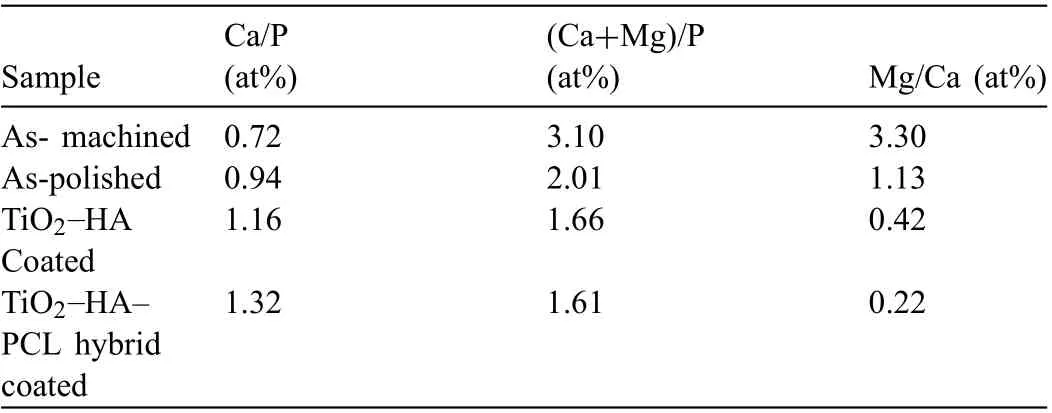
Table 3 Atomic ratios of Ca, P and Mg of corrosion product on as-machined, aspolished, TiO2-HA coated and TiO2-HA-PCL coated ZM21 Mg samples after immersion in SBF for 28 days.
These Ca/P values suggested the formation of calcium deficien hydroxyapatite on the surfaces. Such kind of calcium deficiencie have been reported due to the dissolution of Mg from the substrate partly replacing Ca in the apatite structure [61] . Thus Ca/P ratio was the related to release of Mg ions by the substrate. TiO2-HA-PCL coating has acted as an effective barrier between SBF and substrate. The highest Ca/P ratio of 1.32 and lowest Mg/Ca ratio of 0.22 among all the samples clearly indicated that the TiO2-HA-PCL coated sample has allowed the minimum amount of Mg ions leaching from the substrate followed by TiO2-HA coated sample having second highest Ca/P ratio (1.16) and second lowest Mg/Ca ratio of 0.42. Since the Ca/P ratio was 0.72 and 0.94 for as-machined and as-polished samples respectively, therefore,it was inferred that the corrosion product on the bare Mg alloy surface did not offer an effective barrier. Thus more and more Mg ions continued to leach from the substrate.As a consequence, the degradation continued to result in high Mg/Ca ratios of 3.30 and 1.13 in as-machined and as-polished samples, respectively.
The FT-IR of samples immersed in SBF for 28 days were shown in Fig 19. In TiO2-HA-PCL hybrid coated sample,the bands present at 962 cm-1, 1033-1095 cm-1and 565 and 603 cm-1represents ν1PO43-, ν3PO43-, ν4PO43-.Band appearance at 1596 cm-1was due to the presence of lattice water, while band at 3698 cm-1represents vibration modes of OH group. This represents good mapping with the HA characteristic bands. As compared to hybrid sample, continuous decrease in the intensity of PO43-and OHwas observed for corrosion product of TiO2-HA, as-polished and as-machined ZM21 samples.Moreover,the broadening of ν3PO43-(1033-1095 cm-1)band in order of TiO2-HA-PCL<TiO2-HA<as-polished<as-machined ZM21 samples was observed. These effects are typically suggesting that structurally disordered Ca-Def HA is formed as corrosion product[35,62,63].
Fig. 20 showed the XRD spectra of TiO2-HA-PCL hybrid coated, TiO2-HA coated, as-polished and as-machined ZM21 samples immersed in SBF for 28 days. Along with characteristic peaks of HA, the presence of Mg-PO43-compounds signifie the formation of Mg substituted Ca-Def HA [64].
3.5. Corrosion mechanism
The degradation phenomena of bare Mg surface can be explained as Mg comes in contact with SBF, following reaction will occur The presence of Cl-in the SBF transforms Mg(OH)2into MgCl2.
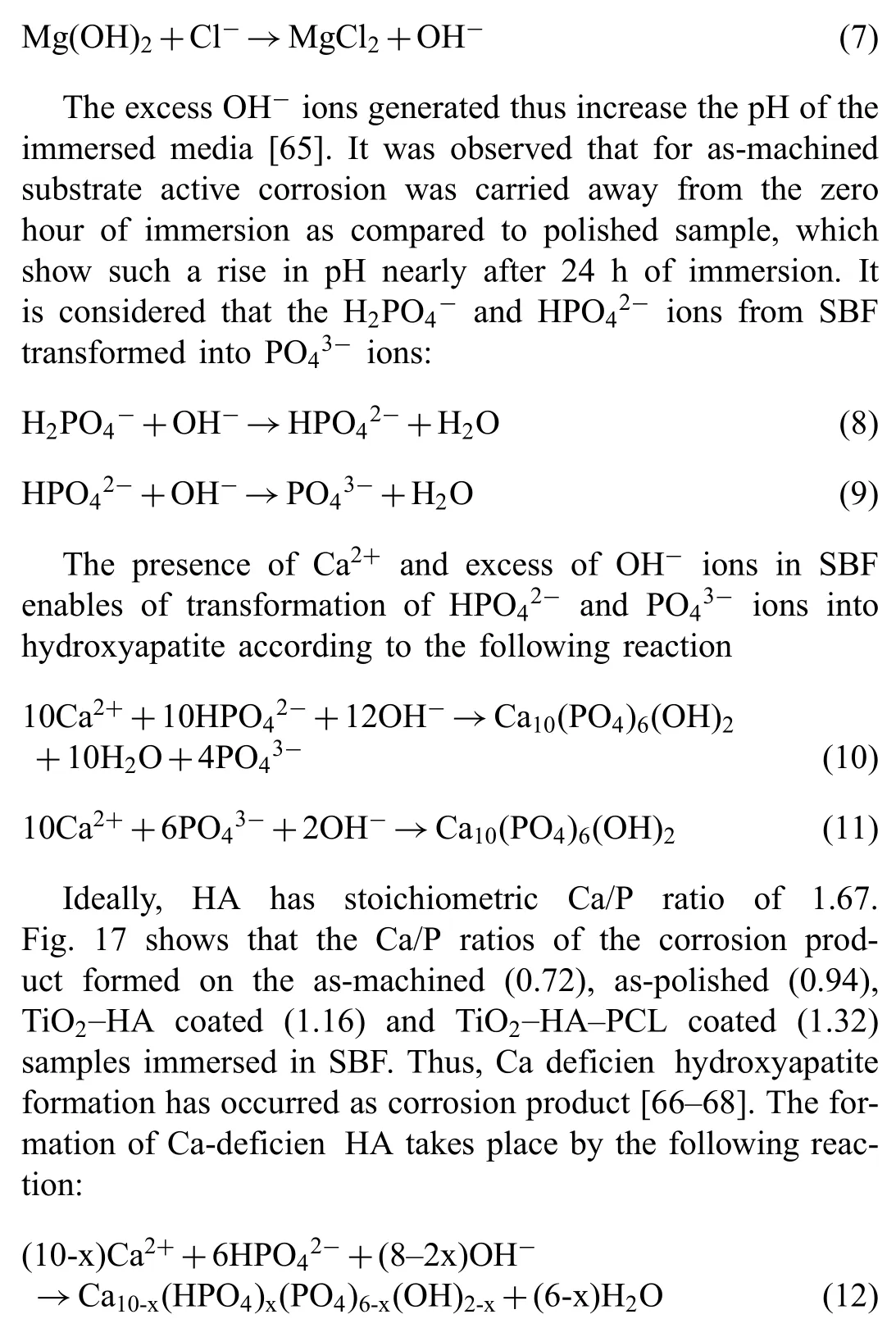
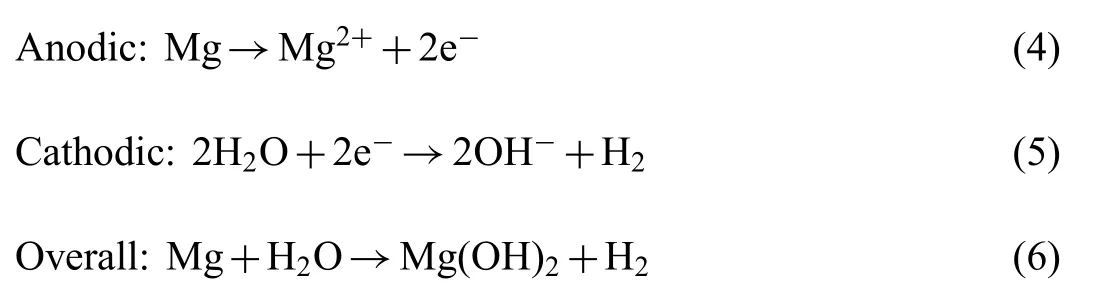
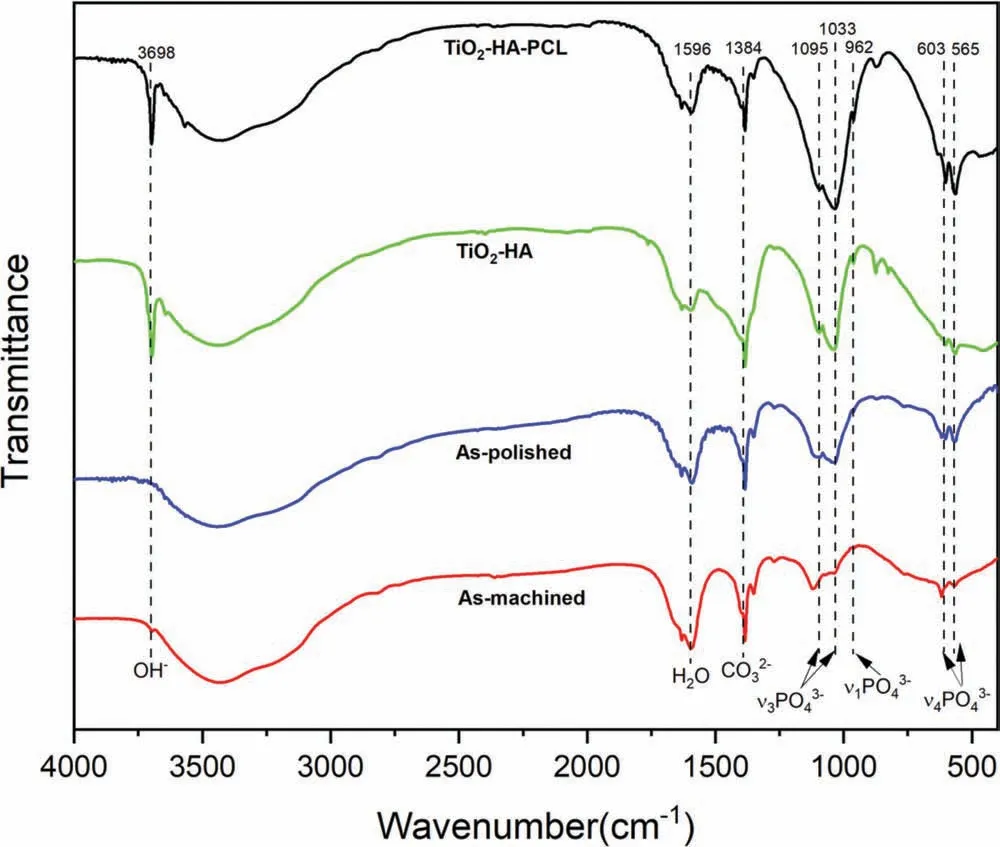
Fig. 19. FT-IR spectra of corrosion product deposited over as-machined, as-polished, TiO2-HA coated and TiO2-HA-PCL hybrid coated ZM21 Mg samples after immersion in SBF for 28 days.
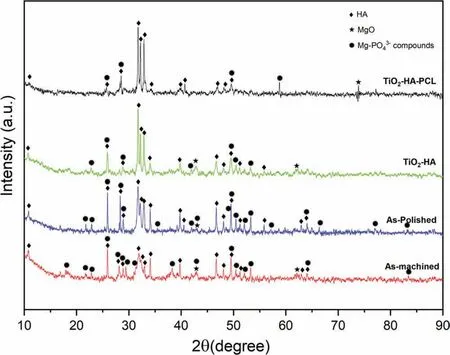
Fig. 20. XRD analysis of corrosion product deposited over as-machined, as-polished, TiO2-HA coated and TiO2-HA-PCL hybrid coated ZM21 Mg samples after immersion in SBF for 28 days.
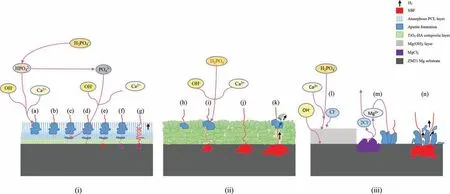
Fig. 21. Schematic illustration of the Adsorption mechanism of (i) TiO2-HA-PCL hybrid coated, (ii) TiO2-HA coated and (iii) as machined/polished ZM21 Mg substrate immersed in SBF.
Mg2+ions possess most dominating bivalent cationic substitution in apatite [69]. The size of Mg2+ions (0.069nm)relatively smaller than Ca2+ions (0.099nm), which facilitates the Mg2+ions to substitute Ca2+ions in the apatite[70,71] . Lattice distortion caused by substitution results into poorly crystalline, highly soluble, destabilized Mg substituted Ca-deficien hydroxyapatite formation [72]. This enables the fl w of electrolyte through its amorphous structure as shown in Fig. 18. Thus, Eq. (12) can be modifie to show the formation as:
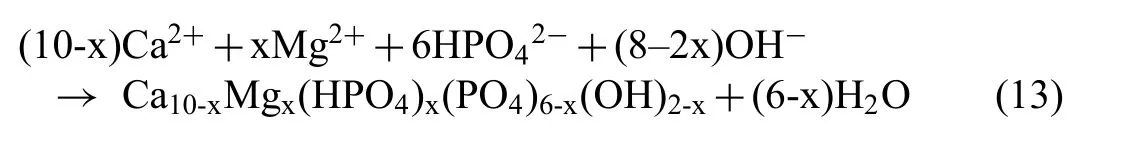
However, as a consequence of reduced interaction between the Mg substrate and SBF for the coated samples, the release of Mg2+from the substrate is significantl reduced resulting in the formation of corrosion products having higher Ca/P ratio and effective corrosion barrier.
The mechanism of effective corrosion resistance displayed by the hybrid coating is suggested as follows. The electrolyte firs enters into the topological pores of PCL layer where the corrosion product formation initiates Fig. 21(i.a). The corrosion product formed within the pores help the interlocking of pores from many coating layers [73] . Thus, hydroxyapatite so formed was not only stable structurally but also acts as obstacle to delay the advancing aggressive ions. Few aggressive ions which manage to escape through the apatite layer are encountered with the permeable matrix of the PCL Fig. 21(i.b). The restricted number of ions which manage to diffuse through the PCL matrix reached at the underneath bioactive TiO2-HA composite layer. Apatite formation may start in the pores and cracks of the TiO2-HA composite layer due to the presence of HA there, which acts yet another barrier to the movement of ions Fig. 21(i.c). As a result of barriers at various layer only a few outnumbered aggressive ions can succeed to reach to the Mg substrate Fig. 21 (i. d). Once the aggressive ions reach the substrate, an active corrosion starts here according to reaction (1) and (2) which results into evolution of H2gas Fig. 21(i.e,f). Evolved H2gas easily escapes out from the pores in the TiO2-HA layer and surface pores of the PCL fil thereby causing little or no damage or delamination of the coating. The intricate route accompanied by multilevel barriers for the fl w of electrolyte, greatly delays the interaction between Mg substrate and aggressive ions Fig. 21(i.g). In the case of TiO2-HA coating, the electrolyte can reach the substrate either diffusing though corrosion product Fig. 21 (ii.i) or by throughout pores/cracks in the composite layer Fig. 21 (ii.j). Thus, the corrosion process starts fairly early in TiO2-HA coated sample than in TiO2-HAPCL coating. Additionally, the escaping H2cause cracks in the corrosion product Fig. 21 (ii.k). In case of as-machined and as-polished ZM21Mg substrates, no such effective corrosion resistance mechanism is observed. On substrate/SBF interaction, once the oxide layer formed Fig. 21 (iii.l) is deteriorated by the active corrosion. Although, apatite formation occurred on the Mg alloy surface Fig. 21 (iii.m) but it does not provide effective shielding towards aggressive ions. The corrosion product formed is highly unstable both structurally as well as chemically. The corrosion rate observed are exceedingly high that led to large volume of H2evolution. The abandoned H2evolution further cause severe damage to the corrosion product formed on surface Fig. 21 (iii.n) thereby exposing fresh Mg surface to advance corrosion.
4. Conclusions
i TiO2-HA-PCL hybrid coatings with the top layer of interconnected microporous PCL were deposited on ZM21 alloy by sol-gel dip-coating method combined with nonsolvent induced phase separation technique.
ii The adhesion strength of TiO2-HA-PCL hybrid coating was 1.5 times more than TiO2-HA composite coating because of bonding of PCL by hydrogen bonding with HA in addition to electrostatic bonding with the Mg substrate.The loss of adhesion strength of hybrid coating after 28 days of immersion in SBF was nearly half (15.11%) of that observed for TiO2-HA coating (about 27.69%).
iii The TiO2-HA-PCL hybrid coating provided adequate protection to the Mg alloy in the presence of SBF and consequently delayed its degradation.
iv The Ca/P ratio of apatite deposited on TiO2-HA-PCL hybrid coating suggested its bioactivity,bone growth and corrosion resistance better than TiO2-HA coating.
Declaration of Competing Interest
The authors declare that they have no conflic of interest.
Acknowledgment
This work is funded under the research grant (File no.EMR/2016/001581) sponsored by SERB, DST, India.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- Effect of yttrium modificatio on the corrosion behavior of AZ63 magnesium alloy in sodium chloride solution
- Effect of lithium content on the mechanical and corrosion behaviors of HCP binary Mg-Li alloys
- Poly caprolactone/titanium dioxide nanofibe coating on AM50 alloy for biomedical application
- New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys
- Pore characterization of PM Mg-0.6Ca alloy and its degradation behavior under physiological conditions
