An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
2021-05-21BarsharaniPriyadarshiniTanaswiniPatraTapasRanjanSahoo
Barsharani Priyadarshini, Tanaswini Patra, Tapas Ranjan Sahoo
Department of Chemistry, School of Applied Sciences, KIIT deemed to be University, Bhubaneswar 24, Odisha, India
Received 29 April 2020; received in revised form 16 August 2020; accepted 6 September 2020
Available online 2 October 2020
Abstract
Keywords: Adsorption; Congo red; Trypan blue; MgO NPs; Kinetics; Isotherm.
1. Introduction
Industrial waste water containing its by-products and toxic dye effluent generated through different chemical reactions,oxidation and hydrolysis have raised serious environmental threats [1,2]. The effluent discharged from such industries like paper, textile, leather, plastics, printing and cosmetics,contaminated with the water bodies cause high chemical oxygen demand(COD),preventing the penetration of light,which leads to carcinogenic and mutagenic effects on plants, humans and aquatic lives. Among these effluents the organic dyes are one of the major groups of pollutants in waste water since, these dyes do not readily degrade under aerobic conditions. From different countries, around 1.6 tons of dye-containing waste water is being drained into various water bodies per year, without being properly treated. These toxic dyes disseminate ill-favored color to water having high degree of chemical, microbiological and photolytic stability.Among several toxic dyes, Congo Red (CR) and Trypan Blue(TB) dyes introduce many harmful effects in the environment. CR is used in paper, printing, leather, plastic industries and widely used for the dyeing process in textile industries.It is also known as 3,30-([1,10-biphenyl]-4.40-diyl) bis (4-amino-1-aminonaphthalene sulphonic) having molecular formula C32H22N6Na2O6S2and molecular weight of 696.7g mol-1. The degradation of CR from contaminated waste water is difficul due to its complex structure. CR could cause serious environmental hazard such as eutrophication and endanger human life with diseases like somnolence, irritation to skin and eyes and respiratory problems. Whereas TB is an azo dye, which is used as a direct dye for cotton textiles[3] and in the fiel of bio-sciences, it is applied as a vital stain to selectively color dead cells or tissues blue. Therefore, the separation of CR and TB dyes from waste water effluent is of major concern, before it mixes with that of the unpolluted natural water bodies.
For purificatio of waste water contaminated with these dyes, various techniques are being implemented, for example adsorption, photo degradation, chemical oxidation, coagulation, electrochemical oxidation and microbiological treatment etc. Among all these techniques, adsorption has been extensively adopted and is superior to other techniques due to its simpler operation, high efficien y, economy and convenience.These properties took adsorption to the forefront of the research interest as an effective novel water treatment process[4,5].
There are many adsorbents which are used to remove dyes from wastewater. The adsorbent which are extensively used are activated carbon, composites of graphene etc. These are porous materials which make them effective adsorbents. But the main disadvantage is that, these are very costly for which,they are rarely considered as economically feasible adsorbent.Many researchers have mostly used activated carbon as an adsorbent, but the need for a high operating system and its initial cost makes it less practicable as an adsorbent. Some cost-effective adsorbents such as clay including sepiolite,bentonite, zeolite, and periodic mesoporous titanium phosphonate[6-9] etc. are used for removal of toxic dyes from aqueous solution.Currently,metal oxide nanoparticles have gained significan interest among scientist as potential adsorbent for removal of dyes from waste water [10] due to its high surface area, large number of active sites, high surface to volume ratio and unusual lattice planes. Among all known metal oxide nanoparticles, magnesium oxide (MgO) has become a distinctive material because of its uniqueness in high ionic character,crystal structure, simple stoichiometry and its novelty in different applications, such as electronics, adsorption, catalysis and many other field [11,12]. There are also some metal oxides which can act as nanoadsorbents like ZnO, SnO2, Fe2O3,etc. [13]. But, the microwave-assisted combustion synthesized MgO nanoparticles are proved to be better nanoadsorbents over other porous metal oxides. Because, these have very small crystallite size and the morphology is homogeneous which increases the surface area to volume ratio [14]. An observation of recent research of porous material in adsorption of dyes showed that the pore diameter of MgO NPs were found to be around 3-4nm with a specifi surface area around 150-180 m2g-1due to which MgO NPs play a significan role in the removal of dyes from aqueous solution. Furthermore, the pH of the point of zero charge (pHpzc) of MgO NPs is 12.4, which makes the NPs a desirable adsorbent for adsorption of anionic dyes due to its electrostatic attraction mechanism.MgO nanoparticles also behave as destructive adsorbent in aqueous solution for the complex dye compounds and are very less toxic.The molecules that can adsorb another chemical substance on its surface which can degrade the original chemical to some other compounds having low toxicity are called destructive adsorbents. The complex dye molecules get adsorbed on the surface of the MgO NPs where the reaction occurs, which degrade the original dye compounds with lower toxicity [15,16]. It releases Mg2+ions to the water bodies which do not cause toxicity. The amount of Reactive Oxygen Species (ROS) generated will determine the biological toxicity of a chemical compound. There was no such change in ROS was observed under 2-10mg/L as reported by Ma et al. [17]. So, these are not toxic for the environment.Different physicochemical techniques have been adopted to synthesize MgO nanoparticles such as precipitation, chemical vapor deposition, solvothermal, microwave combustion, sonochemical methods etc. [18-24]. In comparison with the other conventional techniques, the microwave-assisted combustion synthesis has the advantage of producing smaller particle size metal oxides with high purity within short span of time[25,26]. Hence, we have employed microwave-assisted combustion route for the synthesis of MgO NPs.
The goal of this work is to study the removal of Congo red (CR) and Trypan blue (TB) from aqueous solution by MgO nanoparticles. An efficien and comparative study of the adsorption process was investigated on the basis of thermodynamics, kinetics and isotherm results.
2. Materials and methodology
2.1. Synthesis
MgO nanoparticles were prepared by microwave-assisted combustion synthesis under the influenc of microwave irradiation at a power of 900W taking an oxidizer Mg(NO3)2.6H2O and glycine as fuel, with minimum quantity of distilled water in a beaker (100ml). During the process of microwave heating, the redox solution was boiled followed by dehydration and ignition producing an exothermic reaction with a flam firml continue for 2-3s. This synthesis process took place instantly, and the obtained residue was in the powder form as well as crystalline in nature.
2.2. Adsorption experimental procedure
Batch test for the adsorption of CR and TB on MgO nanoparticles were carried out on a magnetic stirrer by taking 100ml flas containing 50ml of solution and a suitable dose of MgO nanoparticles.The adsorption efficien y of MgO nanoparticle was examined through different parameters like initial concentration of both dyes, pH, temperature, adsorbent dose and agitation speed. Then the adsorbent was filtrate out after the predetermined time intervals and was analysed by a double beam UV-Vis spectrophotometer (Shimadzu, UV-1800, Japan) at 496nm for CR and 607nm for TB dye.
The quantity of CR and TB adsorbed by MgO nanoparticles at the state of equilibrium at time t are qe(mmol g-1), and qt(mmol g-1). Their values were calculated using Eqs. (1) and (2), respectively,

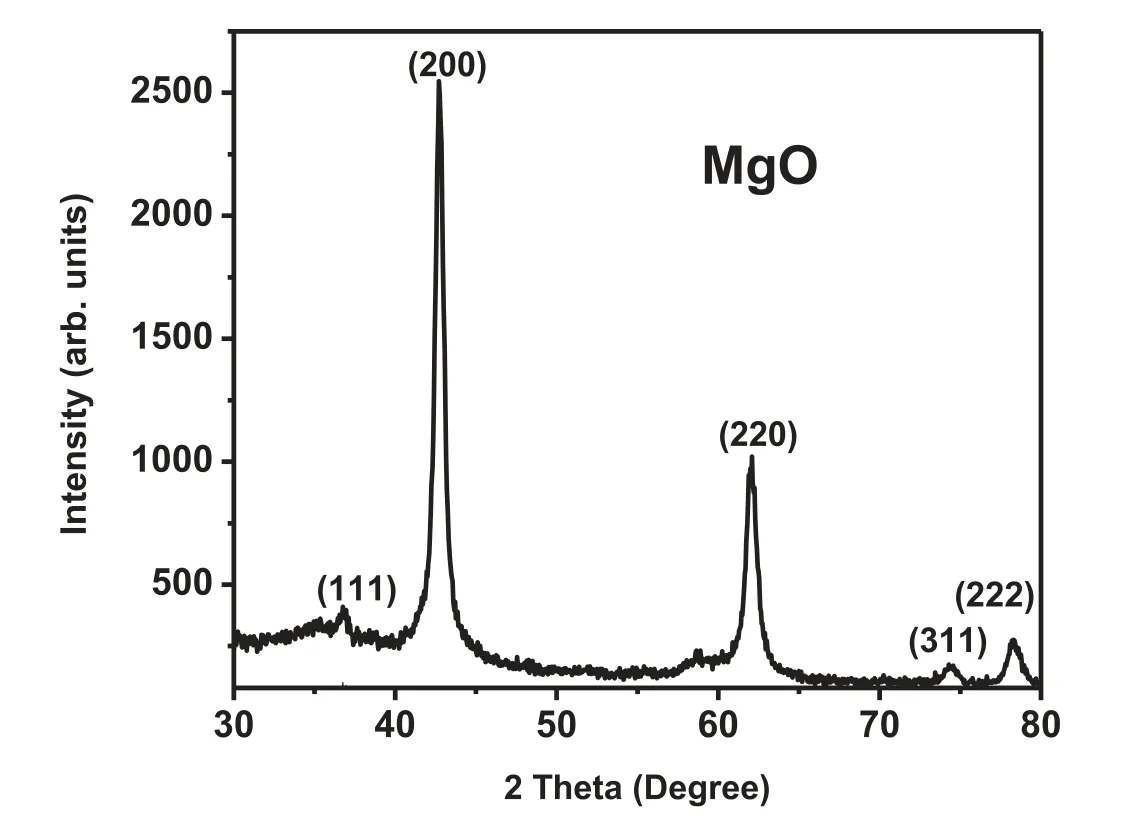
Fig. 1. XRD pattern of MgO.
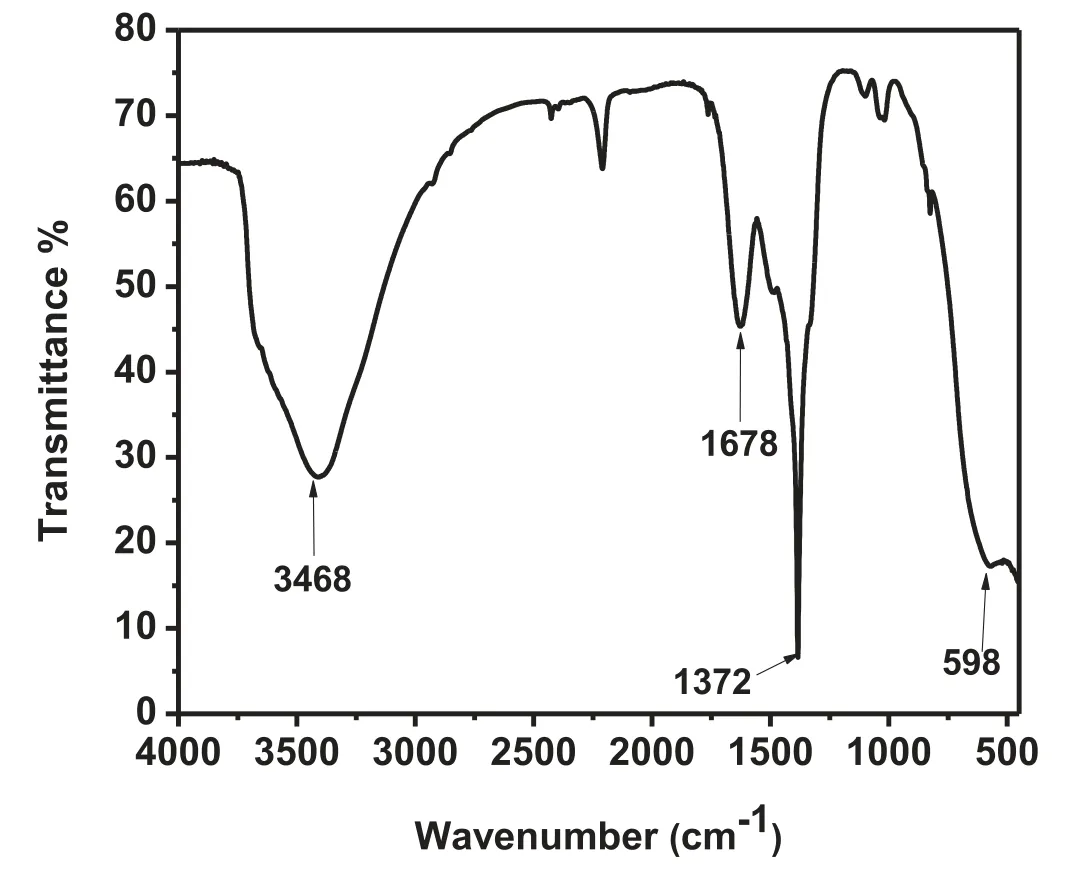
Fig. 2. FTIR of MgO nanoparticles.

The removal percentage of CR and TB dye adsorbed by MgO nanoparticles from the aqueous solution was obtained by using Eq. (3):

Where C0, Ct(mol L-1) and Ce(mol L-1) are the initial dye concentration,concentration at time t and concentration of the dyes at equilibrium state, respectively, whereas the mass of the dyes is m (g) and V (mL) is the solution volume.
3. Results and discussion
3.1. Characterization
Fig. 1 shows the XRD (X-Ray Diffraction) pattern of the sample prepared by microwave-assisted combustion synthesis.All the diffraction peaks correspond to the face-centered cubic phase of MgO (JCPDS card No: 01-1235) [27]. No characteristic peak of Mg (OH)2or any other impurity was observed in XRD pattern confirmin the crystallographic pureness of the sample. The average of crystallite size of the particle was determined from the XRD pattern, by using the Debye-Scherrer’s equation (Eq. (4)) [28-31].

Where D, θ, λ and β are the crystallite size, Bragg diffraction angle, X-ray wavelength and FWHM (full width half maxima) of the diffraction peaks, respectively. The average crystallite size for MgO nanoparticles was found to be 18nm,as calculated from Debye-Scherrer equation.
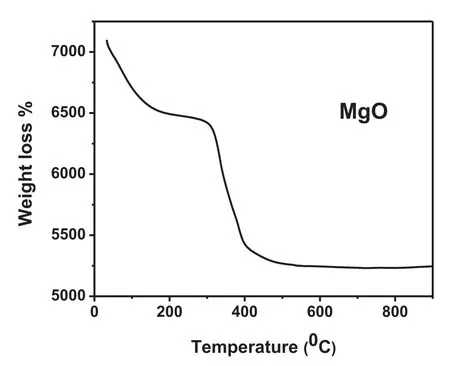
Fig. 3. TGA of MgO nanoparticles.
The FTIR spectra confirme the presence of various functional groups in the adsorbent material,which may be responsible for the process of adsorption with the dye molecules.The FTIR study showed (Fig. 2) a broad band at around 598 cm-1which was attributed to the metal-oxygen bending vibration band and the band at 3468 cm-1was the stretching frequency of H-O-H bond. The broad band peak near 1372 cm-1was because of C=O stretching frequency suggesting the existence of aromatic ring.The spectra at 1678 cm-1indicated the asymmetrical stretching vibration band of carbonyl group [32].
The decomposition temperature of the material was investigated using thermogravimetric analysis (TGA) as shown in the Fig. 3. The TGA curve of MgO was obtained in normal atmospheric condition, with heating rate of 10°C/min. The analysis indicated the loss of mass occurred in two steps, in which the firs step is from 50°C to 320°C,which may be due to the liberation of the water molecules adsorbed on the surface. The second step comes in the range of 320°C-450°C,which may be attributed to the dehydroxylation of Mg (OH)2,signifying the decomposition of Mg (OH)2to MgO.
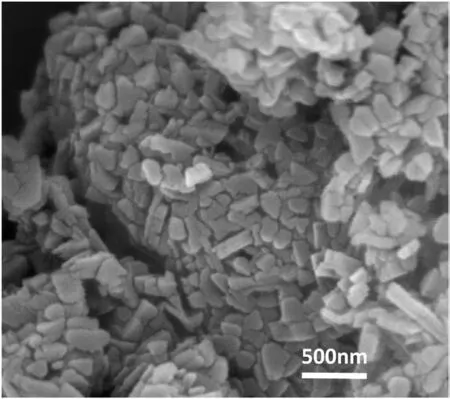
Fig. 4. FESEM of MgO nanoparticles.
In order to study the morphology of the nanoparticles, FESEM image was taken. The FESEM analysis (Fig. 4), exhibited the aggregation of small rod like structured nanoparticles. Such morphology may be attributed to the fact that, the free ions (NO3-, OH-) might have adsorbed on the crystallographic plane of the solid sample surface through loose coordination with Mg+2ion or through hydrogen bonding, during the synthesis of the as-prepared sample. The grain size of the NPs was found to be around 60-85nm. [32-34]
3.2. Adsorption studies
A batch test for the adsorption of CR and TB was performed by using MgO nanoparticles as adsorbent varying different parameters like the quantity of adsorbent, agitation speed, initial concentration of dye, temperature and pH, in aqueous medium.
3.2.1. Initial dye concentration
The effect of various concentration of dye, on the capacity of adsorption of dyes on MgO nanoparticles was studied under equilibrium conditions. In Fig. 5(a) a similar profil for adsorption of dye was obtained for both the dyes (CR and TB), where by the increase in the dye concentration, the adsorption capacity decreased. The maximum adsorption effi ciency of the dye on MgO nanoparticles reached 99.66% and 99.79% for Congo red and Trypan blue, respectively, with the maximum contact time of 120 min. The rate of adsorption became slower by increase in dye concentration from 15 to 45ppm. This is because, at constant amount of adsorbent,the number of active functional sites is limited. The surface could become saturated with increase in concentration of dye at fi ed adsorbent dose. This can also be explained as, the agglomeration of dye molecules and the depletion in the thermal mobility due to aggregation of adsorbed dye molecules lead to the decrease of the adsorption rate. Hence, a lower dye concentration may enhance the process of the adsorption.Accordingly, 25ppm of dye concentration for CR and TB was decided as the appropriate concentration for subsequent experimental studies.
3.2.2. Adsorbent dose
The quantity of adsorbent dose is considered as a prime factor to determine the maximum potential of adsorption of the solid catalyst. A swift adsorption of pollutants in a short time span, determines the capacity of the solid sample in the removal of various toxic dyes. The effect of solid catalyst on the removal of TB and CR dye was explored in the range of 10mg-30mg at normal pH 5.4 with optimum concentration of dyes and fi ed contact time. The analysis of the adsorption results revealed that the efficien y of removal for CR dye was from 77 to 99% and that for TB was 62% to 99%,respectively, with increase of catalyst from 10mg-30mg.
3.2.3. Effect of pH
The effect of adsorption process was carried out at different pH, which reflecte the ability of dye molecule binding to solid surface. This phenomenon is seemingly to know the influenc of pH on the surface characteristics of the solid catalyst as well as ionization or dissociation of the dye molecules.The solution pH can affect the molecular structure of both the adsorbate and adsorbent. The presence of different functional groups on the surface of both adsorbent and adsorbate may produce different charges at various pH range being protonated/deprotonated leading to electrostatic interaction between the charged adsorbate and adsorbent. The experimental values showed that, the adsorption process rises immaculately up to 99% when the pH is 4 in case of TB and at normal pH for CR i.e., the pH of dye (Fig. 5(c)).
In case of TB, at low pH, the H+ion concentration increases leading to a favourable adsorption process due to the strong electrostatic force of attraction between SO3-and H+ions. Whereas in case of CR, when the solution is highly acidic, that would resist the ionization of Na+of CR, resulting in decrease of adsorption rate [35].
3.2.4. Agitation speed
The effect of various agitation speed on the adsorption of CR and TB dye onto MgO NPs was investigated by varying the speed of agitation from 200 to 600rpm at different contact time (0-120min), 20mg of sample and 25ppm of dye concentration at room temperature. The adsorption percentage was maximum at 200rpm for CR and 600rpm for TB,respectively, as evident from the Fig. 5(d). Beyond which the adsorption decreased due to the mass transfer of adsorbate to the internal surface of MgO nanoparticles became lower.Hence, further experiments were performed keeping the agitation speed fi ed at 200rpm for CR and 600rpm for TB.
3.3. Thermodynamics
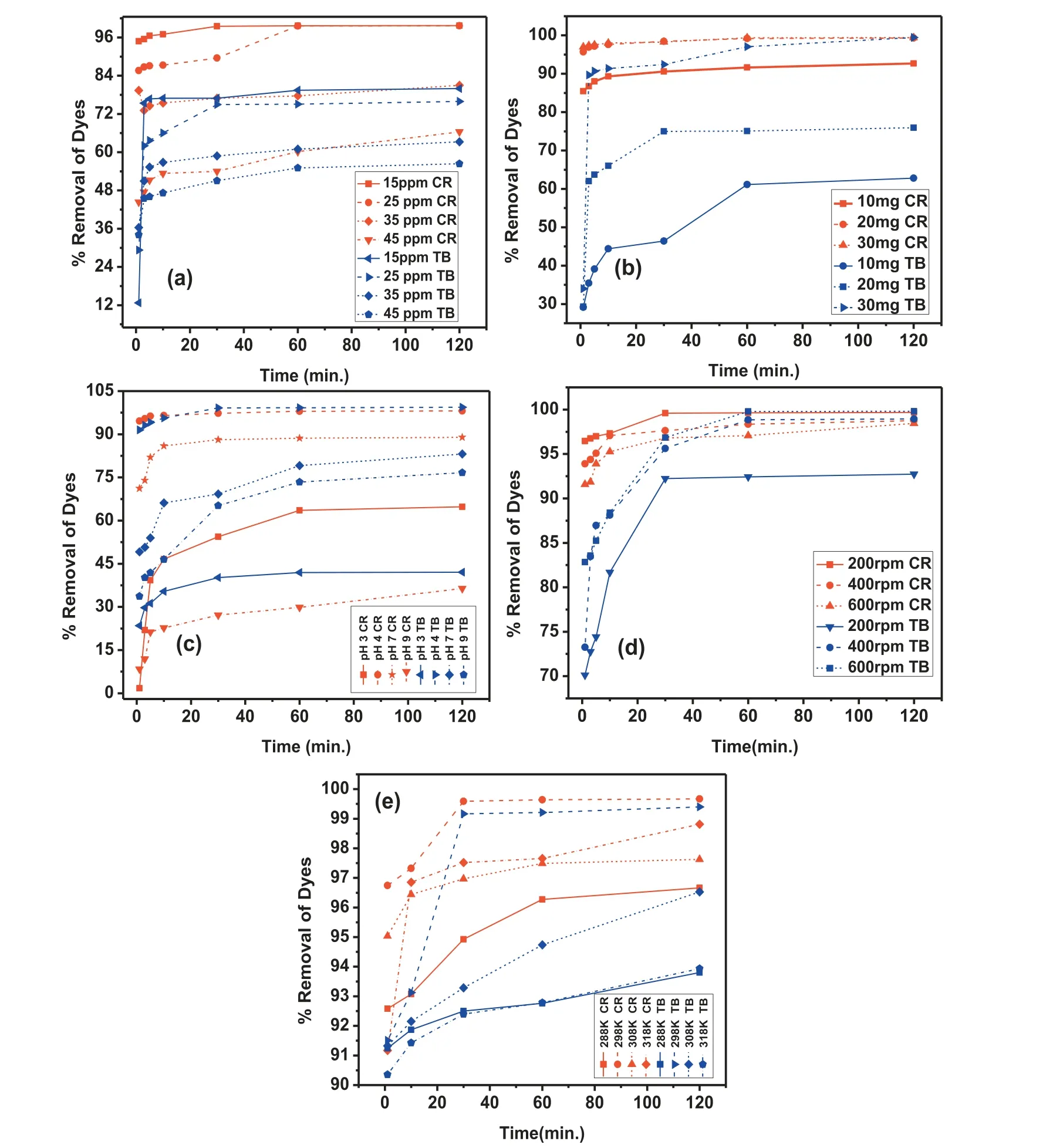
Fig. 5. (a) Initial concentration (b) Adsorbent dose (c) pH (d) Agitation Speed (e) Temperature variation of CR and TB.
The energy as well as the entropy of the reaction were vital,in order to understand the adsorption kinetics.At different temperature conditions, the effect of temperature on adsorption of 25mg L-1for TB and CR dyes on MgO nanoparticle was investigated. The results of this study revealed that the adsorption efficien y decreased when the temperature increased from 288 to 318K. This is because of the decrease in tendency of dye molecules to interact with the solid sample[36]. The reason may be ascribed to two factors i) weakening the bond between the molecules of dyes and the active sites on the solid sample and ii) the increase in dye solubility with temperature [35]. From the data plotted in Fig. 6, the equilibrium constant (Kc) of adsorption study was evaluated using Eq. (5).

Where CAe, and Ceare the dye concentrations at equilibrium in MgO phase and in solution phase, respectively, was calculated from respective plots. The Kcvalue was increased when temperature increased as shown in Fig. 6(b) and (d).
The mechanism of adsorption of CR and TB onto MgO NPs, was studied through change in different parameters such as standard free energy (ΔG0), enthalpy (ΔH0) and entropy(ΔS0) and considered to establish the spontaneous adsorption process by the following thermodynamic relations in Eqs.(6)-(8).

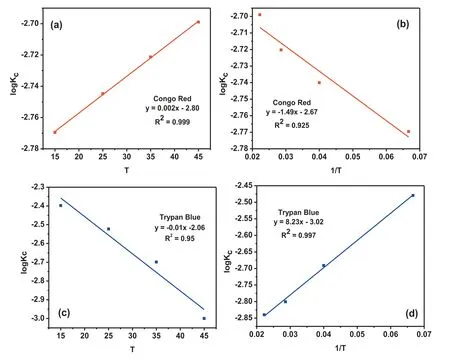
Fig. 6. Effect of temperature on adsorption of CR and TB (a) Equilibrium constant for CR on MgO as function of temperature (b) Van’t Hoff plot for exchange of CR with MgO nanoparticles (c) Equilibrium constant for TB on MgO as function of temperature (d) Van’t Hoff plot for exchange of TB with MgO nanoparticles.
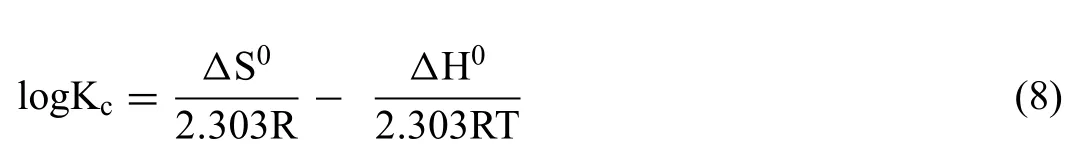
where Kcand CAe(mg L-1) is the equilibrium constant and the amount of CR and TB adsorbed on solid sample at equilibrium, respectively, Ce(mg L-1) is the dye concentration at equilibrium, T and R is the absolute temperature, and gas constant (8.314J.mol-1K-1), respectively.
The van’t Hoff relationship equation i.e. Eq. (10), helps to plot logKcvs 1/T which showed straight line for both TB and CR in Fig. 6(b) and (d). The adsorption of Trypan blue (TB)dye is maximum at pH 4 due to formation of hydrogen bond through electrostatic interaction with adsorbent molecules. By increase in the temperature the adsorption of TB dye decreases because of breaking of hydrogen bond which leads to the desorption of dye molecules from the adsorbent surface and reduces the formation of product. Due to which the equilibrium of the reaction predominated by the reactant species causes shifting of reaction equilibrium in the backward direction. This observation signifie that with increase in temperature, the equilibrium constant (Kc) decreases in case of TB adsorption and showed an inclined graph in the plot of log Kc Vs 1/T in Fig. 6(d). But in case of Congo red (CR), the covalent bond between the dye molecules and the hydroxyl group of the adsorbent is stronger than the weak hydrogen bond between molecules of Trypan blue and the adsorbent [37].On increasing temperature, the Kc value of CR adsorption reaction also increases, which indicated that in the adsorbentadsorbate bond as well as the formation of product is not affected by temperature and the equilibrium shifted towards the forward direction. The thermodynamic parameters ΔH0and ΔS0values were calculated from the intercept and slope of the plot of logKcvs 1/T, respectively, and listed in Table 1.The negative values of ΔG0(Table 1) of MgO NPs showed the process of adsorption is feasible. The negative values of ΔH0revealed an exothermic process of adsorptions whereas the positive values of ΔS0described the randomness of the adsorption process.
3.4. Kinetics studies
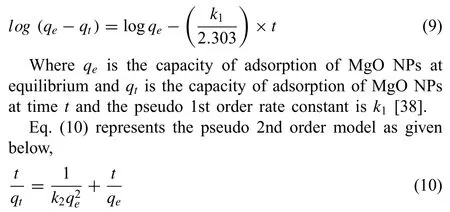
Kinetics is an important study for the process of adsorption because it can calculate the rate at which a waste product can be separated from the aqueous solution and also providesimportant data for a better understanding the process of adsorption mechanism. In order to understand the mechanism of adsorption of CR and TB onto MgO nanoparticles, experimentally obtained kinetic data were fitte to different models like pseudo 1st order,pseudo 2nd order as well as intraparticle diffusion model. Pseudo 1st order kinetic model signifie the relation of rate of change of solute up take has relation with that of the difference in saturation concentration and with that of time, which is usually applicable for the early part of the adsorption process. The pseudo 2nd order model suggests the rate limiting step to be of chemisorptions type. Mostly, the pseudo 1st order and 2nd order kinetics model are commonly applied, as shown in Fig. 7.

Table 1 Thermodynamics parameters of TB and CR adsorption on MgO NPs at different temperatures.
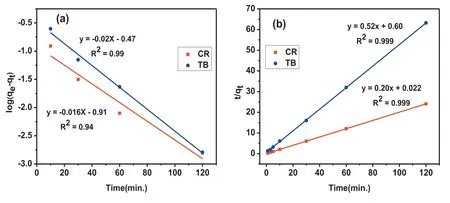
Fig. 7. (a) 1st order kinetics (b) 2nd order kinetics of CR and TB dyes.
The pseudo 1st order rate equation, also known as Lagergren’s equation (Eq. (9)) is given by:
Where k2is the rate constant of pseudo 2nd order kinetic model [39].
At earlier stages via external fil diffusion, the adsorption of CR and TB ions onto MgO nanoparticles may be controlled and later it may also be controlled by the diffusion of particles into the adsorbent.The likelihood of resistance to intraparticle diffusion was identifie by using the following model [40] as mentioned in Eq. (11):

Where Xiand Kpare the thickness of the boundary layer of solid sample and the rate constant of intraparticle diffusion model, respectively.
The correlation coefficien (R) values for kinetic models(both pseudo 1st order and 2nd order) and intraparticle diffusion model are given in Table 2 signifying that the adsorption rate of TB and CR dye on the nanoparticles can be more correctly explained using the pseudo 1st order equation than that of the pseudo 2nd order equation. The mechanism of adsorption depended on both solid catalyst as well as the dye solution and the rate limiting step may be consider as of chemisorption type, as is evident from the best fittin to the pseudo 2nd order kinetics.
The intraparticle diffusion model (Fig. 8) also showed a higher R2value of 0.970 for TB than that of 0.864 for CR,which indicates that the diffusion of TB molecules is more significan than the diffusion of CR molecules onto MgO nanoparticles. This can be described through the molecularmass of both the dyes, as the molecular mass of TB is less than CR dye, so the diffusion of TB is easier than CR.
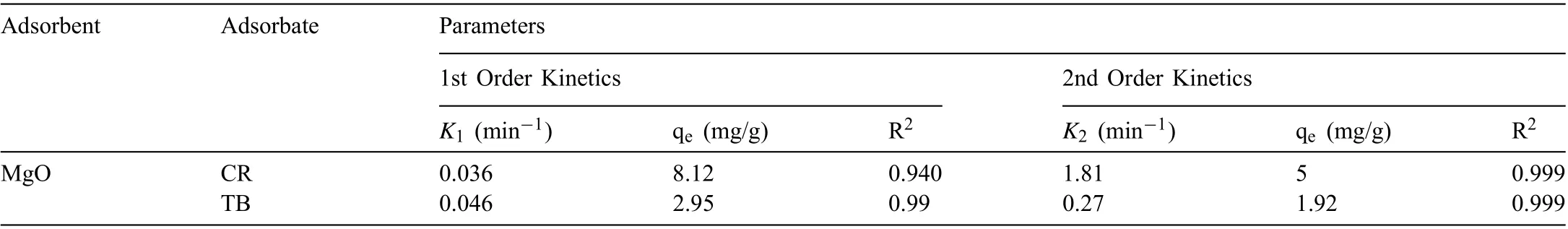
Table 2 Kinetic parameters of TB and CR dye adsorption on MgO NPs.
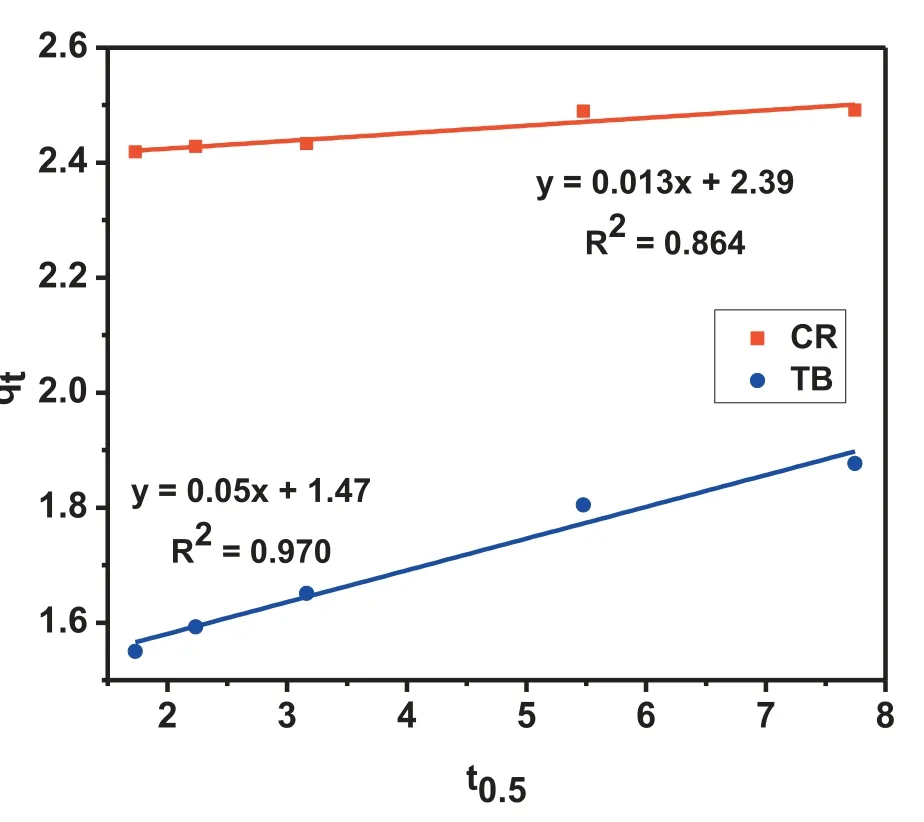
Fig. 8. Intraparticles diffusion plot of CR and TB.
3.5. Adsorption isotherm
Isotherm is the study of correlation between the concentration of the adsorbate in the liquid phase as well as in the solid phase at equilibrium condition.The graphs obtained from Freundlich, Langmuir and Temkin models are given in Figs. 9 and 10, respectively. Adsorption isotherm experiments were carried through some batch tests by taking 20mg of adsorbent and 25ppm dye concentration at the respective suitable pH and rpm values obtained for CR and TB dyes. The Langmuir isotherm is based on the theory of surface homogeneity of the adsorbent, where all adsorption sites are similar and possess equivalent energy. The adsorption process between the MgO nanoparticle and the dye molecule should possess identical adsorption activation energy and demonstrate the monolayer formation of toxic organic dye molecule on the surface of that metal oxide nanoparticles. The linear form of Langmuir equation is:

Where the concentration of solution at equilibrium condition is Ce, qeand qmare the quantity of dye adsorbed at the state of equilibrium and the maximum capacity of adsorption of dyes on the solid sample in order to formation of monolayer coverage on the solid surface, the Langmuir constant is represented as b and is related to the free energy of adsorption. The qmvalue and constant b are calculated from the slope and the intercept of the linear plot of Ceversus Ce/qe.The Freundlich isotherm was used to explain the heterogeneous surface of the material which was not restricted to monolayer formation.The linear form of the Freundlich equation is:

Where kFis a Freundlich constant, which corresponds to the adsorption capacity, 1/n is a constant which symbolizes the adsorption intensity of the process and Ce(mg L-1) is the concentration of the dye at the state of equilibrium. The values of kfand 1/n are calculated from the linear plot of log qeversus log Ce. The phenomenon of adsorption of different toxic materials by heterogeneous systems is usually described by Temkin model, where the adsorption heat decreases linearly due to the adsorbate-adsorbent interactions and presence of uniformly distributed adsorption binding energies. The linear form of Temkin relationship can be given as:

Where, k1(RT/b) and k2(L.mg-1) are represented the heat of adsorption and the binding constant at equilibrium condition, respectively, and b is the Temkin constant.
The graph between log qeversus log Ceshows a straight line with an intercept and a slope, which is equal to log Kfand 1/n, respectively. The favorability of adsorbent/adsorbate system is indicated by the magnitude of exponent ‘n’, if 1/n value lies between 1 and 10. The parameters of the isotherm models were calculated and represented in Table 3. The degree of adsorption is related to the Kfvalue. Compounds having greater value of Kf,have higher affinit towards adsorbent than those having low Kfvalue. The co-relation coefficien R2values from Freundlich adsorption model were found to be 0.999 for TB and 0.995 for CR which were more than the R2values of Langmuir adsorption model, which specifie that the Freundlich model was better fitte to adsorption of both the dyes. The 1/n value was calculated to be 1.002 and 1.01 for CR and TB, respectively, suggesting a multilayer adsorption of dyes onto the heterogeneous surface of MgO NPs.The R2values from Temkin model (Fig 10) were found to be 0.994 and 0.998 for CR and TB, respectively, confirmin the heterogeneous adsorption system and exhibiting the linear decrease of heat of adsorption due to adsorbent-adsorbate interaction.

Fig. 9. (a) Langmuir plot of CR (b) Freundlich plot of CR (c) Langmuir plot of TB (d) Freundlich plot of TB.
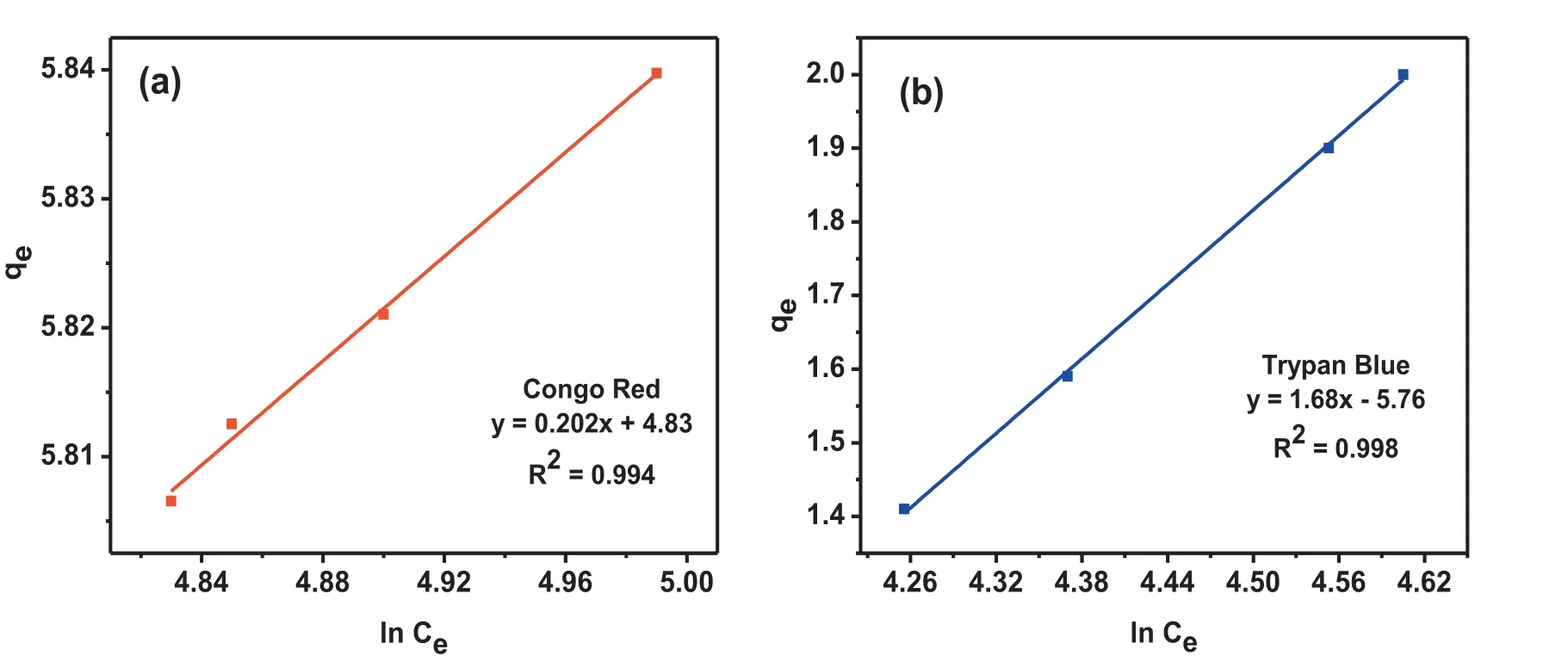
Fig. 10. (a) Temkin plot of CR (b) Temkin plot of CR.
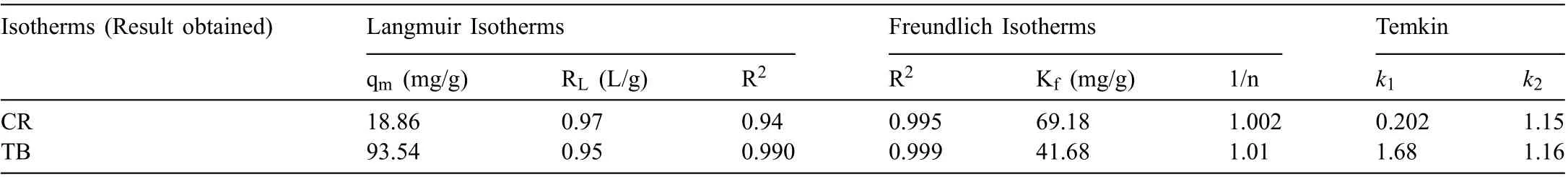
Table 3 Isotherm results for dyes (CR and TB) adsorption on MgO.
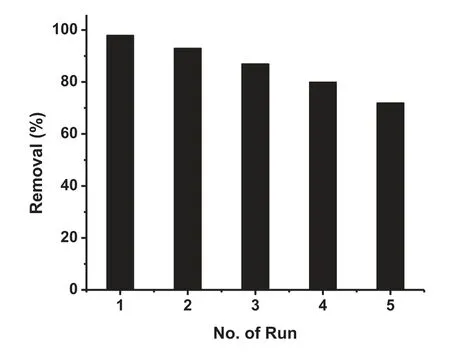
Fig. 11. Recyclability of MgO NPs for the removal of the dye.
4. Reusability of MgO nanoparticles
MgO is nontoxic in nature and high porosity of our sample is the main advantage for the efficien adsorption process. Recyclability is also an essential prerequisite for sustainable and economic applications of an adsorbent. So, the reusability of MgO NPs was further explored by investigating the adsorption capacity in a number of adsorption-desorption cycles.Fig. 11 shows the recyclability of MgO NPs for the removal of the dye. We have recycled the adsorbent for fi e cycles and it was observed that even in the fift cycle, the adsorbent is removing 72% of the dye from the aqueous solution.
5. Conclusion
MgO nanoparticles were successfully synthesized by microwave-assisted combustion route. Powder XRD pattern confirme the cubic phase of MgO nanoparticles. Further the material was characterized by FTIR, TGA and FESEM techniques. The removal potential of CR and TB dyes by MgO NPs was compared. The adsorption was found to be most efficien for 0.2mg/L adsorbent dose at an acidic pH 3-4, for 25mg/L concentration of dye solution.At the optimum condition, the efficien y of removal of both the dyes was found to be more than 98%. The mechanism of adsorption of CR and TB dye onto MgO nanoparticles was confirme by the kinetics, isotherm and thermodynamics studies. The negative values of ΔH0(-0.028 kJmol-1for CR and -0.158 kJmol-1for TB) corresponded to the exothermic process and also the positive values of ΔS0(0.051 and 0.058 Jmol-1K-1for CR and TB, respectively) revealed an increased randomness at the interface of the dye and adsorbent during the process of adsorption. The experimental equilibrium data revealed that the Freundlich isotherm (R2=0.999 and 0.995 for TB and CR,respectively) was best fitte with reflectin the heterogeneous surface of the nanoparticles. The kinetics data analysis confirme that the adsorption of Congo Red and Trypan Blue dye is of pseudo 2nd-order and chemisorption type. In this study,the adsorption potential of MgO nanoparticles is promising at a maximum loading capacity of 136mg/g and 132mg/g for CR and TB dye, respectively, and therefore, it can have applications as eco-friendly adsorbent for removal of hazardous dyes from contaminated waste water.
Declaration of Competing Interest
The authors declare that there is no conflic of interest.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- Investigating TiO2-HA-PCL hybrid coating as an efficien corrosion resistant barrier of ZM21 Mg alloy✩
- Effect of yttrium modificatio on the corrosion behavior of AZ63 magnesium alloy in sodium chloride solution
- Effect of lithium content on the mechanical and corrosion behaviors of HCP binary Mg-Li alloys
- Poly caprolactone/titanium dioxide nanofibe coating on AM50 alloy for biomedical application
- New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys
